

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein (Hepacivirus C) | BDBM50485494 (CHEMBL2063089) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease D168V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485491 (CHEMBL2063088) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease D168V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485492 (Grazoprevir | Grazoprevir monohydrate | MK-5172 | ...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease D168V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485491 (CHEMBL2063088) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156T mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485494 (CHEMBL2063089) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156T mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485492 (Grazoprevir | Grazoprevir monohydrate | MK-5172 | ...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156T mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485494 (CHEMBL2063089) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485491 (CHEMBL2063088) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485490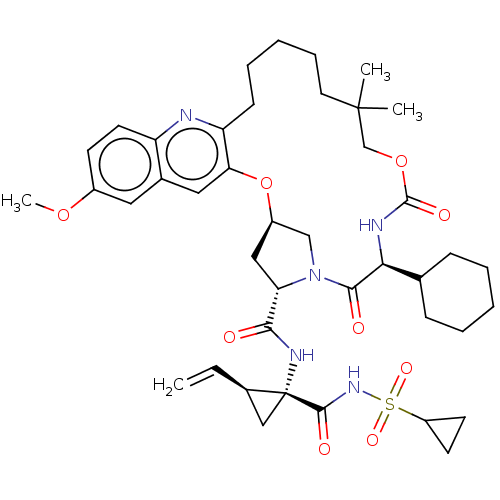 (CHEMBL2063087) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156T mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485492 (Grazoprevir | Grazoprevir monohydrate | MK-5172 | ...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485495 (CHEMBL2063085) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156T mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50326055 ((1R,21S,24S)-21-tert-Butyl-N-((1R,2R)-1-{[(cyclopr...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156T mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485489 (CHEMBL1672609 | MK-1220) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease D168V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485493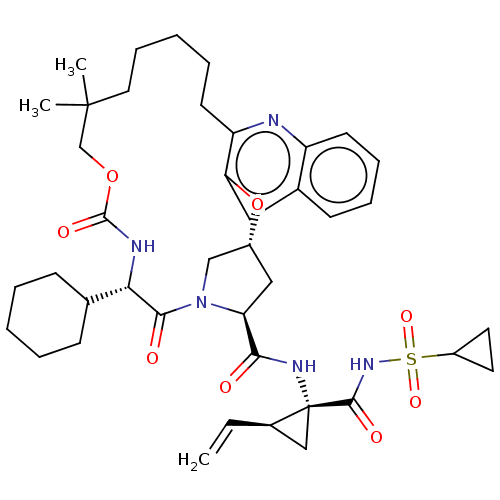 (CHEMBL2063086) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156T mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485490 (CHEMBL2063087) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50326055 ((1R,21S,24S)-21-tert-Butyl-N-((1R,2R)-1-{[(cyclopr...