 Found 3112 hits with Last Name = 'gill' and Initial = 'm'
Found 3112 hits with Last Name = 'gill' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50565986

(CHEMBL4789639)Show SMILES [2H]C([2H])([2H])NC(=O)c1cnc(NC(=O)C2CC2)cc1Nc1cccc(-c2ncc(F)cn2)c1OC | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TYK2 JH2 domain (unknown origin) by HTRF assay based Morrison titration analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01698
BindingDB Entry DOI: 10.7270/Q2XW4PJX |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM16318
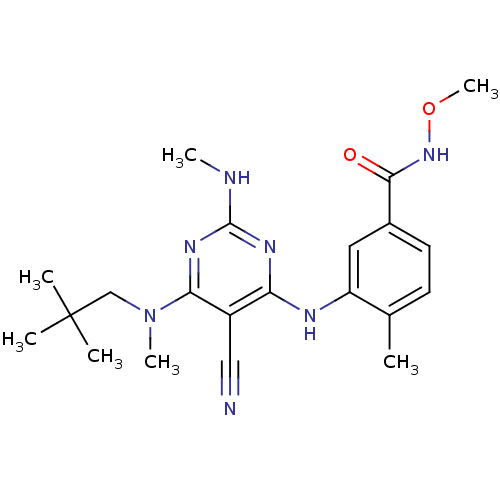
(3-({5-cyano-6-[(2,2-dimethylpropyl)(methyl)amino]-...)Show SMILES CNc1nc(Nc2cc(ccc2C)C(=O)NOC)c(C#N)c(n1)N(C)CC(C)(C)C Show InChI InChI=1S/C21H29N7O2/c1-13-8-9-14(19(29)27-30-7)10-16(13)24-17-15(11-22)18(26-20(23-5)25-17)28(6)12-21(2,3)4/h8-10H,12H2,1-7H3,(H,27,29)(H2,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0470 | -58.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company
| Assay Description
The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... |
J Med Chem 48: 6261-70 (2005)
Article DOI: 10.1021/jm0503594
BindingDB Entry DOI: 10.7270/Q25X276T |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM16319
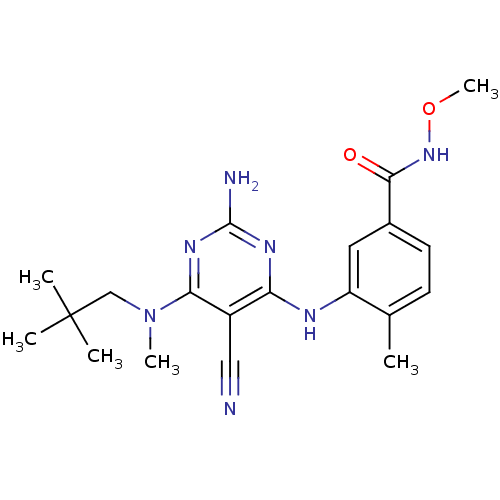
(3-({2-amino-5-cyano-6-[(2,2-dimethylpropyl)(methyl...)Show SMILES CONC(=O)c1ccc(C)c(Nc2nc(N)nc(N(C)CC(C)(C)C)c2C#N)c1 Show InChI InChI=1S/C20H27N7O2/c1-12-7-8-13(18(28)26-29-6)9-15(12)23-16-14(10-21)17(25-19(22)24-16)27(5)11-20(2,3)4/h7-9H,11H2,1-6H3,(H,26,28)(H3,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | -58.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company
| Assay Description
The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... |
J Med Chem 48: 6261-70 (2005)
Article DOI: 10.1021/jm0503594
BindingDB Entry DOI: 10.7270/Q25X276T |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM16317
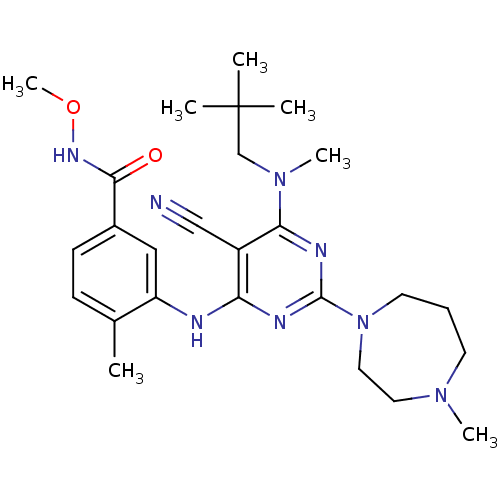
(3-({5-cyano-6-[(2,2-dimethylpropyl)(methyl)amino]-...)Show SMILES CONC(=O)c1ccc(C)c(Nc2nc(nc(N(C)CC(C)(C)C)c2C#N)N2CCCN(C)CC2)c1 Show InChI InChI=1S/C26H38N8O2/c1-18-9-10-19(24(35)31-36-7)15-21(18)28-22-20(16-27)23(33(6)17-26(2,3)4)30-25(29-22)34-12-8-11-32(5)13-14-34/h9-10,15H,8,11-14,17H2,1-7H3,(H,31,35)(H,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0570 | -57.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company
| Assay Description
The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... |
J Med Chem 48: 6261-70 (2005)
Article DOI: 10.1021/jm0503594
BindingDB Entry DOI: 10.7270/Q25X276T |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM16329
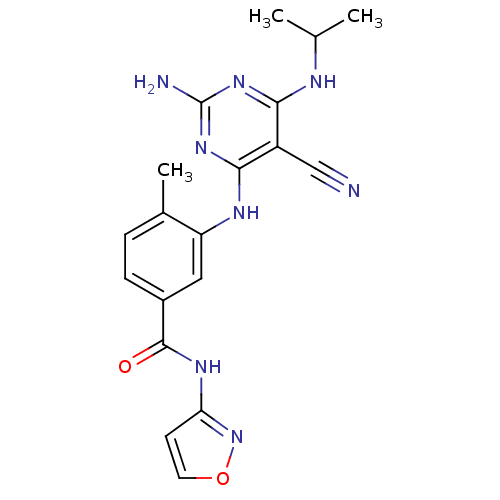
(3-({2-amino-5-cyano-6-[(1-methylethyl)amino]pyrimi...)Show SMILES CC(C)Nc1nc(N)nc(Nc2cc(ccc2C)C(=O)Nc2ccon2)c1C#N Show InChI InChI=1S/C19H20N8O2/c1-10(2)22-16-13(9-20)17(26-19(21)25-16)23-14-8-12(5-4-11(14)3)18(28)24-15-6-7-29-27-15/h4-8,10H,1-3H3,(H,24,27,28)(H4,21,22,23,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0570 | -57.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company
| Assay Description
The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... |
J Med Chem 48: 6261-70 (2005)
Article DOI: 10.1021/jm0503594
BindingDB Entry DOI: 10.7270/Q25X276T |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50017721

