 Found 393 hits with Last Name = 'gonzalez-lopez de turiso' and Initial = 'f'
Found 393 hits with Last Name = 'gonzalez-lopez de turiso' and Initial = 'f' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50396616
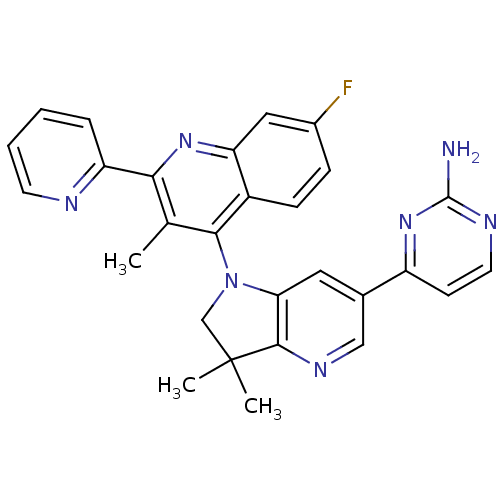
(CHEMBL2171930 | US8765940, 4-(1-(7-fluoro-3-methyl...)Show SMILES Cc1c(nc2cc(F)ccc2c1N1CC(C)(C)c2ncc(cc12)-c1ccnc(N)n1)-c1ccccn1 Show InChI InChI=1S/C28H24FN7/c1-16-24(21-6-4-5-10-31-21)34-22-13-18(29)7-8-19(22)25(16)36-15-28(2,3)26-23(36)12-17(14-33-26)20-9-11-32-27(30)35-20/h4-14H,15H2,1-3H3,(H2,30,32,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta using phosphatidylinositol-4,5-bisphosphate by ATP bioluminescence assay |
J Med Chem 55: 7667-85 (2012)
Article DOI: 10.1021/jm300679u
BindingDB Entry DOI: 10.7270/Q2J38TQ0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50396610
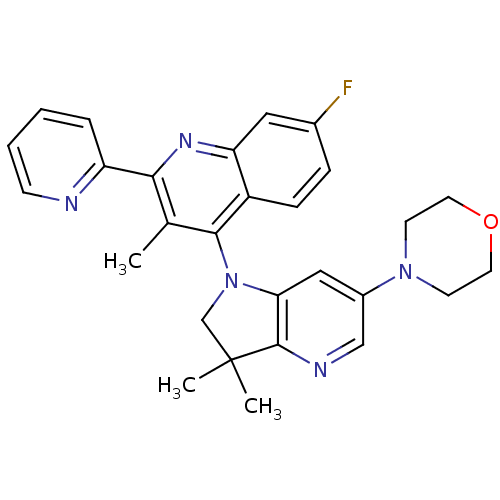
(CHEMBL2171929 | US8765940, 4-(3,3-dimethyl-6-(4-mo...)Show SMILES Cc1c(nc2cc(F)ccc2c1N1CC(C)(C)c2ncc(cc12)N1CCOCC1)-c1ccccn1 Show InChI InChI=1S/C28H28FN5O/c1-18-25(22-6-4-5-9-30-22)32-23-14-19(29)7-8-21(23)26(18)34-17-28(2,3)27-24(34)15-20(16-31-27)33-10-12-35-13-11-33/h4-9,14-16H,10-13,17H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta using phosphatidylinositol-4,5-bisphosphate by ATP bioluminescence assay |
J Med Chem 55: 7667-85 (2012)
Article DOI: 10.1021/jm300679u
BindingDB Entry DOI: 10.7270/Q2J38TQ0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50396611
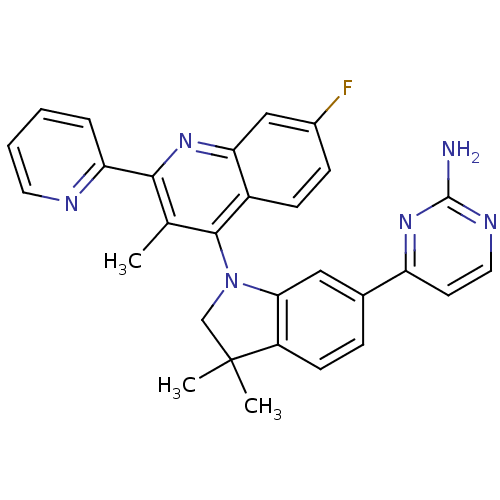
(CHEMBL2171927 | US8765940, 4-(1-(7-fluoro-3-methyl...)Show SMILES Cc1c(nc2cc(F)ccc2c1N1CC(C)(C)c2ccc(cc12)-c1ccnc(N)n1)-c1ccccn1 Show InChI InChI=1S/C29H25FN6/c1-17-26(23-6-4-5-12-32-23)34-24-15-19(30)8-9-20(24)27(17)36-16-29(2,3)21-10-7-18(14-25(21)36)22-11-13-33-28(31)35-22/h4-15H,16H2,1-3H3,(H2,31,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta using phosphatidylinositol-4,5-bisphosphate by ATP bioluminescence assay |
J Med Chem 55: 7667-85 (2012)
Article DOI: 10.1021/jm300679u
BindingDB Entry DOI: 10.7270/Q2J38TQ0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50396614
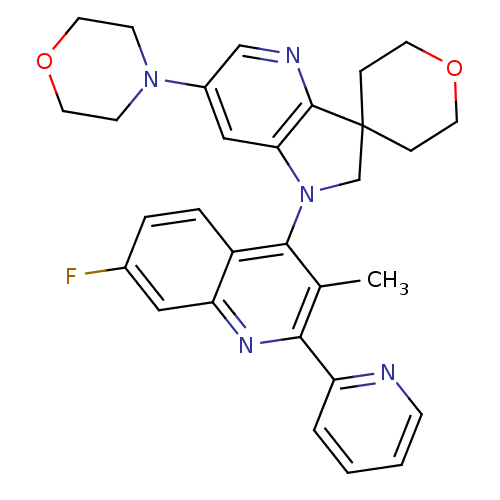
(CHEMBL2171931 | US8765940, 1'-(7-fluoro-3-meth...)Show SMILES Cc1c(nc2cc(F)ccc2c1N1CC2(CCOCC2)c2ncc(cc12)N1CCOCC1)-c1ccccn1 Show InChI InChI=1S/C30H30FN5O2/c1-20-27(24-4-2-3-9-32-24)34-25-16-21(31)5-6-23(25)28(20)36-19-30(7-12-37-13-8-30)29-26(36)17-22(18-33-29)35-10-14-38-15-11-35/h2-6,9,16-18H,7-8,10-15,19H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta using phosphatidylinositol-4,5-bisphosphate by ATP bioluminescence assay |
J Med Chem 55: 7667-85 (2012)
Article DOI: 10.1021/jm300679u
BindingDB Entry DOI: 10.7270/Q2J38TQ0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50396616

(CHEMBL2171930 | US8765940, 4-(1-(7-fluoro-3-methyl...)Show SMILES Cc1c(nc2cc(F)ccc2c1N1CC(C)(C)c2ncc(cc12)-c1ccnc(N)n1)-c1ccccn1 Show InChI InChI=1S/C28H24FN7/c1-16-24(21-6-4-5-10-31-21)34-22-13-18(29)7-8-19(22)25(16)36-15-28(2,3)26-23(36)12-17(14-33-26)20-9-11-32-27(30)35-20/h4-14H,15H2,1-3H3,(H2,30,32,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta using phosphatidylinositol-4,5-bisphosphate by ATP bioluminescence assay |
J Med Chem 55: 7667-85 (2012)
Article DOI: 10.1021/jm300679u
BindingDB Entry DOI: 10.7270/Q2J38TQ0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50396616

(CHEMBL2171930 | US8765940, 4-(1-(7-fluoro-3-methyl...)Show SMILES Cc1c(nc2cc(F)ccc2c1N1CC(C)(C)c2ncc(cc12)-c1ccnc(N)n1)-c1ccccn1 Show InChI InChI=1S/C28H24FN7/c1-16-24(21-6-4-5-10-31-21)34-22-13-18(29)7-8-19(22)25(16)36-15-28(2,3)26-23(36)12-17(14-33-26)20-9-11-32-27(30)35-20/h4-14H,15H2,1-3H3,(H2,30,32,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma by ATP bioluminescence assay |
J Med Chem 55: 7667-85 (2012)
Article DOI: 10.1021/jm300679u
BindingDB Entry DOI: 10.7270/Q2J38TQ0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50396610

