 Found 244 hits with Last Name = 'gottfries' and Initial = 'j'
Found 244 hits with Last Name = 'gottfries' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940]
(Rattus norvegicus (rat)) | BDBM24198
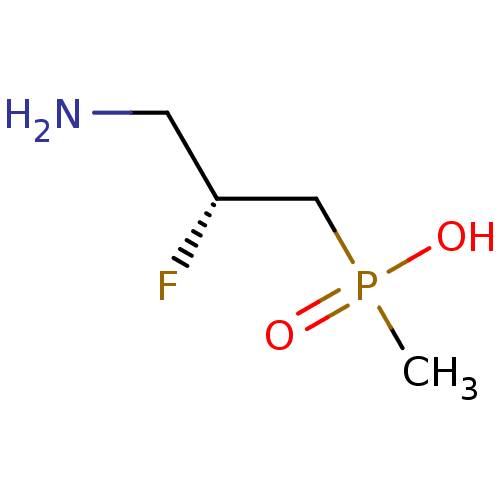
(3-aminopropylphosphinic derivative, (R)-8 | [(2R)-...)Show InChI InChI=1S/C4H11FNO2P/c1-9(7,8)3-4(5)2-6/h4H,2-3,6H2,1H3,(H,7,8)/t4-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30 | -47.3 | n/a | n/a | 14 | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... |
J Med Chem 51: 4315-20 (2008)
Article DOI: 10.1021/jm701425k
BindingDB Entry DOI: 10.7270/Q2M32T2T |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940]
(Rattus norvegicus (rat)) | BDBM24195
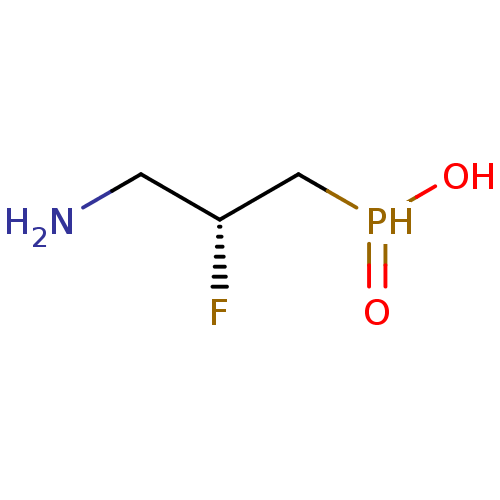
(3-aminopropylphosphinic derivative, (R)-7 | AZD335...)Show InChI InChI=1S/C3H9FNO2P/c4-3(1-5)2-8(6)7/h3,8H,1-2,5H2,(H,6,7)/t3-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.10 | -46.9 | n/a | n/a | 8.64 | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... |
J Med Chem 51: 4315-20 (2008)
Article DOI: 10.1021/jm701425k
BindingDB Entry DOI: 10.7270/Q2M32T2T |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940]
(Rattus norvegicus (rat)) | BDBM24193
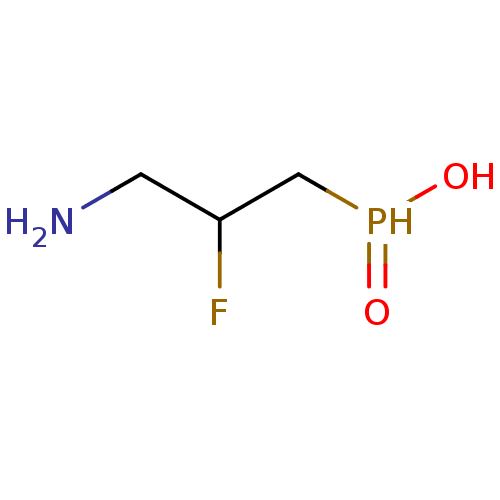
((3-amino-2-fluoropropyl)phosphinic acid | 3-aminop...)Show InChI InChI=1S/C3H9FNO2P/c4-3(1-5)2-8(6)7/h3,8H,1-2,5H2,(H,6,7) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | -45.2 | n/a | n/a | 15 | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... |
J Med Chem 51: 4315-20 (2008)
Article DOI: 10.1021/jm701425k
BindingDB Entry DOI: 10.7270/Q2M32T2T |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940]
(Rattus norvegicus (rat)) | BDBM24196
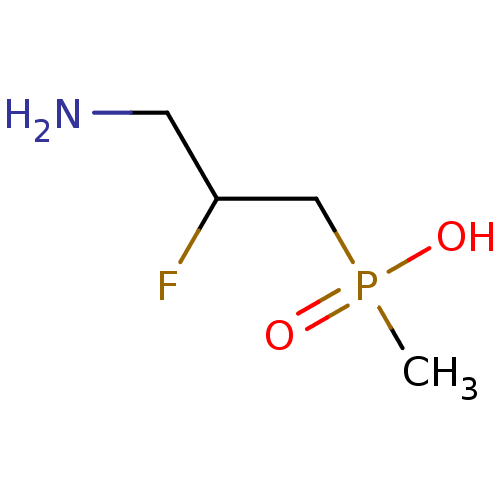
((3-amino-2-fluoropropyl)(methyl)phosphinic acid | ...)Show InChI InChI=1S/C4H11FNO2P/c1-9(7,8)3-4(5)2-6/h4H,2-3,6H2,1H3,(H,7,8) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | -44.4 | n/a | n/a | 23 | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... |
J Med Chem 51: 4315-20 (2008)
Article DOI: 10.1021/jm701425k
BindingDB Entry DOI: 10.7270/Q2M32T2T |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940]
(Rattus norvegicus (rat)) | BDBM24184
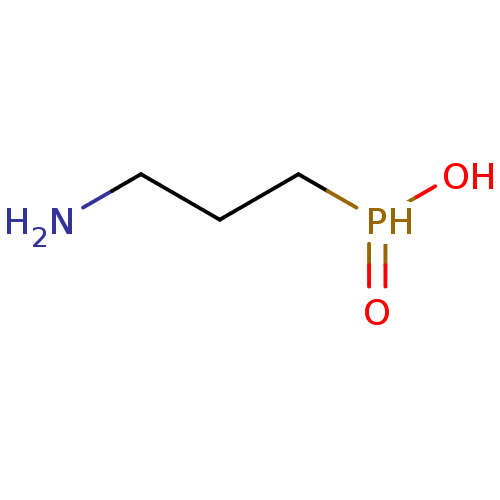
((3-aminopropyl)phosphinic acid | 3-aminopropylphos...)Show InChI InChI=1S/C3H10NO2P/c4-2-1-3-7(5)6/h7H,1-4H2,(H,5,6) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 15 | -44.2 | n/a | n/a | 19 | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... |
J Med Chem 51: 4315-20 (2008)
Article DOI: 10.1021/jm701425k
BindingDB Entry DOI: 10.7270/Q2M32T2T |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940]
(Rattus norvegicus (rat)) | BDBM24185
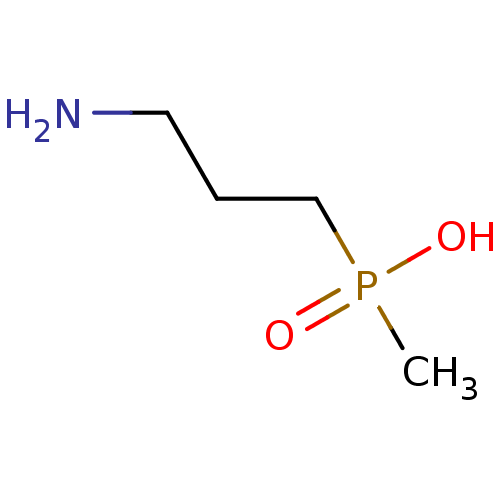
((3-aminopropyl)(methyl)phosphinic acid | 3-Apmpa |...)Show InChI InChI=1S/C4H12NO2P/c1-8(6,7)4-2-3-5/h2-5H2,1H3,(H,6,7) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 33 | -42.3 | n/a | n/a | 41 | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... |
J Med Chem 51: 4315-20 (2008)
Article DOI: 10.1021/jm701425k
BindingDB Entry DOI: 10.7270/Q2M32T2T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940]
