

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM3386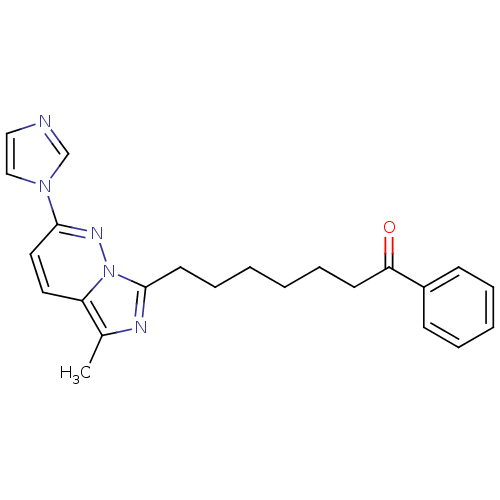 (7-[2-(1H-Imidazol-l-yl)-5-methylimidazo[1,5-b]pyri...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 3784-94 (1993) Article DOI: 10.1021/jm00076a005 BindingDB Entry DOI: 10.7270/Q2PR7T57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50470139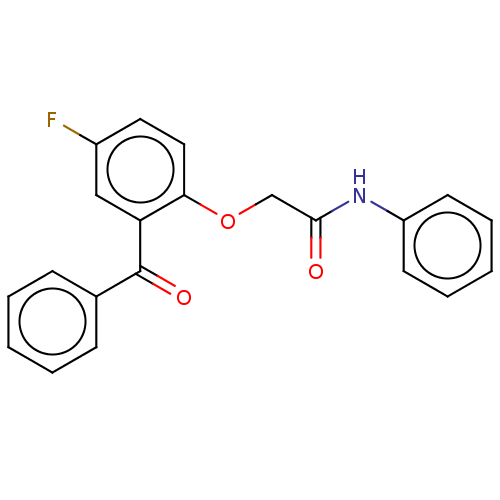 (CHEMBL46745) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.59 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 38: 1657-65 (1995) Article DOI: 10.1021/jm00010a010 BindingDB Entry DOI: 10.7270/Q27947DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50470155 (CHEMBL288971) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 38: 1657-65 (1995) Article DOI: 10.1021/jm00010a010 BindingDB Entry DOI: 10.7270/Q27947DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50470136 (CHEMBL296939 | GF-128590) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 27.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 38: 1657-65 (1995) Article DOI: 10.1021/jm00010a010 BindingDB Entry DOI: 10.7270/Q27947DP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50470145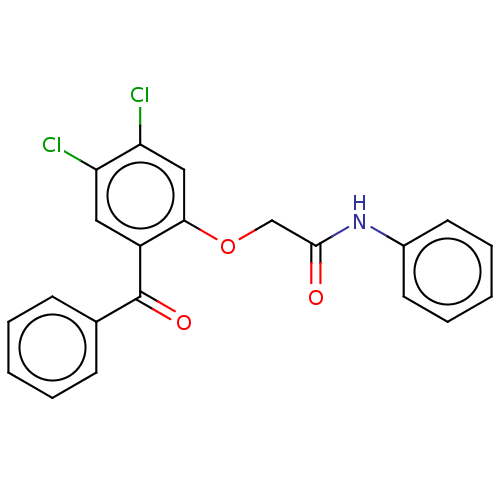 (CHEMBL46925) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 38: 1657-65 (1995) Article DOI: 10.1021/jm00010a010 BindingDB Entry DOI: 10.7270/Q27947DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM3383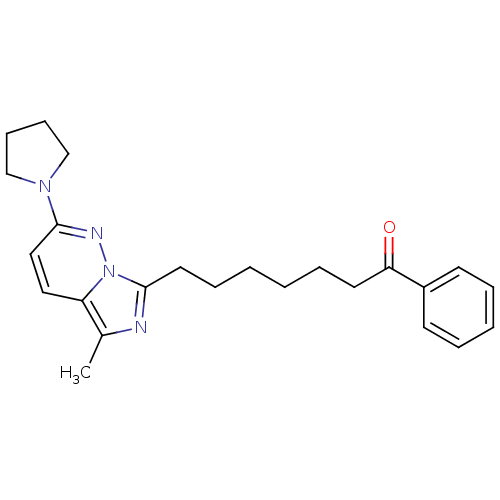 (7-[5-Methyl-2-(1-pyrrolidinyl)imidazo[1,5-b]pyrida...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 3784-94 (1993) Article DOI: 10.1021/jm00076a005 BindingDB Entry DOI: 10.7270/Q2PR7T57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM3385 (7-[5-methyl-2-(1H-pyrrol-1-yl)imidazo[1,5-a]pyrida...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 3784-94 (1993) Article DOI: 10.1021/jm00076a005 BindingDB Entry DOI: 10.7270/Q2PR7T57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM3379 (7-[5-Methyl-2-(methylthio)imidazo[1,5-b]pyridazin-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Glaxo Group Research Ltd. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 3784-94 (1993) Article DOI: 10.1021/jm00076a005 BindingDB Entry DOI: 10.7270/Q2PR7T57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM3392 (7-[5-methyl-2-(5-methyl-1H-imidazol-1-yl)imidazo[1...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 3784-94 (1993) Article DOI: 10.1021/jm00076a005 BindingDB Entry DOI: 10.7270/Q2PR7T57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM3388 (7-[5-methyl-2-(1H-1,2,4-triazol-1-yl)imidazo[1,5-a...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 3784-94 (1993) Article DOI: 10.1021/jm00076a005 BindingDB Entry DOI: 10.7270/Q2PR7T57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50470146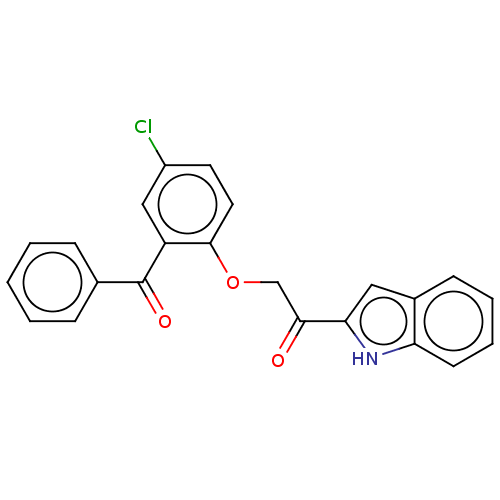 (CHEMBL417183) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 161 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 38: 1657-65 (1995) Article DOI: 10.1021/jm00010a010 BindingDB Entry DOI: 10.7270/Q27947DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50470138 (CHEMBL46650) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 207 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 38: 1657-65 (1995) Article DOI: 10.1021/jm00010a010 BindingDB Entry DOI: 10.7270/Q27947DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM3395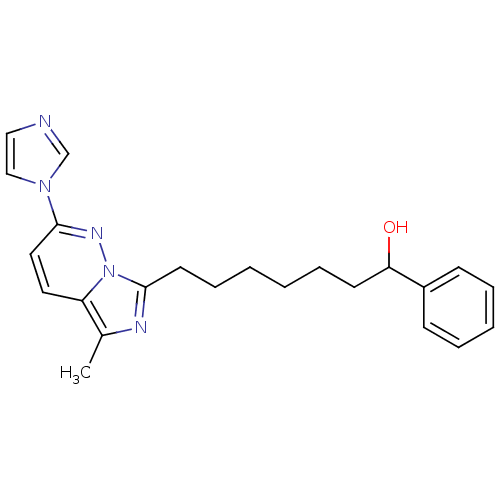 (7-[2-(1H-imidazol-1-yl)-5-methylimidazo[1,5-a]pyri...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 3784-94 (1993) Article DOI: 10.1021/jm00076a005 BindingDB Entry DOI: 10.7270/Q2PR7T57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM3382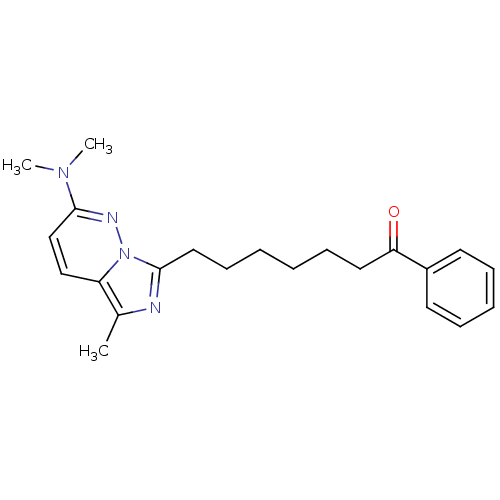 (7-[2-(Dimethylamino)-5-methylimidazo[1,5-b]pyridaz...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 3784-94 (1993) Article DOI: 10.1021/jm00076a005 BindingDB Entry DOI: 10.7270/Q2PR7T57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM3374 (7-{2-chloroimidazo[1,5-a]pyridazin-7-yl}-1-phenylh...