(1-Methyl-4-(5H-dibenzo(a,d)cycloheptenylidene)pipe...)Show SMILES [#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1/c2ccccc2-[#6]=[#6]-c2ccccc-12 |c:16| Show InChI InChI=1S/C21H21N/c1-22-14-12-18(13-15-22)21-19-8-4-2-6-16(19)10-11-17-7-3-5-9-20(17)21/h2-11H,12-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Libre de Bruxelles
Curated by PDSP Ki Database
| |
Eur J Biochem 224: 489-95 (1994)
Article DOI: 10.1111/j.1432-1033.1994.00489.x
BindingDB Entry DOI: 10.7270/Q2H70DB4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50320375
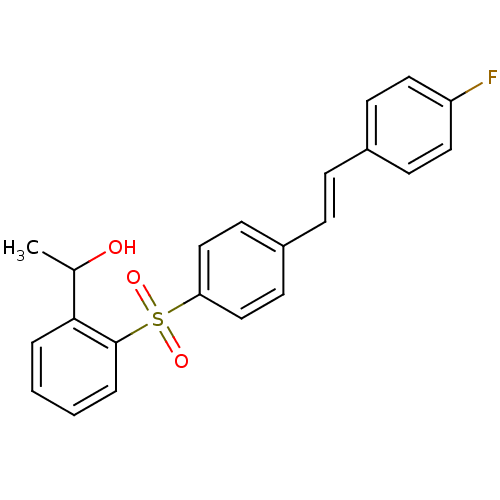
((E)-1-(2-(4-(4-fluorostyryl)phenylsulfonyl)phenyl)...)Show SMILES CC(O)c1ccccc1S(=O)(=O)c1ccc(\C=C\c2ccc(F)cc2)cc1 Show InChI InChI=1S/C22H19FO3S/c1-16(24)21-4-2-3-5-22(21)27(25,26)20-14-10-18(11-15-20)7-6-17-8-12-19(23)13-9-17/h2-16,24H,1H3/b7-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme
Curated by ChEMBL
| Assay Description
Displacement of [3H]- ketanserin from human 5HT2A receptor expressed CHO cells |
Bioorg Med Chem Lett 20: 3708-12 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.090
BindingDB Entry DOI: 10.7270/Q2RX9C89 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(Homo sapiens (Human)) | BDBM9019

(CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...)Show InChI InChI=1S/C13H16N2O2/c1-9(16)14-6-5-10-8-15-13-4-3-11(17-2)7-12(10)13/h3-4,7-8,15H,5-6H2,1-2H3,(H,14,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of the 2-[125I]- iodomelatonin binding to Melatonin receptor type 1A expressed in CHO cells |
J Med Chem 40: 4195-8 (1998)
Article DOI: 10.1021/jm970437q
BindingDB Entry DOI: 10.7270/Q2930S90 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(Homo sapiens (Human)) | BDBM29611

(2-Iodomelatonin | CHEMBL289233 | Melatonin,2-Iodo)Show InChI InChI=1S/C13H15IN2O2/c1-8(17)15-6-5-10-11-7-9(18-2)3-4-12(11)16-13(10)14/h3-4,7,16H,5-6H2,1-2H3,(H,15,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of 2-[125I]-Iodomelatonin from human Melatonin receptor type 1A expressed in CHO cells |
Bioorg Med Chem Lett 7: 2409-2414 (1997)
Article DOI: 10.1016/S0960-894X(97)00444-7
BindingDB Entry DOI: 10.7270/Q25T3KFF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Melatonin receptor type 1A
(Homo sapiens (Human)) | BDBM50035202
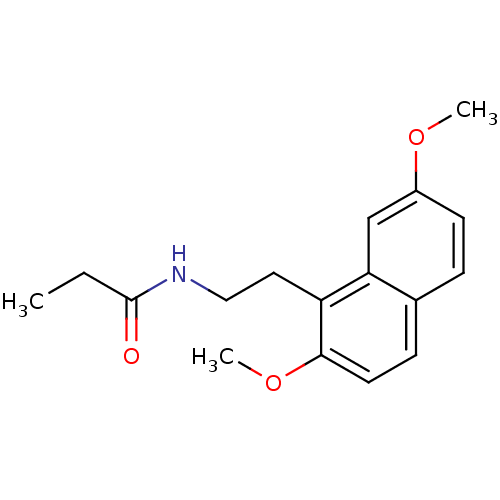
(CHEMBL291857 | N-[2-(2,7-Dimethoxy-naphthalen-1-yl...)Show InChI InChI=1S/C17H21NO3/c1-4-17(19)18-10-9-14-15-11-13(20-2)7-5-12(15)6-8-16(14)21-3/h5-8,11H,4,9-10H2,1-3H3,(H,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of 2-[125I]-Iodomelatonin from human Melatonin receptor type 1A expressed in CHO cells |
Bioorg Med Chem Lett 7: 2409-2414 (1997)
Article DOI: 10.1016/S0960-894X(97)00444-7
BindingDB Entry DOI: 10.7270/Q25T3KFF |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(Homo sapiens (Human)) | BDBM50035202

(CHEMBL291857 | N-[2-(2,7-Dimethoxy-naphthalen-1-yl...)Show InChI InChI=1S/C17H21NO3/c1-4-17(19)18-10-9-14-15-11-13(20-2)7-5-12(15)6-8-16(14)21-3/h5-8,11H,4,9-10H2,1-3H3,(H,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of the 2-[125I]- iodomelatonin binding to Melatonin receptor type 1A expressed in CHO cells |
J Med Chem 40: 4195-8 (1998)
Article DOI: 10.1021/jm970437q
BindingDB Entry DOI: 10.7270/Q2930S90 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50017721

(1-Methyl-4-(5H-dibenzo(a,d)cycloheptenylidene)pipe...)Show SMILES [#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1/c2ccccc2-[#6]=[#6]-c2ccccc-12 |c:16| Show InChI InChI=1S/C21H21N/c1-22-14-12-18(13-15-22)21-19-8-4-2-6-16(19)10-11-17-7-3-5-9-20(17)21/h2-11H,12-15H2,1H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Libre de Bruxelles
Curated by PDSP Ki Database
| |
Eur J Biochem 224: 489-95 (1994)
Article DOI: 10.1111/j.1432-1033.1994.00489.x
BindingDB Entry DOI: 10.7270/Q2H70DB4 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM9019

(CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...)Show InChI InChI=1S/C13H16N2O2/c1-9(16)14-6-5-10-8-15-13-4-3-11(17-2)7-12(10)13/h3-4,7-8,15H,5-6H2,1-2H3,(H,14,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of the 2-[125I]- iodomelatonin binding to Melatonin receptor type 1B expressed in CHO cells |
J Med Chem 40: 4195-8 (1998)
Article DOI: 10.1021/jm970437q
BindingDB Entry DOI: 10.7270/Q2930S90 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(Homo sapiens (Human)) | BDBM9019

(CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...)Show InChI InChI=1S/C13H16N2O2/c1-9(16)14-6-5-10-8-15-13-4-3-11(17-2)7-12(10)13/h3-4,7-8,15H,5-6H2,1-2H3,(H,14,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against human Melatonin receptor type 1A by displacement of [125I]-iodomelatonin stably expressed in CHO cells |
Bioorg Med Chem Lett 7: 2177-2180 (1997)
Article DOI: 10.1016/S0960-894X(97)00392-2
BindingDB Entry DOI: 10.7270/Q208659R |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM16320
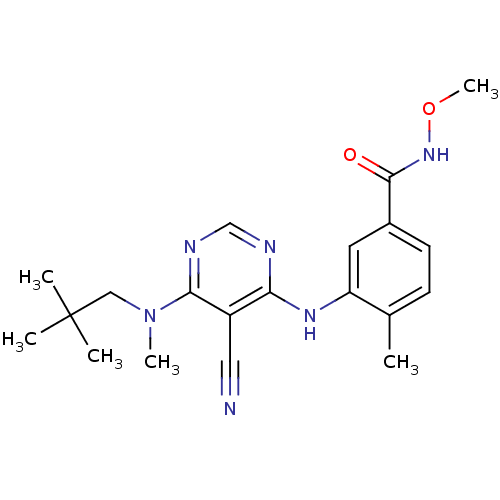
(3-({5-cyano-6-[(2,2-dimethylpropyl)(methyl)amino]p...)Show SMILES CONC(=O)c1ccc(C)c(Nc2ncnc(N(C)CC(C)(C)C)c2C#N)c1 Show InChI InChI=1S/C20H26N6O2/c1-13-7-8-14(19(27)25-28-6)9-16(13)24-17-15(10-21)18(23-12-22-17)26(5)11-20(2,3)4/h7-9,12H,11H2,1-6H3,(H,25,27)(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.150 | -55.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company
| Assay Description
The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... |
J Med Chem 48: 6261-70 (2005)
Article DOI: 10.1021/jm0503594
BindingDB Entry DOI: 10.7270/Q25X276T |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM29611

(2-Iodomelatonin | CHEMBL289233 | Melatonin,2-Iodo)Show InChI InChI=1S/C13H15IN2O2/c1-8(17)15-6-5-10-11-7-9(18-2)3-4-12(11)16-13(10)14/h3-4,7,16H,5-6H2,1-2H3,(H,15,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for binding affinity against human Melatonin receptor type 1B in CHO cells |
Bioorg Med Chem Lett 7: 2409-2414 (1997)
Article DOI: 10.1016/S0960-894X(97)00444-7
BindingDB Entry DOI: 10.7270/Q25T3KFF |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM16330
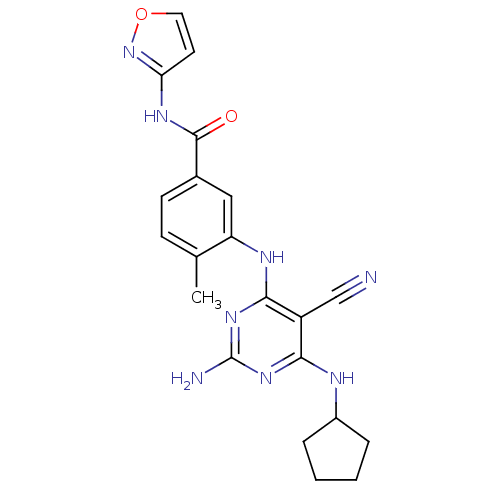
(3-{[2-amino-5-cyano-6-(cyclopentylamino)pyrimidin-...)Show SMILES Cc1ccc(cc1Nc1nc(N)nc(NC2CCCC2)c1C#N)C(=O)Nc1ccon1 Show InChI InChI=1S/C21H22N8O2/c1-12-6-7-13(20(30)26-17-8-9-31-29-17)10-16(12)25-19-15(11-22)18(27-21(23)28-19)24-14-4-2-3-5-14/h6-10,14H,2-5H2,1H3,(H,26,29,30)(H4,23,24,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | -55.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company
| Assay Description
The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... |
J Med Chem 48: 6261-70 (2005)
Article DOI: 10.1021/jm0503594
BindingDB Entry DOI: 10.7270/Q25X276T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50108690
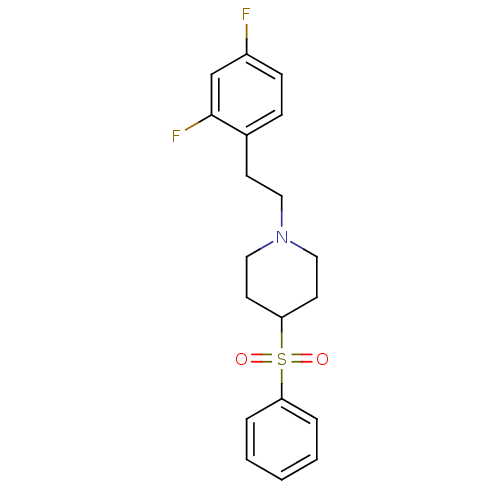
(1-(2,4-difluorophenethyl)-4-(phenylsulfonyl)piperi...)Show SMILES Fc1ccc(CCN2CCC(CC2)S(=O)(=O)c2ccccc2)c(F)c1 Show InChI InChI=1S/C19H21F2NO2S/c20-16-7-6-15(19(21)14-16)8-11-22-12-9-18(10-13-22)25(23,24)17-4-2-1-3-5-17/h1-7,14,18H,8-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Binding affinity against rat 5-hydroxytryptamine 2A receptor |
Bioorg Med Chem Lett 15: 3665-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.104
BindingDB Entry DOI: 10.7270/Q2HM580P |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM50179929

(2'-Fluoro-5'-[8-fluoro-7-(1-hydroxy-1-methyl-ethyl...)Show SMILES CC(C)(O)c1ccn2c(cnc2c1F)-c1ccc(F)c(c1)-c1ccccc1C#N Show InChI InChI=1S/C23H17F2N3O/c1-23(2,29)18-9-10-28-20(13-27-22(28)21(18)25)14-7-8-19(24)17(11-14)16-6-4-3-5-15(16)12-26/h3-11,13,29H,1-2H3 | UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Displacement of [3H]Ro 15-1788 from human GABA-Aalpha1 receptor plus beta3gamma2 expressed in mouse L(tk-) cells |
Bioorg Med Chem Lett 16: 1518-22 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.037
BindingDB Entry DOI: 10.7270/Q2HM582K |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(Homo sapiens (Human)) | BDBM9019

(CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...)Show InChI InChI=1S/C13H16N2O2/c1-9(16)14-6-5-10-8-15-13-4-3-11(17-2)7-12(10)13/h3-4,7-8,15H,5-6H2,1-2H3,(H,14,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of 2-[125I]-Iodomelatonin from human Melatonin receptor type 1A expressed in CHO cells |
Bioorg Med Chem Lett 7: 2409-2414 (1997)
Article DOI: 10.1016/S0960-894X(97)00444-7
BindingDB Entry DOI: 10.7270/Q25T3KFF |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50320373

((E)-3-(4-(2,4-difluorostyryl)phenylsulfonyl)benzam...)Show SMILES NC(=O)c1cccc(c1)S(=O)(=O)c1ccc(\C=C\c2ccc(F)cc2F)cc1 Show InChI InChI=1S/C21H15F2NO3S/c22-17-9-8-15(20(23)13-17)7-4-14-5-10-18(11-6-14)28(26,27)19-3-1-2-16(12-19)21(24)25/h1-13H,(H2,24,25)/b7-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme
Curated by ChEMBL
| Assay Description
Displacement of [3H]- ketanserin from human 5HT2A receptor expressed CHO cells |
Bioorg Med Chem Lett 20: 3708-12 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.090
BindingDB Entry DOI: 10.7270/Q2RX9C89 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50320376
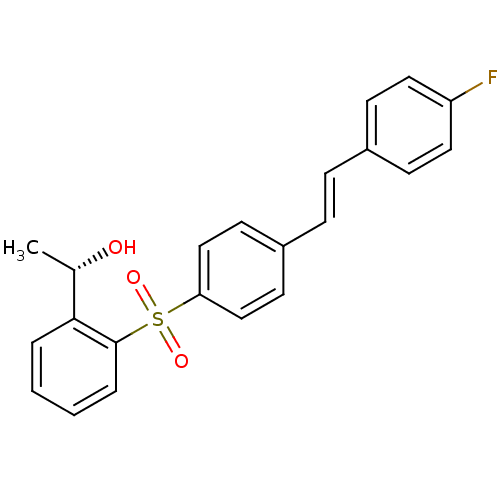
((S,E)-1-(2-(4-(4-fluorostyryl)phenylsulfonyl)pheny...)Show SMILES C[C@H](O)c1ccccc1S(=O)(=O)c1ccc(\C=C\c2ccc(F)cc2)cc1 |r| Show InChI InChI=1S/C22H19FO3S/c1-16(24)21-4-2-3-5-22(21)27(25,26)20-14-10-18(11-15-20)7-6-17-8-12-19(23)13-9-17/h2-16,24H,1H3/b7-6+/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme
Curated by ChEMBL
| Assay Description
Displacement of [3H]- ketanserin from human 5HT2A receptor expressed CHO cells |
Bioorg Med Chem Lett 20: 3708-12 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.090
BindingDB Entry DOI: 10.7270/Q2RX9C89 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM9019

(CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...)Show InChI InChI=1S/C13H16N2O2/c1-9(16)14-6-5-10-8-15-13-4-3-11(17-2)7-12(10)13/h3-4,7-8,15H,5-6H2,1-2H3,(H,14,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against human Melatonin receptor type 1B by displacement of [125I]-iodomelatonin stably expressed in CHO cells |
Bioorg Med Chem Lett 7: 2177-2180 (1997)
Article DOI: 10.1016/S0960-894X(97)00392-2
BindingDB Entry DOI: 10.7270/Q208659R |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM9019

(CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...)Show InChI InChI=1S/C13H16N2O2/c1-9(16)14-6-5-10-8-15-13-4-3-11(17-2)7-12(10)13/h3-4,7-8,15H,5-6H2,1-2H3,(H,14,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of 2-[125I]-Iodomelatonin binding to human Melatonin receptor type 1B expressed in CHO cells |
Bioorg Med Chem Lett 7: 2409-2414 (1997)
Article DOI: 10.1016/S0960-894X(97)00444-7
BindingDB Entry DOI: 10.7270/Q25T3KFF |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50169847
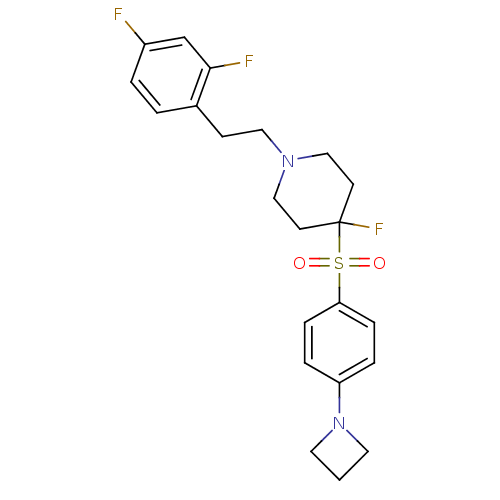
(4-(4-Azetidin-1-yl-benzenesulfonyl)-1-[2-(2,4-difl...)Show SMILES Fc1ccc(CCN2CCC(F)(CC2)S(=O)(=O)c2ccc(cc2)N2CCC2)c(F)c1 Show InChI InChI=1S/C22H25F3N2O2S/c23-18-3-2-17(21(24)16-18)8-13-26-14-9-22(25,10-15-26)30(28,29)20-6-4-19(5-7-20)27-11-1-12-27/h2-7,16H,1,8-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Binding affinity against rat 5-hydroxytryptamine 2A receptor |
Bioorg Med Chem Lett 15: 3665-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.104
BindingDB Entry DOI: 10.7270/Q2HM580P |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50169848
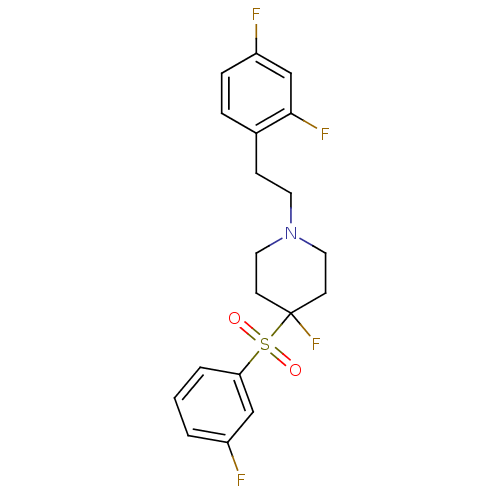
(1-[2-(2,4-Difluoro-phenyl)-ethyl]-4-fluoro-4-(3-fl...)Show SMILES Fc1cccc(c1)S(=O)(=O)C1(F)CCN(CCc2ccc(F)cc2F)CC1 Show InChI InChI=1S/C19H19F4NO2S/c20-15-2-1-3-17(12-15)27(25,26)19(23)7-10-24(11-8-19)9-6-14-4-5-16(21)13-18(14)22/h1-5,12-13H,6-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Binding affinity against human 5-hydroxytryptamine 2A receptor |
Bioorg Med Chem Lett 15: 3665-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.104
BindingDB Entry DOI: 10.7270/Q2HM580P |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity of compound was determined against to human cannabinoid receptor 2 in chinese hamster ovary cells |
J Med Chem 46: 2110-6 (2003)
Article DOI: 10.1021/jm020329q
BindingDB Entry DOI: 10.7270/Q2J67HPG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50128931
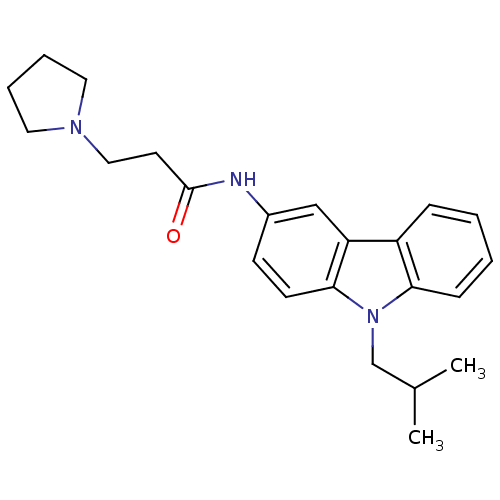
(CHEMBL61880 | N-(9-Isobutyl-9H-carbazol-3-yl)-3-py...)Show InChI InChI=1S/C23H29N3O/c1-17(2)16-26-21-8-4-3-7-19(21)20-15-18(9-10-22(20)26)24-23(27)11-14-25-12-5-6-13-25/h3-4,7-10,15,17H,5-6,11-14,16H2,1-2H3,(H,24,27) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity towards human neuropeptide Y receptor type 5 using 125[I]-PYY as radioligand |
Bioorg Med Chem Lett 13: 1989-92 (2003)
BindingDB Entry DOI: 10.7270/Q2057F9D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50095027
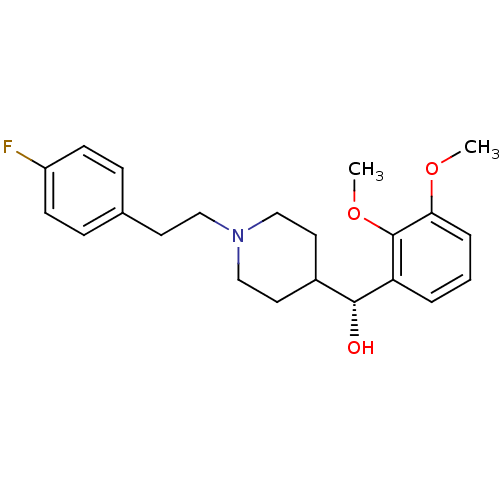
((2,3-Dimethoxy-phenyl)-{1-[2-(4-fluoro-phenyl)-eth...)Show SMILES COc1cccc([C@H](O)C2CCN(CCc3ccc(F)cc3)CC2)c1OC |r| Show InChI InChI=1S/C22H28FNO3/c1-26-20-5-3-4-19(22(20)27-2)21(25)17-11-14-24(15-12-17)13-10-16-6-8-18(23)9-7-16/h3-9,17,21,25H,10-15H2,1-2H3/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme
Curated by ChEMBL
| Assay Description
Displacement of [3H]- ketanserin from human 5HT2A receptor expressed CHO cells |
Bioorg Med Chem Lett 20: 3708-12 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.090
BindingDB Entry DOI: 10.7270/Q2RX9C89 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50292411
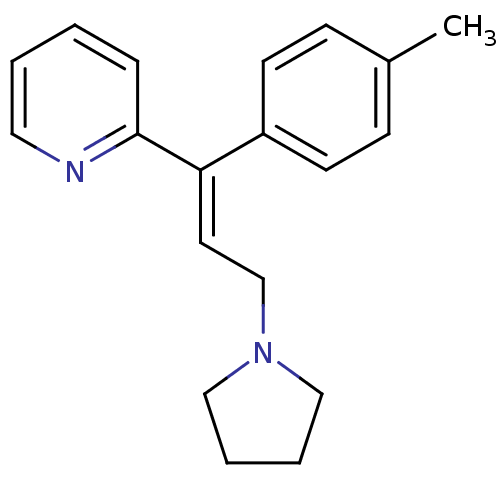
((E)-2-(3-(pyrrolidin-1-yl)-1-p-tolylprop-1-enyl)py...)Show InChI InChI=1S/C19H22N2/c1-16-7-9-17(10-8-16)18(19-6-2-3-12-20-19)11-15-21-13-4-5-14-21/h2-3,6-12H,4-5,13-15H2,1H3/b18-11+ | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Libre de Bruxelles
Curated by PDSP Ki Database
| |
Eur J Biochem 224: 489-95 (1994)
Article DOI: 10.1111/j.1432-1033.1994.00489.x
BindingDB Entry DOI: 10.7270/Q2H70DB4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50169840
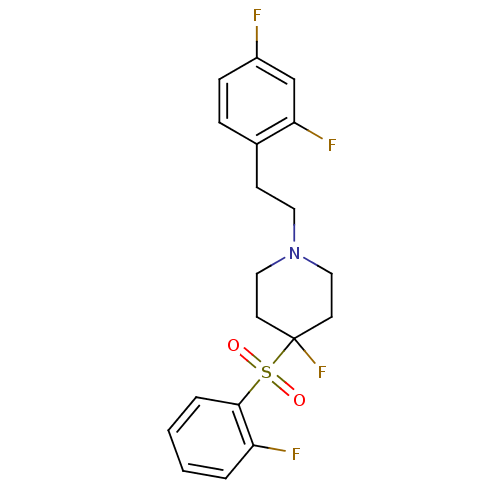
(1-[2-(2,4-Difluoro-phenyl)-ethyl]-4-fluoro-4-(2-fl...)Show SMILES Fc1ccc(CCN2CCC(F)(CC2)S(=O)(=O)c2ccccc2F)c(F)c1 Show InChI InChI=1S/C19H19F4NO2S/c20-15-6-5-14(17(22)13-15)7-10-24-11-8-19(23,9-12-24)27(25,26)18-4-2-1-3-16(18)21/h1-6,13H,7-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Binding affinity against human 5-hydroxytryptamine 2A receptor |
Bioorg Med Chem Lett 15: 3665-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.104
BindingDB Entry DOI: 10.7270/Q2HM580P |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-3/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM50179929