(Rattus norvegicus (rat)) | BDBM24191

((3-amino-2-oxopropyl)phosphinic acid | (3-amino-2-...)Show InChI InChI=1S/C3H8NO3P/c4-1-3(5)2-8(6)7/h8H,1-2,4H2,(H,6,7) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 48 | -41.4 | n/a | n/a | 81 | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... |
J Med Chem 51: 4315-20 (2008)
Article DOI: 10.1021/jm701425k
BindingDB Entry DOI: 10.7270/Q2M32T2T |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940]
(Rattus norvegicus (rat)) | BDBM24186
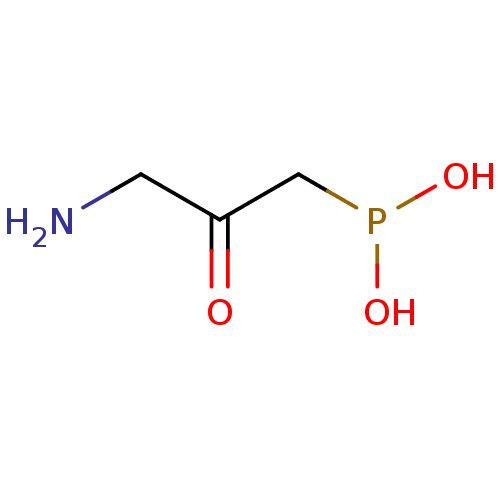
((3-amino-2-hydroxypropyl)phosphinic acid | 3-amino...)Show InChI InChI=1S/C3H8NO3P/c4-1-3(5)2-8(6)7/h6-7H,1-2,4H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | -41.3 | n/a | n/a | 130 | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... |
J Med Chem 51: 4315-20 (2008)
Article DOI: 10.1021/jm701425k
BindingDB Entry DOI: 10.7270/Q2M32T2T |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940]
(Rattus norvegicus (rat)) | BDBM24194

(3-aminopropylphosphinic derivative, (S)-7 | [(2S)-...)Show InChI InChI=1S/C3H9FNO2P/c4-3(1-5)2-8(6)7/h3,8H,1-2,5H2,(H,6,7)/t3-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 70 | -40.4 | n/a | n/a | 250 | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... |
J Med Chem 51: 4315-20 (2008)
Article DOI: 10.1021/jm701425k
BindingDB Entry DOI: 10.7270/Q2M32T2T |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940]
(Rattus norvegicus (rat)) | BDBM24186

((3-amino-2-hydroxypropyl)phosphinic acid | 3-amino...)Show InChI InChI=1S/C3H8NO3P/c4-1-3(5)2-8(6)7/h6-7H,1-2,4H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 94 | -39.7 | n/a | n/a | 220 | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... |
J Med Chem 51: 4315-20 (2008)
Article DOI: 10.1021/jm701425k
BindingDB Entry DOI: 10.7270/Q2M32T2T |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940]
(Rattus norvegicus (rat)) | BDBM24189

(3-aminopropylphosphinic derivative, (R)-4 | 3-amin...)Show InChI InChI=1S/C4H12NO3P/c1-9(7,8)3-4(6)2-5/h7-9H,2-3,5H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 180 | -38.1 | n/a | n/a | 150 | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... |
J Med Chem 51: 4315-20 (2008)
Article DOI: 10.1021/jm701425k
BindingDB Entry DOI: 10.7270/Q2M32T2T |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940]
(Rattus norvegicus (rat)) | BDBM24192
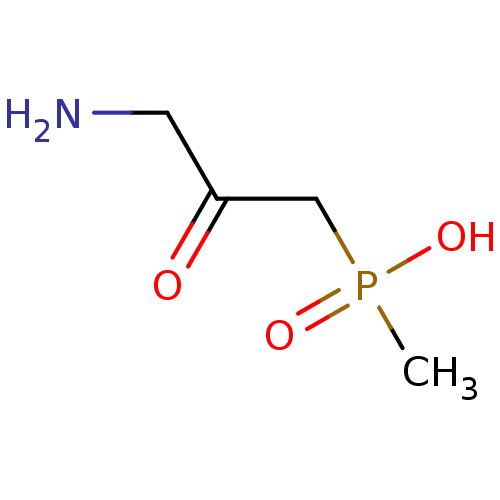
((3-amino-2-oxopropyl)(methyl)phosphinic acid | (3-...)Show InChI InChI=1S/C4H10NO3P/c1-9(7,8)3-4(6)2-5/h2-3,5H2,1H3,(H,7,8) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 200 | -37.9 | n/a | n/a | 270 | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... |
J Med Chem 51: 4315-20 (2008)
Article DOI: 10.1021/jm701425k
BindingDB Entry DOI: 10.7270/Q2M32T2T |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940]
(Rattus norvegicus (rat)) | BDBM24182
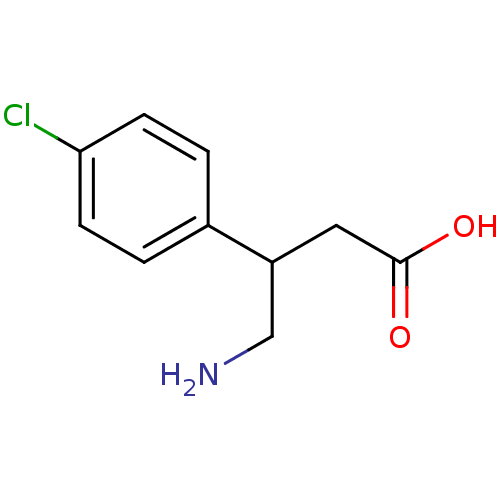
(4-amino-3-(4-chlorophenyl)butanoic acid | Baclofen...)Show InChI InChI=1S/C10H12ClNO2/c11-9-3-1-7(2-4-9)8(6-12)5-10(13)14/h1-4,8H,5-6,12H2,(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 220 | -37.6 | n/a | n/a | 750 | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... |
