

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neprilysin (Homo sapiens (Human)) | BDBM50407297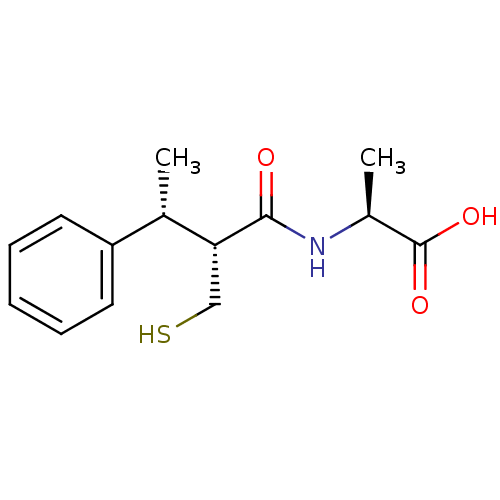 (CHEMBL2052008) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vivo inhibitory potency against neutral endopeptidase by displacement of [3H]-HACBOGly binding in mouse kidney | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50407297 (CHEMBL2052008) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vivo inhibitory potency against neutral endopeptidase by displacement of [3H]-HACBOGly binding in mouse kidney | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50407299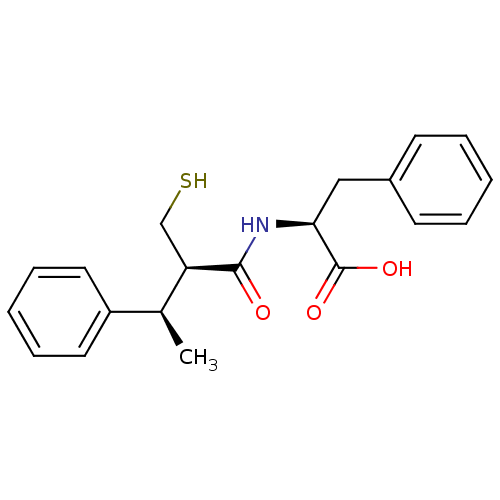 (CHEMBL2052007) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vivo inhibitory potency against neutral endopeptidase by displacement of [3H]-HACBOGly binding in mouse kidney | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50407299 (CHEMBL2052007) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vivo inhibitory potency against neutral endopeptidase by displacement of [3H]-HACBOGly binding in mouse kidney | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50407299 (CHEMBL2052007) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lung | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50407299 (CHEMBL2052007) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lung | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50407298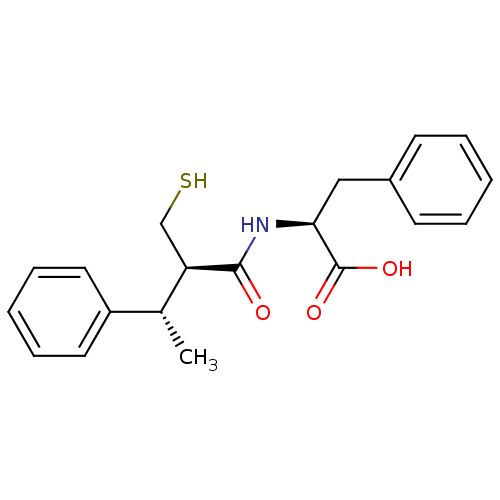 (CHEMBL2051772) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vivo inhibitory potency against neutral endopeptidase by displacement of [3H]-HACBOGly binding in mouse kidney | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50407298 (CHEMBL2051772) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lung | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50407297 (CHEMBL2052008) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lung | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50407297 (CHEMBL2052008) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lung | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50051796 ((2S,5R)-5-(2-Hydroxy-phenyl)-1-{2-[(S)-2-mercapto-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vitro inhibition of rat angiotensin I converting enzyme | J Med Chem 39: 2594-608 (1996) Article DOI: 10.1021/jm950783c BindingDB Entry DOI: 10.7270/Q25B0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50051798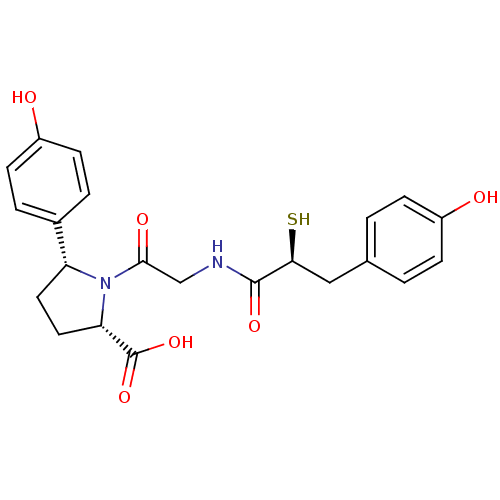 ((2S,5R)-5-(4-Hydroxy-phenyl)-1-{2-[(S)-3-(4-hydrox...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vitro inhibition of rat angiotensin I converting enzyme | J Med Chem 39: 2594-608 (1996) Article DOI: 10.1021/jm950783c BindingDB Entry DOI: 10.7270/Q25B0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50051784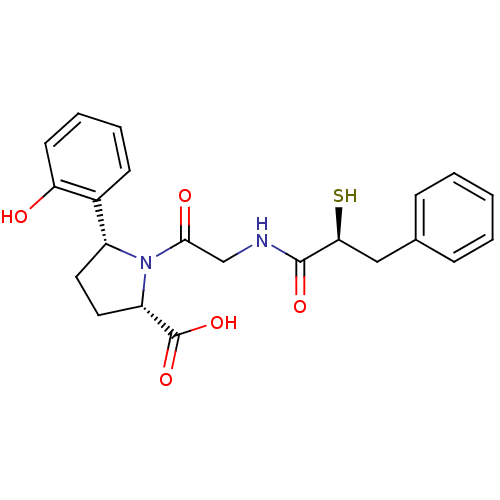 ((2S,5R)-5-(2-Hydroxy-phenyl)-1-[2-((S)-2-mercapto-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vitro inhibition of rat angiotensin I converting enzyme | J Med Chem 39: 2594-608 (1996) Article DOI: 10.1021/jm950783c BindingDB Entry DOI: 10.7270/Q25B0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50051785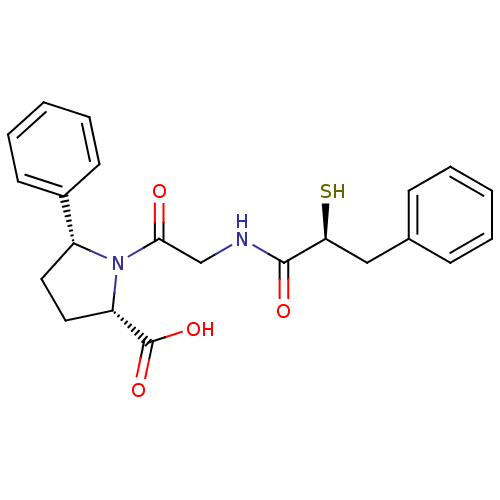 ((2S,5R)-1-[2-((S)-2-Mercapto-3-phenyl-propionylami...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vitro inhibition of rat angiotensin I converting enzyme | J Med Chem 39: 2594-608 (1996) Article DOI: 10.1021/jm950783c BindingDB Entry DOI: 10.7270/Q25B0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50051780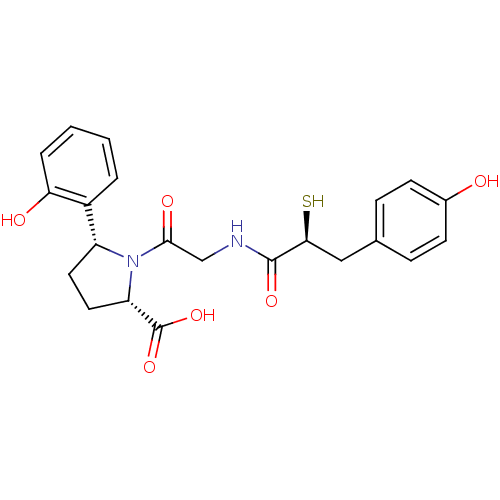 ((2S,5R)-5-(2-Hydroxy-phenyl)-1-{2-[(S)-3-(4-hydrox...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vitro inhibition of rat angiotensin I converting enzyme | J Med Chem 39: 2594-608 (1996) Article DOI: 10.1021/jm950783c BindingDB Entry DOI: 10.7270/Q25B0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50051791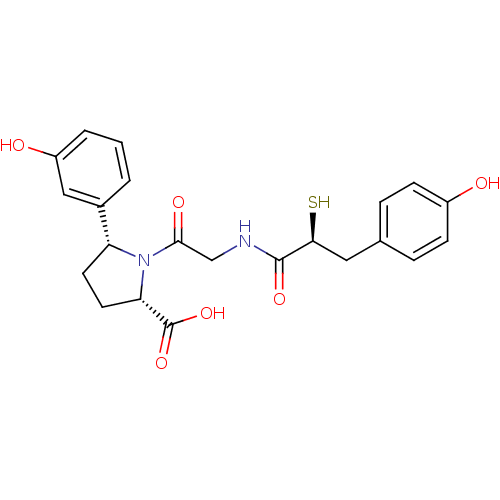 ((2S,5R)-5-(3-Hydroxy-phenyl)-1-{2-[(S)-3-(4-hydrox...