(2'-Fluoro-5'-[8-fluoro-7-(1-hydroxy-1-methyl-ethyl...)Show SMILES CC(C)(O)c1ccn2c(cnc2c1F)-c1ccc(F)c(c1)-c1ccccc1C#N Show InChI InChI=1S/C23H17F2N3O/c1-23(2,29)18-9-10-28-20(13-27-22(28)21(18)25)14-7-8-19(24)17(11-14)16-6-4-3-5-15(16)12-26/h3-11,13,29H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Displacement of [3H]Ro 15-1788 from human GABA-Aalpha3 receptor plus beta3gamma2 expressed in mouse L(tk-) cells |
Bioorg Med Chem Lett 16: 1518-22 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.037
BindingDB Entry DOI: 10.7270/Q2HM582K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50108690

(1-(2,4-difluorophenethyl)-4-(phenylsulfonyl)piperi...)Show SMILES Fc1ccc(CCN2CCC(CC2)S(=O)(=O)c2ccccc2)c(F)c1 Show InChI InChI=1S/C19H21F2NO2S/c20-16-7-6-15(19(21)14-16)8-11-22-12-9-18(10-13-22)25(23,24)17-4-2-1-3-5-17/h1-7,14,18H,8-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Binding affinity against human 5-hydroxytryptamine 2A receptor |
Bioorg Med Chem Lett 15: 3665-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.104
BindingDB Entry DOI: 10.7270/Q2HM580P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22541

(Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...)Show SMILES NC(SCCCc1cnc[nH]1)=NCc1ccc(Cl)cc1 |w:11.12| Show InChI InChI=1S/C14H17ClN4S/c15-12-5-3-11(4-6-12)8-18-14(16)20-7-1-2-13-9-17-10-19-13/h3-6,9-10H,1-2,7-8H2,(H2,16,18)(H,17,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.339 | -54.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
VU University Amsterdam
| Assay Description
Ligand displacement assays were performed on CHO cells membranes expressing hH3R. Retained radioactivity was determined by liquid scintillation count... |
J Med Chem 51: 2944-53 (2008)
Article DOI: 10.1021/jm7014149
BindingDB Entry DOI: 10.7270/Q24F1P2W |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM50035202

(CHEMBL291857 | N-[2-(2,7-Dimethoxy-naphthalen-1-yl...)Show InChI InChI=1S/C17H21NO3/c1-4-17(19)18-10-9-14-15-11-13(20-2)7-5-12(15)6-8-16(14)21-3/h5-8,11H,4,9-10H2,1-3H3,(H,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of the 2-[125I]- iodomelatonin binding to Melatonin receptor type 1B expressed in CHO cells |
J Med Chem 40: 4195-8 (1998)
Article DOI: 10.1021/jm970437q
BindingDB Entry DOI: 10.7270/Q2930S90 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM50035202

(CHEMBL291857 | N-[2-(2,7-Dimethoxy-naphthalen-1-yl...)Show InChI InChI=1S/C17H21NO3/c1-4-17(19)18-10-9-14-15-11-13(20-2)7-5-12(15)6-8-16(14)21-3/h5-8,11H,4,9-10H2,1-3H3,(H,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of 2-[125I]-Iodomelatonin binding to human Melatonin receptor type 1B expressed in CHO cells |
Bioorg Med Chem Lett 7: 2409-2414 (1997)
Article DOI: 10.1016/S0960-894X(97)00444-7
BindingDB Entry DOI: 10.7270/Q25T3KFF |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(Homo sapiens (Human)) | BDBM50290238
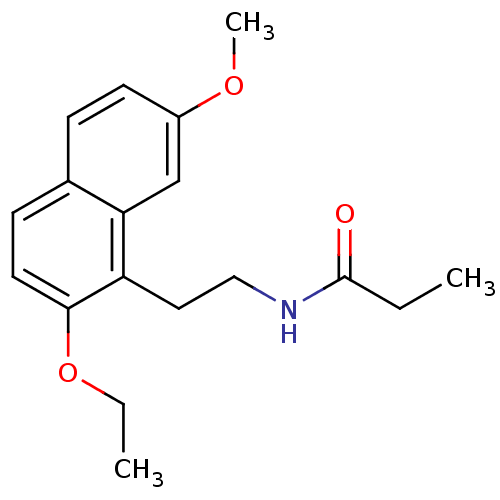
(CHEMBL80736 | N-[2-(2-Ethoxy-7-methoxy-naphthalen-...)Show InChI InChI=1S/C18H23NO3/c1-4-18(20)19-11-10-15-16-12-14(21-3)8-6-13(16)7-9-17(15)22-5-2/h6-9,12H,4-5,10-11H2,1-3H3,(H,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of 2-[125I]-Iodomelatonin from human Melatonin receptor type 1A expressed in CHO cells |
Bioorg Med Chem Lett 7: 2409-2414 (1997)
Article DOI: 10.1016/S0960-894X(97)00444-7
BindingDB Entry DOI: 10.7270/Q25T3KFF |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50169842
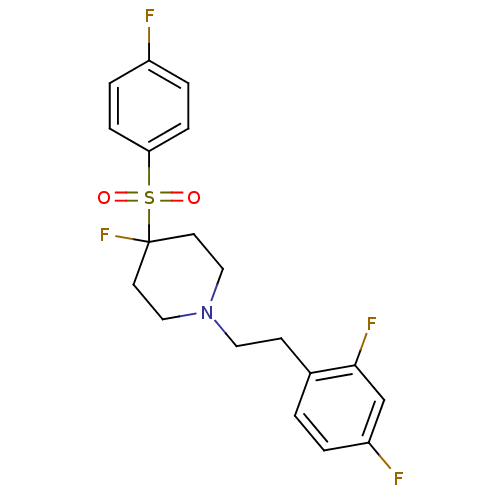
(1-(2,4-difluorophenethyl)-4-fluoro-4-(4-fluorophen...)Show SMILES Fc1ccc(cc1)S(=O)(=O)C1(F)CCN(CCc2ccc(F)cc2F)CC1 Show InChI InChI=1S/C19H19F4NO2S/c20-15-3-5-17(6-4-15)27(25,26)19(23)8-11-24(12-9-19)10-7-14-1-2-16(21)13-18(14)22/h1-6,13H,7-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Binding affinity against human 5-hydroxytryptamine 2A receptor |
Bioorg Med Chem Lett 15: 3665-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.104
BindingDB Entry DOI: 10.7270/Q2HM580P |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50169842