J Med Chem 51: 4315-20 (2008)
Article DOI: 10.1021/jm701425k
BindingDB Entry DOI: 10.7270/Q2M32T2T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940]
(Rattus norvegicus (rat)) | BDBM24197
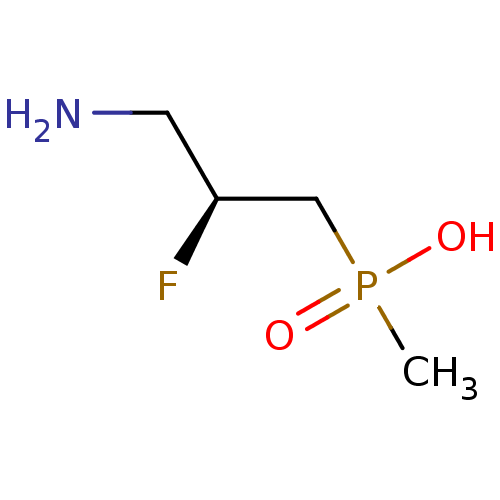
(3-aminopropylphosphinic derivative, (S)-8 | [(2S)-...)Show InChI InChI=1S/C4H11FNO2P/c1-9(7,8)3-4(5)2-6/h4H,2-3,6H2,1H3,(H,7,8)/t4-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 480 | -35.7 | n/a | n/a | 1.70E+3 | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... |
J Med Chem 51: 4315-20 (2008)
Article DOI: 10.1021/jm701425k
BindingDB Entry DOI: 10.7270/Q2M32T2T |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940]
(Rattus norvegicus (rat)) | BDBM24189

(3-aminopropylphosphinic derivative, (R)-4 | 3-amin...)Show InChI InChI=1S/C4H12NO3P/c1-9(7,8)3-4(6)2-5/h7-9H,2-3,5H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 840 | -34.3 | n/a | n/a | 600 | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... |
J Med Chem 51: 4315-20 (2008)
Article DOI: 10.1021/jm701425k
BindingDB Entry DOI: 10.7270/Q2M32T2T |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940]
(Rattus norvegicus (rat)) | BDBM24186

((3-amino-2-hydroxypropyl)phosphinic acid | 3-amino...)Show InChI InChI=1S/C3H8NO3P/c4-1-3(5)2-8(6)7/h6-7H,1-2,4H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10E+3 | -32.1 | n/a | n/a | 1.10E+3 | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... |
J Med Chem 51: 4315-20 (2008)
Article DOI: 10.1021/jm701425k
BindingDB Entry DOI: 10.7270/Q2M32T2T |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM29388
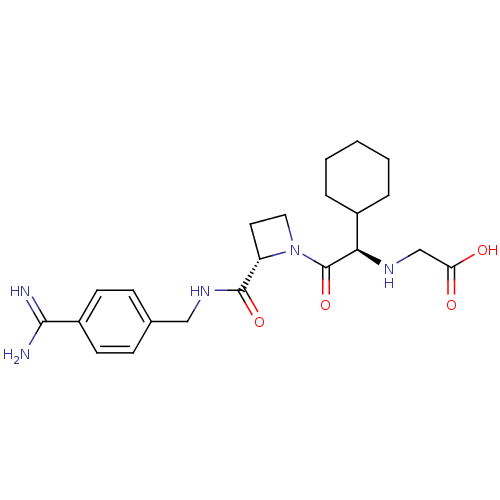
(Exanta | Melagatran | US11584714, Compound 999)Show SMILES NC(=N)c1ccc(CNC(=O)[C@@H]2CCN2C(=O)[C@H](NCC(O)=O)C2CCCCC2)cc1 Show InChI InChI=1S/C22H31N5O4/c23-20(24)16-8-6-14(7-9-16)12-26-21(30)17-10-11-27(17)22(31)19(25-13-18(28)29)15-4-2-1-3-5-15/h6-9,15,17,19,25H,1-5,10-13H2,(H3,23,24)(H,26,30)(H,28,29)/t17-,19+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kalmar
| Assay Description
The thrombin inhibitor potency (IC50) was measured with a chromogenic substrate method in a robotic microplate processor, using 96-well, half-volume ... |
J Med Chem 52: 2708-15 (2009)
Article DOI: 10.1021/jm8011849
BindingDB Entry DOI: 10.7270/Q2H130B7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
J Nat Prod 68: 985-91 (2005)
Article DOI: 10.1021/np049655u
BindingDB Entry DOI: 10.7270/Q27S7PN5 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50422375
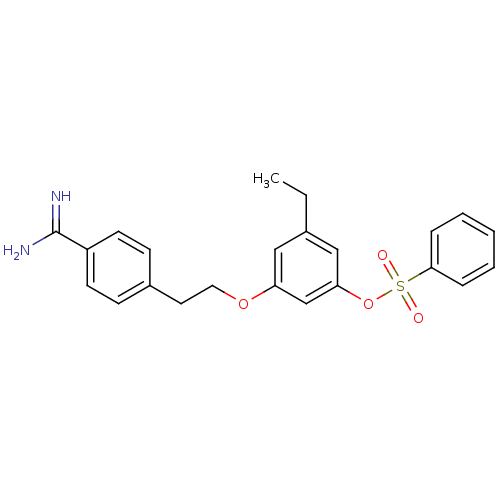
(CHEMBL116616)Show SMILES CCc1cc(OCCc2ccc(cc2)C(N)=N)cc(OS(=O)(=O)c2ccccc2)c1 Show InChI InChI=1S/C23H24N2O4S/c1-2-17-14-20(28-13-12-18-8-10-19(11-9-18)23(24)25)16-21(15-17)29-30(26,27)22-6-4-3-5-7-22/h3-11,14-16H,2,12-13H2,1H3,(H3,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
J Med Chem 44: 3424-39 (2001)
BindingDB Entry DOI: 10.7270/Q2D21ZWN |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50347503
