

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50294783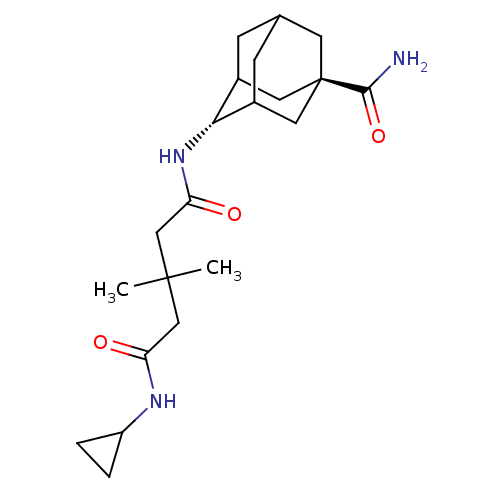 (3,3-Dimethyl-pentanedioic acid(5-carbamoyl-adamant...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck-Serono Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli assessed as cortisol level after 150 mins by HTRF assay | Bioorg Med Chem Lett 19: 2674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.140 BindingDB Entry DOI: 10.7270/Q2JQ112H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50294781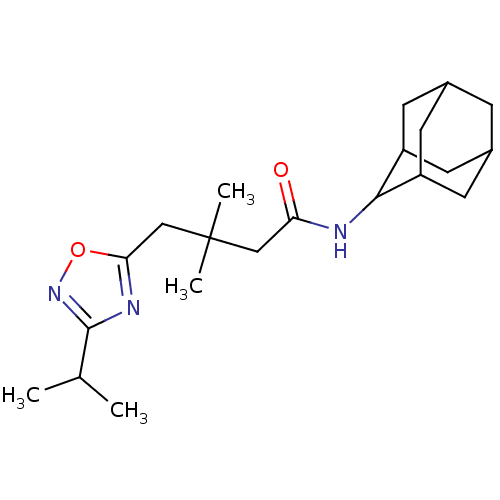 (CHEMBL552159 | N-Adamantan-2-yl-4-(3-isopropyl-[1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck-Serono Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli assessed as cortisol level after 150 mins by HTRF assay | Bioorg Med Chem Lett 19: 2674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.140 BindingDB Entry DOI: 10.7270/Q2JQ112H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50294764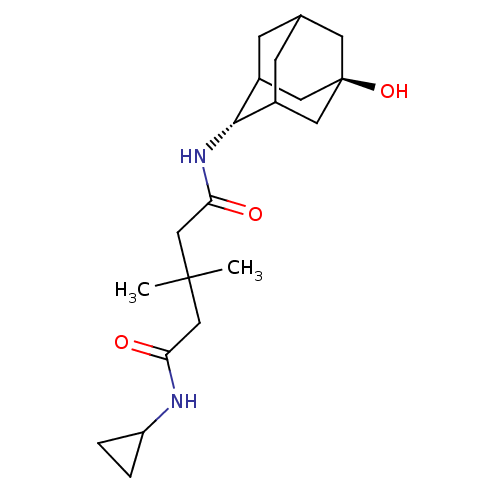 (3,3-Dimethyl-pentanedioic acid cyclopropylamide(5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck-Serono Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli assessed as cortisol level after 150 mins by HTRF assay | Bioorg Med Chem Lett 19: 2674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.140 BindingDB Entry DOI: 10.7270/Q2JQ112H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50294771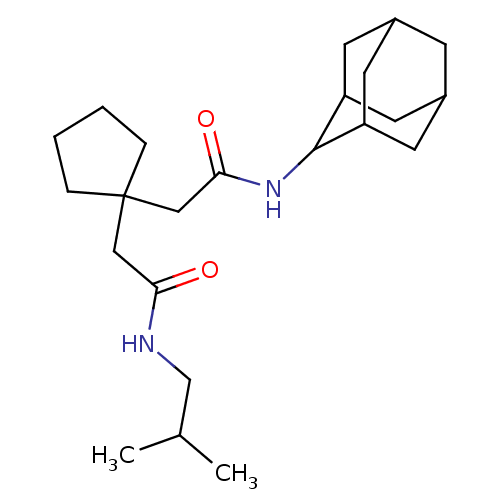 (CHEMBL549419 | N-Adamantan-2-yl-2-[1-(isobutylcarb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck-Serono Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli assessed as cortisol level after 150 mins by HTRF assay | Bioorg Med Chem Lett 19: 2674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.140 BindingDB Entry DOI: 10.7270/Q2JQ112H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50294767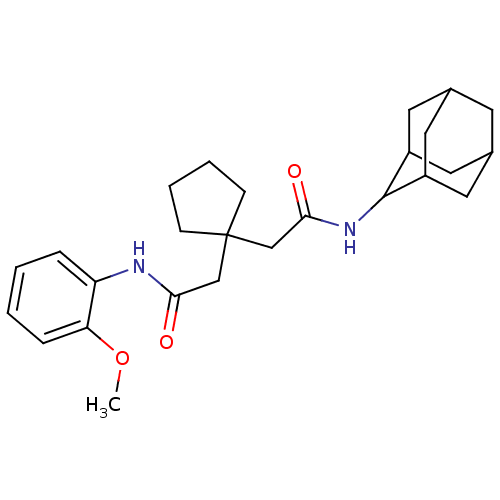 (CHEMBL562113 | N-Adamantan-2-yl-2-{1-[(2-methoxy-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck-Serono Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli assessed as cortisol level after 150 mins by HTRF assay | Bioorg Med Chem Lett 19: 2674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.140 BindingDB Entry DOI: 10.7270/Q2JQ112H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50294783 (3,3-Dimethyl-pentanedioic acid(5-carbamoyl-adamant...