 Found 8824 hits with Last Name = 'gu' and Initial = 'f'
Found 8824 hits with Last Name = 'gu' and Initial = 'f' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Somatostatin receptor type 4
(RAT) | BDBM82253
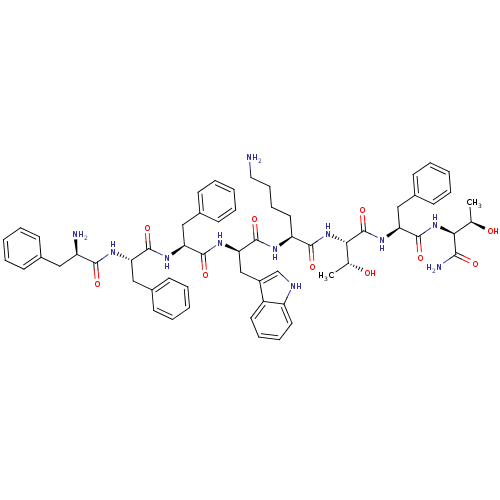
(BIM 23052 | CAS_133073-82-2)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](N)Cc1ccccc1)[C@@H](C)O)C(N)=O |r| Show InChI InChI=1S/C61H75N11O10/c1-37(73)52(54(64)75)71-60(81)50(34-42-25-13-6-14-26-42)70-61(82)53(38(2)74)72-56(77)47(29-17-18-30-62)66-59(80)51(35-43-36-65-46-28-16-15-27-44(43)46)69-58(79)49(33-41-23-11-5-12-24-41)68-57(78)48(32-40-21-9-4-10-22-40)67-55(76)45(63)31-39-19-7-3-8-20-39/h3-16,19-28,36-38,45,47-53,65,73-74H,17-18,29-35,62-63H2,1-2H3,(H2,64,75)(H,66,80)(H,67,76)(H,68,78)(H,69,79)(H,70,82)(H,71,81)(H,72,77)/t37-,38-,45-,47+,48+,49+,50+,51-,52+,53+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tulane University
Curated by PDSP Ki Database
| |
Peptides 15: 1421-4 (1994)
Article DOI: 10.1016/0196-9781(94)90118-x
BindingDB Entry DOI: 10.7270/Q24748C1 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(RAT) | BDBM82255
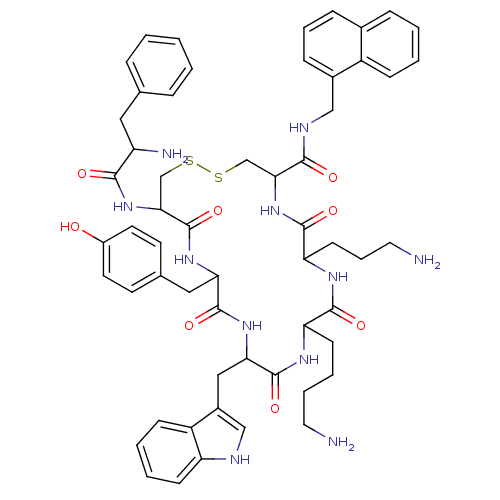
(D-Phe-Cys-Tyr-D-Trp-Lys-Abu-Cys-Nal-NH | NC8-12)Show SMILES NCCCCC1NC(=O)C(Cc2c[nH]c3ccccc23)NC(=O)C(Cc2ccc(O)cc2)NC(=O)C(CSSCC(NC(=O)C(CCCN)NC1=O)C(=O)NCc1cccc2ccccc12)NC(=O)C(N)Cc1ccccc1 Show InChI InChI=1S/C57H69N11O8S2/c58-26-9-8-20-45-53(72)63-46(21-11-27-59)54(73)68-49(52(71)62-31-38-16-10-15-37-14-4-5-17-41(37)38)33-77-78-34-50(67-51(70)43(60)28-35-12-2-1-3-13-35)57(76)65-47(29-36-22-24-40(69)25-23-36)55(74)66-48(56(75)64-45)30-39-32-61-44-19-7-6-18-42(39)44/h1-7,10,12-19,22-25,32,43,45-50,61,69H,8-9,11,20-21,26-31,33-34,58-60H2,(H,62,71)(H,63,72)(H,64,75)(H,65,76)(H,66,74)(H,67,70)(H,68,73) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tulane University
Curated by PDSP Ki Database
| |
Peptides 15: 1421-4 (1994)
Article DOI: 10.1016/0196-9781(94)90118-x
BindingDB Entry DOI: 10.7270/Q24748C1 |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50162774