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Glaxo Group Research Ltd. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 3784-94 (1993) Article DOI: 10.1021/jm00076a005 BindingDB Entry DOI: 10.7270/Q2PR7T57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM3373 (( 1RS)-2-Chloro-5-methyl-alpha-phenyl-7-imidazo[1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Glaxo Group Research Ltd. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 3784-94 (1993) Article DOI: 10.1021/jm00076a005 BindingDB Entry DOI: 10.7270/Q2PR7T57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50470151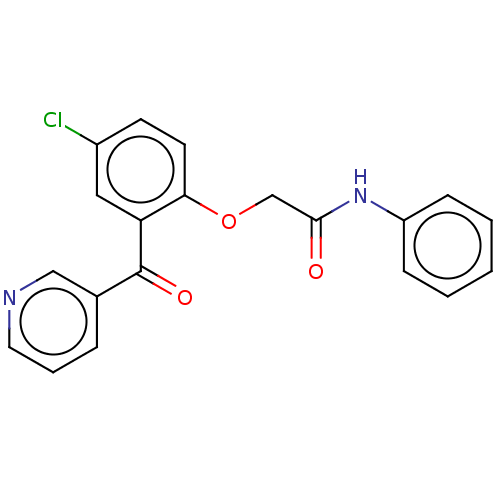 (CHEMBL297010) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 436 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 38: 1657-65 (1995) Article DOI: 10.1021/jm00010a010 BindingDB Entry DOI: 10.7270/Q27947DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50470144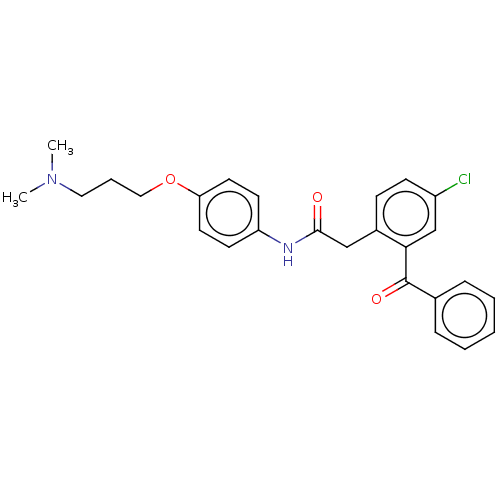 (CHEMBL43263) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 443 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 38: 1657-65 (1995) Article DOI: 10.1021/jm00010a010 BindingDB Entry DOI: 10.7270/Q27947DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM3393 (7-[5-methyl-2-(4-methyl-1H-imidazol-1-yl)imidazo[1...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 3784-94 (1993) Article DOI: 10.1021/jm00076a005 BindingDB Entry DOI: 10.7270/Q2PR7T57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM3377 (7-(5-Methylimidazo[1,5-b]pyridazin-7-yl)-l-phenyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Glaxo Group Research Ltd. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 3784-94 (1993) Article DOI: 10.1021/jm00076a005 BindingDB Entry DOI: 10.7270/Q2PR7T57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM3389 (7-[5-methyl-2-(1H-1,2,3-triazol-1-yl)imidazo[1,5-a...