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck-Serono Curated by ChEMBL | Assay Description Inhibition of 11beta-HSD1 in mouse liver microsome | Bioorg Med Chem Lett 19: 2674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.140 BindingDB Entry DOI: 10.7270/Q2JQ112H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50294780 (CHEMBL560849 | N-Adamantan-2-yl-4-(3-cyclopropyl-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck-Serono Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli assessed as cortisol level after 150 mins by HTRF assay | Bioorg Med Chem Lett 19: 2674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.140 BindingDB Entry DOI: 10.7270/Q2JQ112H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50294770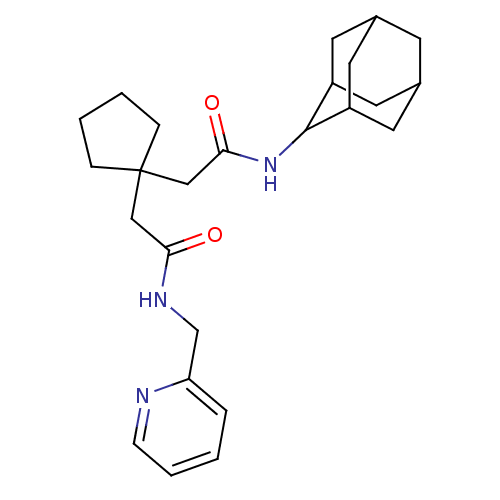 (CHEMBL560250 | N-Adamantan-2-yl-2-(1-{[(pyridin-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck-Serono Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli assessed as cortisol level after 150 mins by HTRF assay | Bioorg Med Chem Lett 19: 2674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.140 BindingDB Entry DOI: 10.7270/Q2JQ112H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50294765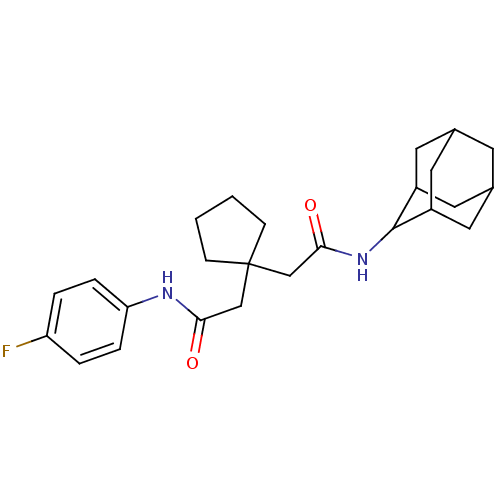 (CHEMBL551306 | N-Adamantan-2-yl-2-{1-[(4-fluoro-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck-Serono Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli assessed as cortisol level after 150 mins by HTRF assay | Bioorg Med Chem Lett 19: 2674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.140 BindingDB Entry DOI: 10.7270/Q2JQ112H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50294769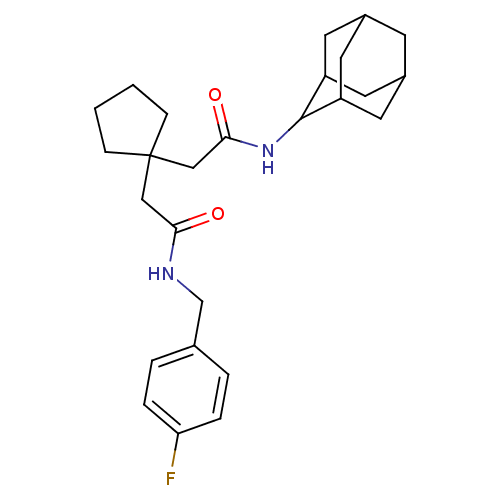 (CHEMBL564500 | N-Adamantan-2-yl-2-{1-[(4-fluoro-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck-Serono Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli assessed as cortisol level after 150 mins by HTRF assay | Bioorg Med Chem Lett 19: 2674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.140 BindingDB Entry DOI: 10.7270/Q2JQ112H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50294766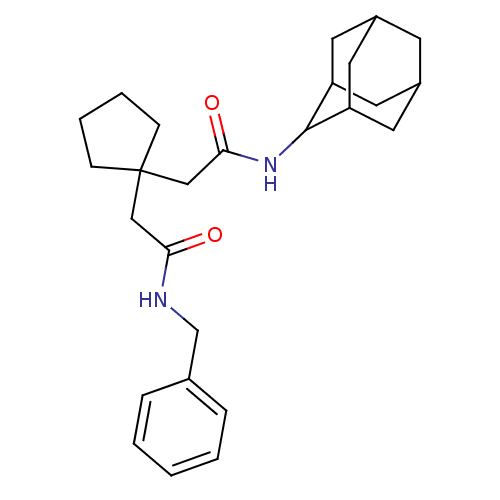 (CHEMBL562112 | N-Adamantan-2-yl-2-[1-(benzylcarbam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck-Serono Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli assessed as cortisol level after 150 mins by HTRF assay | Bioorg Med Chem Lett 19: 2674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.140 BindingDB Entry DOI: 10.7270/Q2JQ112H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Rattus norvegicus (rat)) | BDBM50294783 (3,3-Dimethyl-pentanedioic acid(5-carbamoyl-adamant...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck-Serono Curated by ChEMBL | Assay Description Inhibition of 11beta-HSD1 in rat liver microsome | Bioorg Med Chem Lett 19: 2674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.140 BindingDB Entry DOI: 10.7270/Q2JQ112H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50294773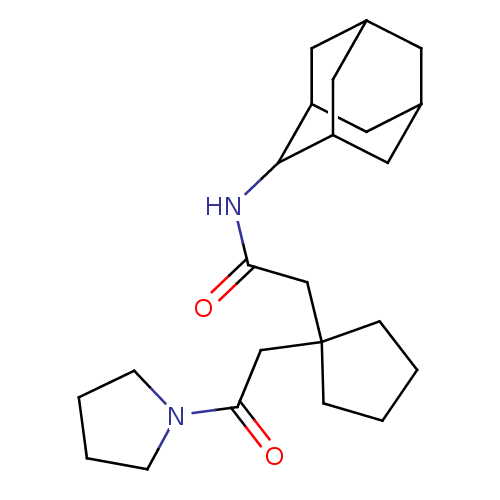 (CHEMBL564367 | N-Adamantan-2-yl-2-[1-(2-oxo-2-pyrr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck-Serono Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli assessed as cortisol level after 150 mins by HTRF assay | Bioorg Med Chem Lett 19: 2674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.140 BindingDB Entry DOI: 10.7270/Q2JQ112H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50294768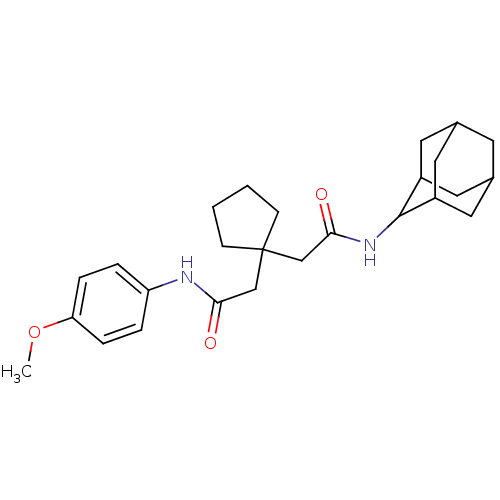 (CHEMBL551973 | N-Adamantan-2-yl-2-{1-[(4-methoxy-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck-Serono Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli assessed as cortisol level after 150 mins by HTRF assay | Bioorg Med Chem Lett 19: 2674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.140 BindingDB Entry DOI: 10.7270/Q2JQ112H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50294779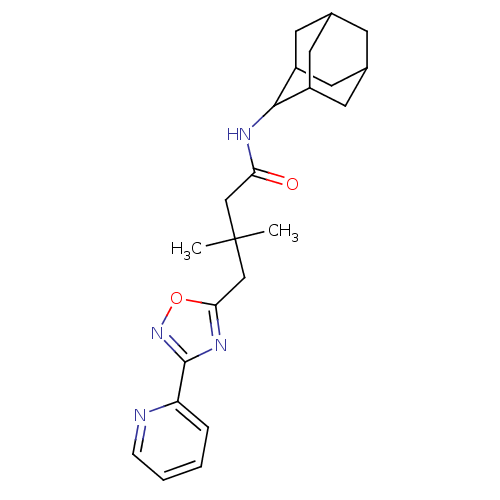 (CHEMBL550036 | N-Adamantan-2-yl-3,3-dimethyl-4-(3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck-Serono Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli assessed as cortisol level after 150 mins by HTRF assay | Bioorg Med Chem Lett 19: 2674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.140 BindingDB Entry DOI: 10.7270/Q2JQ112H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50294774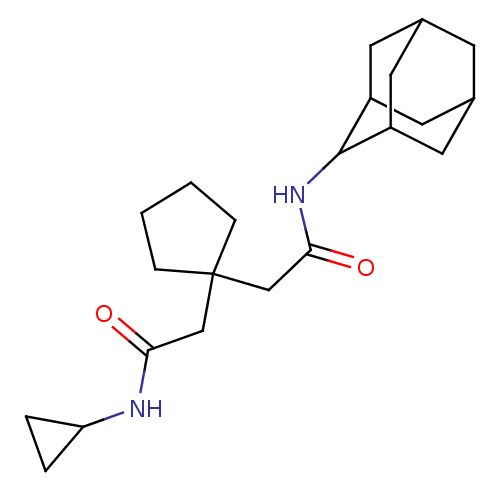 (CHEMBL560570 | N-Adamantan-2-yl-2-(1-cyclopropylca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck-Serono Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli assessed as cortisol level after 150 mins by HTRF assay | Bioorg Med Chem Lett 19: 2674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.140 BindingDB Entry DOI: 10.7270/Q2JQ112H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50294764 (3,3-Dimethyl-pentanedioic acid cyclopropylamide(5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck-Serono Curated by ChEMBL | Assay Description Inhibition of 11beta-HSD1 in mouse liver microsome | Bioorg Med Chem Lett 19: 2674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.140 BindingDB Entry DOI: 10.7270/Q2JQ112H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50294776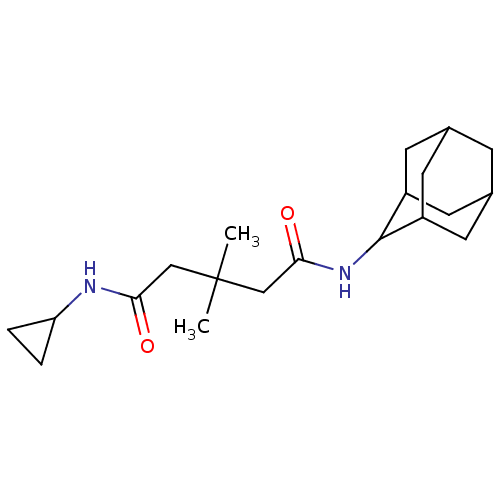 (3,3-Dimethyl-pentanedioic acid adamantan-2-ylamide...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck-Serono Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli assessed as cortisol level after 150 mins by HTRF assay | Bioorg Med Chem Lett 19: 2674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.140 BindingDB Entry DOI: 10.7270/Q2JQ112H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50294772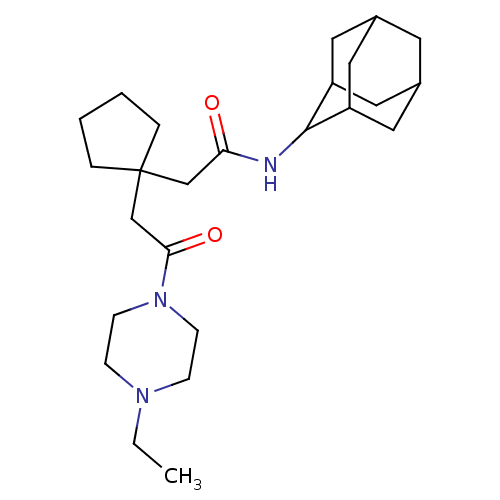 (CHEMBL560449 | N-Adamantan-2-yl-2-{1-[2-(4-ethyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck-Serono Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli assessed as cortisol level after 150 mins by HTRF assay | Bioorg Med Chem Lett 19: 2674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.140 BindingDB Entry DOI: 10.7270/Q2JQ112H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50294778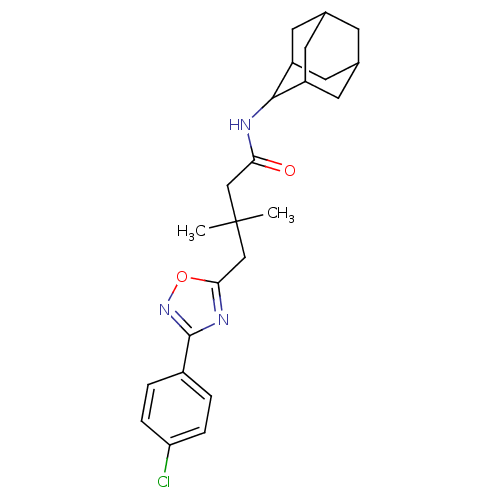 (CHEMBL565064 | N-Adamantan-2-yl-4-[3-(4-chloro-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck-Serono Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli assessed as cortisol level after 150 mins by HTRF assay | Bioorg Med Chem Lett 19: 2674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.140 BindingDB Entry DOI: 10.7270/Q2JQ112H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50294777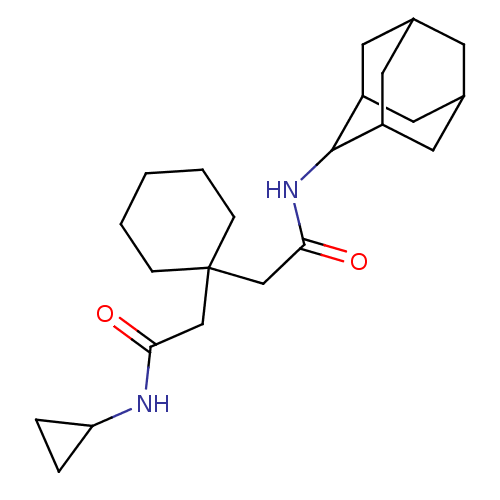 (CHEMBL562937 | N-Adamantan-2-yl-2-(1-cyclopropylca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck-Serono Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli assessed as cortisol level after 150 mins by HTRF assay | Bioorg Med Chem Lett 19: 2674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.140 BindingDB Entry DOI: 10.7270/Q2JQ112H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50294785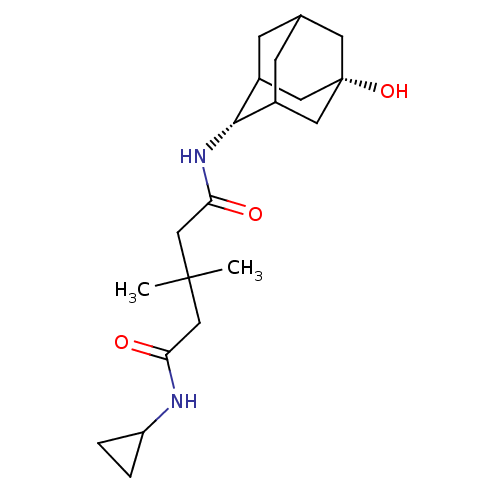 (3,3-Dimethyl-pentanedioic acid cyclopropylamide(5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck-Serono Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli assessed as cortisol level after 150 mins by HTRF assay | Bioorg Med Chem Lett 19: 2674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.140 BindingDB Entry DOI: 10.7270/Q2JQ112H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Rattus norvegicus (rat)) | BDBM50294764 (3,3-Dimethyl-pentanedioic acid cyclopropylamide(5-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck-Serono Curated by ChEMBL | Assay Description Inhibition of 11beta-HSD1 in rat liver microsome | Bioorg Med Chem Lett 19: 2674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.140 BindingDB Entry DOI: 10.7270/Q2JQ112H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50294775 (CHEMBL549555 | Pentanedioic acid adamantan-2-ylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck-Serono Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli assessed as cortisol level after 150 mins by HTRF assay | Bioorg Med Chem Lett 19: 2674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.140 BindingDB Entry DOI: 10.7270/Q2JQ112H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50294782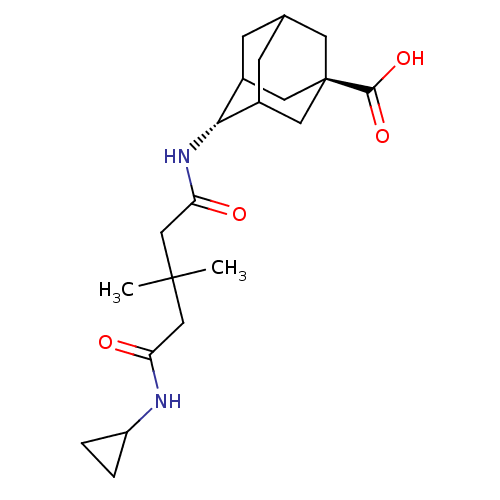 (4-(4-Cyclopropylcarbamoyl-3,3-dimethyl-butyrylamin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck-Serono Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli assessed as cortisol level after 150 mins by HTRF assay | Bioorg Med Chem Lett 19: 2674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.140 BindingDB Entry DOI: 10.7270/Q2JQ112H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50294784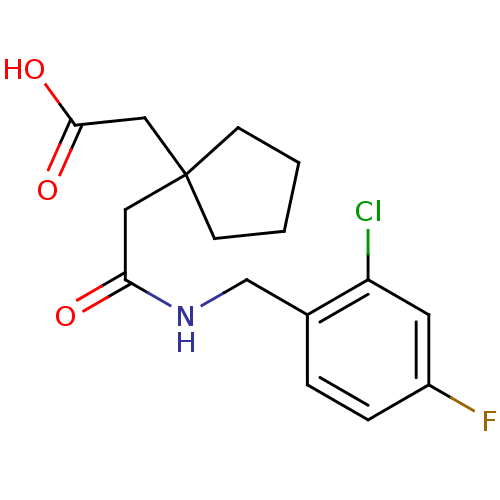 (2-(1-(2-(2-chloro-4-fluorobenzylamino)-2-oxoethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck-Serono Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 | Bioorg Med Chem Lett 19: 2674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.140 BindingDB Entry DOI: 10.7270/Q2JQ112H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50294786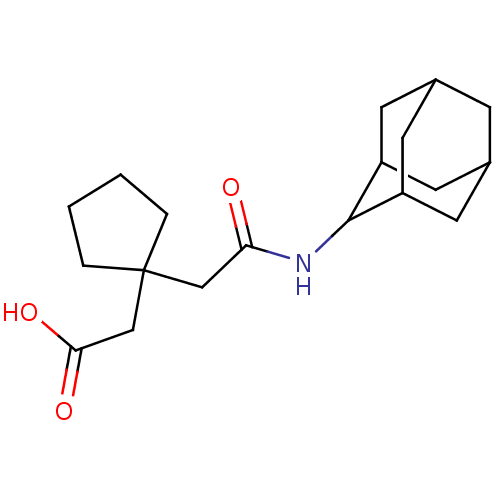 (CHEMBL562600 | [1-(Adamantan-2-ylcarbamoylmethyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck-Serono Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 | Bioorg Med Chem Lett 19: 2674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.140 BindingDB Entry DOI: 10.7270/Q2JQ112H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM394274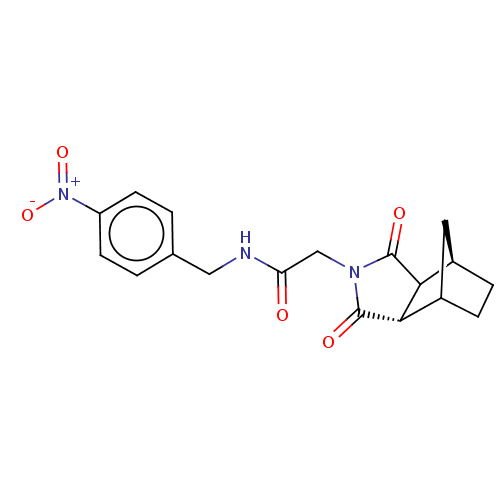 (US9975902, Example 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC | Assay Description The binding capacity of the compounds was measured using a competition Fluorescence-Polarisation based assay with fluorescine labelled cyclosporin. | J Med Chem 52: 2724-32 (2009) BindingDB Entry DOI: 10.7270/Q26W9DD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM394273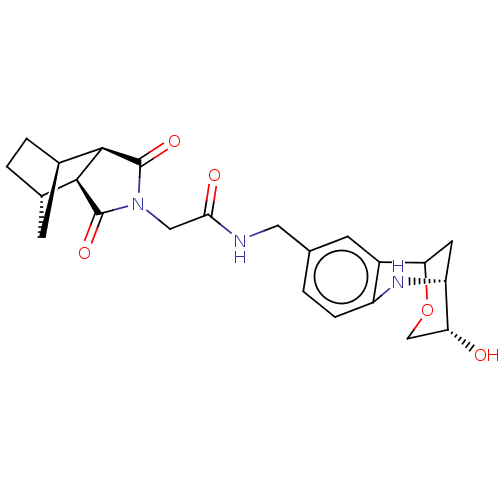 (US9975902, Example 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC | Assay Description The binding capacity of the compounds was measured using a competition Fluorescence-Polarisation based assay with fluorescine labelled cyclosporin. | J Med Chem 52: 2724-32 (2009) BindingDB Entry DOI: 10.7270/Q26W9DD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM394272 (US9975902, Example 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC | Assay Description The peptidyl-proline isomerase activity (PPase) was determined by using a PPase-chymotrypsin coupled assay with suc-AAPF-p-NA as substrated and color... | J Med Chem 52: 2724-32 (2009) BindingDB Entry DOI: 10.7270/Q26W9DD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM394272 (US9975902, Example 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC | Assay Description The binding capacity of the compounds was measured using a competition Fluorescence-Polarisation based assay with fluorescine labelled cyclosporin. | J Med Chem 52: 2724-32 (2009) BindingDB Entry DOI: 10.7270/Q26W9DD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM394270 (US9975902, Example 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC | Assay Description The binding capacity of the compounds was measured using a competition Fluorescence-Polarisation based assay with fluorescine labelled cyclosporin. | J Med Chem 52: 2724-32 (2009) BindingDB Entry DOI: 10.7270/Q26W9DD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50294783 (3,3-Dimethyl-pentanedioic acid(5-carbamoyl-adamant...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck-Serono Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD2 expressed in Escherichia coli assessed as cortisol level after 60 mins by HTRF assay | Bioorg Med Chem Lett 19: 2674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.140 BindingDB Entry DOI: 10.7270/Q2JQ112H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50294764 (3,3-Dimethyl-pentanedioic acid cyclopropylamide(5-...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck-Serono Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD2 expressed in Escherichia coli assessed as cortisol level after 60 mins by HTRF assay | Bioorg Med Chem Lett 19: 2674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.140 BindingDB Entry DOI: 10.7270/Q2JQ112H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM394280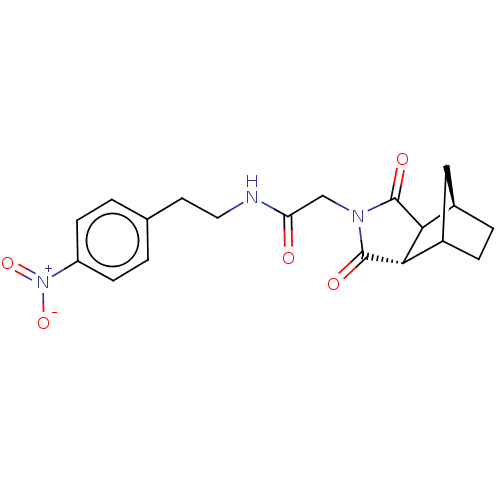 (US9975902, Example 10) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC | Assay Description The binding capacity of the compounds was measured using a competition Fluorescence-Polarisation based assay with fluorescine labelled cyclosporin. | J Med Chem 52: 2724-32 (2009) BindingDB Entry DOI: 10.7270/Q26W9DD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM394279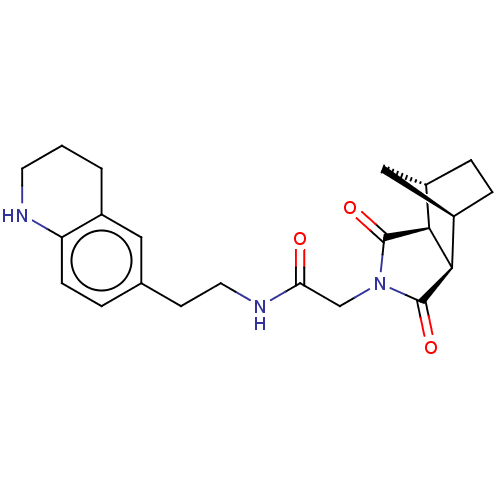 (US9975902, Example 9) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC | Assay Description The binding capacity of the compounds was measured using a competition Fluorescence-Polarisation based assay with fluorescine labelled cyclosporin. | J Med Chem 52: 2724-32 (2009) BindingDB Entry DOI: 10.7270/Q26W9DD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM394278 (US9975902, Example 8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC | Assay Description The binding capacity of the compounds was measured using a