(CHEMBL2171929 | US8765940, 4-(3,3-dimethyl-6-(4-mo...)Show SMILES Cc1c(nc2cc(F)ccc2c1N1CC(C)(C)c2ncc(cc12)N1CCOCC1)-c1ccccn1 Show InChI InChI=1S/C28H28FN5O/c1-18-25(22-6-4-5-9-30-22)32-23-14-19(29)7-8-21(23)26(18)34-17-28(2,3)27-24(34)15-20(16-31-27)33-10-12-35-13-11-33/h4-9,14-16H,10-13,17H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta using phosphatidylinositol-4,5-bisphosphate by ATP bioluminescence assay |
J Med Chem 55: 7667-85 (2012)
Article DOI: 10.1021/jm300679u
BindingDB Entry DOI: 10.7270/Q2J38TQ0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50396614

(CHEMBL2171931 | US8765940, 1'-(7-fluoro-3-meth...)Show SMILES Cc1c(nc2cc(F)ccc2c1N1CC2(CCOCC2)c2ncc(cc12)N1CCOCC1)-c1ccccn1 Show InChI InChI=1S/C30H30FN5O2/c1-20-27(24-4-2-3-9-32-24)34-25-16-21(31)5-6-23(25)28(20)36-19-30(7-12-37-13-8-30)29-26(36)17-22(18-33-29)35-10-14-38-15-11-35/h2-6,9,16-18H,7-8,10-15,19H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta using phosphatidylinositol-4,5-bisphosphate by ATP bioluminescence assay |
J Med Chem 55: 7667-85 (2012)
Article DOI: 10.1021/jm300679u
BindingDB Entry DOI: 10.7270/Q2J38TQ0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50396611

(CHEMBL2171927 | US8765940, 4-(1-(7-fluoro-3-methyl...)Show SMILES Cc1c(nc2cc(F)ccc2c1N1CC(C)(C)c2ccc(cc12)-c1ccnc(N)n1)-c1ccccn1 Show InChI InChI=1S/C29H25FN6/c1-17-26(23-6-4-5-12-32-23)34-24-15-19(30)8-9-20(24)27(17)36-16-29(2,3)21-10-7-18(14-25(21)36)22-11-13-33-28(31)35-22/h4-15H,16H2,1-3H3,(H2,31,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta using phosphatidylinositol-4,5-bisphosphate by ATP bioluminescence assay |
J Med Chem 55: 7667-85 (2012)
Article DOI: 10.1021/jm300679u
BindingDB Entry DOI: 10.7270/Q2J38TQ0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50396613

(CHEMBL2171924 | US8765940, 1-(7-fluoro-3-methyl-2-...)Show SMILES Cc1c(nc2cc(F)ccc2c1N1CC2(CS(=O)(=O)C2)c2ccc(cc12)N1CCOCC1)-c1ccccn1 Show InChI InChI=1S/C29H27FN4O3S/c1-19-27(24-4-2-3-9-31-24)32-25-14-20(30)5-7-22(25)28(19)34-16-29(17-38(35,36)18-29)23-8-6-21(15-26(23)34)33-10-12-37-13-11-33/h2-9,14-15H,10-13,16-18H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta using phosphatidylinositol-4,5-bisphosphate by ATP bioluminescence assay |
J Med Chem 55: 7667-85 (2012)
Article DOI: 10.1021/jm300679u
BindingDB Entry DOI: 10.7270/Q2J38TQ0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50396611

(CHEMBL2171927 | US8765940, 4-(1-(7-fluoro-3-methyl...)Show SMILES Cc1c(nc2cc(F)ccc2c1N1CC(C)(C)c2ccc(cc12)-c1ccnc(N)n1)-c1ccccn1 Show InChI InChI=1S/C29H25FN6/c1-17-26(23-6-4-5-12-32-23)34-24-15-19(30)8-9-20(24)27(17)36-16-29(2,3)21-10-7-18(14-25(21)36)22-11-13-33-28(31)35-22/h4-15H,16H2,1-3H3,(H2,31,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma by ATP bioluminescence assay |
J Med Chem 55: 7667-85 (2012)
Article DOI: 10.1021/jm300679u
BindingDB Entry DOI: 10.7270/Q2J38TQ0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50396610

(CHEMBL2171929 | US8765940, 4-(3,3-dimethyl-6-(4-mo...)Show SMILES Cc1c(nc2cc(F)ccc2c1N1CC(C)(C)c2ncc(cc12)N1CCOCC1)-c1ccccn1 Show InChI InChI=1S/C28H28FN5O/c1-18-25(22-6-4-5-9-30-22)32-23-14-19(29)7-8-21(23)26(18)34-17-28(2,3)27-24(34)15-20(16-31-27)33-10-12-35-13-11-33/h4-9,14-16H,10-13,17H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma by ATP bioluminescence assay |
J Med Chem 55: 7667-85 (2012)
Article DOI: 10.1021/jm300679u
BindingDB Entry DOI: 10.7270/Q2J38TQ0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50396617

(CHEMBL2171925 | US8765940, (1-(7-fluoro-3-methyl-2...)Show SMILES Cc1c(nc2cc(F)ccc2c1N1CC(CO)(CO)c2ccc(cc12)N1CCOCC1)-c1ccccn1 Show InChI InChI=1S/C29H29FN4O3/c1-19-27(24-4-2-3-9-31-24)32-25-14-20(30)5-7-22(25)28(19)34-16-29(17-35,18-36)23-8-6-21(15-26(23)34)33-10-12-37-13-11-33/h2-9,14-15,35-36H,10-13,16-18H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 154 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta using phosphatidylinositol-4,5-bisphosphate by ATP bioluminescence assay |
J Med Chem 55: 7667-85 (2012)
Article DOI: 10.1021/jm300679u
BindingDB Entry DOI: 10.7270/Q2J38TQ0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50396617

(CHEMBL2171925 | US8765940, (1-(7-fluoro-3-methyl-2...)Show SMILES Cc1c(nc2cc(F)ccc2c1N1CC(CO)(CO)c2ccc(cc12)N1CCOCC1)-c1ccccn1 Show InChI InChI=1S/C29H29FN4O3/c1-19-27(24-4-2-3-9-31-24)32-25-14-20(30)5-7-22(25)28(19)34-16-29(17-35,18-36)23-8-6-21(15-26(23)34)33-10-12-37-13-11-33/h2-9,14-15,35-36H,10-13,16-18H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 269 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta using phosphatidylinositol-4,5-bisphosphate by ATP bioluminescence assay |
J Med Chem 55: 7667-85 (2012)
Article DOI: 10.1021/jm300679u
BindingDB Entry DOI: 10.7270/Q2J38TQ0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50396615
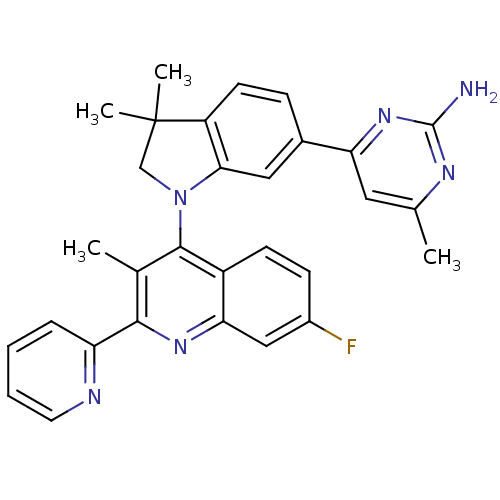
(CHEMBL2171928 | US8765940, 4-(1-(7-fluoro-3-methyl...)Show SMILES Cc1cc(nc(N)n1)-c1ccc2c(c1)N(CC2(C)C)c1c(C)c(nc2cc(F)ccc12)-c1ccccn1 Show InChI InChI=1S/C30H27FN6/c1-17-13-24(36-29(32)34-17)19-8-11-22-26(14-19)37(16-30(22,3)4)28-18(2)27(23-7-5-6-12-33-23)35-25-15-20(31)9-10-21(25)28/h5-15H,16H2,1-4H3,(H2,32,34,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 364 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma by ATP bioluminescence assay |
J Med Chem 55: 7667-85 (2012)
Article DOI: 10.1021/jm300679u
BindingDB Entry DOI: 10.7270/Q2J38TQ0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50396613