(ABT-199 | US11420968, Example ABT-199 | Venetoclax)Show SMILES CC1(C)CCC(CN2CCN(CC2)c2ccc(C(=O)NS(=O)(=O)c3ccc(NCC4CCOCC4)c(c3)N(=O)=O)c(Oc3cnc4[nH]ccc4c3)c2)=C(C1)c1ccc(Cl)cc1 |c:57| Show InChI InChI=1S/C45H50ClN7O7S/c1-45(2)15-11-33(39(26-45)31-3-5-34(46)6-4-31)29-51-17-19-52(20-18-51)35-7-9-38(42(24-35)60-36-23-32-12-16-47-43(32)49-28-36)44(54)50-61(57,58)37-8-10-40(41(25-37)53(55)56)48-27-30-13-21-59-22-14-30/h3-10,12,16,23-25,28,30,48H,11,13-15,17-22,26-27,29H2,1-2H3,(H,47,49)(H,50,54) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BCL2 (unknown origin) by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00242
BindingDB Entry DOI: 10.7270/Q2KD22HB |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(RAT) | BDBM82256
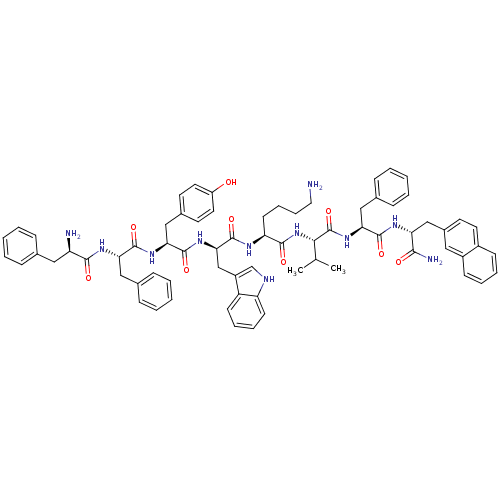
(BIM 23056 | CAS_150155-61-6 | D-Phe-Phe-Tyr-D-Trp-...)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](N)Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H](Cc1ccc2ccccc2c1)C(N)=O |r| Show InChI InChI=1S/C71H81N11O9/c1-44(2)63(71(91)81-61(39-47-22-10-5-11-23-47)67(87)77-58(64(74)84)41-49-29-32-50-24-12-13-25-51(50)36-49)82-66(86)57(28-16-17-35-72)76-70(90)62(42-52-43-75-56-27-15-14-26-54(52)56)80-69(89)60(40-48-30-33-53(83)34-31-48)79-68(88)59(38-46-20-8-4-9-21-46)78-65(85)55(73)37-45-18-6-3-7-19-45/h3-15,18-27,29-34,36,43-44,55,57-63,75,83H,16-17,28,35,37-42,72-73H2,1-2H3,(H2,74,84)(H,76,90)(H,77,87)(H,78,85)(H,79,88)(H,80,89)(H,81,91)(H,82,86)/t55-,57+,58-,59+,60+,61+,62-,63+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tulane University
Curated by PDSP Ki Database
| |
Peptides 15: 1421-4 (1994)
Article DOI: 10.1016/0196-9781(94)90118-x
BindingDB Entry DOI: 10.7270/Q24748C1 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(RAT) | BDBM82257
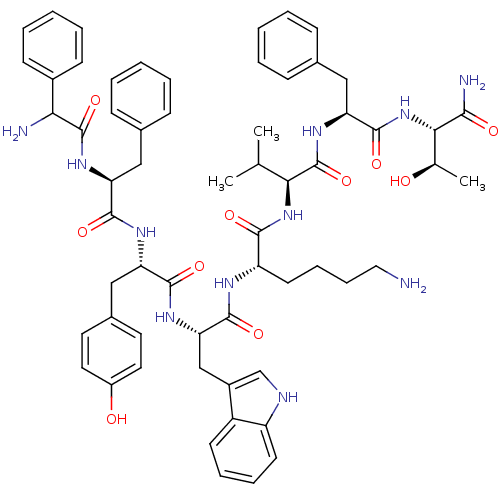
(D-Phe-Phe-Tyr-D-Trp-Lys-Val-Phe-Thr-NH2 | DC-25-12)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)C(N)c1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H]([C@@H](C)O)C(N)=O Show InChI InChI=1S/C61H75N11O10/c1-36(2)52(61(82)70-49(32-39-19-9-5-10-20-39)59(80)72-53(37(3)73)54(64)75)71-55(76)46(25-15-16-30-62)66-58(79)50(34-42-35-65-45-24-14-13-23-44(42)45)68-56(77)47(33-40-26-28-43(74)29-27-40)67-57(78)48(31-38-17-7-4-8-18-38)69-60(81)51(63)41-21-11-6-12-22-41/h4-14,17-24,26-29,35-37,46-53,65,73-74H,15-16,25,30-34,62-63H2,1-3H3,(H2,64,75)(H,66,79)(H,67,78)(H,68,77)(H,69,81)(H,70,82)(H,71,76)(H,72,80)/t37-,46+,47+,48+,49+,50+,51?,52+,53+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tulane University