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vitro inhibition of rat angiotensin I converting enzyme | J Med Chem 39: 2594-608 (1996) Article DOI: 10.1021/jm950783c BindingDB Entry DOI: 10.7270/Q25B0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50051803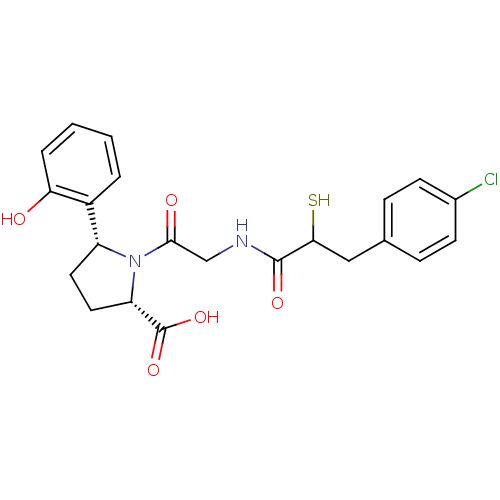 ((2S,5R)-1-{2-[3-(4-Chloro-phenyl)-2-mercapto-propi...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vitro inhibition of rat angiotensin I converting enzyme | J Med Chem 39: 2594-608 (1996) Article DOI: 10.1021/jm950783c BindingDB Entry DOI: 10.7270/Q25B0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50051784 ((2S,5R)-5-(2-Hydroxy-phenyl)-1-[2-((S)-2-mercapto-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vitro inhibition of neutral endopeptidase | J Med Chem 39: 2594-608 (1996) Article DOI: 10.1021/jm950783c BindingDB Entry DOI: 10.7270/Q25B0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50051803 ((2S,5R)-1-{2-[3-(4-Chloro-phenyl)-2-mercapto-propi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vitro inhibition of neutral endopeptidase | J Med Chem 39: 2594-608 (1996) Article DOI: 10.1021/jm950783c BindingDB Entry DOI: 10.7270/Q25B0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50041071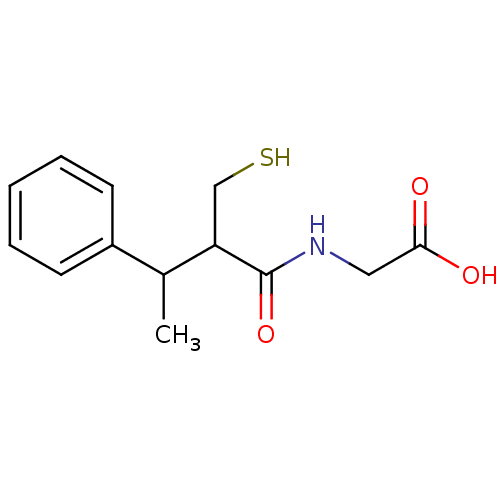 ((2-Mercaptomethyl-3-phenyl-butyrylamino)-acetic ac...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vivo inhibitory potency against neutral endopeptidase by displacement of [3H]-HACBOGly binding in mouse kidney | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50051800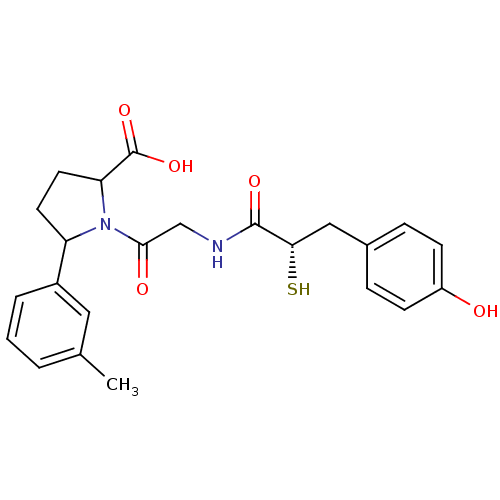 (1-{2-[(S)-3-(4-Hydroxy-phenyl)-2-mercapto-propiony...