(CHEMBL2171924 | US8765940, 1-(7-fluoro-3-methyl-2-...)Show SMILES Cc1c(nc2cc(F)ccc2c1N1CC2(CS(=O)(=O)C2)c2ccc(cc12)N1CCOCC1)-c1ccccn1 Show InChI InChI=1S/C29H27FN4O3S/c1-19-27(24-4-2-3-9-31-24)32-25-14-20(30)5-7-22(25)28(19)34-16-29(17-38(35,36)18-29)23-8-6-21(15-26(23)34)33-10-12-37-13-11-33/h2-9,14-15H,10-13,16-18H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 395 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta using phosphatidylinositol-4,5-bisphosphate by ATP bioluminescence assay |
J Med Chem 55: 7667-85 (2012)
Article DOI: 10.1021/jm300679u
BindingDB Entry DOI: 10.7270/Q2J38TQ0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50396615

(CHEMBL2171928 | US8765940, 4-(1-(7-fluoro-3-methyl...)Show SMILES Cc1cc(nc(N)n1)-c1ccc2c(c1)N(CC2(C)C)c1c(C)c(nc2cc(F)ccc12)-c1ccccn1 Show InChI InChI=1S/C30H27FN6/c1-17-13-24(36-29(32)34-17)19-8-11-22-26(14-19)37(16-30(22,3)4)28-18(2)27(23-7-5-6-12-33-23)35-25-15-20(31)9-10-21(25)28/h5-15H,16H2,1-4H3,(H2,32,34,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 449 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta using phosphatidylinositol-4,5-bisphosphate by ATP bioluminescence assay |
J Med Chem 55: 7667-85 (2012)
Article DOI: 10.1021/jm300679u
BindingDB Entry DOI: 10.7270/Q2J38TQ0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50396614

(CHEMBL2171931 | US8765940, 1'-(7-fluoro-3-meth...)Show SMILES Cc1c(nc2cc(F)ccc2c1N1CC2(CCOCC2)c2ncc(cc12)N1CCOCC1)-c1ccccn1 Show InChI InChI=1S/C30H30FN5O2/c1-20-27(24-4-2-3-9-32-24)34-25-16-21(31)5-6-23(25)28(20)36-19-30(7-12-37-13-8-30)29-26(36)17-22(18-33-29)35-10-14-38-15-11-35/h2-6,9,16-18H,7-8,10-15,19H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 509 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma by ATP bioluminescence assay |
J Med Chem 55: 7667-85 (2012)
Article DOI: 10.1021/jm300679u
BindingDB Entry DOI: 10.7270/Q2J38TQ0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50396616

(CHEMBL2171930 | US8765940, 4-(1-(7-fluoro-3-methyl...)Show SMILES Cc1c(nc2cc(F)ccc2c1N1CC(C)(C)c2ncc(cc12)-c1ccnc(N)n1)-c1ccccn1 Show InChI InChI=1S/C28H24FN7/c1-16-24(21-6-4-5-10-31-21)34-22-13-18(29)7-8-19(22)25(16)36-15-28(2,3)26-23(36)12-17(14-33-26)20-9-11-32-27(30)35-20/h4-14H,15H2,1-3H3,(H2,30,32,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha by ATP bioluminescence assay |
J Med Chem 55: 7667-85 (2012)
Article DOI: 10.1021/jm300679u
BindingDB Entry DOI: 10.7270/Q2J38TQ0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50396610

(CHEMBL2171929 | US8765940, 4-(3,3-dimethyl-6-(4-mo...)Show SMILES Cc1c(nc2cc(F)ccc2c1N1CC(C)(C)c2ncc(cc12)N1CCOCC1)-c1ccccn1 Show InChI InChI=1S/C28H28FN5O/c1-18-25(22-6-4-5-9-30-22)32-23-14-19(29)7-8-21(23)26(18)34-17-28(2,3)27-24(34)15-20(16-31-27)33-10-12-35-13-11-33/h4-9,14-16H,10-13,17H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha by ATP bioluminescence assay |
J Med Chem 55: 7667-85 (2012)
Article DOI: 10.1021/jm300679u
BindingDB Entry DOI: 10.7270/Q2J38TQ0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50396614

(CHEMBL2171931 | US8765940, 1'-(7-fluoro-3-meth...)Show SMILES Cc1c(nc2cc(F)ccc2c1N1CC2(CCOCC2)c2ncc(cc12)N1CCOCC1)-c1ccccn1 Show InChI InChI=1S/C30H30FN5O2/c1-20-27(24-4-2-3-9-32-24)34-25-16-21(31)5-6-23(25)28(20)36-19-30(7-12-37-13-8-30)29-26(36)17-22(18-33-29)35-10-14-38-15-11-35/h2-6,9,16-18H,7-8,10-15,19H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 3.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha by ATP bioluminescence assay |
J Med Chem 55: 7667-85 (2012)
Article DOI: 10.1021/jm300679u
BindingDB Entry DOI: 10.7270/Q2J38TQ0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50396611

(CHEMBL2171927 | US8765940, 4-(1-(7-fluoro-3-methyl...)Show SMILES Cc1c(nc2cc(F)ccc2c1N1CC(C)(C)c2ccc(cc12)-c1ccnc(N)n1)-c1ccccn1 Show InChI InChI=1S/C29H25FN6/c1-17-26(23-6-4-5-12-32-23)34-24-15-19(30)8-9-20(24)27(17)36-16-29(2,3)21-10-7-18(14-25(21)36)22-11-13-33-28(31)35-22/h4-15H,16H2,1-3H3,(H2,31,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha by ATP bioluminescence assay |
J Med Chem 55: 7667-85 (2012)
Article DOI: 10.1021/jm300679u
BindingDB Entry DOI: 10.7270/Q2J38TQ0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50396613

(CHEMBL2171924 | US8765940, 1-(7-fluoro-3-methyl-2-...)Show SMILES Cc1c(nc2cc(F)ccc2c1N1CC2(CS(=O)(=O)C2)c2ccc(cc12)N1CCOCC1)-c1ccccn1 Show InChI InChI=1S/C29H27FN4O3S/c1-19-27(24-4-2-3-9-31-24)32-25-14-20(30)5-7-22(25)28(19)34-16-29(17-38(35,36)18-29)23-8-6-21(15-26(23)34)33-10-12-37-13-11-33/h2-9,14-15H,10-13,16-18H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma by ATP bioluminescence assay |
J Med Chem 55: 7667-85 (2012)
Article DOI: 10.1021/jm300679u
BindingDB Entry DOI: 10.7270/Q2J38TQ0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50396613

(CHEMBL2171924 | US8765940, 1-(7-fluoro-3-methyl-2-...)Show SMILES Cc1c(nc2cc(F)ccc2c1N1CC2(CS(=O)(=O)C2)c2ccc(cc12)N1CCOCC1)-c1ccccn1 Show InChI InChI=1S/C29H27FN4O3S/c1-19-27(24-4-2-3-9-31-24)32-25-14-20(30)5-7-22(25)28(19)34-16-29(17-38(35,36)18-29)23-8-6-21(15-26(23)34)33-10-12-37-13-11-33/h2-9,14-15H,10-13,16-18H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha by ATP bioluminescence assay |
J Med Chem 55: 7667-85 (2012)
Article DOI: 10.1021/jm300679u
BindingDB Entry DOI: 10.7270/Q2J38TQ0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50396617

(CHEMBL2171925 | US8765940, (1-(7-fluoro-3-methyl-2...)Show SMILES Cc1c(nc2cc(F)ccc2c1N1CC(CO)(CO)c2ccc(cc12)N1CCOCC1)-c1ccccn1 Show InChI InChI=1S/C29H29FN4O3/c1-19-27(24-4-2-3-9-31-24)32-25-14-20(30)5-7-22(25)28(19)34-16-29(17-35,18-36)23-8-6-21(15-26(23)34)33-10-12-37-13-11-33/h2-9,14-15,35-36H,10-13,16-18H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma by ATP bioluminescence assay |
J Med Chem 55: 7667-85 (2012)
Article DOI: 10.1021/jm300679u
BindingDB Entry DOI: 10.7270/Q2J38TQ0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50396615