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 3784-94 (1993) Article DOI: 10.1021/jm00076a005 BindingDB Entry DOI: 10.7270/Q2PR7T57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM3378 (7-(2-Methoxy-5-methylimidazo[1,5-b]pyridazin-7-y1)...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 640 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Glaxo Group Research Ltd. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 3784-94 (1993) Article DOI: 10.1021/jm00076a005 BindingDB Entry DOI: 10.7270/Q2PR7T57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50470148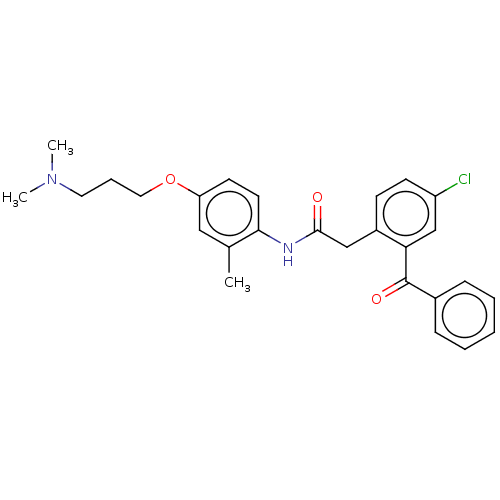 (CHEMBL42078) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 752 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 38: 1657-65 (1995) Article DOI: 10.1021/jm00010a010 BindingDB Entry DOI: 10.7270/Q27947DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM3365 (7-(2-Chloro-5-methylimidazo[1,5-b]pyridazin-7-yl)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 840 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Glaxo Group Research Ltd. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 3784-94 (1993) Article DOI: 10.1021/jm00076a005 BindingDB Entry DOI: 10.7270/Q2PR7T57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50470137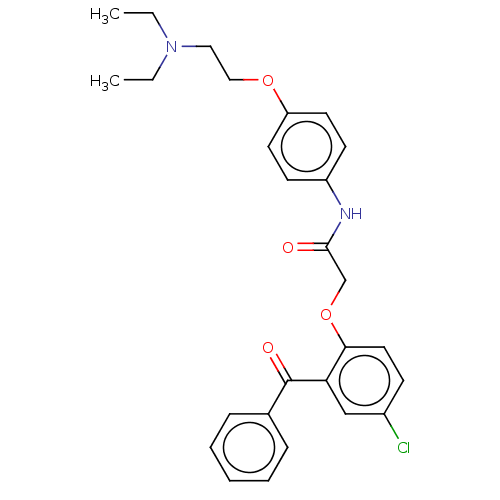 (CHEMBL45100) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 935 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 38: 1657-65 (1995) Article DOI: 10.1021/jm00010a010 BindingDB Entry DOI: 10.7270/Q27947DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM3391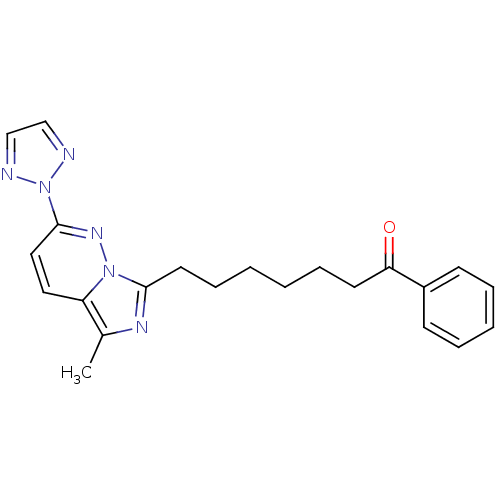 (7-[5-methyl-2-(2H-1,2,3-triazol-2-yl)imidazo[1,5-a...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 3784-94 (1993) Article DOI: 10.1021/jm00076a005 BindingDB Entry DOI: 10.7270/Q2PR7T57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM3363 (5-{2-chloro-5-methylimidazo[1,5-a]pyridazin-7-yl}p...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Glaxo Group Research Ltd. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 3784-94 (1993) Article DOI: 10.1021/jm00076a005 BindingDB Entry DOI: 10.7270/Q2PR7T57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50470166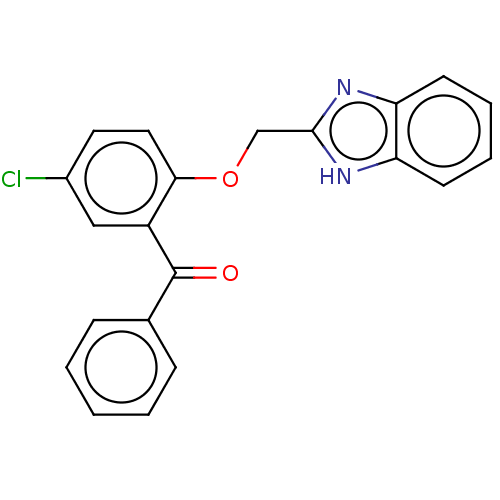 (CHEMBL44002) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 38: 1657-65 (1995) Article DOI: 10.1021/jm00010a010 BindingDB Entry DOI: 10.7270/Q27947DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071777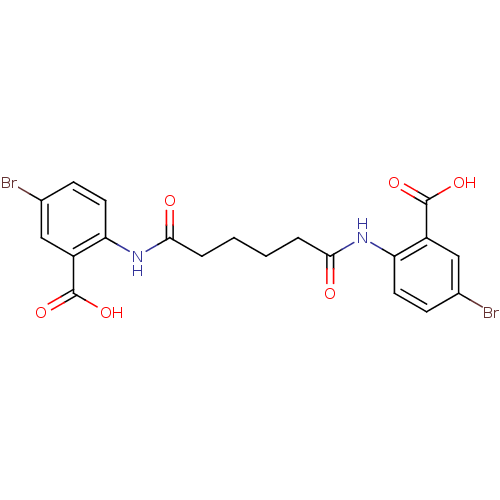 (5-bromo-2-({6-[(4-bromo-2-carboxyphenyl)amino]-6-o...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-1 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071776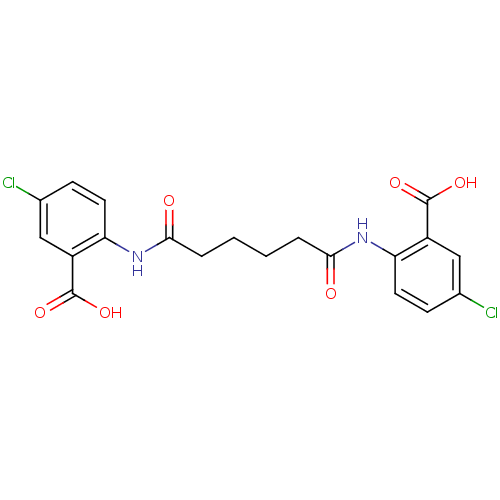 (2-({6-[(2-carboxy-4-chlorophenyl)amino]-6-oxohexan...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-2 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM3375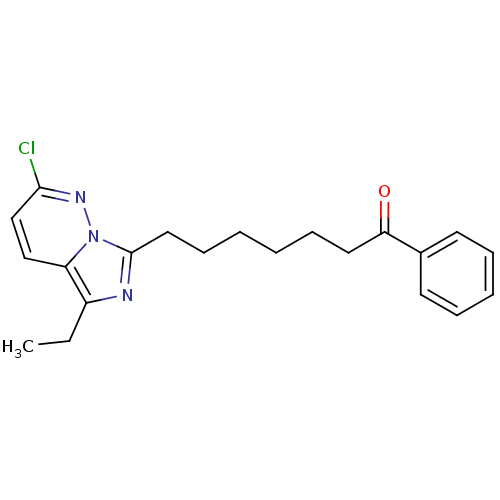 (7-{2-chloro-5-ethylimidazo[1,5-a]pyridazin-7-yl}-1...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Glaxo Group Research Ltd. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 3784-94 (1993) Article DOI: 10.1021/jm00076a005 BindingDB Entry DOI: 10.7270/Q2PR7T57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM3364 (7-[5-(benzyloxy)pentyl]-2-chloro-5-methylimidazo[1...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Glaxo Group Research Ltd. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 3784-94 (1993) Article DOI: 10.1021/jm00076a005 BindingDB Entry DOI: 10.7270/Q2PR7T57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM3394 (7-[5-methyl-2-(2-methyl-1H-imidazol-1-yl)imidazo[1...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 3784-94 (1993) Article DOI: 10.1021/jm00076a005 BindingDB Entry DOI: 10.7270/Q2PR7T57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM3390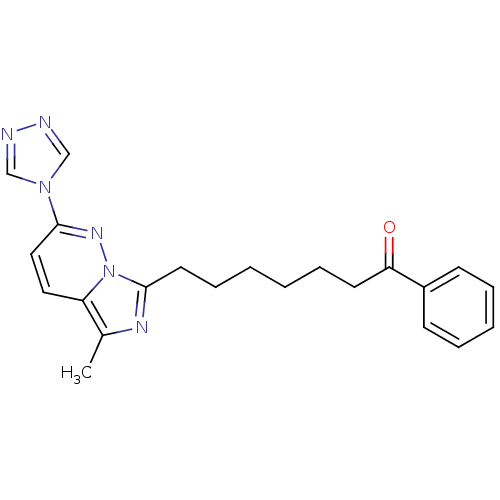 (7-[5-methyl-2-(4H-1,2,4-triazol-4-yl)imidazo[1,5-a...