(RADICICOL)Show SMILES C[C@@H]1C[C@@H]2O[C@@H]2C=CC=CC(=O)Cc2c(Cl)c(O)cc(O)c2C(=O)O1 |r,w:9.10,7.8| Show InChI InChI=1S/C18H17ClO6/c1-9-6-15-14(25-15)5-3-2-4-10(20)7-11-16(18(23)24-9)12(21)8-13(22)17(11)19/h2-5,8-9,14-15,21-22H,6-7H2,1H3/t9-,14-,15+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
J Nat Prod 68: 985-91 (2005)
Article DOI: 10.1021/np049655u
BindingDB Entry DOI: 10.7270/Q27S7PN5 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50241049

(CHEMBL463763 | Triptolide, 1 | triptolide)Show SMILES CC(C)[C@]12O[C@H]1[C@@H]1O[C@]11[C@]3(O[C@H]3C[C@H]3C4=C(CC[C@]13C)C(=O)OC4)[C@@H]2O |r,c:17| Show InChI InChI=1S/C20H24O6/c1-8(2)18-13(25-18)14-20(26-14)17(3)5-4-9-10(7-23-15(9)21)11(17)6-12-19(20,24-12)16(18)22/h8,11-14,16,22H,4-7H2,1-3H3/t11-,12-,13-,14-,16+,17-,18-,19+,20+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
J Nat Prod 68: 985-91 (2005)
Article DOI: 10.1021/np049655u
BindingDB Entry DOI: 10.7270/Q27S7PN5 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50422376

(CHEMBL116149)Show SMILES COc1c(Cl)cc(Cl)cc1S(=O)(=O)Oc1cccc(OCCc2ccc(cc2)C(N)=N)c1 Show InChI InChI=1S/C22H20Cl2N2O5S/c1-29-21-19(24)11-16(23)12-20(21)32(27,28)31-18-4-2-3-17(13-18)30-10-9-14-5-7-15(8-6-14)22(25)26/h2-8,11-13H,9-10H2,1H3,(H3,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
J Med Chem 44: 3424-39 (2001)
BindingDB Entry DOI: 10.7270/Q2D21ZWN |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50240612
(CHEMBL306946 | GNF-PF-2691 | Indolo[2,1-b]quinazol...)Show InChI InChI=1S/C15H8N2O2/c18-13-10-6-2-4-8-12(10)17-14(13)16-11-7-3-1-5-9(11)15(17)19/h1-8H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
J Nat Prod 68: 985-91 (2005)
Article DOI: 10.1021/np049655u
BindingDB Entry DOI: 10.7270/Q27S7PN5 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50422368
(CHEMBL117266)Show SMILES Cc1cccc(c1)S(=O)(=O)Oc1cc(C)cc(OCCc2ccc(C(N)=N)c(O)c2)c1 Show InChI InChI=1S/C23H24N2O5S/c1-15-4-3-5-20(12-15)31(27,28)30-19-11-16(2)10-18(14-19)29-9-8-17-6-7-21(23(24)25)22(26)13-17/h3-7,10-14,26H,8-9H2,1-2H3,(H3,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 135 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
J Med Chem 44: 3424-39 (2001)
BindingDB Entry DOI: 10.7270/Q2D21ZWN |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50029593

(CHEMBL7162 | N-(2-(cyclohexyloxy)-4-nitrophenyl)me...)Show InChI InChI=1S/C13H18N2O5S/c1-21(18,19)14-12-8-7-10(15(16)17)9-13(12)20-11-5-3-2-4-6-11/h7-9,11,14H,2-6H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
J Nat Prod 68: 985-91 (2005)
Article DOI: 10.1021/np049655u
BindingDB Entry DOI: 10.7270/Q27S7PN5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM50422377
(CHEMBL116971)Show SMILES CCc1cc(OCCc2ccc(C(N)=N)c(O)c2)cc(OS(=O)(=O)c2cc(Cl)cc(Cl)c2OC)c1 Show InChI InChI=1S/C24H24Cl2N2O6S/c1-3-14-8-17(33-7-6-15-4-5-19(24(27)28)21(29)10-15)13-18(9-14)34-35(30,31)22-12-16(25)11-20(26)23(22)32-2/h4-5,8-13,29H,3,6-7H2,1-2H3,(H3,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 288 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
J Med Chem 44: 3424-39 (2001)
BindingDB Entry DOI: 10.7270/Q2D21ZWN |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50131046