(CHEMBL2171928 | US8765940, 4-(1-(7-fluoro-3-methyl...)Show SMILES Cc1cc(nc(N)n1)-c1ccc2c(c1)N(CC2(C)C)c1c(C)c(nc2cc(F)ccc12)-c1ccccn1 Show InChI InChI=1S/C30H27FN6/c1-17-13-24(36-29(32)34-17)19-8-11-22-26(14-19)37(16-30(22,3)4)28-18(2)27(23-7-5-6-12-33-23)35-25-15-20(31)9-10-21(25)28/h5-15H,16H2,1-4H3,(H2,32,34,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta using phosphatidylinositol-4,5-bisphosphate by ATP bioluminescence assay |
J Med Chem 55: 7667-85 (2012)
Article DOI: 10.1021/jm300679u
BindingDB Entry DOI: 10.7270/Q2J38TQ0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50396615

(CHEMBL2171928 | US8765940, 4-(1-(7-fluoro-3-methyl...)Show SMILES Cc1cc(nc(N)n1)-c1ccc2c(c1)N(CC2(C)C)c1c(C)c(nc2cc(F)ccc12)-c1ccccn1 Show InChI InChI=1S/C30H27FN6/c1-17-13-24(36-29(32)34-17)19-8-11-22-26(14-19)37(16-30(22,3)4)28-18(2)27(23-7-5-6-12-33-23)35-25-15-20(31)9-10-21(25)28/h5-15H,16H2,1-4H3,(H2,32,34,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha by ATP bioluminescence assay |
J Med Chem 55: 7667-85 (2012)
Article DOI: 10.1021/jm300679u
BindingDB Entry DOI: 10.7270/Q2J38TQ0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50396617