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease D168V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485495 (CHEMBL2063085) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485493 (CHEMBL2063086) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50326055 ((1R,21S,24S)-21-tert-Butyl-N-((1R,2R)-1-{[(cyclopr...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485489 (CHEMBL1672609 | MK-1220) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156T mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485489 (CHEMBL1672609 | MK-1220) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Homo sapiens (Human)) | BDBM50352268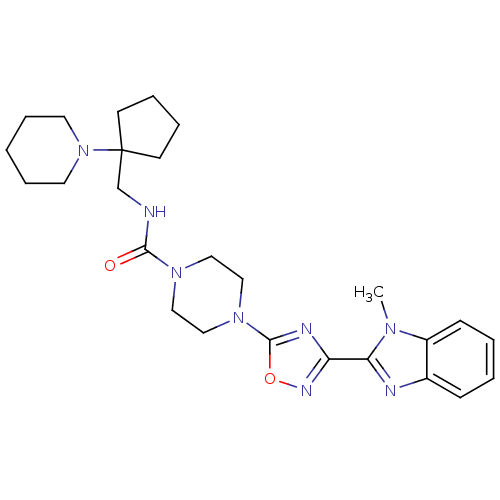 (CHEMBL1822467) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Rome Curated by ChEMBL | Assay Description Displacement of BODIPY-cyclopamine from Smo expressed in COS-1 cells after 4 to 6 hrs by Flow Cytometry analysis in presence of 2% fetal calf serum | Bioorg Med Chem Lett 21: 5283-8 (2011) Article DOI: 10.1016/j.bmcl.2011.07.030 BindingDB Entry DOI: 10.7270/Q24F1R35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Homo sapiens (Human)) | BDBM50352268 (CHEMBL1822467) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Rome Curated by ChEMBL | Assay Description Displacement of BODIPY-cyclopamine from Smo expressed in COS-1 cells after 4 to 6 hrs by Flow Cytometry analysis in presence of 20% normal human seru... | Bioorg Med Chem Lett 21: 5283-8 (2011) Article DOI: 10.1016/j.bmcl.2011.07.030 BindingDB Entry DOI: 10.7270/Q24F1R35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50169942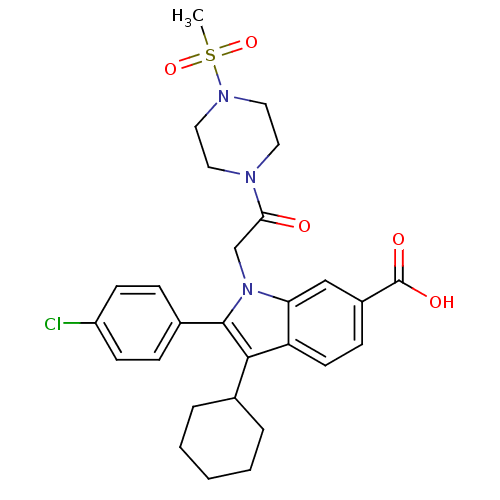 (2-(4-Chloro-phenyl)-3-cyclohexyl-1-[2-(4-methanesu...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against by hepatitis C virus NS5B polymerase | J Med Chem 48: 4547-57 (2005) Article DOI: 10.1021/jm050056+ BindingDB Entry DOI: 10.7270/Q24B30VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50169930 (2-(4-Chloro-phenyl)-3-cyclohexyl-1-[(cyclopropylme...