(8,13-Dihydro-7H-indolo[2',3':3,4]pyrido[2,1-b]quin...)Show InChI InChI=1S/C18H13N3O/c22-18-13-6-2-4-8-15(13)20-17-16-12(9-10-21(17)18)11-5-1-3-7-14(11)19-16/h1-8,19H,9-10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
J Nat Prod 68: 985-91 (2005)
Article DOI: 10.1021/np049655u
BindingDB Entry DOI: 10.7270/Q27S7PN5 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50269635
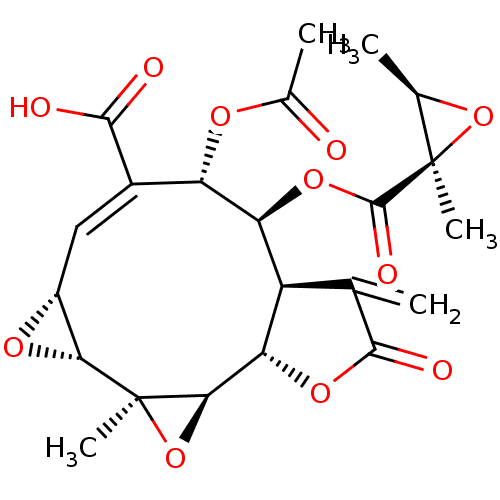
(CHEMBL463571 | leucanthin B)Show SMILES C[C@H]1O[C@@]1(C)C(=O)O[C@H]1[C@@H]2[C@H](OC(=O)C2=C)[C@H]2O[C@@]2(C)[C@@H]2O[C@@H]2\C=C(/[C@@H]1OC(C)=O)C(O)=O |r,c:27| Show InChI InChI=1S/C22H24O11/c1-7-12-14(31-20(27)21(4)8(2)32-21)13(28-9(3)23)10(18(24)25)6-11-16(29-11)22(5)17(33-22)15(12)30-19(7)26/h6,8,11-17H,1H2,2-5H3,(H,24,25)/b10-6+/t8-,11-,12-,13+,14+,15+,16-,17-,21-,22+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
J Nat Prod 68: 985-91 (2005)
Article DOI: 10.1021/np049655u
BindingDB Entry DOI: 10.7270/Q27S7PN5 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50269625
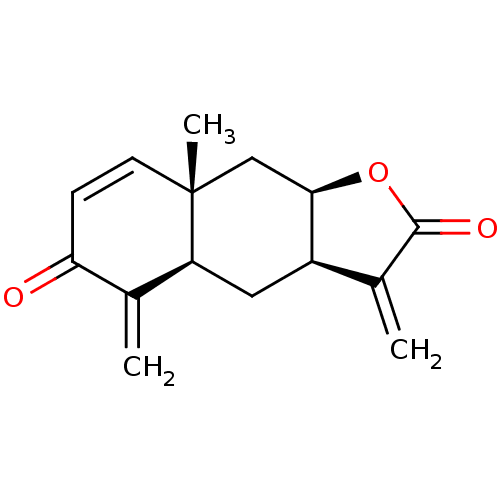
(CHEMBL512400 | encelin)Show SMILES C[C@@]12C[C@H]3OC(=O)C(=C)[C@H]3C[C@H]1C(=C)C(=O)C=C2 |r,c:18| Show InChI InChI=1S/C15H16O3/c1-8-10-6-11-9(2)12(16)4-5-15(11,3)7-13(10)18-14(8)17/h4-5,10-11,13H,1-2,6-7H2,3H3/t10-,11+,13-,15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
J Nat Prod 68: 985-91 (2005)
Article DOI: 10.1021/np049655u
BindingDB Entry DOI: 10.7270/Q27S7PN5 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50422369
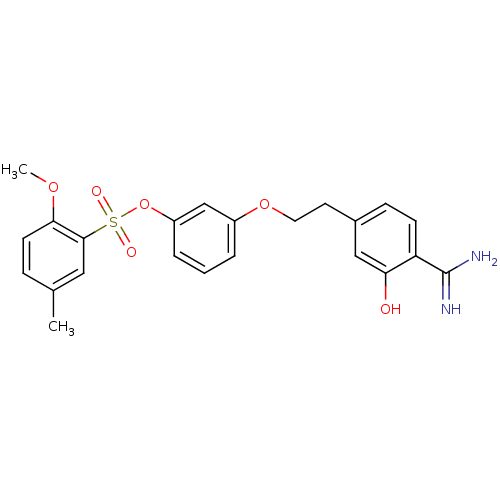
(CHEMBL114124)Show SMILES COc1ccc(C)cc1S(=O)(=O)Oc1cccc(OCCc2ccc(C(N)=N)c(O)c2)c1 Show InChI InChI=1S/C23H24N2O6S/c1-15-6-9-21(29-2)22(12-15)32(27,28)31-18-5-3-4-17(14-18)30-11-10-16-7-8-19(23(24)25)20(26)13-16/h3-9,12-14,26H,10-11H2,1-2H3,(H3,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 575 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
J Med Chem 44: 3424-39 (2001)
BindingDB Entry DOI: 10.7270/Q2D21ZWN |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50208612
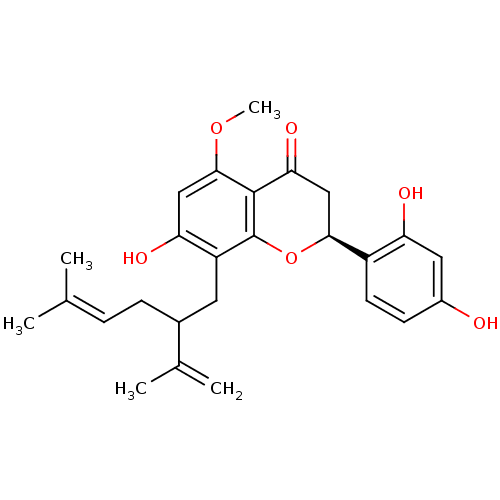
((-)-kurarinone | (2S)-2-(2,4-dihydroxyphenyl)-7-hy...)Show SMILES [#6]-[#8]-c1cc(-[#8])c(-[#6]-[#6](-[#6]\[#6]=[#6](\[#6])-[#6])-[#6](-[#6])=[#6])c2-[#8]-[#6@@H](-[#6]-[#6](=O)-c12)-c1ccc(-[#8])cc1-[#8] |r| Show InChI InChI=1S/C26H30O6/c1-14(2)6-7-16(15(3)4)10-19-21(29)12-24(31-5)25-22(30)13-23(32-26(19)25)18-9-8-17(27)11-20(18)28/h6,8-9,11-12,16,23,27-29H,3,7,10,13H2,1-2,4-5H3/t16?,23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
J Nat Prod 68: 985-91 (2005)
Article DOI: 10.1021/np049655u
BindingDB Entry DOI: 10.7270/Q27S7PN5 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50366787
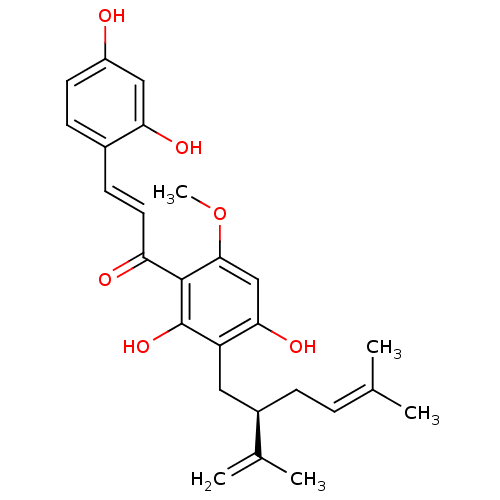
(KURAIDIN)Show SMILES [#6]-[#8]-c1cc(-[#8])c(-[#6]-[#6@@H](-[#6]\[#6]=[#6](\[#6])-[#6])-[#6](-[#6])=[#6])c(-[#8])c1-[#6](=O)\[#6]=[#6]\c1ccc(-[#8])cc1-[#8] Show InChI InChI=1S/C26H30O6/c1-15(2)6-7-18(16(3)4)12-20-23(30)14-24(32-5)25(26(20)31)21(28)11-9-17-8-10-19(27)13-22(17)29/h6,8-11,13-14,18,27,29-31H,3,7,12H2,1-2,4-5H3/b11-9+/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
J Nat Prod 68: 985-91 (2005)
Article DOI: 10.1021/np049655u
BindingDB Entry DOI: 10.7270/Q27S7PN5 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50377905