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vitro inhibition of neutral endopeptidase | J Med Chem 39: 2594-608 (1996) Article DOI: 10.1021/jm950783c BindingDB Entry DOI: 10.7270/Q25B0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50051790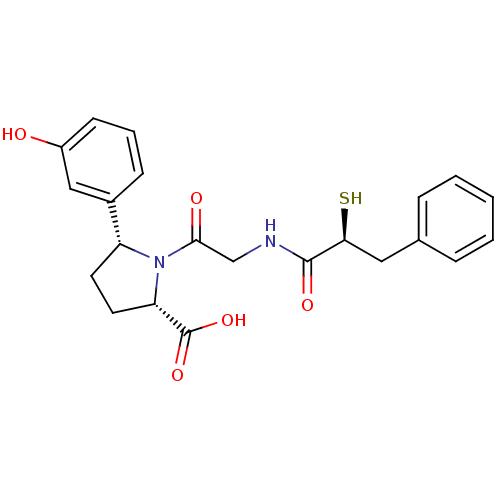 ((2S,5R)-5-(3-Hydroxy-phenyl)-1-[2-((S)-2-mercapto-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vitro inhibition of rat angiotensin I converting enzyme | J Med Chem 39: 2594-608 (1996) Article DOI: 10.1021/jm950783c BindingDB Entry DOI: 10.7270/Q25B0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50051785 ((2S,5R)-1-[2-((S)-2-Mercapto-3-phenyl-propionylami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vitro inhibition of neutral endopeptidase | J Med Chem 39: 2594-608 (1996) Article DOI: 10.1021/jm950783c BindingDB Entry DOI: 10.7270/Q25B0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50051791 ((2S,5R)-5-(3-Hydroxy-phenyl)-1-{2-[(S)-3-(4-hydrox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vitro inhibition of neutral endopeptidase | J Med Chem 39: 2594-608 (1996) Article DOI: 10.1021/jm950783c BindingDB Entry DOI: 10.7270/Q25B0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50051794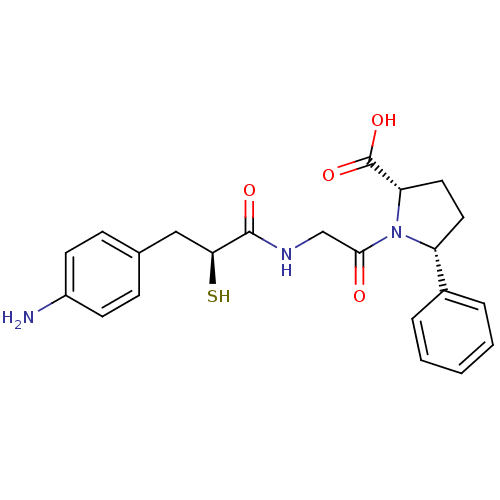 ((2S,5R)-1-{2-[(S)-3-(4-Amino-phenyl)-2-mercapto-pr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vitro inhibition of neutral endopeptidase | J Med Chem 39: 2594-608 (1996) Article DOI: 10.1021/jm950783c BindingDB Entry DOI: 10.7270/Q25B0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50041067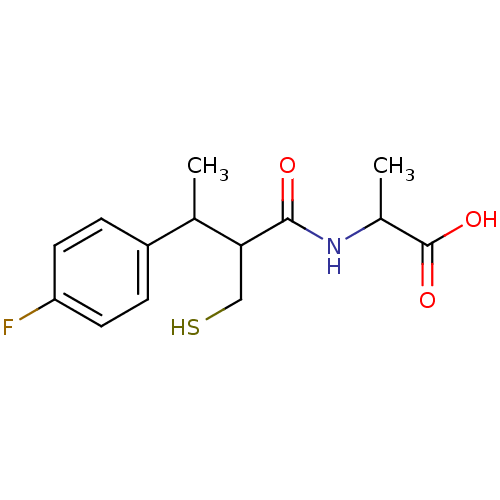 (2-[3-(4-Fluoro-phenyl)-2-mercaptomethyl-butyrylami...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vivo inhibitory potency against neutral endopeptidase by displacement of [3H]-HACBOGly binding in mouse kidney | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50041069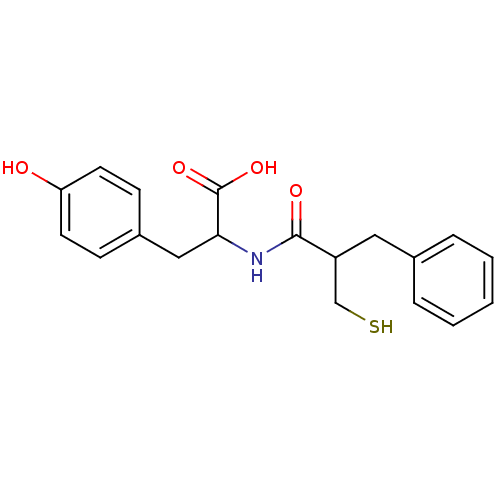 (3-(4-Hydroxy-phenyl)-2-(2-mercaptomethyl-3-phenyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vivo inhibitory potency against neutral endopeptidase by displacement of [3H]-HACBOGly binding in mouse kidney | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50407297 (CHEMBL2052008) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vivo inhibitory potency against neutral endopeptidase by displacement of [3H]-HACBOGly binding in mouse kidney | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50041058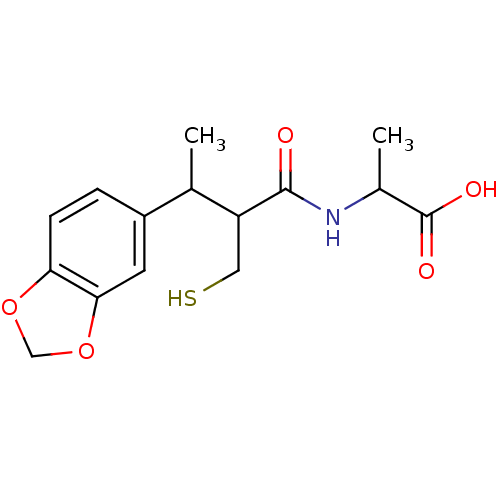 (2-(3-Benzo[1,3]dioxol-5-yl-2-mercaptomethyl-butyry...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vivo inhibitory potency against neutral endopeptidase by displacement of [3H]-HACBOGly binding in mouse kidney | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50051799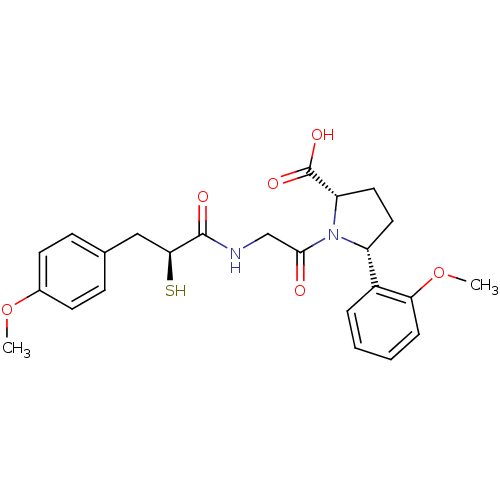 ((2S,5R)-1-{2-[(S)-2-Mercapto-3-(4-methoxy-phenyl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vitro inhibition of rat angiotensin I converting enzyme | J Med Chem 39: 2594-608 (1996) Article DOI: 10.1021/jm950783c BindingDB Entry DOI: 10.7270/Q25B0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50051796 ((2S,5R)-5-(2-Hydroxy-phenyl)-1-{2-[(S)-2-mercapto-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vitro inhibition of neutral endopeptidase | J Med Chem 39: 2594-608 (1996) Article DOI: 10.1021/jm950783c BindingDB Entry DOI: 10.7270/Q25B0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50051804 ((2S,5R)-1-[2-((S)-2-Mercapto-3-phenyl-propionylami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vitro inhibition of neutral endopeptidase | J Med Chem 39: 2594-608 (1996) Article DOI: 10.1021/jm950783c BindingDB Entry DOI: 10.7270/Q25B0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50051790 ((2S,5R)-5-(3-Hydroxy-phenyl)-1-[2-((S)-2-mercapto-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vitro inhibition of neutral