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against by hepatitis C virus NS5B polymerase | J Med Chem 48: 4547-57 (2005) Article DOI: 10.1021/jm050056+ BindingDB Entry DOI: 10.7270/Q24B30VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Homo sapiens (Human)) | BDBM50352269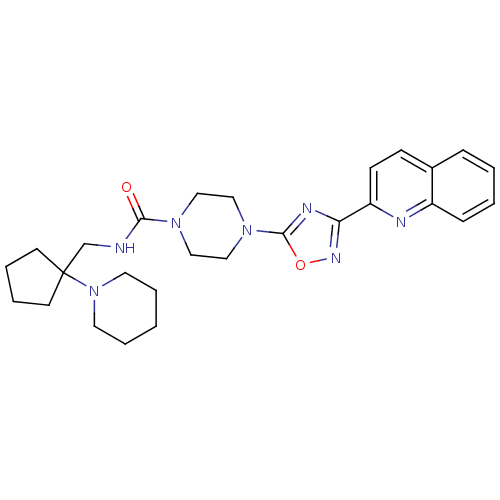 (CHEMBL1822466) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Rome Curated by ChEMBL | Assay Description Displacement of BODIPY-cyclopamine from Smo expressed in COS-1 cells after 4 to 6 hrs by Flow Cytometry analysis in presence of 2% fetal calf serum | Bioorg Med Chem Lett 21: 5283-8 (2011) Article DOI: 10.1016/j.bmcl.2011.07.030 BindingDB Entry DOI: 10.7270/Q24F1R35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50169922 (3-Cyclohexyl-1-[2-(4-diethylamino-piperidin-1-yl)-...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus NS5B polymerase enzyme; Range=6-9 nM | J Med Chem 48: 4547-57 (2005) Article DOI: 10.1021/jm050056+ BindingDB Entry DOI: 10.7270/Q24B30VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50169906 (3-Cyclohexyl-1-[2-(2-diethylaminomethyl-morpholin-...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus NS5B polymerase enzyme; Range=6-9 nM | J Med Chem 48: 4547-57 (2005) Article DOI: 10.1021/jm050056+ BindingDB Entry DOI: 10.7270/Q24B30VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50169904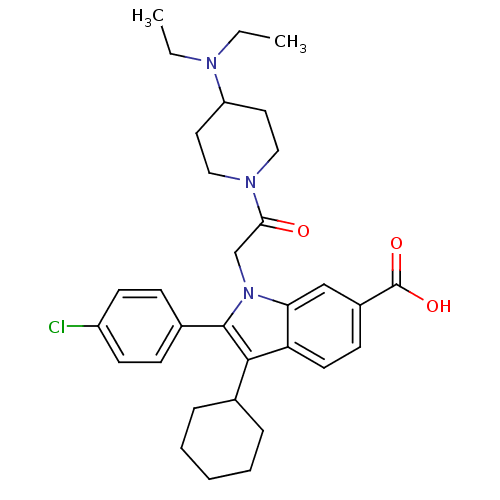 (2-(4-Chloro-phenyl)-3-cyclohexyl-1-[2-(4-diethylam...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus NS5B polymerase enzyme; Range=6-9 nM | J Med Chem 48: 4547-57 (2005) Article DOI: 10.1021/jm050056+ BindingDB Entry DOI: 10.7270/Q24B30VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50169899 (3-Cyclohexyl-1-[2-(4-dimethylamino-piperidin-1-yl)...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus NS5B polymerase enzyme; Range=6-9 nM | J Med Chem 48: 4547-57 (2005) Article DOI: 10.1021/jm050056+ BindingDB Entry DOI: 10.7270/Q24B30VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50169911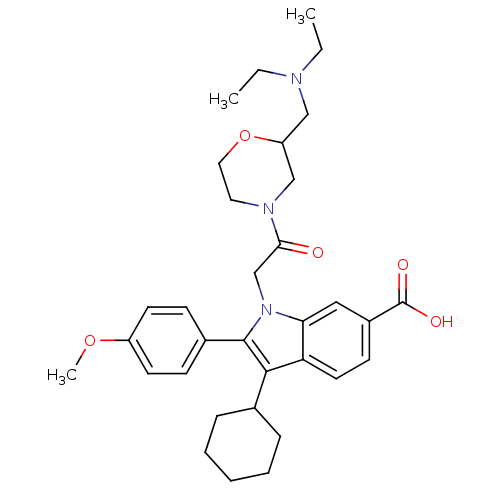 (3-Cyclohexyl-1-[2-(2-diethylaminomethyl-morpholin-...