Curated by PDSP Ki Database
| |
Peptides 15: 1421-4 (1994)
Article DOI: 10.1016/0196-9781(94)90118-x
BindingDB Entry DOI: 10.7270/Q24748C1 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50082556

((S)-2,2-Dimethyl-4-[4-(pyridin-4-yloxy)-benzenesul...)Show SMILES CC1(C)SCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C18H21N3O5S2/c1-18(2)16(17(22)20-23)21(11-12-27-18)28(24,25)15-5-3-13(4-6-15)26-14-7-9-19-10-8-14/h3-10,16,23H,11-12H2,1-2H3,(H,20,22)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of collagenase (Matrix metalloprotease-13) |
J Med Chem 42: 4547-62 (1999)
BindingDB Entry DOI: 10.7270/Q2D79C32 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(RAT) | BDBM81767

(15-28-Somatostatin-28 | CAS_38916-34-6 | CB6417646...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O |r| Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,62-,63-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tulane University
Curated by PDSP Ki Database
| |
Peptides 15: 1421-4 (1994)
Article DOI: 10.1016/0196-9781(94)90118-x
BindingDB Entry DOI: 10.7270/Q24748C1 |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM50546262
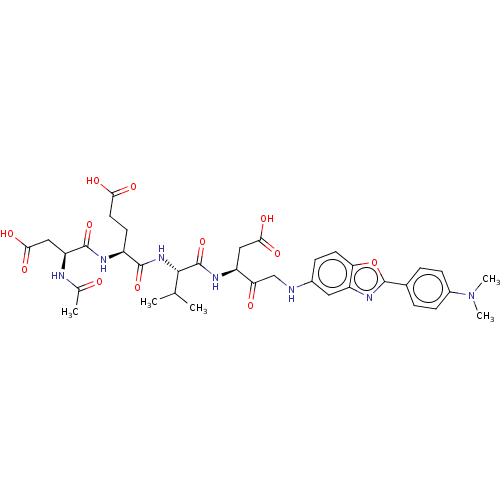
(CHEMBL4751195)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(O)=O)NC(C)=O)C(=O)N[C@@H](CC(O)=O)C(=O)CNc1ccc2oc(nc2c1)-c1ccc(cc1)N(C)C |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of caspase-3 (unknown origin) using Ac-DEVD-AMCA as substrate incubated for 5 mins by Dixon plot analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00242
BindingDB Entry DOI: 10.7270/Q2KD22HB |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50082556

((S)-2,2-Dimethyl-4-[4-(pyridin-4-yloxy)-benzenesul...)Show SMILES CC1(C)SCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C18H21N3O5S2/c1-18(2)16(17(22)20-23)21(11-12-27-18)28(24,25)15-5-3-13(4-6-15)26-14-7-9-19-10-8-14/h3-10,16,23H,11-12H2,1-2H3,(H,20,22)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of gelatinase-A (Matrix metalloprotease-2) |
