

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein (Hepatitis C virus) | BDBM50158804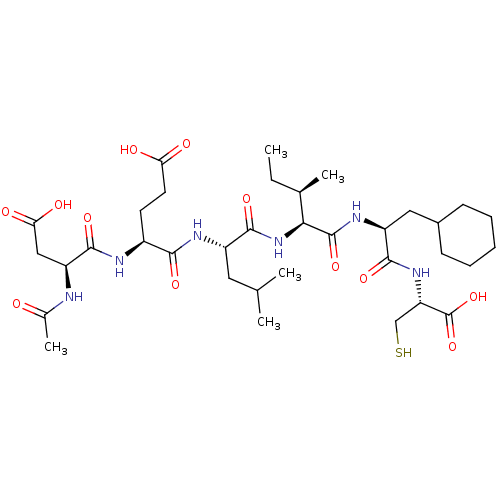 (AcAsp-D-Gla-Leu-Ile-Cha-Cys | CHEMBL179084) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Inhibitory constant against hepatitis C virus NS3 protease | J Med Chem 48: 1-20 (2005) Article DOI: 10.1021/jm0400101 BindingDB Entry DOI: 10.7270/Q2XP75Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50110121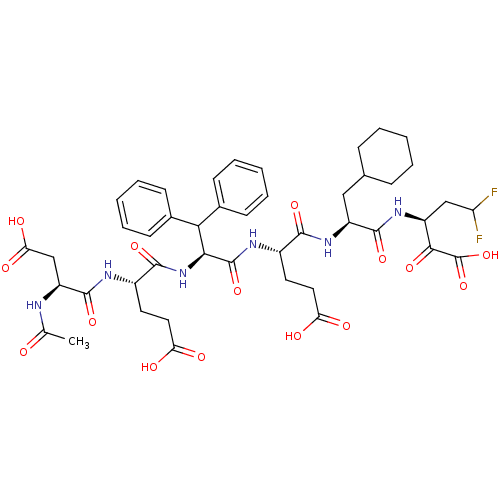 (3-[2-(2-{2-[2-(2-Acetylamino-3-carboxy-propionylam...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Inhibitory constant against hepatitis C virus NS3 protease | J Med Chem 48: 1-20 (2005) Article DOI: 10.1021/jm0400101 BindingDB Entry DOI: 10.7270/Q2XP75Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50158796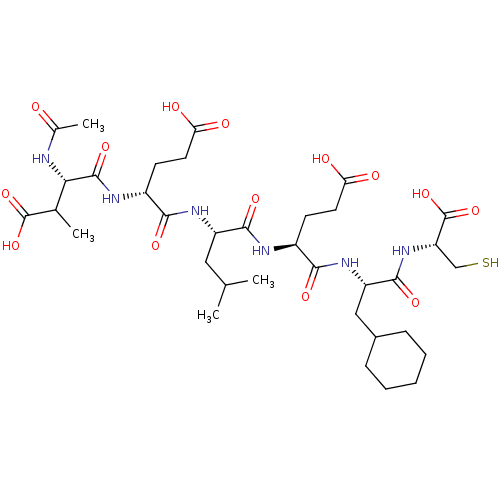 (AcAsp-D-Glu-Leu-Glu-Cha-Cys | CHEMBL360025) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Inhibitory constant against hepatitis C virus NS3 protease | J Med Chem 48: 1-20 (2005) Article DOI: 10.1021/jm0400101 BindingDB Entry DOI: 10.7270/Q2XP75Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50084685 (4-(2-Acetylamino-3-carboxy-propionylamino)-4-(1-{3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Inhibitory constant against hepatitis C virus NS3 protease | J Med Chem 48: 1-20 (2005) Article DOI: 10.1021/jm0400101 BindingDB Entry DOI: 10.7270/Q2XP75Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50158798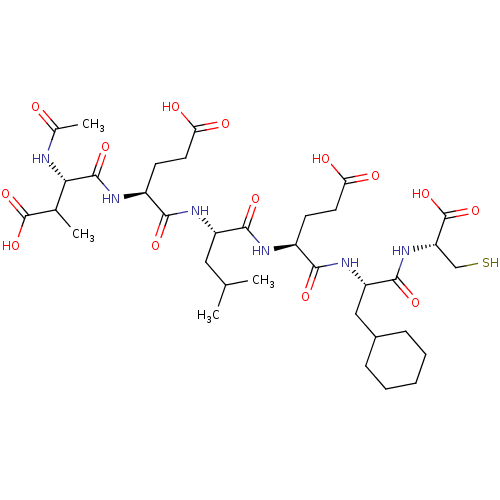 (AcAsp-Glu-Leu-Glu-Cha-Cys | CHEMBL178986) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Inhibitory constant against hepatitis C virus NS3 protease | J Med Chem 48: 1-20 (2005) Article DOI: 10.1021/jm0400101 BindingDB Entry DOI: 10.7270/Q2XP75Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50158793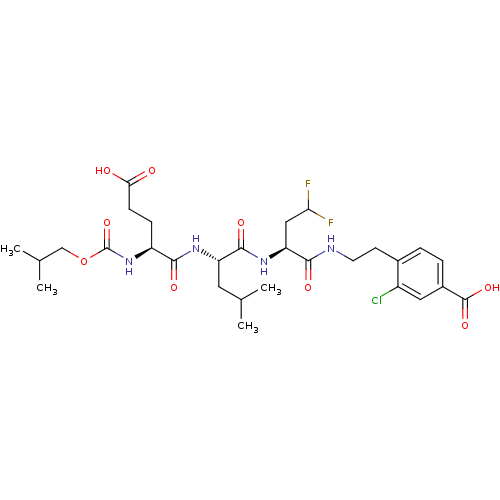 (4-(2-{(S)-2-[(S)-2-((S)-4-Carboxy-2-isobutoxycarbo...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | MMDB Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Inhibitory constant against hepatitis C virus NS3 protease | J Med Chem 48: 1-20 (2005) Article DOI: 10.1021/jm0400101 BindingDB Entry DOI: 10.7270/Q2XP75Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50084634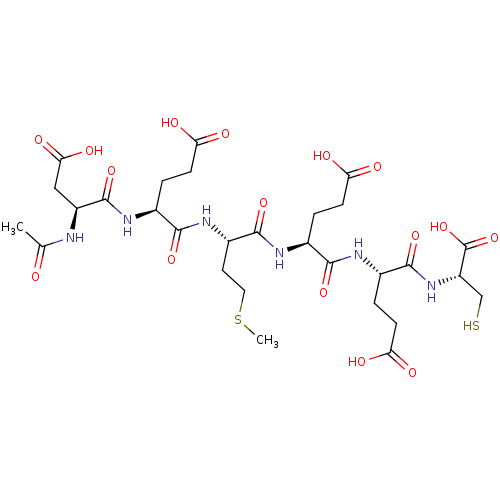 (4-(2-Acetylamino-3-carboxy-propionylamino)-4-(1-{3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Inhibitory constant against hepatitis C virus NS3 protease | J Med Chem 48: 1-20 (2005) Article DOI: 10.1021/jm0400101 BindingDB Entry DOI: 10.7270/Q2XP75Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50145828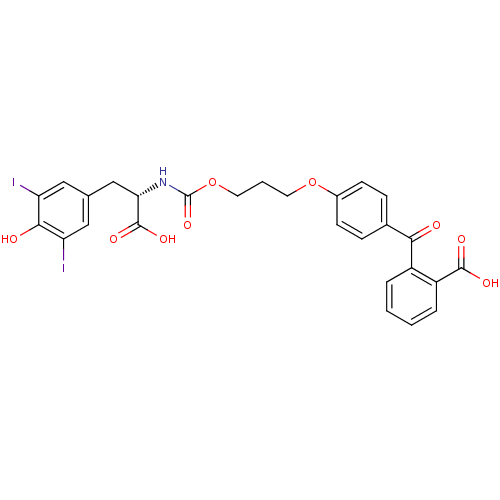 (2-(4-{3-[(S)-1-Carboxy-2-(4-hydroxy-3,5-diiodo-phe...