competition Fluorescence-Polarisation based assay with fluorescine labelled cyclosporin. | J Med Chem 52: 2724-32 (2009) BindingDB Entry DOI: 10.7270/Q26W9DD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM394277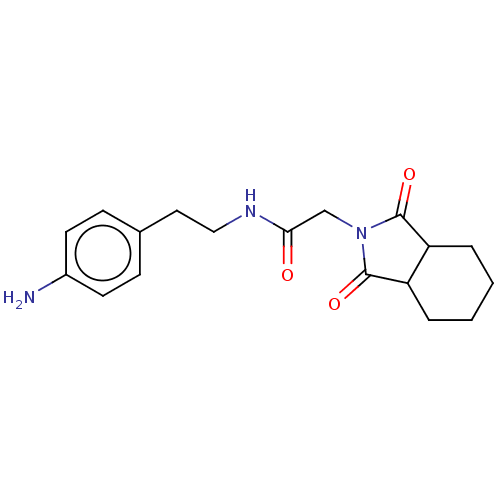 (US9975902, Example 7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC | Assay Description The binding capacity of the compounds was measured using a competition Fluorescence-Polarisation based assay with fluorescine labelled cyclosporin. | J Med Chem 52: 2724-32 (2009) BindingDB Entry DOI: 10.7270/Q26W9DD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM394276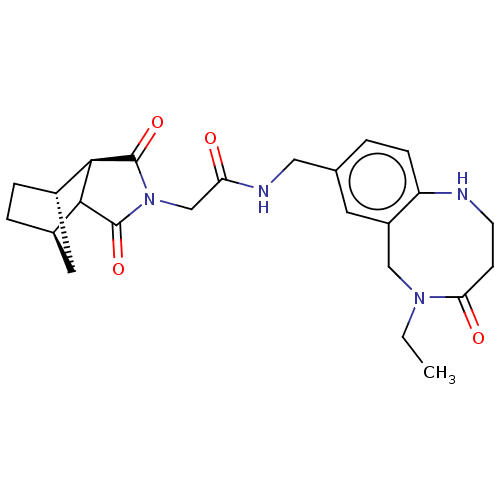 (US9975902, Example 6) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC | Assay Description The binding capacity of the compounds was measured using a competition Fluorescence-Polarisation based assay with fluorescine labelled cyclosporin. | J Med Chem 52: 2724-32 (2009) BindingDB Entry DOI: 10.7270/Q26W9DD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM394275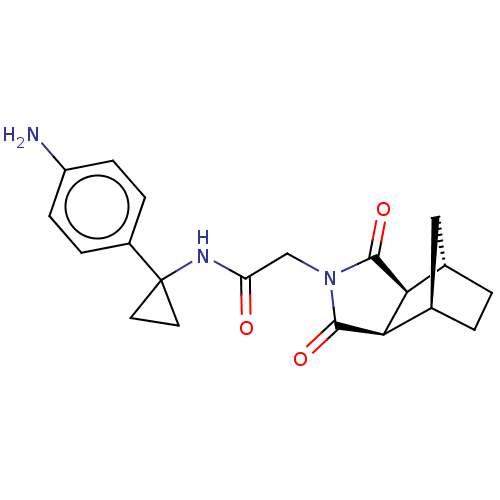 (US9975902, Example 5) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC | Assay Description The binding capacity of the compounds was measured using a competition Fluorescence-Polarisation based assay with fluorescine labelled cyclosporin. | J Med Chem 52: 2724-32 (2009) BindingDB Entry DOI: 10.7270/Q26W9DD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM394281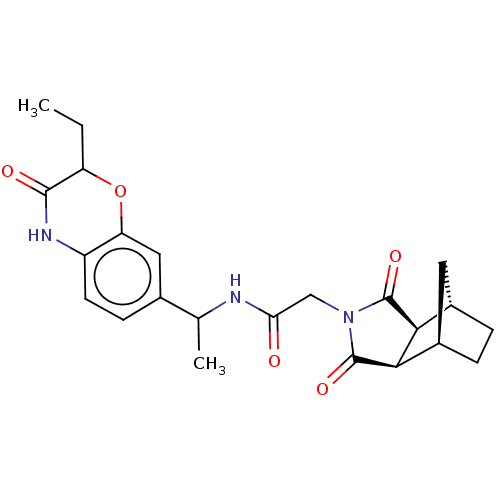 (US9975902, Example 11) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC | Assay Description The binding capacity of the compounds was measured using a competition Fluorescence-Polarisation based assay with fluorescine labelled cyclosporin. | J Med Chem 52: 2724-32 (2009) BindingDB Entry DOI: 10.7270/Q26W9DD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM394274 (US9975902, Example 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC | Assay Description The peptidyl-proline isomerase activity (PPase) was determined by using a PPase-chymotrypsin coupled assay with suc-AAPF-p-NA as substrated and color... | J Med Chem 52: 2724-32 (2009) BindingDB Entry DOI: 10.7270/Q26W9DD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||