(CHEMBL2171925 | US8765940, (1-(7-fluoro-3-methyl-2...)Show SMILES Cc1c(nc2cc(F)ccc2c1N1CC(CO)(CO)c2ccc(cc12)N1CCOCC1)-c1ccccn1 Show InChI InChI=1S/C29H29FN4O3/c1-19-27(24-4-2-3-9-31-24)32-25-14-20(30)5-7-22(25)28(19)34-16-29(17-35,18-36)23-8-6-21(15-26(23)34)33-10-12-37-13-11-33/h2-9,14-15,35-36H,10-13,16-18H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha by ATP bioluminescence assay |
J Med Chem 55: 7667-85 (2012)
Article DOI: 10.1021/jm300679u
BindingDB Entry DOI: 10.7270/Q2J38TQ0 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50448966
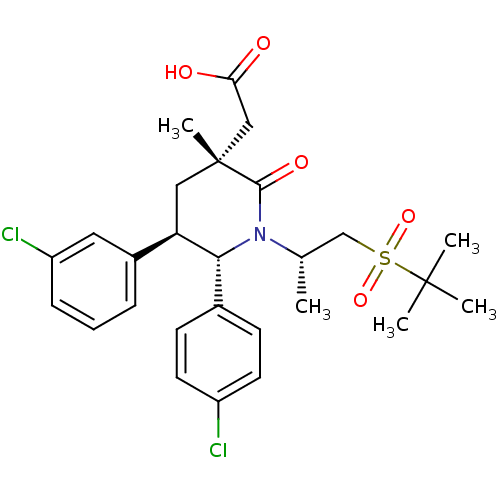
(CHEMBL3125534)Show SMILES C[C@@H](CS(=O)(=O)C(C)(C)C)N1[C@@H]([C@H](C[C@](C)(CC(O)=O)C1=O)c1cccc(Cl)c1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C27H33Cl2NO5S/c1-17(16-36(34,35)26(2,3)4)30-24(18-9-11-20(28)12-10-18)22(19-7-6-8-21(29)13-19)14-27(5,25(30)33)15-23(31)32/h6-13,17,22,24H,14-16H2,1-5H3,(H,31,32)/t17-,22+,24+,27+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human GST-thrombin-tagged MDM2 assessed as inhibition of interaction with human p53 after 1 hr by HTRF assay |
J Med Chem 57: 1454-72 (2014)
Article DOI: 10.1021/jm401753e
BindingDB Entry DOI: 10.7270/Q24M960Z |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50448967
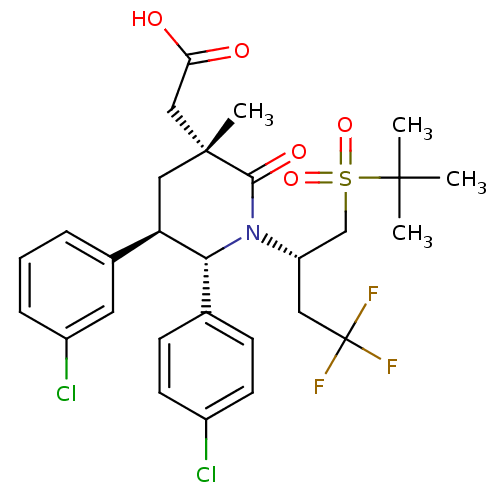
(CHEMBL3125533)Show SMILES CC(C)(C)S(=O)(=O)C[C@H](CC(F)(F)F)N1[C@@H]([C@H](C[C@](C)(CC(O)=O)C1=O)c1cccc(Cl)c1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C28H32Cl2F3NO5S/c1-26(2,3)40(38,39)16-21(13-28(31,32)33)34-24(17-8-10-19(29)11-9-17)22(18-6-5-7-20(30)12-18)14-27(4,25(34)37)15-23(35)36/h5-12,21-22,24H,13-16H2,1-4H3,(H,35,36)/t21-,22+,24+,27+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human GST-thrombin-tagged MDM2 assessed as inhibition of interaction with human p53 after 1 hr by HTRF assay |
J Med Chem 57: 1454-72 (2014)
Article DOI: 10.1021/jm401753e
BindingDB Entry DOI: 10.7270/Q24M960Z |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50448962
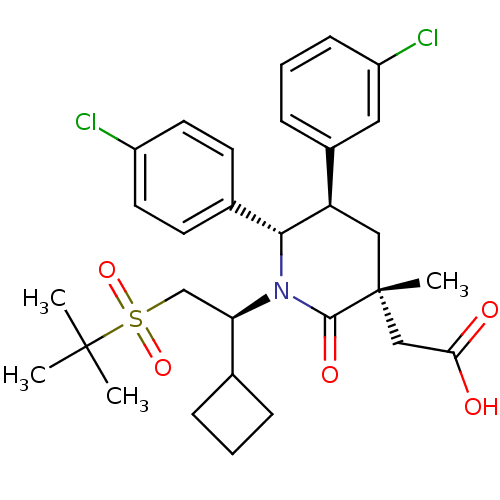
(CHEMBL3125538)Show SMILES CC(C)(C)S(=O)(=O)C[C@H](C1CCC1)N1[C@@H]([C@H](C[C@](C)(CC(O)=O)C1=O)c1cccc(Cl)c1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C30H37Cl2NO5S/c1-29(2,3)39(37,38)18-25(19-7-5-8-19)33-27(20-11-13-22(31)14-12-20)24(21-9-6-10-23(32)15-21)16-30(4,28(33)36)17-26(34)35/h6,9-15,19,24-25,27H,5,7-8,16-18H2,1-4H3,(H,34,35)/t24-,25-,27-,30-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human GST-thrombin-tagged MDM2 assessed as inhibition of interaction with human p53 after 1 hr by HTRF assay |
J Med Chem 57: 1454-72 (2014)
Article DOI: 10.1021/jm401753e
BindingDB Entry DOI: 10.7270/Q24M960Z |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50448958
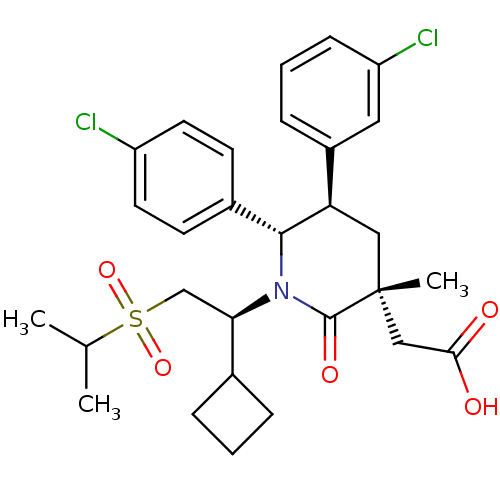
(CHEMBL3125698)Show SMILES CC(C)S(=O)(=O)C[C@H](C1CCC1)N1[C@@H]([C@H](C[C@](C)(CC(O)=O)C1=O)c1cccc(Cl)c1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C29H35Cl2NO5S/c1-18(2)38(36,37)17-25(19-6-4-7-19)32-27(20-10-12-22(30)13-11-20)24(21-8-5-9-23(31)14-21)15-29(3,28(32)35)16-26(33)34/h5,8-14,18-19,24-25,27H,4,6-7,15-17H2,1-3H3,(H,33,34)/t24-,25-,27-,29-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human GST-thrombin-tagged MDM2 assessed as inhibition of interaction with human p53 after 1 hr by HTRF assay |
J Med Chem 57: 1454-72 (2014)
Article DOI: 10.1021/jm401753e
BindingDB Entry DOI: 10.7270/Q24M960Z |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50448963
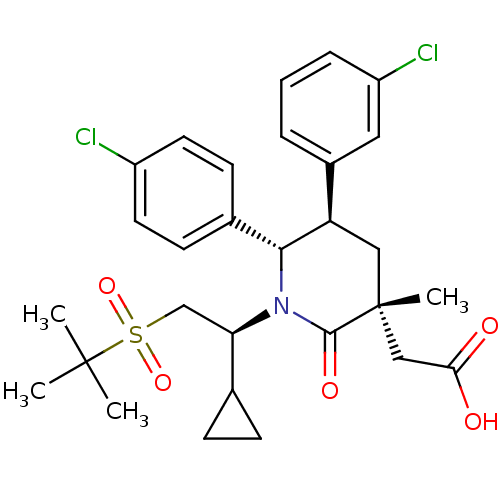
(CHEMBL3125537 | US9296736, 351 | US9593129, Exampl...)Show SMILES CC(C)(C)S(=O)(=O)C[C@H](C1CC1)N1[C@@H]([C@H](C[C@](C)(CC(O)=O)C1=O)c1cccc(Cl)c1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C29H35Cl2NO5S/c1-28(2,3)38(36,37)17-24(18-8-9-18)32-26(19-10-12-21(30)13-11-19)23(20-6-5-7-22(31)14-20)15-29(4,27(32)35)16-25(33)34/h5-7,10-14,18,23-24,26H,8-9,15-17H2,1-4H3,(H,33,34)/t23-,24-,26-,29-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human GST-thrombin-tagged MDM2 assessed as inhibition of interaction with human p53 after 1 hr by HTRF assay |
J Med Chem 57: 1454-72 (2014)
Article DOI: 10.1021/jm401753e
BindingDB Entry DOI: 10.7270/Q24M960Z |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50448936
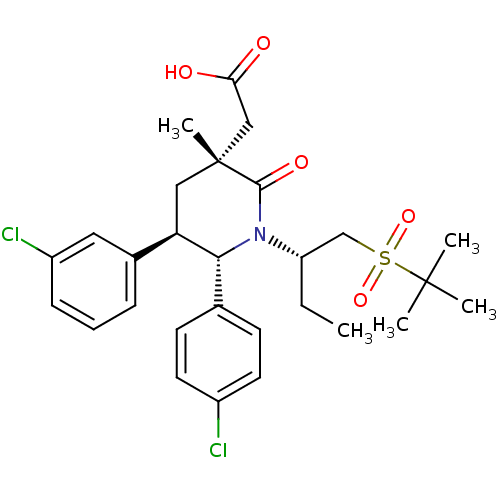