(ENHYDRIN)Show SMILES COC(=O)C1=CCC[C@@]2(C)O[C@@H]2[C@H]2OC(=O)C(=C)[C@@H]2[C@H](OC(=O)[C@@]2(C)O[C@H]2C)[C@H]1OC(C)=O Show InChI InChI=1S/C23H28O10/c1-10-14-16(31-21(27)23(5)11(2)32-23)15(29-12(3)24)13(20(26)28-6)8-7-9-22(4)18(33-22)17(14)30-19(10)25/h8,11,14-18H,1,7,9H2,2-6H3/b13-8+/t11-,14+,15-,16-,17-,18+,22+,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
J Nat Prod 68: 985-91 (2005)
Article DOI: 10.1021/np049655u
BindingDB Entry DOI: 10.7270/Q27S7PN5 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM22369

(4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(C(=O)OC1)c1ccccc1 |t:11| Show InChI InChI=1S/C17H14O4S/c1-22(19,20)14-9-7-12(8-10-14)15-11-21-17(18)16(15)13-5-3-2-4-6-13/h2-10H,11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
J Nat Prod 68: 985-91 (2005)
Article DOI: 10.1021/np049655u
BindingDB Entry DOI: 10.7270/Q27S7PN5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50241453

(1,3,6,7-tetrahydroxy-2,8-bis(3-methylbut-2-enyl)-9...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])cc2oc3cc(-[#8])c(-[#8])c(-[#6]\[#6]=[#6](\[#6])-[#6])c3c(=O)c2c1-[#8] Show InChI InChI=1S/C23H24O6/c1-11(2)5-7-13-15(24)9-18-20(22(13)27)23(28)19-14(8-6-12(3)4)21(26)16(25)10-17(19)29-18/h5-6,9-10,24-27H,7-8H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
J Nat Prod 68: 985-91 (2005)
Article DOI: 10.1021/np049655u
BindingDB Entry DOI: 10.7270/Q27S7PN5 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50194429
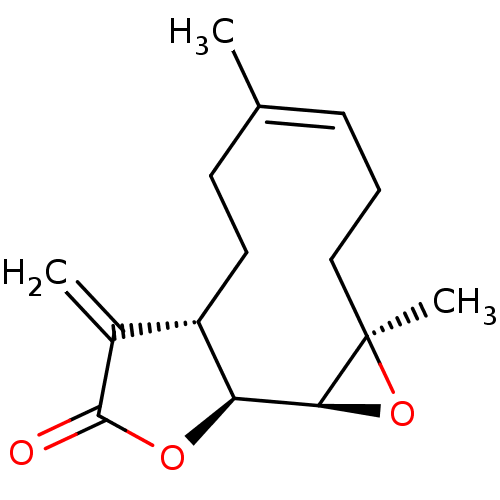
((-)-parthenolide | (1aR,7aS,10aS,10bS,Z)-1a,5-dime...)Show SMILES C\C1=C\CC[C@@]2(C)O[C@H]2[C@H]2OC(=O)C(=C)[C@@H]2CC1 |r,t:1| Show InChI InChI=1S/C15H20O3/c1-9-5-4-8-15(3)13(18-15)12-11(7-6-9)10(2)14(16)17-12/h5,11-13H,2,4,6-8H2,1,3H3/b9-5-/t11-,12-,13-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
J Nat Prod 68: 985-91 (2005)
Article DOI: 10.1021/np049655u
BindingDB Entry DOI: 10.7270/Q27S7PN5 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50422373
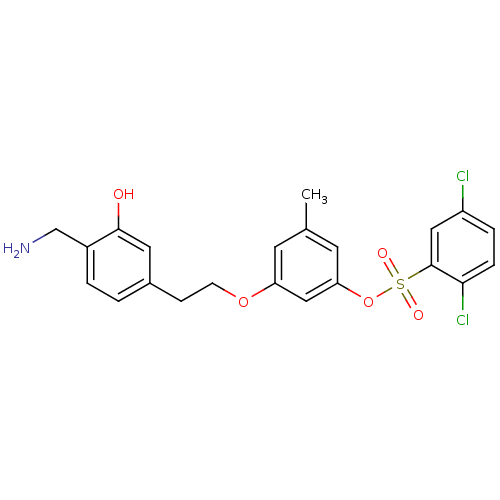
(CHEMBL113711)Show SMILES Cc1cc(OCCc2ccc(CN)c(O)c2)cc(OS(=O)(=O)c2cc(Cl)ccc2Cl)c1 Show InChI InChI=1S/C22H21Cl2NO5S/c1-14-8-18(29-7-6-15-2-3-16(13-25)21(26)10-15)12-19(9-14)30-31(27,28)22-11-17(23)4-5-20(22)24/h2-5,8-12,26H,6-7,13,25H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 933 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
J Med Chem 44: 3424-39 (2001)
BindingDB Entry DOI: 10.7270/Q2D21ZWN |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50269640
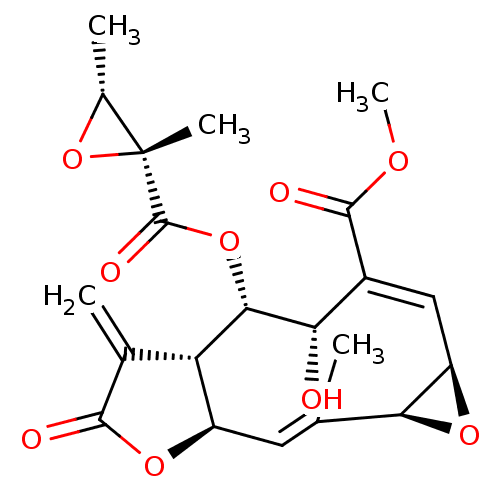
(CHEMBL463559 | melampodin A)Show SMILES COC(=O)C1=C/[C@H]2O[C@H]2\C(C)=C\[C@H]2OC(=O)C(=C)[C@@H]2[C@H](OC(=O)[C@]2(C)O[C@@H]2C)[C@H]\1O |r,t:4,11| Show InChI InChI=1S/C21H24O9/c1-8-6-12-14(9(2)18(23)28-12)17(29-20(25)21(4)10(3)30-21)15(22)11(19(24)26-5)7-13-16(8)27-13/h6-7,10,12-17,22H,2H2,1,3-5H3/b8-6+,11-7+/t10-,12-,13-,14+,15+,16+,17+,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
J Nat Prod 68: 985-91 (2005)
Article DOI: 10.1021/np049655u
BindingDB Entry DOI: 10.7270/Q27S7PN5 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50422371
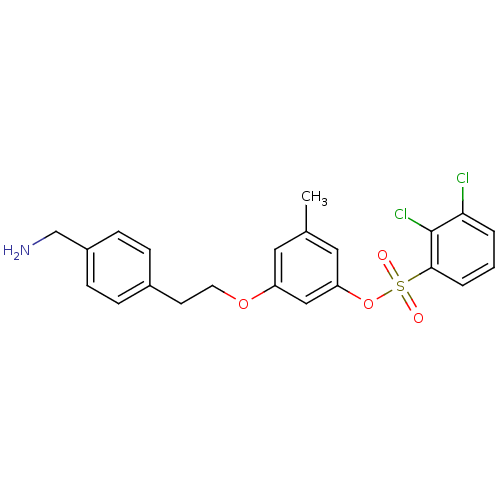