endopeptidase | J Med Chem 39: 2594-608 (1996) Article DOI: 10.1021/jm950783c BindingDB Entry DOI: 10.7270/Q25B0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50041070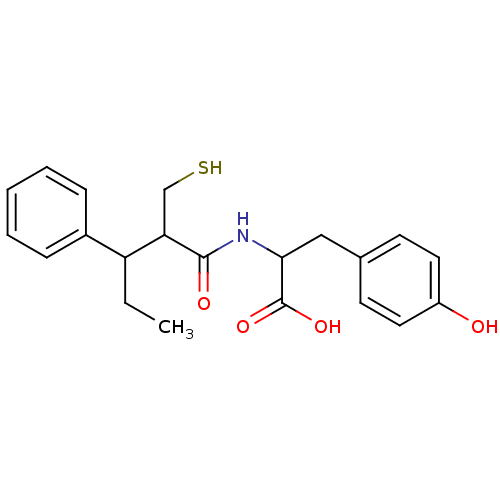 (3-(4-Hydroxy-phenyl)-2-(2-mercaptomethyl-3-phenyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vivo inhibitory potency against neutral endopeptidase by displacement of [3H]-HACBOGly binding in mouse kidney | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50051786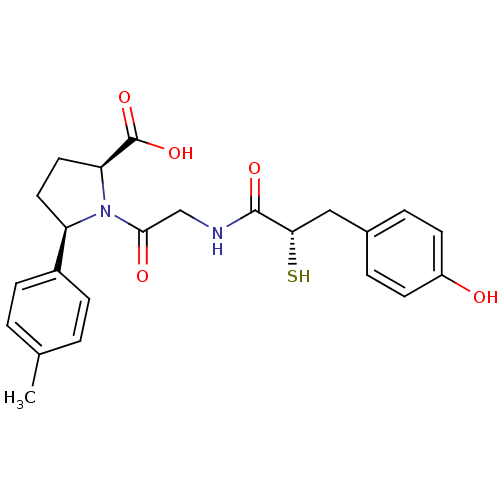 ((2S,5R)-1-{2-[(S)-3-(4-Hydroxy-phenyl)-2-mercapto-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vitro inhibition of rat angiotensin I converting enzyme | J Med Chem 39: 2594-608 (1996) Article DOI: 10.1021/jm950783c BindingDB Entry DOI: 10.7270/Q25B0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50041054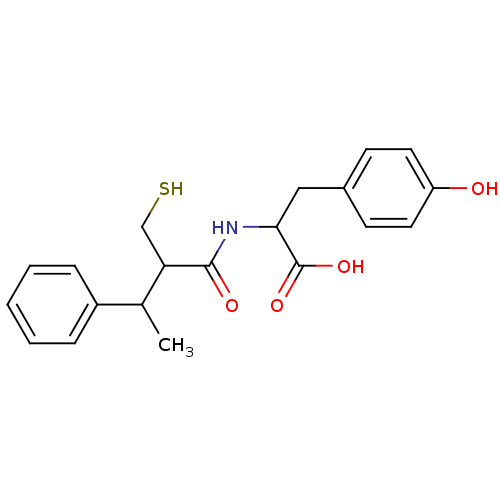 (3-(4-Hydroxy-phenyl)-2-(2-mercaptomethyl-3-phenyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vivo inhibitory potency against neutral endopeptidase by displacement of [3H]-HACBOGly binding in mouse kidney | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Mus musculus) | BDBM50041062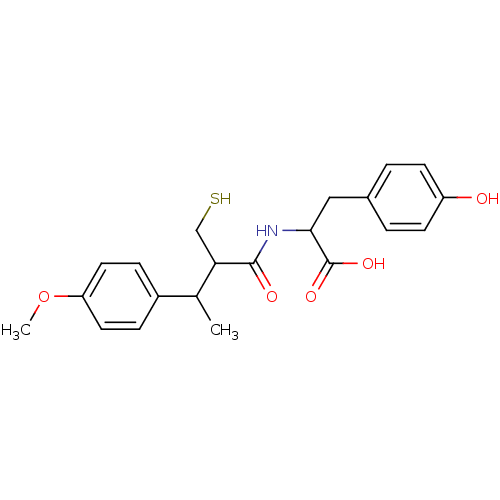 (3-(4-Hydroxy-phenyl)-2-[2-mercaptomethyl-3-(4-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vivo inhibitory potency against Neutral Endopeptidase by measuring the displacement of [3H]-HACBOGly binding in mouse kidney | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50051799 ((2S,5R)-1-{2-[(S)-2-Mercapto-3-(4-methoxy-phenyl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vitro inhibition of neutral endopeptidase | J Med Chem 39: 2594-608 (1996) Article DOI: 10.1021/jm950783c BindingDB Entry DOI: 10.7270/Q25B0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50407847 (CHEMBL2115220) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vitro inhibition of neutral endopeptidase | J Med Chem 39: 2594-608 (1996) Article DOI: 10.1021/jm950783c BindingDB Entry DOI: 10.7270/Q25B0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50051800 (1-{2-[(S)-3-(4-Hydroxy-phenyl)-2-mercapto-propiony...