J Med Chem 42: 4547-62 (1999)
BindingDB Entry DOI: 10.7270/Q2D79C32 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(Homo sapiens (Human)) | BDBM29611

(2-Iodomelatonin | CHEMBL289233 | Melatonin,2-Iodo)Show InChI InChI=1S/C13H15IN2O2/c1-8(17)15-6-5-10-11-7-9(18-2)3-4-12(11)16-13(10)14/h3-4,7,16H,5-6H2,1-2H3,(H,15,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 92: 8734-8 (1995)
Article DOI: 10.1073/pnas.92.19.8734
BindingDB Entry DOI: 10.7270/Q2GH9GGM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Somatostatin receptor type 1
(RAT) | BDBM81767

(15-28-Somatostatin-28 | CAS_38916-34-6 | CB6417646...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O |r| Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,62-,63-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tulane University
Curated by PDSP Ki Database
| |
Peptides 15: 1421-4 (1994)
Article DOI: 10.1016/0196-9781(94)90118-x
BindingDB Entry DOI: 10.7270/Q24748C1 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(RAT) | BDBM82254
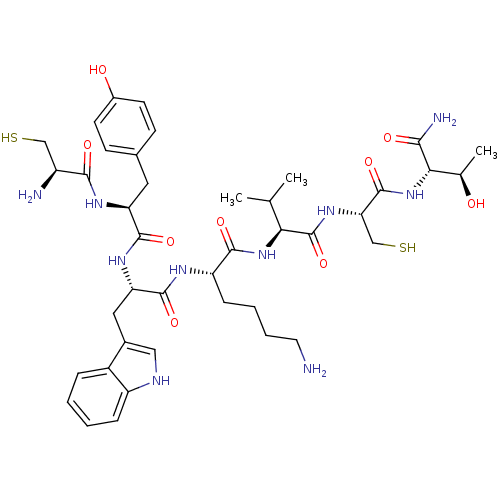
(D-Nal-Cys-Tyr-D-Trp-Lys-Val-Cys-Thr-NH2 | DC-25-10...)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](N)CS)C(=O)N[C@@H](CS)C(=O)N[C@@H]([C@@H](C)O)C(N)=O Show InChI InChI=1S/C41H60N10O9S2/c1-21(2)33(41(60)49-32(20-62)40(59)51-34(22(3)52)35(44)54)50-37(56)29(10-6-7-15-42)46-39(58)31(17-24-18-45-28-9-5-4-8-26(24)28)48-38(57)30(47-36(55)27(43)19-61)16-23-11-13-25(53)14-12-23/h4-5,8-9,11-14,18,21-22,27,29-34,45,52-53,61-62H,6-7,10,15-17,19-20,42-43H2,1-3H3,(H2,44,54)(H,46,58)(H,47,55)(H,48,57)(H,49,60)(H,50,56)(H,51,59)/t22-,27+,29+,30+,31+,32+,33+,34+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tulane University
Curated by PDSP Ki Database
| |
Peptides 15: 1421-4 (1994)
Article DOI: 10.1016/0196-9781(94)90118-x
BindingDB Entry DOI: 10.7270/Q24748C1 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50342706