(CHEMBL419954)Show SMILES Cc1cc(OCCc2ccc(CN)cc2)cc(OS(=O)(=O)c2cccc(Cl)c2Cl)c1 Show InChI InChI=1S/C22H21Cl2NO4S/c1-15-11-18(28-10-9-16-5-7-17(14-25)8-6-16)13-19(12-15)29-30(26,27)21-4-2-3-20(23)22(21)24/h2-8,11-13H,9-10,14,25H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
J Med Chem 44: 3424-39 (2001)
BindingDB Entry DOI: 10.7270/Q2D21ZWN |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50269627
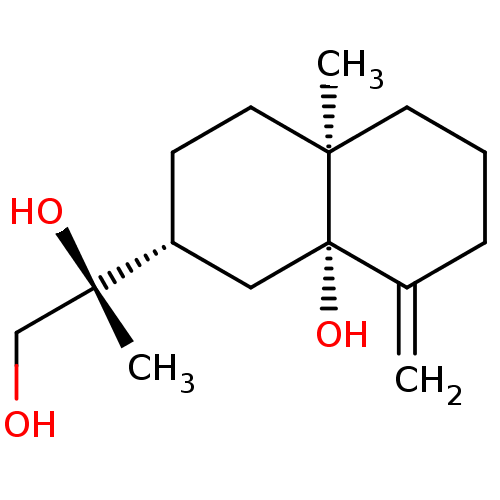
(5-epi-kutdtriol | CHEMBL456310)Show SMILES C[C@](O)(CO)[C@@H]1CC[C@@]2(C)CCCC(=C)[C@@]2(O)C1 |r| Show InChI InChI=1S/C15H26O3/c1-11-5-4-7-13(2)8-6-12(9-15(11,13)18)14(3,17)10-16/h12,16-18H,1,4-10H2,2-3H3/t12-,13-,14+,15+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
J Nat Prod 68: 985-91 (2005)
Article DOI: 10.1021/np049655u
BindingDB Entry DOI: 10.7270/Q27S7PN5 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50269630
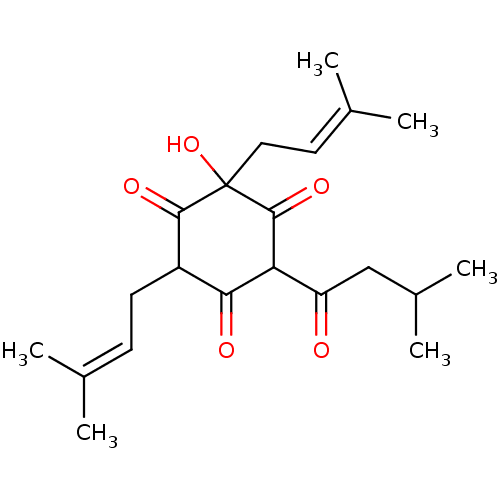
(CHEMBL512227 | humulone)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6](=O)-[#6]-1-[#6](=O)-[#6](-[#6]\[#6]=[#6](\[#6])-[#6])-[#6](=O)C([#8])([#6]\[#6]=[#6](\[#6])-[#6])[#6]-1=O Show InChI InChI=1S/C21H30O5/c1-12(2)7-8-15-18(23)17(16(22)11-14(5)6)20(25)21(26,19(15)24)10-9-13(3)4/h7,9,14-15,17,26H,8,10-11H2,1-6H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
J Nat Prod 68: 985-91 (2005)
Article DOI: 10.1021/np049655u
BindingDB Entry DOI: 10.7270/Q27S7PN5 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM29379
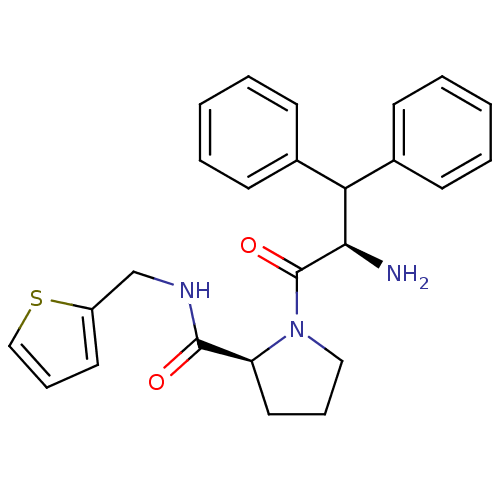
(proline scaffold, 28)Show SMILES N[C@H](C(c1ccccc1)c1ccccc1)C(=O)N1CCC[C@H]1C(=O)NCc1cccs1 |r| Show InChI InChI=1S/C25H27N3O2S/c26-23(22(18-9-3-1-4-10-18)19-11-5-2-6-12-19)25(30)28-15-7-14-21(28)24(29)27-17-20-13-8-16-31-20/h1-6,8-13,16,21-23H,7,14-15,17,26H2,(H,27,29)/t21-,23+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | 270 | n/a | n/a | n/a | n/a | n/a |
University of Kalmar
| Assay Description
SPR experiments where performed with a Biacore A100 using Series S sensor chip CM5 (GE Healthcare Bio-Sciences AB, Uppsala, Sweden). The proteins wer... |
J Med Chem 52: 2708-15 (2009)
Article DOI: 10.1021/jm8011849
BindingDB Entry DOI: 10.7270/Q2H130B7 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50241453

(1,3,6,7-tetrahydroxy-2,8-bis(3-methylbut-2-enyl)-9...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])cc2oc3cc(-[#8])c(-[#8])c(-[#6]\[#6]=[#6](\[#6])-[#6])c3c(=O)c2c1-[#8] Show InChI InChI=1S/C23H24O6/c1-11(2)5-7-13-15(24)9-18-20(22(13)27)23(28)19-14(8-6-12(3)4)21(26)16(25)10-17(19)29-18/h5-6,9-10,24-27H,7-8H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
J Nat Prod 68: 985-91 (2005)
Article DOI: 10.1021/np049655u
BindingDB Entry DOI: 10.7270/Q27S7PN5 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50242257
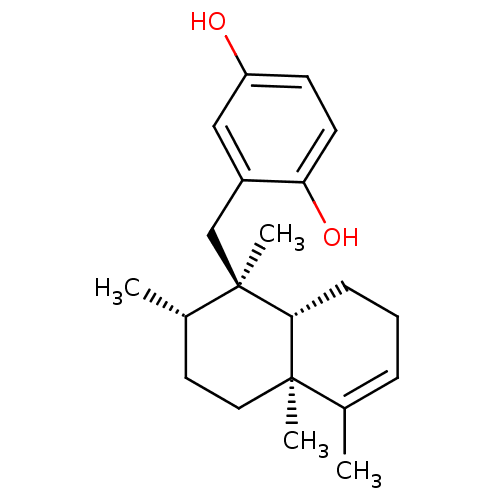
(2-((1R,2S,4aS)-1,2,4a,5-Tetramethyl-1,2,3,4,4a,7,8...)Show SMILES C[C@H]1CC[C@@]2(C)[C@@H](CCC=C2C)[C@]1(C)Cc1cc(O)ccc1O |r,c:9| Show InChI InChI=1S/C21H30O2/c1-14-6-5-7-19-20(14,3)11-10-15(2)21(19,4)13-16-12-17(22)8-9-18(16)23/h6,8-9,12,15,19,22-23H,5,7,10-11,13H2,1-4H3/t15-,19+,20+,21+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