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus NS5B polymerase enzyme; Range=6-9 nM | J Med Chem 48: 4547-57 (2005) Article DOI: 10.1021/jm050056+ BindingDB Entry DOI: 10.7270/Q24B30VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Homo sapiens (Human)) | BDBM50352270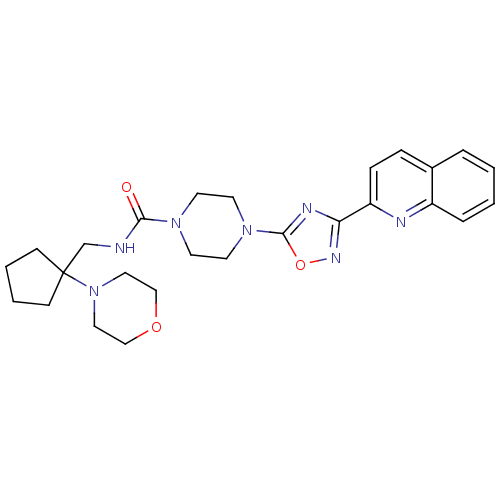 (CHEMBL1822465) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Rome Curated by ChEMBL | Assay Description Displacement of BODIPY-cyclopamine from Smo expressed in COS-1 cells after 4 to 6 hrs by Flow Cytometry analysis in presence of 2% fetal calf serum | Bioorg Med Chem Lett 21: 5283-8 (2011) Article DOI: 10.1016/j.bmcl.2011.07.030 BindingDB Entry DOI: 10.7270/Q24F1R35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Homo sapiens (Human)) | BDBM50352269 (CHEMBL1822466) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Rome Curated by ChEMBL | Assay Description Displacement of BODIPY-cyclopamine from Smo expressed in COS-1 cells after 4 to 6 hrs by Flow Cytometry analysis in presence of 20% normal human seru... | Bioorg Med Chem Lett 21: 5283-8 (2011) Article DOI: 10.1016/j.bmcl.2011.07.030 BindingDB Entry DOI: 10.7270/Q24F1R35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50169903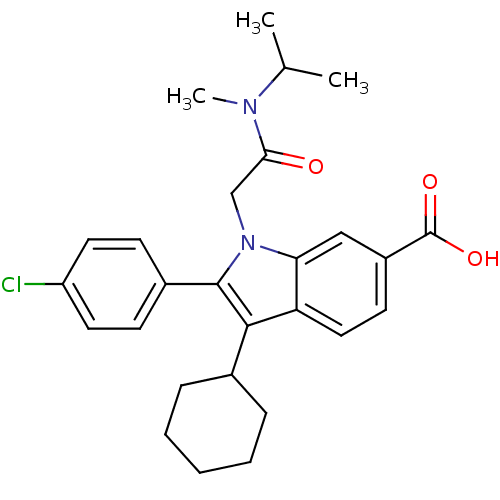 (2-(4-Chloro-phenyl)-3-cyclohexyl-1-[(isopropyl-met...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against by hepatitis C virus NS5B polymerase | J Med Chem 48: 4547-57 (2005) Article DOI: 10.1021/jm050056+ BindingDB Entry DOI: 10.7270/Q24B30VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50169915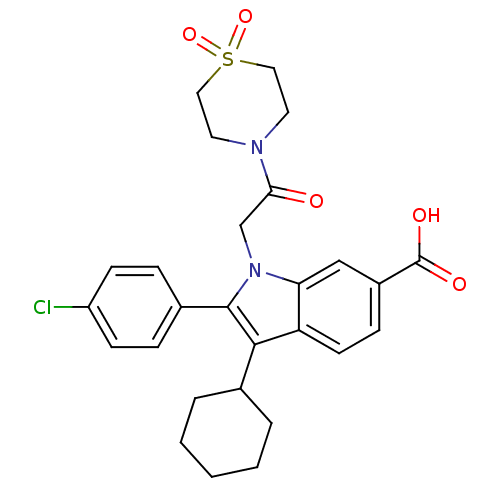 (2-(4-Chloro-phenyl)-3-cyclohexyl-1-[2-(1,1-dioxo-1...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against by hepatitis C virus NS5B polymerase | J Med Chem 48: 4547-57 (2005) Article DOI: 10.1021/jm050056+ BindingDB Entry DOI: 10.7270/Q24B30VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50169920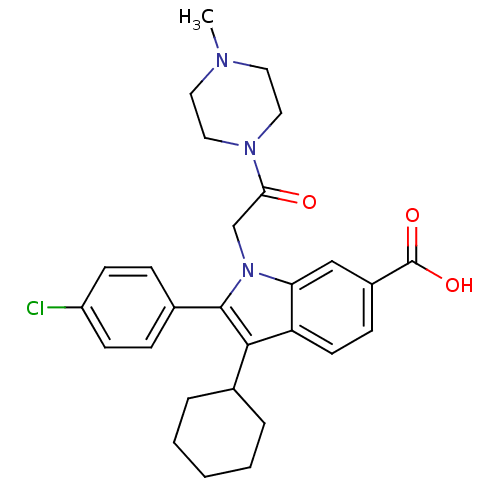 (2-(4-Chloro-phenyl)-3-cyclohexyl-1-[2-(4-methyl-pi...