(CHEMBL3125527 | US9296736, 342 | US9593129, Exampl...)Show SMILES CC[C@@H](CS(=O)(=O)C(C)(C)C)N1[C@@H]([C@H](C[C@](C)(CC(O)=O)C1=O)c1cccc(Cl)c1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C28H35Cl2NO5S/c1-6-22(17-37(35,36)27(2,3)4)31-25(18-10-12-20(29)13-11-18)23(19-8-7-9-21(30)14-19)15-28(5,26(31)34)16-24(32)33/h7-14,22-23,25H,6,15-17H2,1-5H3,(H,32,33)/t22-,23+,25+,28+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human GST-thrombin-tagged MDM2 assessed as inhibition of interaction with human p53 after 1 hr by HTRF assay |
J Med Chem 57: 1454-72 (2014)
Article DOI: 10.1021/jm401753e
BindingDB Entry DOI: 10.7270/Q24M960Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50448945
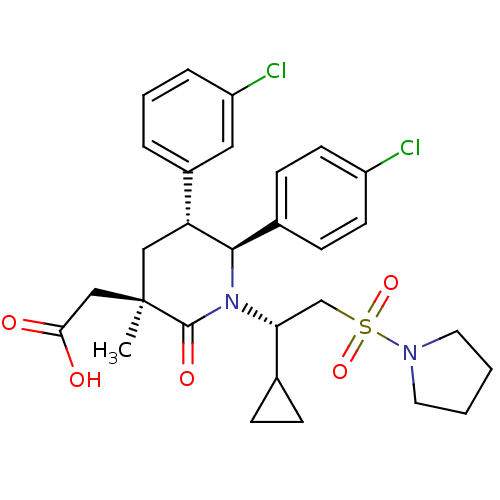
(CHEMBL3125521 | US9296736, 381 | US9593129, Exampl...)Show SMILES C[C@]1(CC(O)=O)C[C@@H]([C@H](N([C@H](CS(=O)(=O)N2CCCC2)C2CC2)C1=O)c1ccc(Cl)cc1)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C29H34Cl2N2O5S/c1-29(17-26(34)35)16-24(21-5-4-6-23(31)15-21)27(20-9-11-22(30)12-10-20)33(28(29)36)25(19-7-8-19)18-39(37,38)32-13-2-3-14-32/h4-6,9-12,15,19,24-25,27H,2-3,7-8,13-14,16-18H2,1H3,(H,34,35)/t24-,25-,27-,29-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human GST-thrombin-tagged MDM2 assessed as inhibition of interaction with human p53 after 1 hr by HTRF assay |
J Med Chem 57: 1454-72 (2014)
Article DOI: 10.1021/jm401753e
BindingDB Entry DOI: 10.7270/Q24M960Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50448942
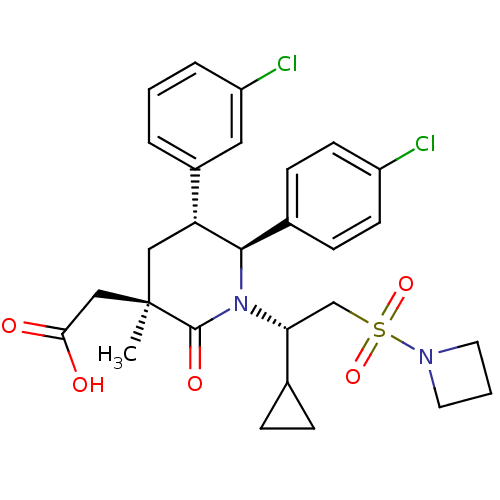
(CHEMBL3125520 | US9296736, 382 | US9593129, Exampl...)Show SMILES C[C@]1(CC(O)=O)C[C@@H]([C@H](N([C@H](CS(=O)(=O)N2CCC2)C2CC2)C1=O)c1ccc(Cl)cc1)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C28H32Cl2N2O5S/c1-28(16-25(33)34)15-23(20-4-2-5-22(30)14-20)26(19-8-10-21(29)11-9-19)32(27(28)35)24(18-6-7-18)17-38(36,37)31-12-3-13-31/h2,4-5,8-11,14,18,23-24,26H,3,6-7,12-13,15-17H2,1H3,(H,33,34)/t23-,24-,26-,28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human GST-thrombin-tagged MDM2 assessed as inhibition of interaction with human p53 after 1 hr by HTRF assay |
J Med Chem 57: 1454-72 (2014)
Article DOI: 10.1021/jm401753e
BindingDB Entry DOI: 10.7270/Q24M960Z |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50448955
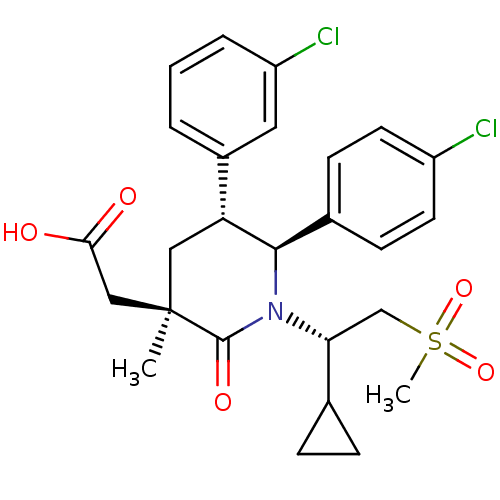
(CHEMBL3125701 | US9296736, 354 | US9593129, Exampl...)Show SMILES C[C@]1(CC(O)=O)C[C@@H]([C@H](N([C@H](CS(C)(=O)=O)C2CC2)C1=O)c1ccc(Cl)cc1)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C26H29Cl2NO5S/c1-26(14-23(30)31)13-21(18-4-3-5-20(28)12-18)24(17-8-10-19(27)11-9-17)29(25(26)32)22(16-6-7-16)15-35(2,33)34/h3-5,8-12,16,21-22,24H,6-7,13-15H2,1-2H3,(H,30,31)/t21-,22-,24-,26-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human GST-thrombin-tagged MDM2 assessed as inhibition of interaction with human p53 after 1 hr by HTRF assay |
J Med Chem 57: 1454-72 (2014)
Article DOI: 10.1021/jm401753e
BindingDB Entry DOI: 10.7270/Q24M960Z |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50448970
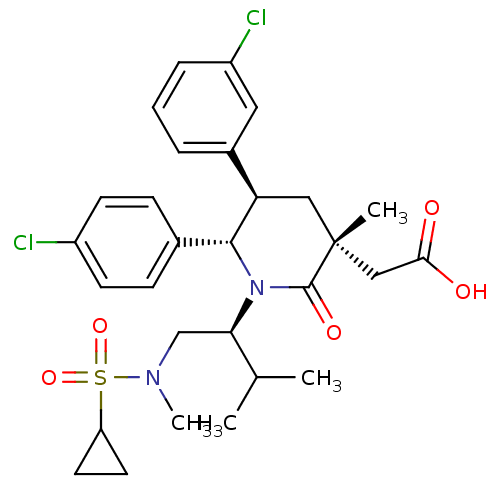
(CHEMBL3125518 | US9296736, 295 | US9593129, Exampl...)Show SMILES CC(C)[C@@H](CN(C)S(=O)(=O)C1CC1)N1[C@@H]([C@H](C[C@](C)(CC(O)=O)C1=O)c1cccc(Cl)c1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C29H36Cl2N2O5S/c1-18(2)25(17-32(4)39(37,38)23-12-13-23)33-27(19-8-10-21(30)11-9-19)24(20-6-5-7-22(31)14-20)15-29(3,28(33)36)16-26(34)35/h5-11,14,18,23-25,27H,12-13,15-17H2,1-4H3,(H,34,35)/t24-,25-,27-,29-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human GST-thrombin-tagged MDM2 assessed as inhibition of interaction with human p53 after 1 hr by HTRF assay |
J Med Chem 57: 1454-72 (2014)
Article DOI: 10.1021/jm401753e
BindingDB Entry DOI: 10.7270/Q24M960Z |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50448969
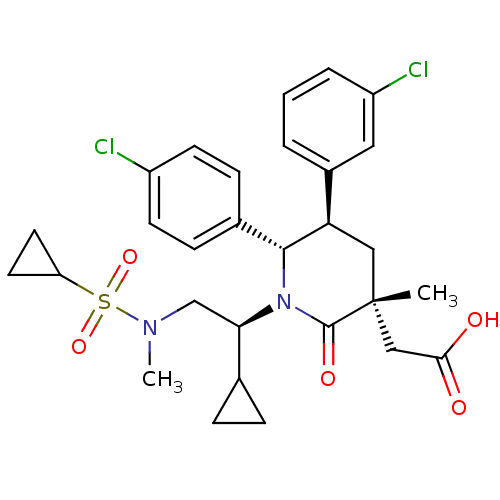
(CHEMBL3125517 | US9296736, 256 | US9593129, Exampl...)Show SMILES CN(C[C@H](C1CC1)N1[C@@H]([C@H](C[C@](C)(CC(O)=O)C1=O)c1cccc(Cl)c1)c1ccc(Cl)cc1)S(=O)(=O)C1CC1 |r| Show InChI InChI=1S/C29H34Cl2N2O5S/c1-29(16-26(34)35)15-24(20-4-3-5-22(31)14-20)27(19-8-10-21(30)11-9-19)33(28(29)36)25(18-6-7-18)17-32(2)39(37,38)23-12-13-23/h3-5,8-11,14,18,23-25,27H,6-7,12-13,15-17H2,1-2H3,(H,34,35)/t24-,25-,27-,29-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human GST-thrombin-tagged MDM2 assessed as inhibition of interaction with human p53 after 1 hr by HTRF assay |