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vitro inhibition of rat angiotensin I converting enzyme | J Med Chem 39: 2594-608 (1996) Article DOI: 10.1021/jm950783c BindingDB Entry DOI: 10.7270/Q25B0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50041061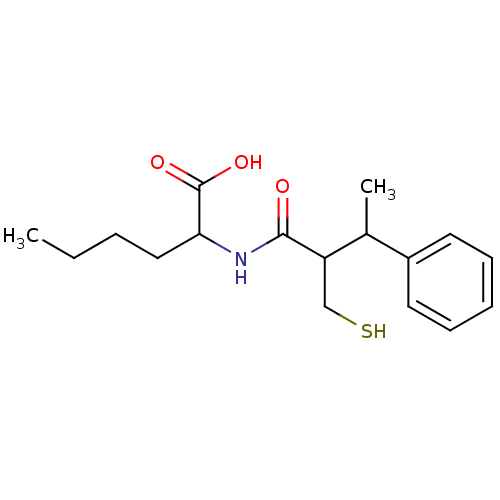 (2-(2-Mercaptomethyl-3-phenyl-butyrylamino)-hexanoi...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vivo inhibitory potency against neutral endopeptidase by displacement of [3H]-HACBOGly binding in mouse kidney | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50051778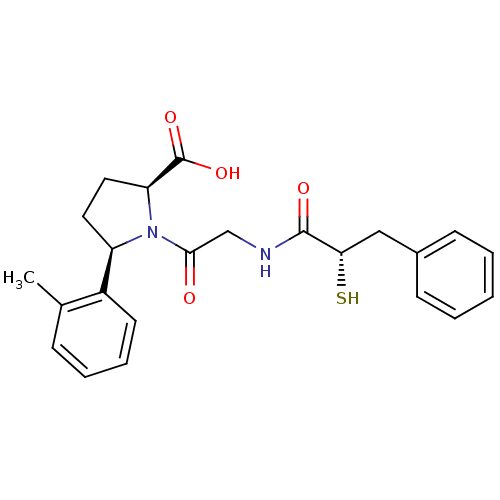 ((2S,5R)-1-[2-((S)-2-Mercapto-3-phenyl-propionylami...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vitro inhibition of rat angiotensin I converting enzyme | J Med Chem 39: 2594-608 (1996) Article DOI: 10.1021/jm950783c BindingDB Entry DOI: 10.7270/Q25B0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50051805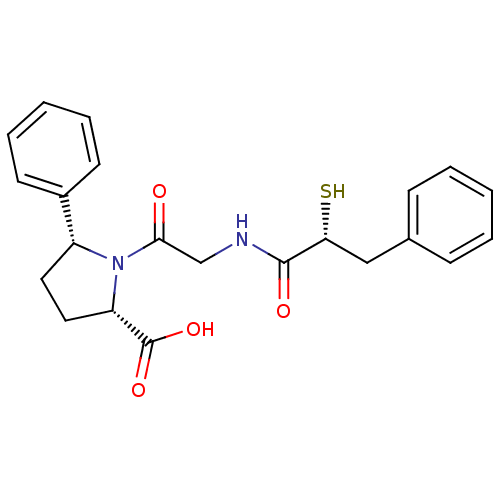 ((2S,5R)-1-[2-((R)-2-Mercapto-3-phenyl-propionylami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vitro inhibition of neutral endopeptidase | J Med Chem 39: 2594-608 (1996) Article DOI: 10.1021/jm950783c BindingDB Entry DOI: 10.7270/Q25B0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50051795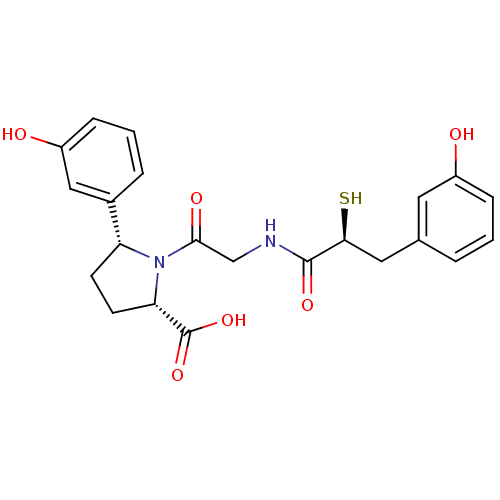 ((2S,5R)-5-(3-Hydroxy-phenyl)-1-{2-[(S)-3-(3-hydrox...