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against by hepatitis C virus NS5B polymerase | J Med Chem 48: 4547-57 (2005) Article DOI: 10.1021/jm050056+ BindingDB Entry DOI: 10.7270/Q24B30VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50169931 (2-(4-Chloro-phenyl)-3-cyclohexyl-1-{[methyl-(1-met...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against by hepatitis C virus NS5B polymerase | J Med Chem 48: 4547-57 (2005) Article DOI: 10.1021/jm050056+ BindingDB Entry DOI: 10.7270/Q24B30VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50169919 (2-(4-Chloro-phenyl)-3-cyclohexyl-1-[2-(4-dimethyla...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against by hepatitis C virus NS5B polymerase | J Med Chem 48: 4547-57 (2005) Article DOI: 10.1021/jm050056+ BindingDB Entry DOI: 10.7270/Q24B30VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Homo sapiens (Human)) | BDBM50352296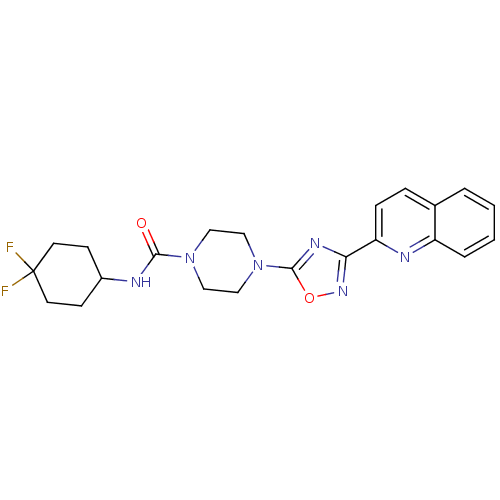 (CHEMBL1822468) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Rome Curated by ChEMBL | Assay Description Displacement of BODIPY-cyclopamine from Smo expressed in COS-1 cells after 4 to 6 hrs by Flow Cytometry analysis in presence of 2% fetal calf serum | Bioorg Med Chem Lett 21: 5283-8 (2011) Article DOI: 10.1016/j.bmcl.2011.07.030 BindingDB Entry DOI: 10.7270/Q24F1R35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Homo sapiens (Human)) | BDBM50352296 (CHEMBL1822468) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Rome Curated by ChEMBL | Assay Description Displacement of BODIPY-cyclopamine from Smo expressed in COS-1 cells after 4 to 6 hrs by Flow Cytometry analysis in presence of 20% normal human seru... | Bioorg Med Chem Lett 21: 5283-8 (2011) Article DOI: 10.1016/j.bmcl.2011.07.030 BindingDB Entry DOI: 10.7270/Q24F1R35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Homo sapiens (Human)) | BDBM50352270 (CHEMBL1822465) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Rome Curated by ChEMBL | Assay Description Displacement of BODIPY-cyclopamine from Smo expressed in COS-1 cells after 4 to 6 hrs by Flow Cytometry analysis in presence of 20% normal human seru... | Bioorg Med Chem Lett 21: 5283-8 (2011) Article DOI: 10.1016/j.bmcl.2011.07.030 BindingDB Entry DOI: 10.7270/Q24F1R35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Homo sapiens (Human)) | BDBM50352271 (CHEMBL1822463) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Rome Curated by ChEMBL | Assay Description Displacement of BODIPY-cyclopamine from Smo expressed in COS-1 cells after 4 to 6 hrs by Flow Cytometry analysis in presence of 20% normal human seru... | Bioorg Med Chem Lett 21: 5283-8 (2011) Article DOI: 10.1016/j.bmcl.2011.07.030 BindingDB Entry DOI: 10.7270/Q24F1R35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Homo sapiens (Human)) | BDBM50352272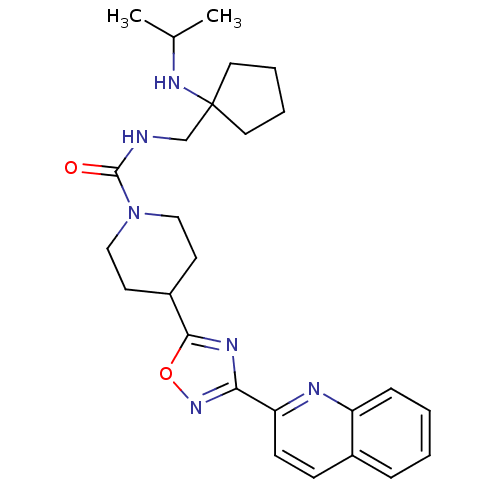 (CHEMBL1822447) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Rome Curated by ChEMBL | Assay Description Displacement of BODIPY-cyclopamine from Smo expressed in COS-1 cells after 4 to 6 hrs by Flow Cytometry analysis in presence of 2% fetal calf serum | Bioorg Med Chem Lett 21: 5283-8 (2011) Article DOI: 10.1016/j.bmcl.2011.07.030 BindingDB Entry DOI: 10.7270/Q24F1R35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Homo sapiens (Human)) | BDBM50352271 (CHEMBL1822463) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Rome Curated by ChEMBL | Assay Description Displacement of BODIPY-cyclopamine from Smo expressed in COS-1 cells after 4 to 6 hrs by Flow Cytometry analysis in presence of 2% fetal calf serum | Bioorg Med Chem Lett 21: 5283-8 (2011) Article DOI: 10.1016/j.bmcl.2011.07.030 BindingDB Entry DOI: 10.7270/Q24F1R35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50169932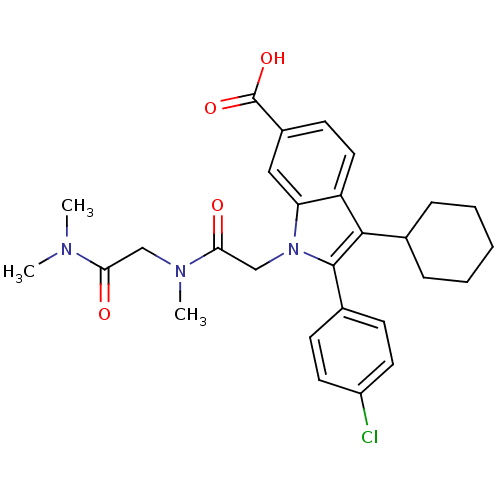 (2-(4-Chloro-phenyl)-3-cyclohexyl-1-[(dimethylcarba...