(3,4-Dihydro-2-[4-(4-(2-methoxyphenyl)piperazin-1-y...)Show SMILES COc1ccccc1N1CCN(CCCCN2CCc3cc(ccc3C2=O)-c2ccncc2)CC1 Show InChI InChI=1S/C29H34N4O2/c1-35-28-7-3-2-6-27(28)32-20-18-31(19-21-32)15-4-5-16-33-17-12-25-22-24(8-9-26(25)29(33)34)23-10-13-30-14-11-23/h2-3,6-11,13-14,22H,4-5,12,15-21H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Santiago de Compostela
Curated by ChEMBL
| Assay Description
Displacement of [3H]Spiperone from human dopamine D3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 2670-4 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.083
BindingDB Entry DOI: 10.7270/Q2GF0TT4 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50342704
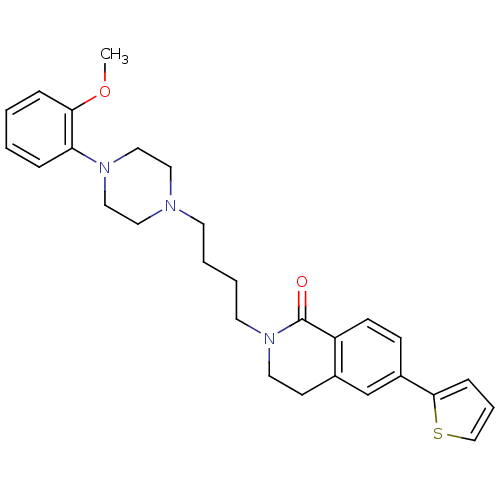
(3,4-Dihydro-2-[4-(4-(2-methoxyphenyl)piperazin-1-y...)Show SMILES COc1ccccc1N1CCN(CCCCN2CCc3cc(ccc3C2=O)-c2cccs2)CC1 Show InChI InChI=1S/C28H33N3O2S/c1-33-26-8-3-2-7-25(26)30-18-16-29(17-19-30)13-4-5-14-31-15-12-22-21-23(27-9-6-20-34-27)10-11-24(22)28(31)32/h2-3,6-11,20-21H,4-5,12-19H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Santiago de Compostela
Curated by ChEMBL
| Assay Description
Displacement of [3H]Spiperone from human dopamine D3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 2670-4 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.083
BindingDB Entry DOI: 10.7270/Q2GF0TT4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50067499

((6aR,10aR)-3(1,1-dimethylheptyl)-9-hydroxymethyl)-...)Show SMILES CCCCCCC(C)(C)c1cc(O)c2[C@@H]3CC(CO)=CC[C@H]3C(C)(C)Oc2c1 |r,c:18| Show InChI InChI=1S/C25H38O3/c1-6-7-8-9-12-24(2,3)18-14-21(27)23-19-13-17(16-26)10-11-20(19)25(4,5)28-22(23)15-18/h10,14-15,19-20,26-27H,6-9,11-13,16H2,1-5H3/t19-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Siena
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant CB2 receptor expressed in african green monkey COS cells by radioligand binding assay |
J Med Chem 52: 2506-14 (2009)
Article DOI: 10.1021/jm8016255
BindingDB Entry DOI: 10.7270/Q2X34XCN |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM29611

(2-Iodomelatonin | CHEMBL289233 | Melatonin,2-Iodo)Show InChI InChI=1S/C13H15IN2O2/c1-8(17)15-6-5-10-11-7-9(18-2)3-4-12(11)16-13(10)14/h3-4,7,16H,5-6H2,1-2H3,(H,15,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 92: 8734-8 (1995)
Article DOI: 10.1073/pnas.92.19.8734