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Inhibitory constant against hepatitis C virus NS3 protease | J Med Chem 48: 1-20 (2005) Article DOI: 10.1021/jm0400101 BindingDB Entry DOI: 10.7270/Q2XP75Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50145844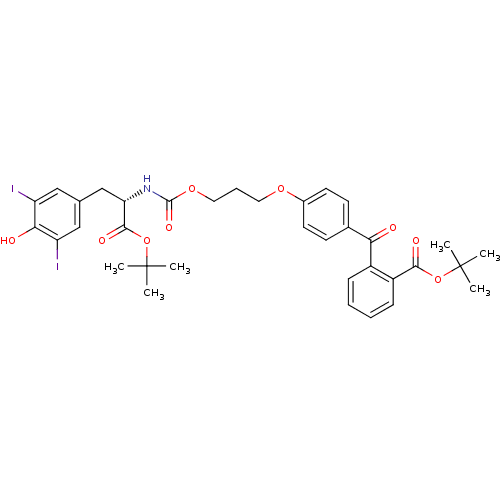 (2-(4-{3-[(S)-1-tert-Butoxycarbonyl-2-(4-hydroxy-3,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Inhibitory constant against hepatitis C virus NS3 protease | J Med Chem 48: 1-20 (2005) Article DOI: 10.1021/jm0400101 BindingDB Entry DOI: 10.7270/Q2XP75Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50126661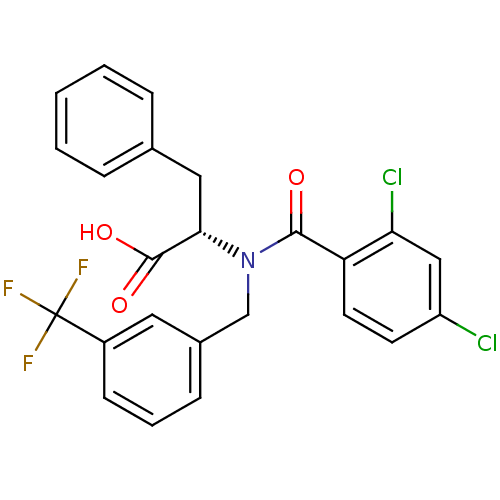 ((2S)-2-[(2,4-DICHLORO-BENZOYL)-(3-TRIFLUOROMETHYL-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Inhibitory constant against hepatitis C virus NS3 protease | J Med Chem 48: 1-20 (2005) Article DOI: 10.1021/jm0400101 BindingDB Entry DOI: 10.7270/Q2XP75Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50126669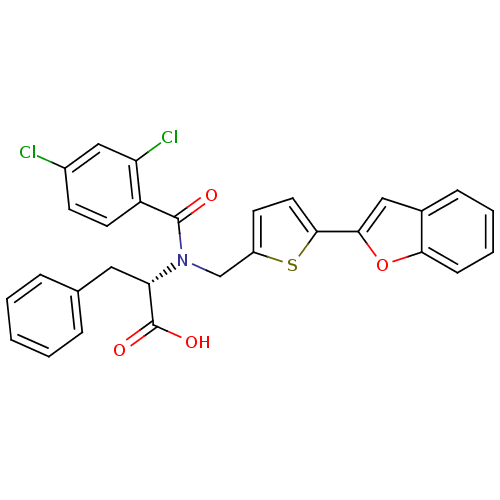 ((2S)-2-[(5-BENZOFURAN-2-YL-THIOPHEN-2-YLMETHYL)-(2...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Inhibitory constant against hepatitis C virus NS3 protease | J Med Chem 48: 1-20 (2005) Article DOI: 10.1021/jm0400101 BindingDB Entry DOI: 10.7270/Q2XP75Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sensory histidine kinase DcuS (Staphylococcus aureus) | BDBM50526860 (CHEMBL4554516) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterans Affairs Eastern Colorado Health Care System Curated by ChEMBL | Assay Description Inhibition of AgrC in mid-exponential phase Staphylococcus aureus RN6390B assessed as beta-lactamase activity incubated for 80 mins by spectrophotome... | J Med Chem 63: 2705-2730 (2020) Article DOI: 10.1021/acs.jmedchem.9b00798 BindingDB Entry DOI: 10.7270/Q2CF9TJ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sensory histidine kinase DcuS (Staphylococcus aureus) | BDBM50020375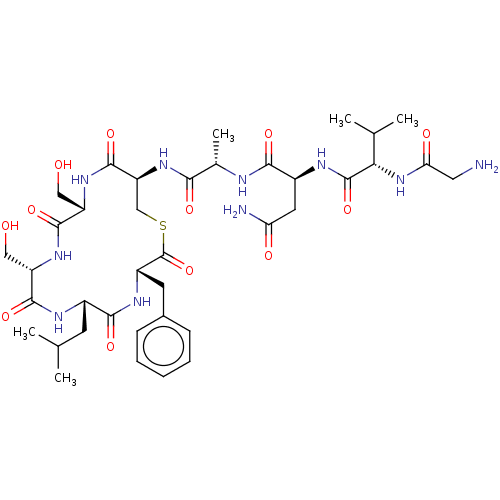 (CHEMBL3289785) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterans Affairs Eastern Colorado Health Care System Curated by ChEMBL | Assay Description Inhibition of AgrC in mid-exponential phase Staphylococcus aureus RN6390B assessed as beta-lactamase activity incubated for 80 mins by spectrophotome... | J Med Chem 63: 2705-2730 (2020) Article DOI: 10.1021/acs.jmedchem.9b00798 BindingDB Entry DOI: 10.7270/Q2CF9TJ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sensory histidine kinase DcuS (Staphylococcus aureus) | BDBM50526861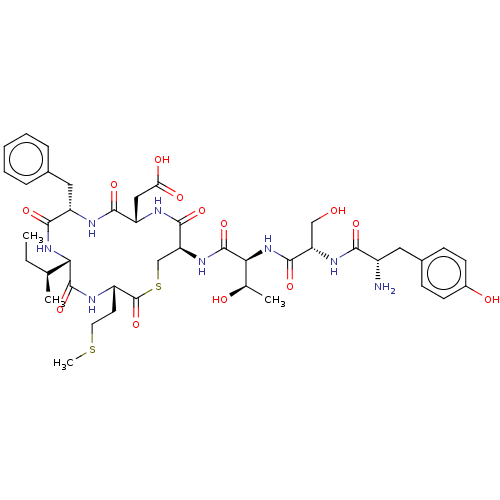 (CHEMBL2337554) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterans Affairs Eastern Colorado Health Care System Curated by ChEMBL | Assay Description Inhibition of AgrC in mid-exponential phase Staphylococcus aureus RN8463 assessed as beta-lactamase activity incubated for 80 mins by spectrophotomet... | J Med Chem 63: 2705-2730 (2020) Article DOI: 10.1021/acs.jmedchem.9b00798 BindingDB Entry DOI: 10.7270/Q2CF9TJ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sensory histidine kinase DcuS (Staphylococcus aureus) | BDBM50020375 (CHEMBL3289785) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterans Affairs Eastern Colorado Health Care System Curated by ChEMBL | Assay Description Inhibition of AgrC in mid-exponential phase Staphylococcus aureus RN8463 assessed as beta-lactamase activity incubated for 80 mins by spectrophotomet... | J Med Chem 63: 2705-2730 (2020) Article DOI: 10.1021/acs.jmedchem.9b00798 BindingDB Entry DOI: 10.7270/Q2CF9TJ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sensory histidine kinase DcuS (Staphylococcus aureus) | BDBM50526861 (CHEMBL2337554) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterans Affairs Eastern Colorado Health Care System Curated by ChEMBL | Assay Description Inhibition of AgrC in mid-exponential phase Staphylococcus aureus SA502A assessed as beta-lactamase activity incubated for 80 mins by spectrophotomet... | J Med Chem 63: 2705-2730 (2020) Article DOI: 10.1021/acs.jmedchem.9b00798 BindingDB Entry DOI: 10.7270/Q2CF9TJ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sensory histidine kinase DcuS (Staphylococcus aureus) | BDBM50526859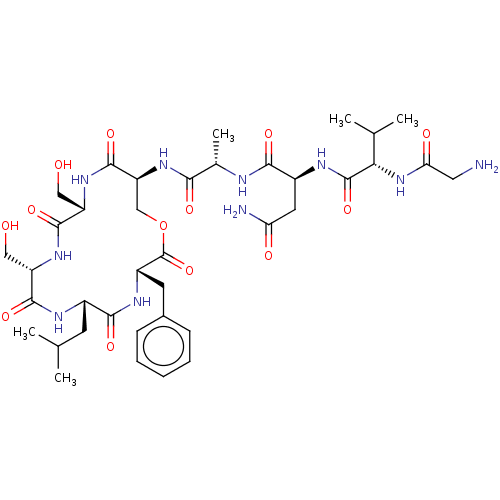 (CHEMBL4518791) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterans Affairs Eastern Colorado Health Care System Curated by ChEMBL | Assay Description Inhibition of AgrC in mid-exponential phase Staphylococcus