(1-(2,4-difluorophenethyl)-4-fluoro-4-(4-fluorophen...)Show SMILES Fc1ccc(cc1)S(=O)(=O)C1(F)CCN(CCc2ccc(F)cc2F)CC1 Show InChI InChI=1S/C19H19F4NO2S/c20-15-3-5-17(6-4-15)27(25,26)19(23)8-11-24(12-9-19)10-7-14-1-2-16(21)13-18(14)22/h1-6,13H,7-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme
Curated by ChEMBL
| Assay Description
Displacement of [3H]- ketanserin from human 5HT2A receptor expressed CHO cells |
Bioorg Med Chem Lett 20: 3708-12 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.090
BindingDB Entry DOI: 10.7270/Q2RX9C89 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22542

(4-(1H-imidazol-4-ylmethyl)piperidine | 4-(1H-imida...)Show InChI InChI=1S/C9H15N3/c1-3-10-4-2-8(1)5-9-6-11-7-12-9/h6-8,10H,1-5H2,(H,11,12) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.407 | -53.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
VU University Amsterdam
| Assay Description
Ligand displacement assays were performed on CHO cells membranes expressing hH3R. Retained radioactivity was determined by liquid scintillation count... |
J Med Chem 51: 2944-53 (2008)
Article DOI: 10.1021/jm7014149
BindingDB Entry DOI: 10.7270/Q24F1P2W |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM50290000
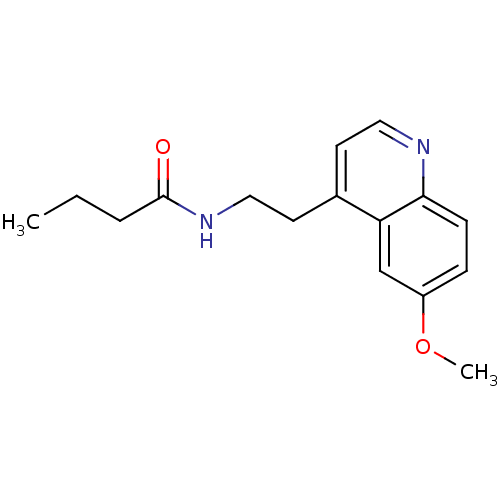
(CHEMBL68864 | N-[2-(6-Methoxy-quinolin-4-yl)-ethyl...)Show InChI InChI=1S/C16H20N2O2/c1-3-4-16(19)18-10-8-12-7-9-17-15-6-5-13(20-2)11-14(12)15/h5-7,9,11H,3-4,8,10H2,1-2H3,(H,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against human Melatonin receptor type 1B by displacement of [125I]-iodomelatonin stably expressed in CHO cells |
Bioorg Med Chem Lett 7: 2177-2180 (1997)
Article DOI: 10.1016/S0960-894X(97)00392-2
BindingDB Entry DOI: 10.7270/Q208659R |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM16325
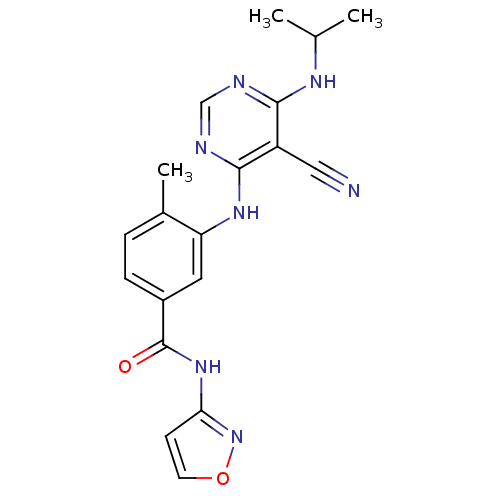
(3-({5-cyano-6-[(1-methylethyl)amino]pyrimidin-4-yl...)Show SMILES CC(C)Nc1ncnc(Nc2cc(ccc2C)C(=O)Nc2ccon2)c1C#N Show InChI InChI=1S/C19H19N7O2/c1-11(2)23-17-14(9-20)18(22-10-21-17)24-15-8-13(5-4-12(15)3)19(27)25-16-6-7-28-26-16/h4-8,10-11H,1-3H3,(H,25,26,27)(H2,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.410 | -53.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company
| Assay Description
The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... |
J Med Chem 48: 6261-70 (2005)
Article DOI: 10.1021/jm0503594
BindingDB Entry DOI: 10.7270/Q25X276T |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50376456
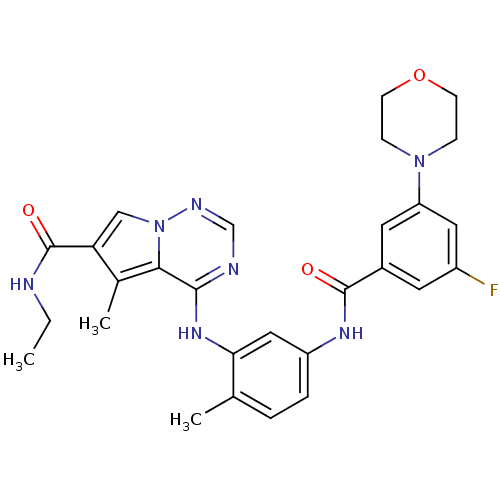
(CHEMBL262592)Show SMILES CCNC(=O)c1cn2ncnc(Nc3cc(NC(=O)c4cc(F)cc(c4)N4CCOCC4)ccc3C)c2c1C Show InChI InChI=1S/C28H30FN7O3/c1-4-30-28(38)23-15-36-25(18(23)3)26(31-16-32-36)34-24-14-21(6-5-17(24)2)33-27(37)19-11-20(29)13-22(12-19)35-7-9-39-10-8-35/h5-6,11-16H,4,7-10H2,1-3H3,(H,30,38)(H,33,37)(H,31,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha |
Bioorg Med Chem Lett 18: 2739-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.067
BindingDB Entry DOI: 10.7270/Q29887X6 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM16324
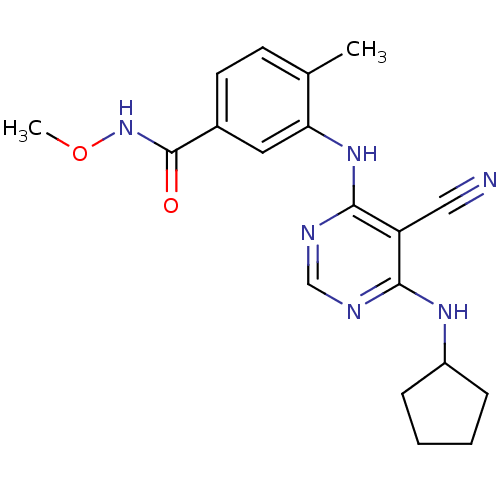
(3-{[5-cyano-6-(cyclopentylamino)pyrimidin-4-yl]ami...)Show SMILES CONC(=O)c1ccc(C)c(Nc2ncnc(NC3CCCC3)c2C#N)c1 Show InChI InChI=1S/C19H22N6O2/c1-12-7-8-13(19(26)25-27-2)9-16(12)24-18-15(10-20)17(21-11-22-18)23-14-5-3-4-6-14/h7-9,11,14H,3-6H2,1-2H3,(H,25,26)(H2,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.420 | -53.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company
| Assay Description
The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... |
J Med Chem 48: 6261-70 (2005)
Article DOI: 10.1021/jm0503594
BindingDB Entry DOI: 10.7270/Q25X276T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50108699
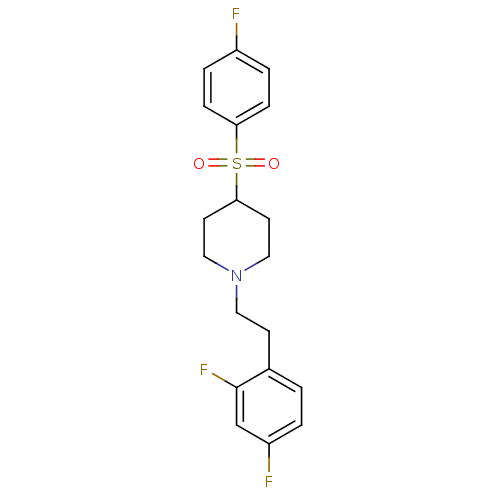
(1-(2,4-difluorophenethyl)-4-(4-fluorophenylsulfony...)Show SMILES Fc1ccc(cc1)S(=O)(=O)C1CCN(CCc2ccc(F)cc2F)CC1 Show InChI InChI=1S/C19H20F3NO2S/c20-15-3-5-17(6-4-15)26(24,25)18-8-11-23(12-9-18)10-7-14-1-2-16(21)13-19(14)22/h1-6,13,18H,7-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Binding affinity against human 5-hydroxytryptamine 2A receptor |
Bioorg Med Chem Lett 15: 3665-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.104
BindingDB Entry DOI: 10.7270/Q2HM580P |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50108699