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vitro inhibition of rat angiotensin I converting enzyme | J Med Chem 39: 2594-608 (1996) Article DOI: 10.1021/jm950783c BindingDB Entry DOI: 10.7270/Q25B0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50051787 ((2S,5R)-1-{2-[(S)-3-(4-Hydroxy-phenyl)-2-mercapto-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vitro inhibition of neutral endopeptidase | J Med Chem 39: 2594-608 (1996) Article DOI: 10.1021/jm950783c BindingDB Entry DOI: 10.7270/Q25B0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50051789 ((2S,5R)-1-{2-[(S)-3-(4-Hydroxy-phenyl)-2-mercapto-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vitro inhibition of neutral endopeptidase | J Med Chem 39: 2594-608 (1996) Article DOI: 10.1021/jm950783c BindingDB Entry DOI: 10.7270/Q25B0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50041059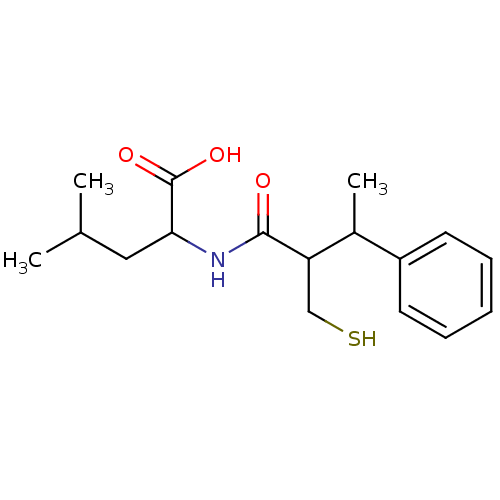 (2-(2-Mercaptomethyl-3-phenyl-butyrylamino)-4-methy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vivo inhibitory potency against neutral endopeptidase by displacement of [3H]-HACBOGly binding in mouse kidney | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50041053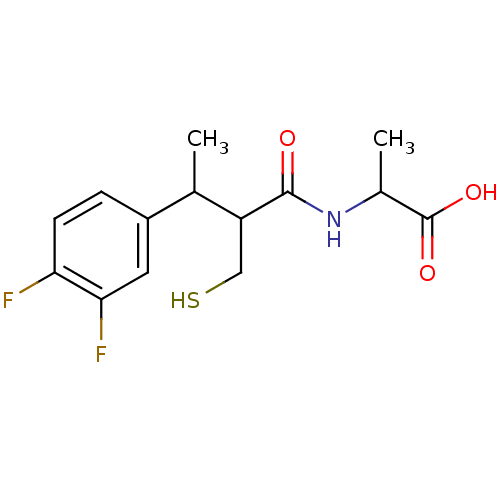 (2-[3-(3,4-Difluoro-phenyl)-2-mercaptomethyl-butyry...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vivo inhibitory potency against neutral endopeptidase by displacement of [3H]-HACBOGly binding in mouse kidney | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50051780 ((2S,5R)-5-(2-Hydroxy-phenyl)-1-{2-[(S)-3-(4-hydrox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vitro inhibition of neutral endopeptidase | J Med Chem 39: 2594-608 (1996) Article DOI: 10.1021/jm950783c BindingDB Entry DOI: 10.7270/Q25B0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50051794 ((2S,5R)-1-{2-[(S)-3-(4-Amino-phenyl)-2-mercapto-pr...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vitro inhibition of rat angiotensin I converting enzyme | J Med Chem 39: 2594-608 (1996) Article DOI: 10.1021/jm950783c BindingDB Entry DOI: 10.7270/Q25B0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 114 total ) | Next | Last >> |