aureus RN6390B assessed as beta-lactamase activity incubated for 80 mins by spectrophotome... | J Med Chem 63: 2705-2730 (2020) Article DOI: 10.1021/acs.jmedchem.9b00798 BindingDB Entry DOI: 10.7270/Q2CF9TJ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50158847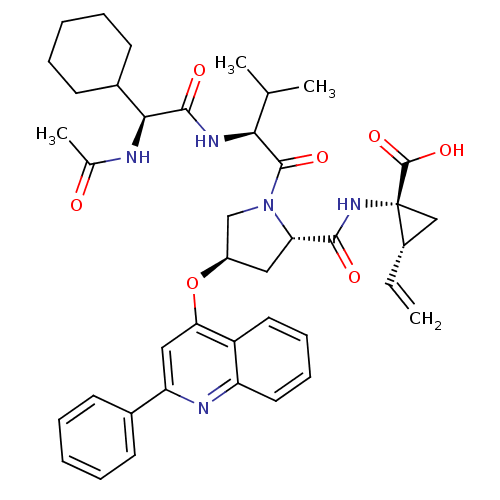 ((R)-1-{[(2S,4R)-1-[(S)-2-((S)-2-Acetylamino-2-cycl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Inhibitory concentration against hepatitis C virus NS3 protease | J Med Chem 48: 1-20 (2005) Article DOI: 10.1021/jm0400101 BindingDB Entry DOI: 10.7270/Q2XP75Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50158823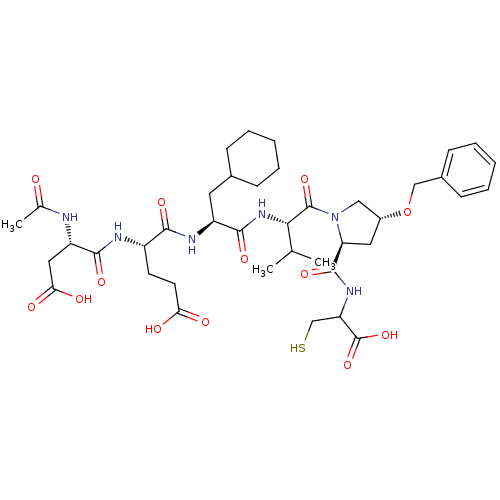 (AcAsp-Glu-Cha-Val-Prb-Cys | CHEMBL179963) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Inhibitory concentration against hepatitis C virus NS3 protease | J Med Chem 48: 1-20 (2005) Article DOI: 10.1021/jm0400101 BindingDB Entry DOI: 10.7270/Q2XP75Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50158837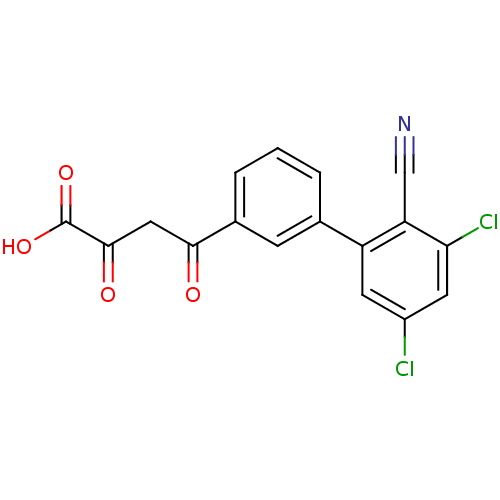 (4-(3'',5''-Dichloro-2''-cyano-biphenyl-3-yl)-2,4-d...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Inhibitory concentration against hepatitis C virus polymerase | J Med Chem 48: 1-20 (2005) Article DOI: 10.1021/jm0400101 BindingDB Entry DOI: 10.7270/Q2XP75Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50158813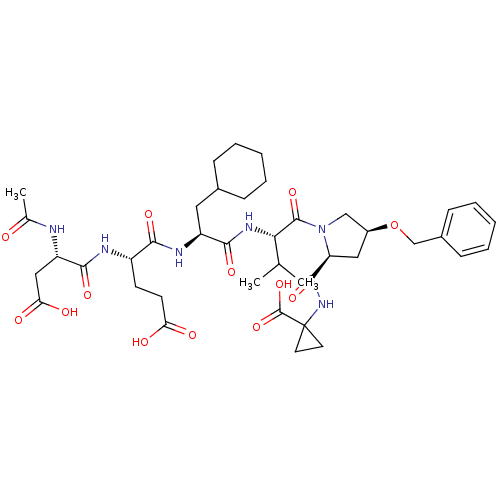 (AcAsp-Glu-Cha-Val-Prb-Cpg | CHEMBL360983) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Inhibitory concentration against hepatitis C virus NS3 protease | J Med Chem 48: 1-20 (2005) Article DOI: 10.1021/jm0400101 BindingDB Entry DOI: 10.7270/Q2XP75Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50370545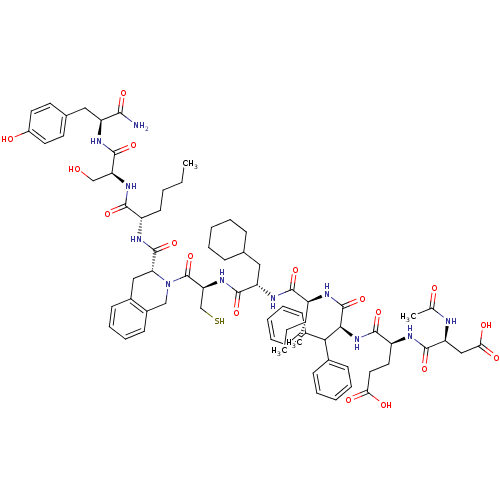 (CHEMBL1791289) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Inhibitory concentration against hepatitis C virus NS3 protease | J Med Chem 48: 1-20 (2005) Article DOI: 10.1021/jm0400101 BindingDB Entry DOI: 10.7270/Q2XP75Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50158845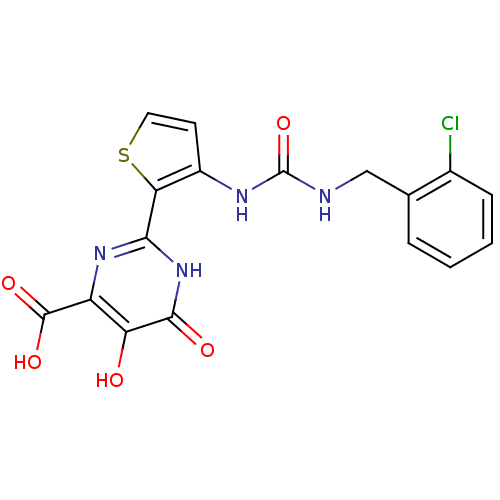 (2-(3-(3-(2-chlorobenzyl)ureido)thiophen-2-yl)-5,6-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Inhibitory concentration against hepatitis C virus polymerase | J Med Chem 48: 1-20 (2005) Article DOI: 10.1021/jm0400101 BindingDB Entry DOI: 10.7270/Q2XP75Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50158839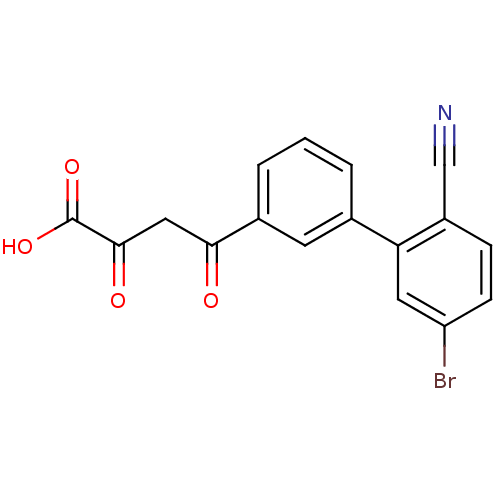 (4-(5''-Bromo-2''-cyano-biphenyl-3-yl)-2,4-dioxo-bu...