(1-(2,4-difluorophenethyl)-4-(4-fluorophenylsulfony...)Show SMILES Fc1ccc(cc1)S(=O)(=O)C1CCN(CCc2ccc(F)cc2F)CC1 Show InChI InChI=1S/C19H20F3NO2S/c20-15-3-5-17(6-4-15)26(24,25)18-8-11-23(12-9-18)10-7-14-1-2-16(21)13-19(14)22/h1-6,13,18H,7-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme
Curated by ChEMBL
| Assay Description
Displacement of [3H]- ketanserin from human 5HT2A receptor expressed CHO cells |
Bioorg Med Chem Lett 20: 3708-12 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.090
BindingDB Entry DOI: 10.7270/Q2RX9C89 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM85093
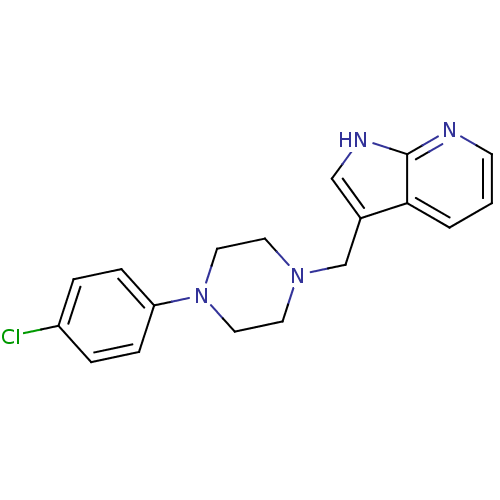
(CAS_3853 | CHEMBL267014 | CHEMBL555670 | L 745,870...)Show InChI InChI=1S/C18H19ClN4/c19-15-3-5-16(6-4-15)23-10-8-22(9-11-23)13-14-12-21-18-17(14)2-1-7-20-18/h1-7,12H,8-11,13H2,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to displace [3H]-spiperone from cloned human dopamine D4 receptor stably expressed in HEK293 cells |
Bioorg Med Chem Lett 9: 585-8 (1999)
BindingDB Entry DOI: 10.7270/Q2NV9HD4 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM85093

(CAS_3853 | CHEMBL267014 | CHEMBL555670 | L 745,870...)Show InChI InChI=1S/C18H19ClN4/c19-15-3-5-16(6-4-15)23-10-8-22(9-11-23)13-14-12-21-18-17(14)2-1-7-20-18/h1-7,12H,8-11,13H2,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards cloned human Dopamine receptor D4 stably expressed in CHO cells was evaluated using [3H]-spiperone as radioligand |
J Med Chem 39: 1941-2 (1996)
Article DOI: 10.1021/jm9600712
BindingDB Entry DOI: 10.7270/Q2MW2G6D |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50376433

(CHEMBL258895)Show SMILES C[C@H](NC(=O)c1cn2ncnc(Nc3cc(NC(=O)c4ccnc(c4)N4CCOCC4)ccc3C)c2c1C)c1ccccc1 Show InChI InChI=1S/C33H34N8O3/c1-21-9-10-26(38-32(42)25-11-12-34-29(17-25)40-13-15-44-16-14-40)18-28(21)39-31-30-22(2)27(19-41(30)36-20-35-31)33(43)37-23(3)24-7-5-4-6-8-24/h4-12,17-20,23H,13-16H2,1-3H3,(H,37,43)(H,38,42)(H,35,36,39)/t23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha |
Bioorg Med Chem Lett 18: 2739-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.067
BindingDB Entry DOI: 10.7270/Q29887X6 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50376434

(CHEMBL408150)Show SMILES C[C@H](NC(=O)c1cn2ncnc(Nc3cc(NC(=O)c4cccc(c4)N4CCOCC4)ccc3C)c2c1C)c1ccccc1 |r| Show InChI InChI=1S/C34H35N7O3/c1-22-12-13-27(38-33(42)26-10-7-11-28(18-26)40-14-16-44-17-15-40)19-30(22)39-32-31-23(2)29(20-41(31)36-21-35-32)34(43)37-24(3)25-8-5-4-6-9-25/h4-13,18-21,24H,14-17H2,1-3H3,(H,37,43)(H,38,42)(H,35,36,39)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha |
Bioorg Med Chem Lett 18: 2739-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.067
BindingDB Entry DOI: 10.7270/Q29887X6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data