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 3784-94 (1993) Article DOI: 10.1021/jm00076a005 BindingDB Entry DOI: 10.7270/Q2PR7T57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM3380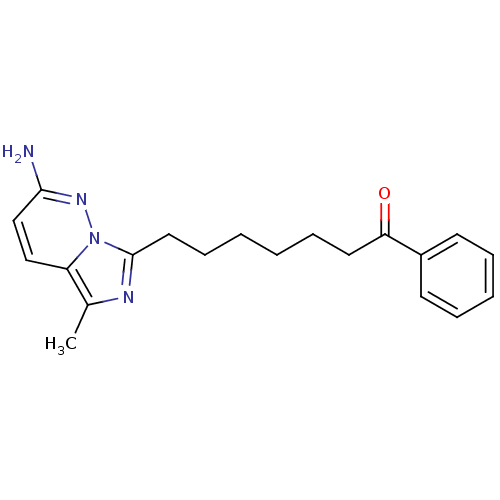 (7-(2-Amino-5-methylimidazo[1,5-b]pyridazin-7-yl)-l...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 3784-94 (1993) Article DOI: 10.1021/jm00076a005 BindingDB Entry DOI: 10.7270/Q2PR7T57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM3369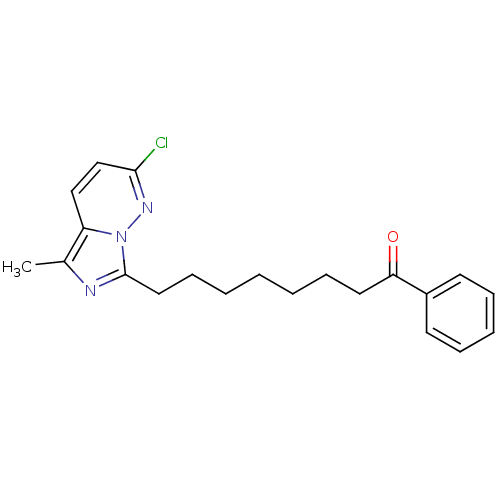 (8-{2-chloro-5-methylimidazo[1,5-a]pyridazin-7-yl}-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Glaxo Group Research Ltd. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 3784-94 (1993) Article DOI: 10.1021/jm00076a005 BindingDB Entry DOI: 10.7270/Q2PR7T57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071777 (5-bromo-2-({6-[(4-bromo-2-carboxyphenyl)amino]-6-o...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-1 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM3387 (7-[5-methyl-2-(1H-pyrazol-1-yl)imidazo[1,5-a]pyrid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 3784-94 (1993) Article DOI: 10.1021/jm00076a005 BindingDB Entry DOI: 10.7270/Q2PR7T57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM3381 (7-[5-methyl-2-(methylamino)imidazo[1,5-a]pyridazin...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 3784-94 (1993) Article DOI: 10.1021/jm00076a005 BindingDB Entry DOI: 10.7270/Q2PR7T57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071766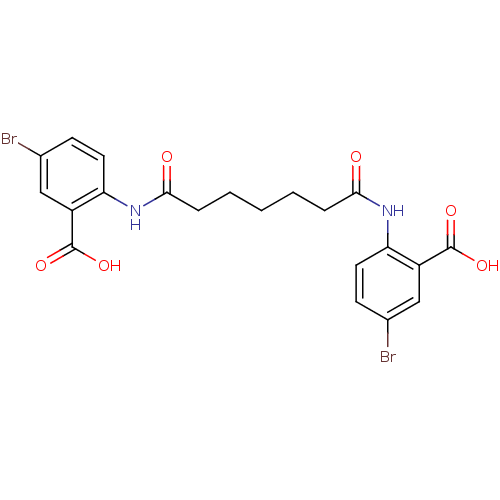 (5-bromo-2-[5-(4-bromo-2-carboxyphenylcarbamoyl)pen...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-2 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM3367 (7-{2-chloro-5-methylimidazo[1,5-a]pyridazin-7-yl}-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Glaxo Group Research Ltd. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 3784-94 (1993) Article DOI: 10.1021/jm00076a005 BindingDB Entry DOI: 10.7270/Q2PR7T57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071768 (2-({6-[(2-carboxy-4-iodophenyl)amino]-6-oxohexanoy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-1 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071765 (2-[4-(2-carboxy-4-methoxyphenylcarbamoyl)butylcarb...