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Inhibitory concentration against hepatitis C virus polymerase | J Med Chem 48: 1-20 (2005) Article DOI: 10.1021/jm0400101 BindingDB Entry DOI: 10.7270/Q2XP75Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50370548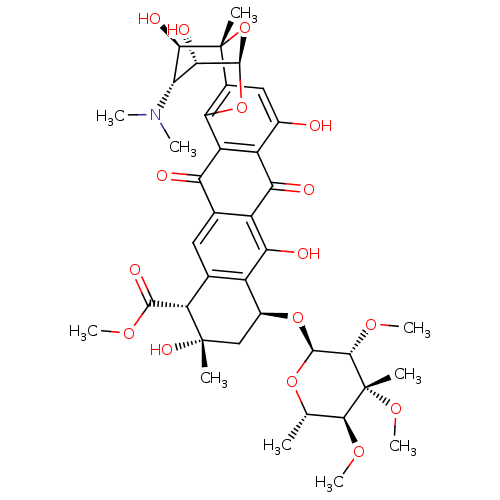 (NOGALAMYCIN) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Inhibitory concentration against hepatitis C virus helicase | J Med Chem 48: 1-20 (2005) Article DOI: 10.1021/jm0400101 BindingDB Entry DOI: 10.7270/Q2XP75Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50158815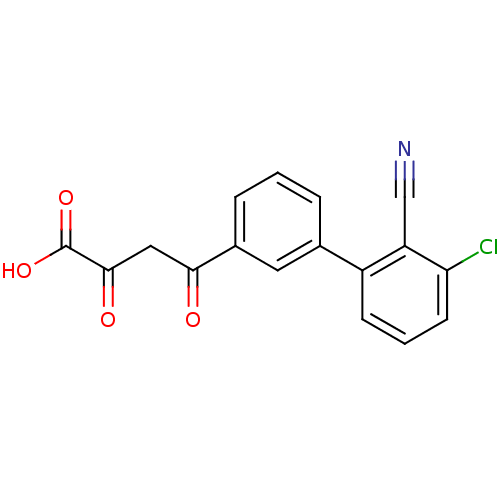 (4-(3''-Chloro-2''-cyano-biphenyl-3-yl)-2,4-dioxo-b...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Inhibitory concentration against hepatitis C virus polymerase | J Med Chem 48: 1-20 (2005) Article DOI: 10.1021/jm0400101 BindingDB Entry DOI: 10.7270/Q2XP75Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50158802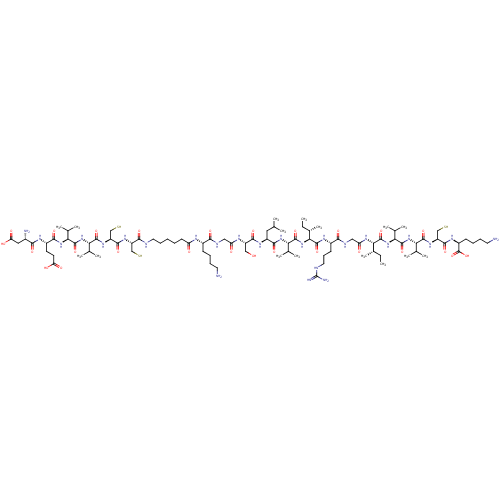 (Asp-Glu-Val-Val-Cys-Cys-L2-Lys-Gly-Ser-Leu-Val-Ile...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Inhibitory concentration against hepatitis C virus NS4A binding region of NS3 protease | J Med Chem 48: 1-20 (2005) Article DOI: 10.1021/jm0400101 BindingDB Entry DOI: 10.7270/Q2XP75Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-structural protein 4A (Hepatitis C virus) | BDBM50158808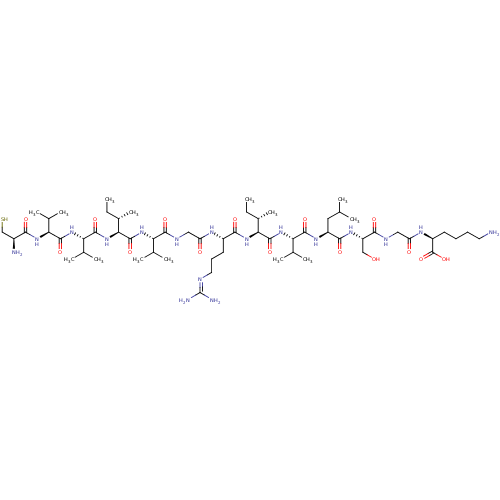 (CHEMBL408166 | Cys-Val-Val-Ile-Val-Gly-Arg-Ile-Val...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Inhibitory concentration against hepatitis C NS4A protease | J Med Chem 48: 1-20 (2005) Article DOI: 10.1021/jm0400101 BindingDB Entry DOI: 10.7270/Q2XP75Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50158811 (CHEMBL428797 | Glu-Asp-Val-Val-Cys-Cys-L1-Lys-Gly-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Inhibitory concentration against hepatitis C virus NS4A binding region of NS3 protease | J Med Chem 48: 1-20 (2005) Article DOI: 10.1021/jm0400101 BindingDB Entry DOI: 10.7270/Q2XP75Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-structural protein 4A (Hepatitis C virus) | BDBM50158824 (CHEMBL438893 | Lys-Gly-Ser-Leu-Val-Ile-Arg-Gly-Val...