J Nat Prod 68: 985-91 (2005)
Article DOI: 10.1021/np049655u
BindingDB Entry DOI: 10.7270/Q27S7PN5 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50234867
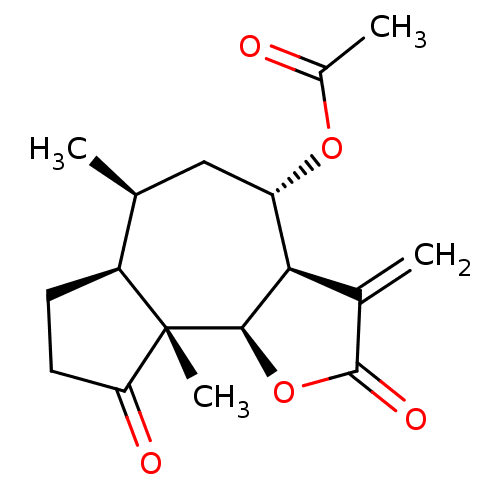
(CHEMBL409627 | confertiflorin)Show SMILES C[C@H]1C[C@H](OC(C)=O)[C@@H]2[C@@H](OC(=O)C2=C)[C@@]2(C)[C@H]1CCC2=O |r| Show InChI InChI=1S/C17H22O5/c1-8-7-12(21-10(3)18)14-9(2)16(20)22-15(14)17(4)11(8)5-6-13(17)19/h8,11-12,14-15H,2,5-7H2,1,3-4H3/t8-,11-,12-,14+,15+,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
J Nat Prod 68: 985-91 (2005)
Article DOI: 10.1021/np049655u
BindingDB Entry DOI: 10.7270/Q27S7PN5 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50240728
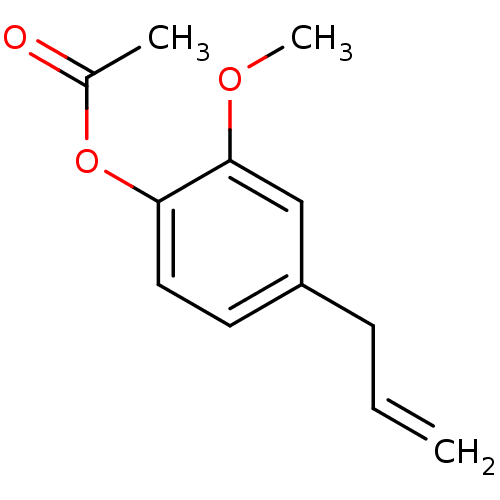
(Acetic acid 4-allyl-2-methoxy-phenyl ester | CHEMB...)Show InChI InChI=1S/C12H14O3/c1-4-5-10-6-7-11(15-9(2)13)12(8-10)14-3/h4,6-8H,1,5H2,2-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
J Nat Prod 68: 985-91 (2005)
Article DOI: 10.1021/np049655u
BindingDB Entry DOI: 10.7270/Q27S7PN5 |
More data for this
Ligand-Target Pair | |
Sortase family protein
(Staphylococcus aureus) | BDBM33322
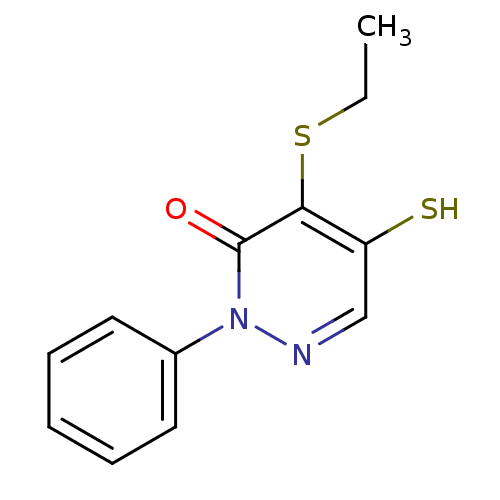
(pyridazinone, 2-9)Show InChI InChI=1S/C12H12N2OS2/c1-2-17-11-10(16)8-13-14(12(11)15)9-6-4-3-5-7-9/h3-8,16H,2H2,1H3 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Gothenburg
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Staphylococcus aureus wild type truncated C-terminal His6 tagged Srt A using Abz-Leu-Pro-Glu-Thr-Gly-Lys(Dnp)-NH2 as substr... |
Bioorg Med Chem 27: (2019)
Article DOI: 10.1016/j.bmc.2019.115043
BindingDB Entry DOI: 10.7270/Q2HM5CWK |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50422366
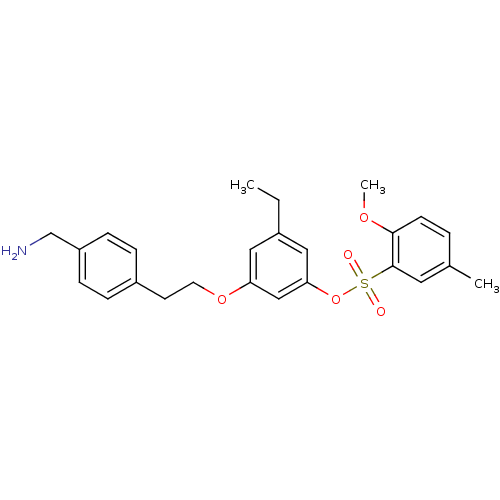
(CHEMBL115413)Show SMILES CCc1cc(OCCc2ccc(CN)cc2)cc(OS(=O)(=O)c2cc(C)ccc2OC)c1 Show InChI InChI=1S/C25H29NO5S/c1-4-19-14-22(30-12-11-20-6-8-21(17-26)9-7-20)16-23(15-19)31-32(27,28)25-13-18(2)5-10-24(25)29-3/h5-10,13-16H,4,11-12,17,26H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
J Med Chem 44: 3424-39 (2001)
BindingDB Entry DOI: 10.7270/Q2D21ZWN |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50259939
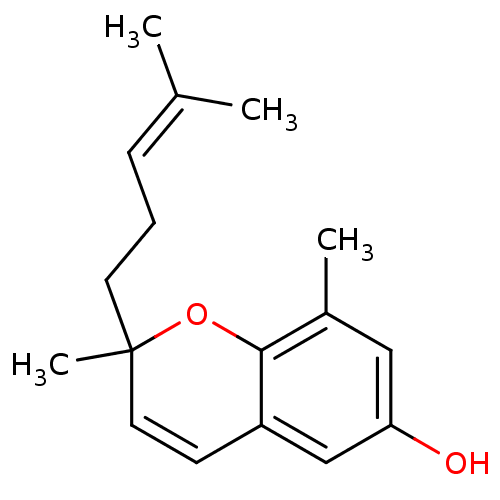
(2,8-dimethyl-6-hydroxy-2-(4-methyl-3-pentenyl)-2h-...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]C1([#6])[#8]-c2c(-[#6])cc(-[#8])cc2-[#6]=[#6]1 |c:18| Show InChI InChI=1S/C17H22O2/c1-12(2)6-5-8-17(4)9-7-14-11-15(18)10-13(3)16(14)19-17/h6-7,9-11,18H,5,8H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
J Nat Prod 68: 985-91 (2005)
Article DOI: 10.1021/np049655u
BindingDB Entry DOI: 10.7270/Q27S7PN5 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50260057
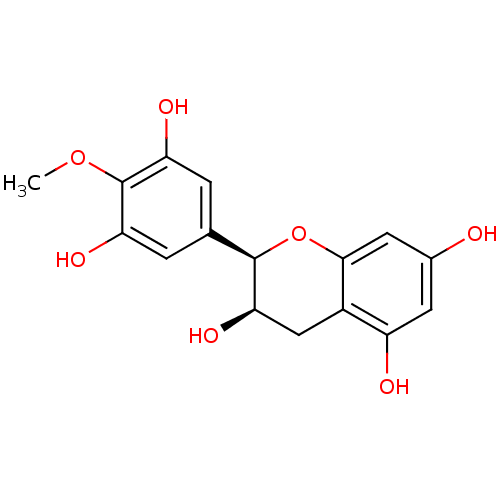
((-)-4'-O-methylepigallocatechin | CHEMBL485460 | o...)Show SMILES COc1c(O)cc(cc1O)[C@H]1Oc2cc(O)cc(O)c2C[C@H]1O |r| Show InChI InChI=1S/C16H16O7/c1-22-16-11(19)2-7(3-12(16)20)15-13(21)6-9-10(18)4-8(17)5-14(9)23-15/h2-5,13,15,17-21H,6H2,1H3/t13-,15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
J Nat Prod 68: 985-91 (2005)
Article DOI: 10.1021/np049655u
BindingDB Entry DOI: 10.7270/Q27S7PN5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data