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against by hepatitis C virus NS5B polymerase | J Med Chem 48: 4547-57 (2005) Article DOI: 10.1021/jm050056+ BindingDB Entry DOI: 10.7270/Q24B30VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50169921 (3-Cyclohexyl-2-[3-(3,3-difluoro-piperidin-1-ylmeth...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against by hepatitis C virus NS5B polymerase | J Med Chem 48: 4547-57 (2005) Article DOI: 10.1021/jm050056+ BindingDB Entry DOI: 10.7270/Q24B30VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50169929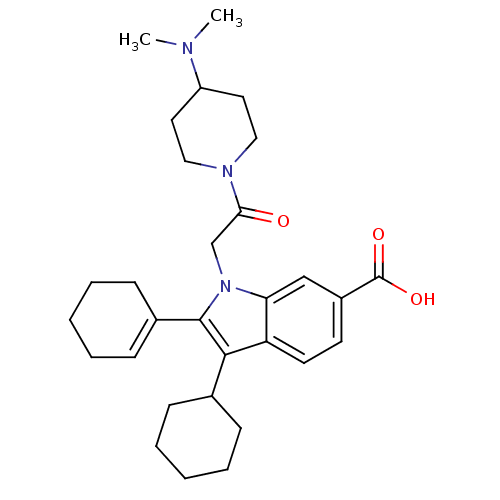 (2-Cyclohex-1-enyl-3-cyclohexyl-1-[2-(4-dimethylami...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against by hepatitis C virus NS5B polymerase | J Med Chem 48: 4547-57 (2005) Article DOI: 10.1021/jm050056+ BindingDB Entry DOI: 10.7270/Q24B30VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50169916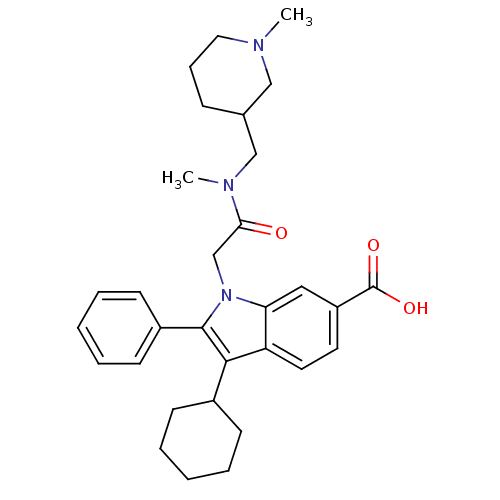 (3-Cyclohexyl-1-{[methyl-(1-methyl-piperidin-3-ylme...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against by hepatitis C virus NS5B polymerase | J Med Chem 48: 4547-57 (2005) Article DOI: 10.1021/jm050056+ BindingDB Entry DOI: 10.7270/Q24B30VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Homo sapiens (Human)) | BDBM50352273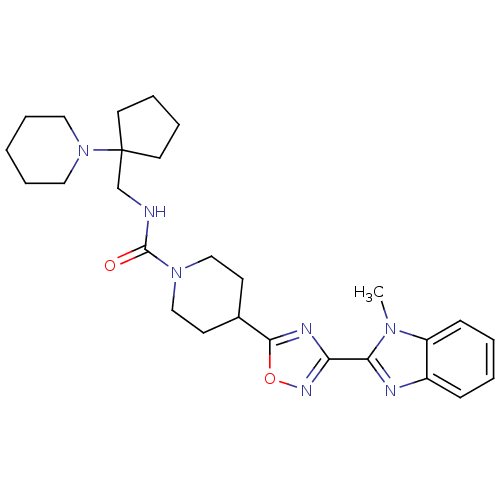 (CHEMBL1822464) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Rome Curated by ChEMBL | Assay Description Displacement of BODIPY-cyclopamine from Smo expressed in COS-1 cells after 4 to 6 hrs by Flow Cytometry analysis in presence of 20% normal human seru... | Bioorg Med Chem Lett 21: 5283-8 (2011) Article DOI: 10.1016/j.bmcl.2011.07.030 BindingDB Entry DOI: 10.7270/Q24F1R35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50169910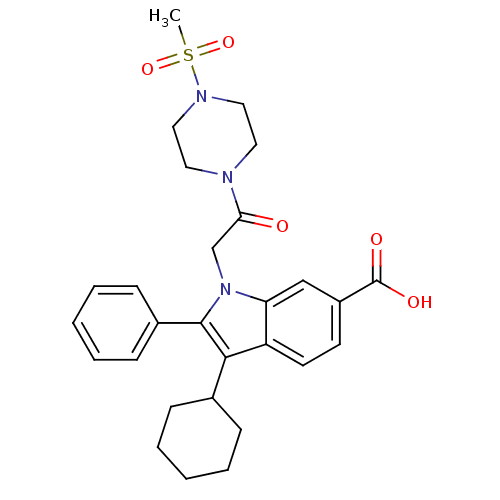 (3-Cyclohexyl-1-[2-(4-methanesulfonyl-piperazin-1-y...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against by hepatitis C virus NS5B polymerase | J Med Chem 48: 4547-57 (2005) Article DOI: 10.1021/jm050056+ BindingDB Entry DOI: 10.7270/Q24B30VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 221 total ) | Next | Last >> |