J Med Chem 57: 1454-72 (2014)
Article DOI: 10.1021/jm401753e
BindingDB Entry DOI: 10.7270/Q24M960Z |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50448938
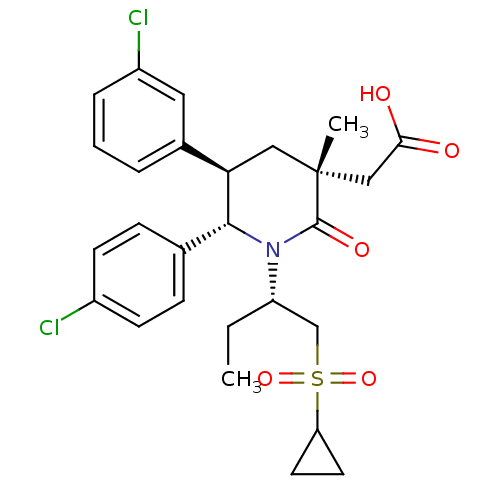
(CHEMBL3125525 | US9296736, 340 | US9593129, Exampl...)Show SMILES CC[C@@H](CS(=O)(=O)C1CC1)N1[C@@H]([C@H](C[C@](C)(CC(O)=O)C1=O)c1cccc(Cl)c1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C27H31Cl2NO5S/c1-3-21(16-36(34,35)22-11-12-22)30-25(17-7-9-19(28)10-8-17)23(18-5-4-6-20(29)13-18)14-27(2,26(30)33)15-24(31)32/h4-10,13,21-23,25H,3,11-12,14-16H2,1-2H3,(H,31,32)/t21-,23+,25+,27+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human GST-thrombin-tagged MDM2 assessed as inhibition of interaction with human p53 after 1 hr by HTRF assay |
J Med Chem 57: 1454-72 (2014)
Article DOI: 10.1021/jm401753e
BindingDB Entry DOI: 10.7270/Q24M960Z |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50448937
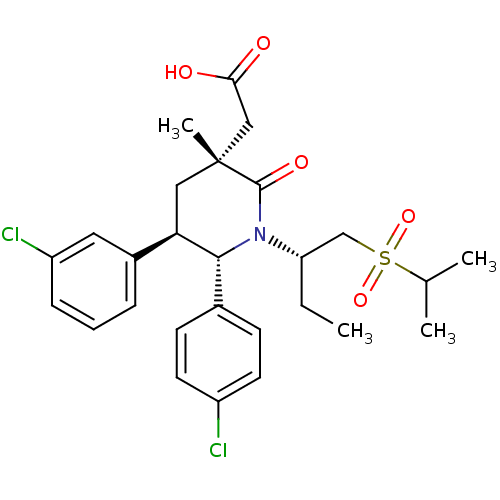
(CHEMBL3125526 | US9296736, 341 | US9593129, Exampl...)Show SMILES CC[C@@H](CS(=O)(=O)C(C)C)N1[C@@H]([C@H](C[C@](C)(CC(O)=O)C1=O)c1cccc(Cl)c1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C27H33Cl2NO5S/c1-5-22(16-36(34,35)17(2)3)30-25(18-9-11-20(28)12-10-18)23(19-7-6-8-21(29)13-19)14-27(4,26(30)33)15-24(31)32/h6-13,17,22-23,25H,5,14-16H2,1-4H3,(H,31,32)/t22-,23+,25+,27+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human GST-thrombin-tagged MDM2 assessed as inhibition of interaction with human p53 after 1 hr by HTRF assay |
J Med Chem 57: 1454-72 (2014)
Article DOI: 10.1021/jm401753e
BindingDB Entry DOI: 10.7270/Q24M960Z |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50448935
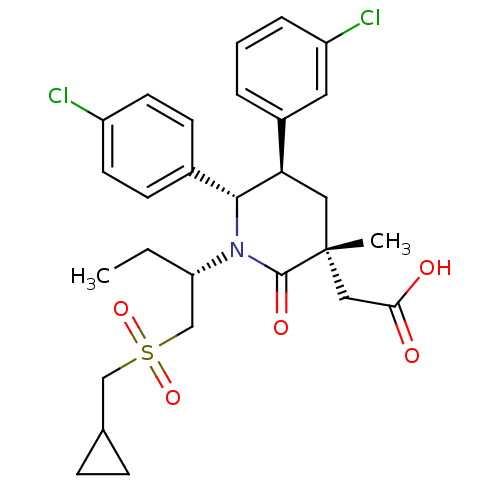
(CHEMBL3125528 | US9296736, 304 | US9593129, Exampl...)Show SMILES CC[C@@H](CS(=O)(=O)CC1CC1)N1[C@@H]([C@H](C[C@](C)(CC(O)=O)C1=O)c1cccc(Cl)c1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C28H33Cl2NO5S/c1-3-23(17-37(35,36)16-18-7-8-18)31-26(19-9-11-21(29)12-10-19)24(20-5-4-6-22(30)13-20)14-28(2,27(31)34)15-25(32)33/h4-6,9-13,18,23-24,26H,3,7-8,14-17H2,1-2H3,(H,32,33)/t23-,24+,26+,28+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human GST-thrombin-tagged MDM2 assessed as inhibition of interaction with human p53 after 1 hr by HTRF assay |
J Med Chem 57: 1454-72 (2014)
Article DOI: 10.1021/jm401753e
BindingDB Entry DOI: 10.7270/Q24M960Z |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50448934
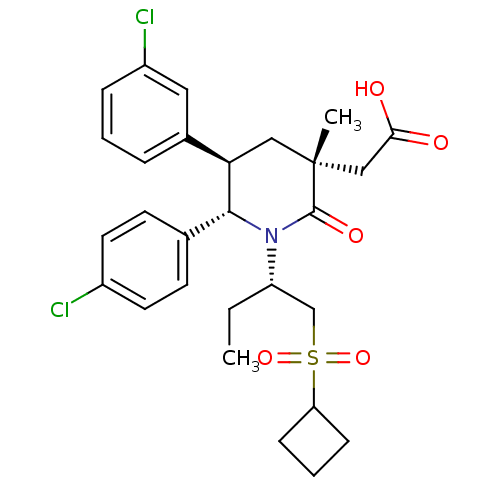
(CHEMBL3125529 | US9296736, 343 | US9593129, Exampl...)Show SMILES CC[C@@H](CS(=O)(=O)C1CCC1)N1[C@@H]([C@H](C[C@](C)(CC(O)=O)C1=O)c1cccc(Cl)c1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C28H33Cl2NO5S/c1-3-22(17-37(35,36)23-8-5-9-23)31-26(18-10-12-20(29)13-11-18)24(19-6-4-7-21(30)14-19)15-28(2,27(31)34)16-25(32)33/h4,6-7,10-14,22-24,26H,3,5,8-9,15-17H2,1-2H3,(H,32,33)/t22-,24+,26+,28+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human GST-thrombin-tagged MDM2 assessed as inhibition of interaction with human p53 after 1 hr by HTRF assay |
J Med Chem 57: 1454-72 (2014)
Article DOI: 10.1021/jm401753e
BindingDB Entry DOI: 10.7270/Q24M960Z |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50448932
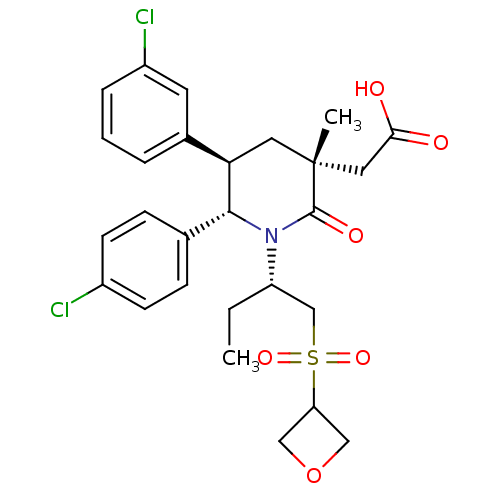
(CHEMBL3125531 | US9296736, 307 | US9593129, Exampl...)Show SMILES CC[C@@H](CS(=O)(=O)C1COC1)N1[C@@H]([C@H](C[C@](C)(CC(O)=O)C1=O)c1cccc(Cl)c1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C27H31Cl2NO6S/c1-3-21(16-37(34,35)22-14-36-15-22)30-25(17-7-9-19(28)10-8-17)23(18-5-4-6-20(29)11-18)12-27(2,26(30)33)13-24(31)32/h4-11,21-23,25H,3,12-16H2,1-2H3,(H,31,32)/t21-,23+,25+,27+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human GST-thrombin-tagged MDM2 assessed as inhibition of interaction with human p53 after 1 hr by HTRF assay |
J Med Chem 57: 1454-72 (2014)
Article DOI: 10.1021/jm401753e
BindingDB Entry DOI: 10.7270/Q24M960Z |
More data for this
Ligand-Target Pair | |
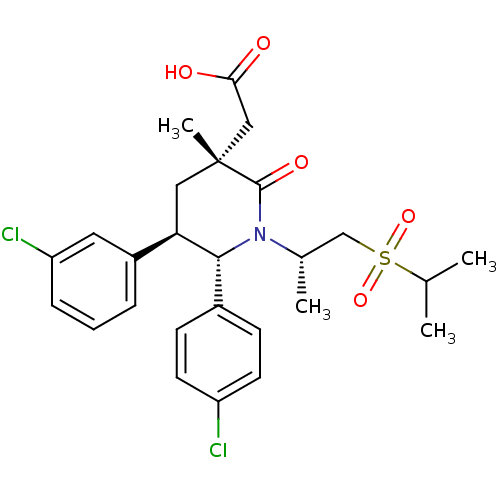
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50448961