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Inhibitory concentration against hepatitis C virus NS4A active site | J Med Chem 48: 1-20 (2005) Article DOI: 10.1021/jm0400101 BindingDB Entry DOI: 10.7270/Q2XP75Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dynamin-2 (Homo sapiens (Human)) | BDBM50316543 (CHEMBL1094197 | N,N'-(Propane-1,3-diyl)bis(6,7-dih...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle Curated by ChEMBL | Assay Description Inhibition of recombinant dynamin 2 expressed in Sf9 cells by malachite green GTPase assay | J Med Chem 53: 4094-102 (2010) Article DOI: 10.1021/jm100119c BindingDB Entry DOI: 10.7270/Q2PZ5903 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50158822 (4-[2-(5-Bromo-2-cyano-phenoxy)-phenyl]-2,4-dioxo-b...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Inhibitory concentration against hepatitis C virus polymerase | J Med Chem 48: 1-20 (2005) Article DOI: 10.1021/jm0400101 BindingDB Entry DOI: 10.7270/Q2XP75Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dynamin-2 (Homo sapiens (Human)) | BDBM50316542 (CHEMBL1098764 | N,N'-(Propane-1,3-diyl)bis(7,8-dih...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle Curated by ChEMBL | Assay Description Inhibition of recombinant dynamin 2 expressed in Sf9 cells by malachite green GTPase assay | J Med Chem 53: 4094-102 (2010) Article DOI: 10.1021/jm100119c BindingDB Entry DOI: 10.7270/Q2PZ5903 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dynamin-2 (Homo sapiens (Human)) | BDBM50316539 (CHEMBL1094850 | N,N'-(Ethane-1,2-diyl)bis(7,8-dihy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle Curated by ChEMBL | Assay Description Inhibition of recombinant dynamin 2 expressed in Sf9 cells by malachite green GTPase assay | J Med Chem 53: 4094-102 (2010) Article DOI: 10.1021/jm100119c BindingDB Entry DOI: 10.7270/Q2PZ5903 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50370549 (CHEMBL609647) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Inhibitory concentration against hepatitis C virus helicase | J Med Chem 48: 1-20 (2005) Article DOI: 10.1021/jm0400101 BindingDB Entry DOI: 10.7270/Q2XP75Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50158820 (CHEMBL414693 | Glu-Asp-Val-Val-Cys-Cys-L1-Cys-Val-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Inhibitory concentration against hepatitis C virus NS4A binding region of NS3 protease | J Med Chem 48: 1-20 (2005) Article DOI: 10.1021/jm0400101 