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-2 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071766 (5-bromo-2-[5-(4-bromo-2-carboxyphenylcarbamoyl)pen...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-1 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM3368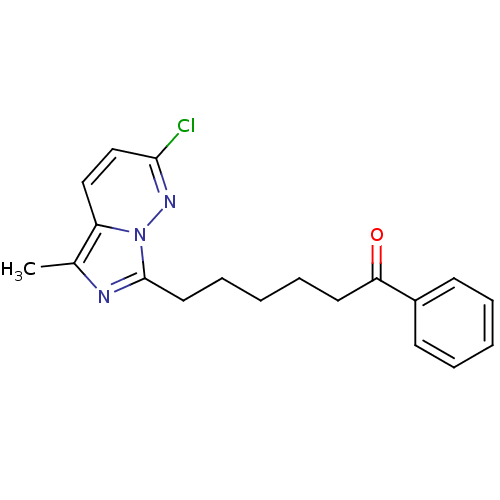 (6-{2-chloro-5-methylimidazo[1,5-a]pyridazin-7-yl}-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Glaxo Group Research Ltd. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 3784-94 (1993) Article DOI: 10.1021/jm00076a005 BindingDB Entry DOI: 10.7270/Q2PR7T57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50470140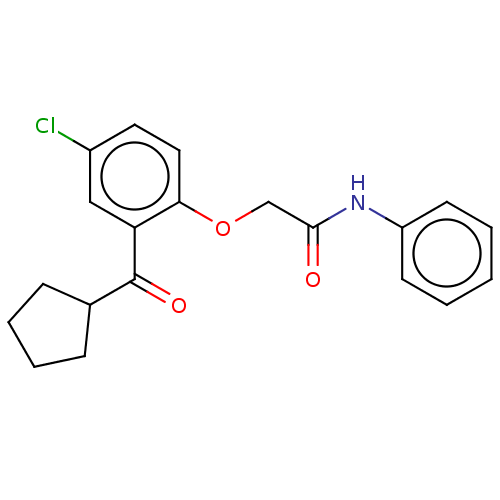 (CHEMBL42066) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 38: 1657-65 (1995) Article DOI: 10.1021/jm00010a010 BindingDB Entry DOI: 10.7270/Q27947DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50470152 (CHEMBL42823) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 38: 1657-65 (1995) Article DOI: 10.1021/jm00010a010 BindingDB Entry DOI: 10.7270/Q27947DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50470168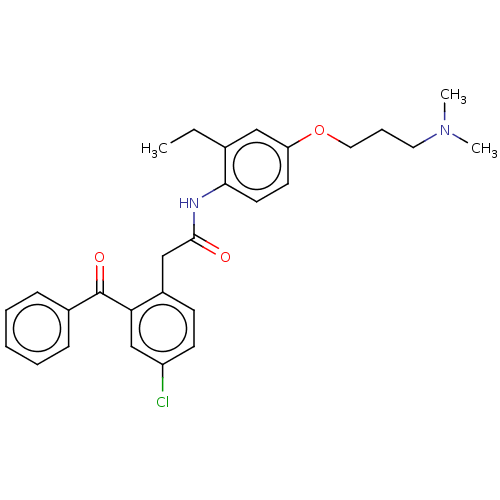 (CHEMBL46977) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 38: 1657-65 (1995) Article DOI: 10.1021/jm00010a010 BindingDB Entry DOI: 10.7270/Q27947DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071771 (5-bromo-2-{[(5-{[(4-bromo-2-carboxyphenyl)amino]ca...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-2 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50470142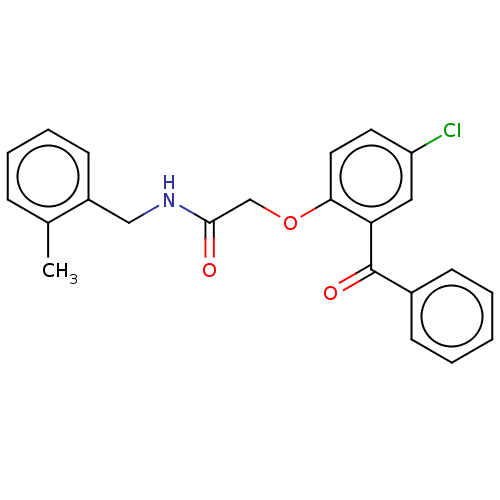 (CHEMBL46675) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 38: 1657-65 (1995) Article DOI: 10.1021/jm00010a010 BindingDB Entry DOI: 10.7270/Q27947DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 105 total ) | Next | Last >> |