(CHEMBL3125539)Show SMILES CC(C)S(=O)(=O)C[C@H](C)N1[C@@H]([C@H](C[C@](C)(CC(O)=O)C1=O)c1cccc(Cl)c1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C26H31Cl2NO5S/c1-16(2)35(33,34)15-17(3)29-24(18-8-10-20(27)11-9-18)22(19-6-5-7-21(28)12-19)13-26(4,25(29)32)14-23(30)31/h5-12,16-17,22,24H,13-15H2,1-4H3,(H,30,31)/t17-,22+,24+,26+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human GST-thrombin-tagged MDM2 assessed as inhibition of interaction with human p53 after 1 hr by HTRF assay |
J Med Chem 57: 1454-72 (2014)
Article DOI: 10.1021/jm401753e
BindingDB Entry DOI: 10.7270/Q24M960Z |
More data for this
Ligand-Target Pair | |
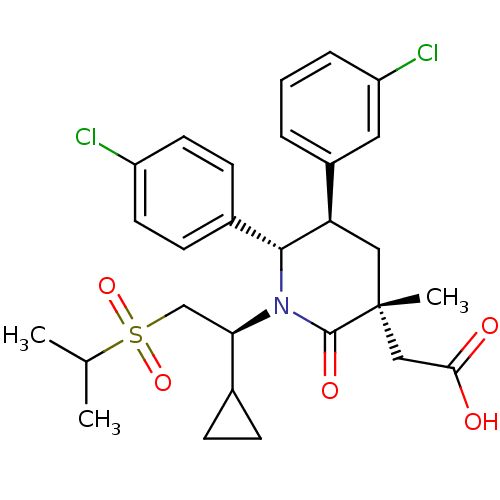
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50448959
(CHEMBL3125697 | US9296736, 350 | US9593129, Exampl...)Show SMILES CC(C)S(=O)(=O)C[C@H](C1CC1)N1[C@@H]([C@H](C[C@](C)(CC(O)=O)C1=O)c1cccc(Cl)c1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C28H33Cl2NO5S/c1-17(2)37(35,36)16-24(18-7-8-18)31-26(19-9-11-21(29)12-10-19)23(20-5-4-6-22(30)13-20)14-28(3,27(31)34)15-25(32)33/h4-6,9-13,17-18,23-24,26H,7-8,14-16H2,1-3H3,(H,32,33)/t23-,24-,26-,28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human GST-thrombin-tagged MDM2 assessed as inhibition of interaction with human p53 after 1 hr by HTRF assay |
J Med Chem 57: 1454-72 (2014)
Article DOI: 10.1021/jm401753e
BindingDB Entry DOI: 10.7270/Q24M960Z |
More data for this
Ligand-Target Pair | |
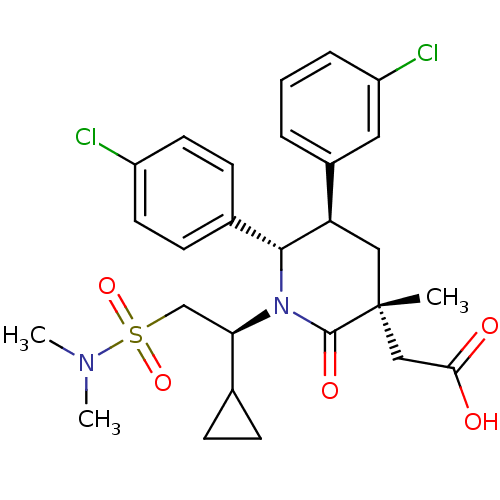
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50448948
(CHEMBL3125519 | US9296736, 377 | US9593129, Exampl...)Show SMILES CN(C)S(=O)(=O)C[C@H](C1CC1)N1[C@@H]([C@H](C[C@](C)(CC(O)=O)C1=O)c1cccc(Cl)c1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C27H32Cl2N2O5S/c1-27(15-24(32)33)14-22(19-5-4-6-21(29)13-19)25(18-9-11-20(28)12-10-18)31(26(27)34)23(17-7-8-17)16-37(35,36)30(2)3/h4-6,9-13,17,22-23,25H,7-8,14-16H2,1-3H3,(H,32,33)/t22-,23-,25-,27-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human GST-thrombin-tagged MDM2 assessed as inhibition of interaction with human p53 after 1 hr by HTRF assay |
J Med Chem 57: 1454-72 (2014)
Article DOI: 10.1021/jm401753e
BindingDB Entry DOI: 10.7270/Q24M960Z |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50448941
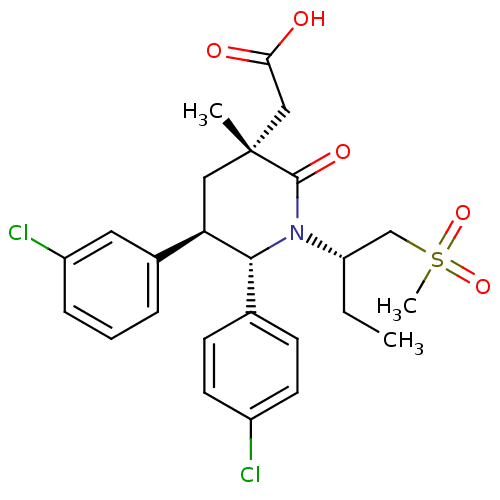
(CHEMBL3125522 | US9296736, 301 | US9593129, Exampl...)Show SMILES CC[C@@H](CS(C)(=O)=O)N1[C@@H]([C@H](C[C@](C)(CC(O)=O)C1=O)c1cccc(Cl)c1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C25H29Cl2NO5S/c1-4-20(15-34(3,32)33)28-23(16-8-10-18(26)11-9-16)21(17-6-5-7-19(27)12-17)13-25(2,24(28)31)14-22(29)30/h5-12,20-21,23H,4,13-15H2,1-3H3,(H,29,30)/t20-,21+,23+,25+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human GST-thrombin-tagged MDM2 assessed as inhibition of interaction with human p53 after 1 hr by HTRF assay |
J Med Chem 57: 1454-72 (2014)
Article DOI: 10.1021/jm401753e
BindingDB Entry DOI: 10.7270/Q24M960Z |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50448933
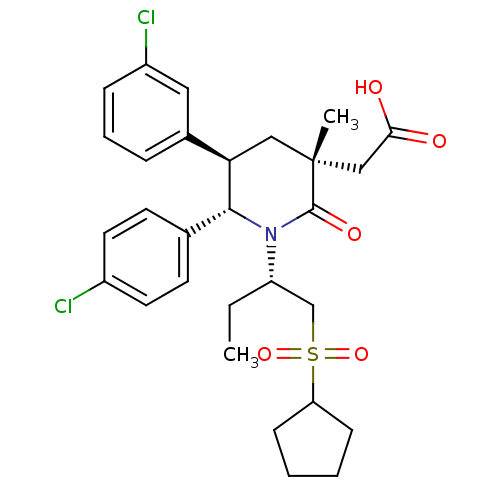
(CHEMBL3125530 | US9296736, 306 | US9593129, Exampl...)Show SMILES CC[C@@H](CS(=O)(=O)C1CCCC1)N1[C@@H]([C@H](C[C@](C)(CC(O)=O)C1=O)c1cccc(Cl)c1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C29H35Cl2NO5S/c1-3-23(18-38(36,37)24-9-4-5-10-24)32-27(19-11-13-21(30)14-12-19)25(20-7-6-8-22(31)15-20)16-29(2,28(32)35)17-26(33)34/h6-8,11-15,23-25,27H,3-5,9-10,16-18H2,1-2H3,(H,33,34)/t23-,25+,27+,29+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human GST-thrombin-tagged MDM2 assessed as inhibition of interaction with human p53 after 1 hr by HTRF assay |
J Med Chem 57: 1454-72 (2014)
Article DOI: 10.1021/jm401753e
BindingDB Entry DOI: 10.7270/Q24M960Z |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50448931
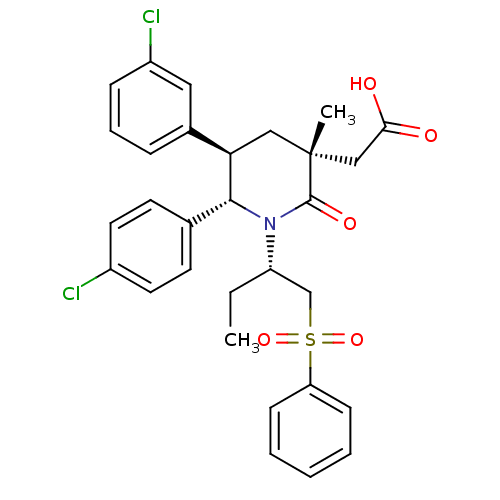
(CHEMBL3125532)Show SMILES CC[C@@H](CS(=O)(=O)c1ccccc1)N1[C@@H]([C@H](C[C@](C)(CC(O)=O)C1=O)c1cccc(Cl)c1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C30H31Cl2NO5S/c1-3-24(19-39(37,38)25-10-5-4-6-11-25)33-28(20-12-14-22(31)15-13-20)26(21-8-7-9-23(32)16-21)17-30(2,29(33)36)18-27(34)35/h4-16,24,26,28H,3,17-19H2,1-2H3,(H,34,35)/t24-,26+,28+,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human GST-thrombin-tagged MDM2 assessed as inhibition of interaction with human p53 after 1 hr by HTRF assay |
J Med Chem 57: 1454-72 (2014)
Article DOI: 10.1021/jm401753e
BindingDB Entry DOI: 10.7270/Q24M960Z |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data