BindingDB Entry DOI: 10.7270/Q2XP75Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-structural protein 4A (Hepatitis C virus) | BDBM50158836 (CHEMBL361380 | Ser-Leu-Val-Ile-Arg-Gly-Val-Ile-Val) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Inhibitory concentration against hepatitis C virus NS4A active site | J Med Chem 48: 1-20 (2005) Article DOI: 10.1021/jm0400101 BindingDB Entry DOI: 10.7270/Q2XP75Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50158795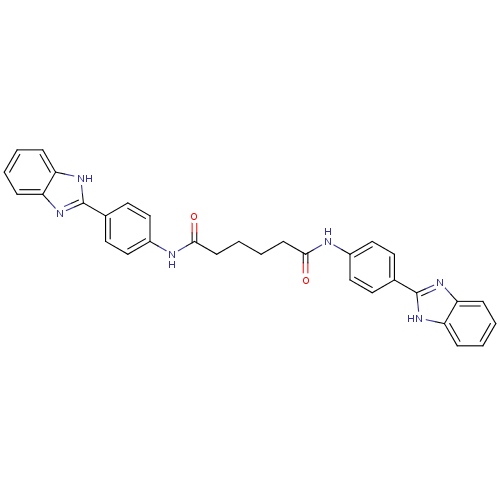 (CHEMBL298210 | Hexanedioic acid bis-{[4-(1H-benzoi...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Inhibitory concentration against hepatitis C virus helicase | J Med Chem 48: 1-20 (2005) Article DOI: 10.1021/jm0400101 BindingDB Entry DOI: 10.7270/Q2XP75Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM79181 (10-[3-(4-methyl-1-piperazinyl)propyl]-2-(trifluoro...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Inhibitory concentration against hepatitis C virus helicase; range 0.6-0.7 | J Med Chem 48: 1-20 (2005) Article DOI: 10.1021/jm0400101 BindingDB Entry DOI: 10.7270/Q2XP75Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50370552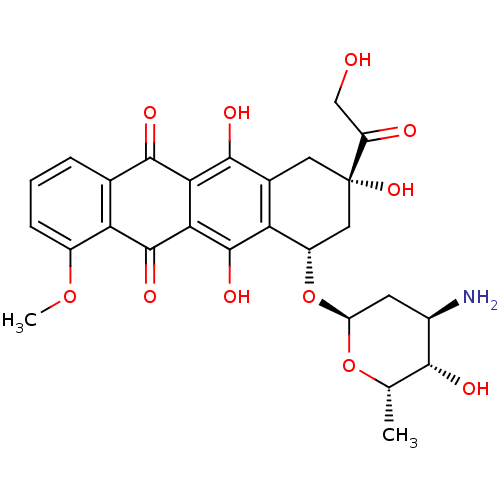 (EPIRUBICIN | Ellence) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Inhibitory concentration against hepatitis C virus helicase | J Med Chem 48: 1-20 (2005) Article DOI: 10.1021/jm0400101 BindingDB Entry DOI: 10.7270/Q2XP75Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM30406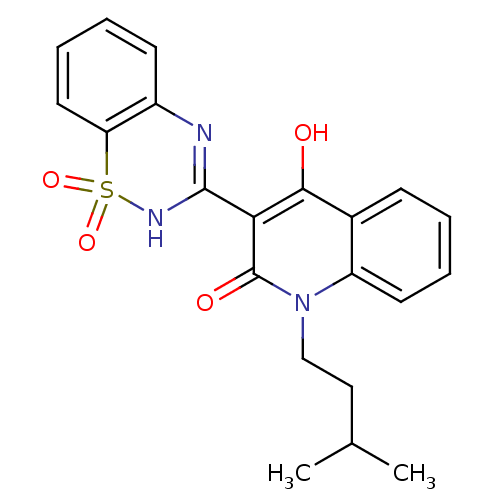 (CHEMBL176058 | benzo[1,2,4]thiadiazine-1,1-dioxide...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Inhibitory concentration against hepatitis C virus polymerase | J Med Chem 48: 1-20 (2005) Article DOI: 10.1021/jm0400101 BindingDB Entry DOI: 10.7270/Q2XP75Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50158821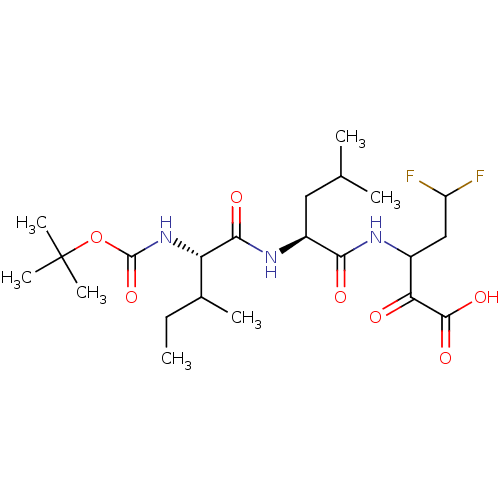 (Boc-Ile-Leu-L-(difluoro)aminobutyric aid | CHEMBL1...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Inhibitory concentration against hepatitis C virus NS3 protease | J Med Chem 48: 1-20 (2005) Article DOI: 10.1021/jm0400101 BindingDB Entry DOI: 10.7270/Q2XP75Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50001839 (CHEMBL428647 | PACLITAXEL | taxol) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Inhibitory concentration against hepatitis C virus helicase | J Med Chem 48: 1-20 (2005) Article DOI: 10.1021/jm0400101 BindingDB Entry DOI: 10.7270/Q2XP75Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50158809 (AcGlu-Asp-Val-Val-Leu-Cys-Iqc-Nle-Thr-TyrNH2 | CHE...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Inhibitory concentration against hepatitis C virus NS3 protease | J Med Chem 48: 1-20 (2005) Article DOI: 10.1021/jm0400101 BindingDB Entry DOI: 10.7270/Q2XP75Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50370547 (CHEMBL1791288) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Inhibitory concentration against hepatitis C virus NS3 protease | J Med Chem 48: 1-20 (2005) Article DOI: 10.1021/jm0400101 BindingDB Entry DOI: 10.7270/Q2XP75Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50370553 (CHEMBL610048) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Inhibitory concentration against hepatitis C virus helicase | J Med Chem 48: 1-20 (2005) Article DOI: 10.1021/jm0400101 BindingDB Entry DOI: 10.7270/Q2XP75Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50370546 (CHEMBL607669) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Inhibitory concentration against hepatitis C virus helicase | J Med Chem 48: 1-20 (2005) Article DOI: 10.1021/jm0400101 BindingDB Entry DOI: 10.7270/Q2XP75Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50158792 (CHEMBL175623 | Cbz-Ile-Leu-L-(difluoro)aminobutyri...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Inhibitory concentration against hepatitis C virus NS3 protease | J Med Chem 48: 1-20 (2005) Article DOI: 10.1021/jm0400101 BindingDB Entry DOI: 10.7270/Q2XP75Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50370550 (CHEMBL1791287) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Inhibitory concentration against hepatitis C virus NS3 protease | J Med Chem 48: 1-20 (2005) Article DOI: 10.1021/jm0400101 BindingDB Entry DOI: 10.7270/Q2XP75Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dynamin-2 (Homo sapiens (Human)) | BDBM50423883 (CHEMBL2312430) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle Curated by ChEMBL | Assay Description Inhibition of dyn2 in human U2OS cells assessed as clathrin-mediated endocytosis of Tfn after 30 mins | J Med Chem 56: 46-59 (2013) Article DOI: 10.1021/jm300844m BindingDB Entry DOI: 10.7270/Q2DJ5GZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 110 total ) | Next | Last >> |