BindingDB Entry DOI: 10.7270/Q2GH9GGM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]ketanserin binding from 5-hydroxytryptamine 2A receptor in rat striatal membrane. |
J Med Chem 42: 2774-97 (1999)
Article DOI: 10.1021/jm981094e
BindingDB Entry DOI: 10.7270/Q2BG2RR8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50350161
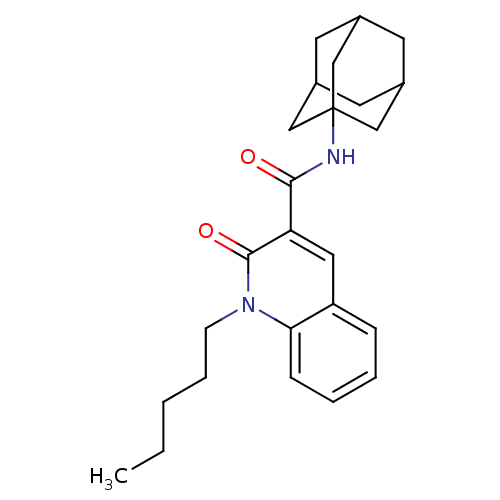
(CHEMBL1814468)Show SMILES CCCCCn1c2ccccc2cc(C(=O)NC23CC4CC(CC(C4)C2)C3)c1=O |TLB:16:17:20.19.24:22,THB:18:19:22:26.17.25,18:17:20.19.24:22,25:17:20:24.23.22,25:23:20:26.18.17| Show InChI InChI=1S/C25H32N2O2/c1-2-3-6-9-27-22-8-5-4-7-20(22)13-21(24(27)29)23(28)26-25-14-17-10-18(15-25)12-19(11-17)16-25/h4-5,7-8,13,17-19H,2-3,6,9-12,14-16H2,1H3,(H,26,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Siena
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK cells |
J Med Chem 54: 5444-53 (2011)
Article DOI: 10.1021/jm200476p
BindingDB Entry DOI: 10.7270/Q2M045S3 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(Homo sapiens (Human)) | BDBM50034110
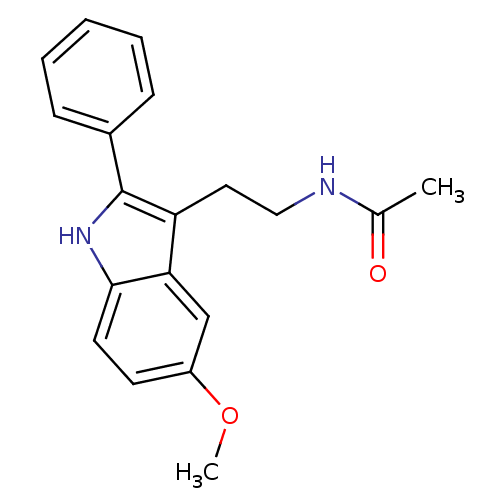
(CHEMBL15060 | Melatonin,2-Phenyl | N-[2-(5-Methoxy...)Show InChI InChI=1S/C19H20N2O2/c1-13(22)20-11-10-16-17-12-15(23-2)8-9-18(17)21-19(16)14-6-4-3-5-7-14/h3-9,12,21H,10-11H2,1-2H3,(H,20,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 92: 8734-8 (1995)
Article DOI: 10.1073/pnas.92.19.8734
BindingDB Entry DOI: 10.7270/Q2GH9GGM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50342715
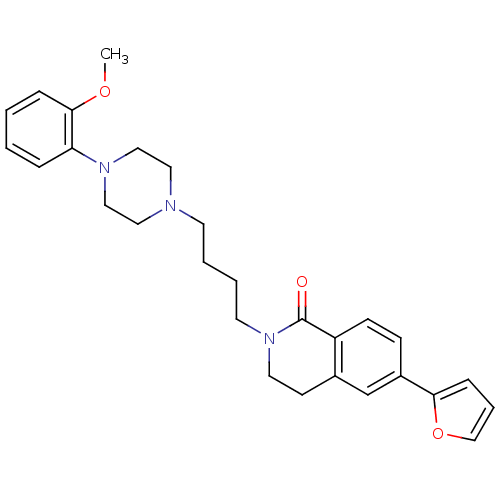
(3,4-Dihydro-6-(2-furyl)-2-[4-(4-(2-methoxyphenyl)p...)Show SMILES COc1ccccc1N1CCN(CCCCN2CCc3cc(ccc3C2=O)-c2ccco2)CC1 Show InChI InChI=1S/C28H33N3O3/c1-33-27-8-3-2-7-25(27)30-18-16-29(17-19-30)13-4-5-14-31-15-12-22-21-23(26-9-6-20-34-26)10-11-24(22)28(31)32/h2-3,6-11,20-21H,4-5,12-19H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Santiago de Compostela
Curated by ChEMBL
| Assay Description
Displacement of [3H]Spiperone from human dopamine D3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 2670-4 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.083
BindingDB Entry DOI: 10.7270/Q2GF0TT4 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM50035179
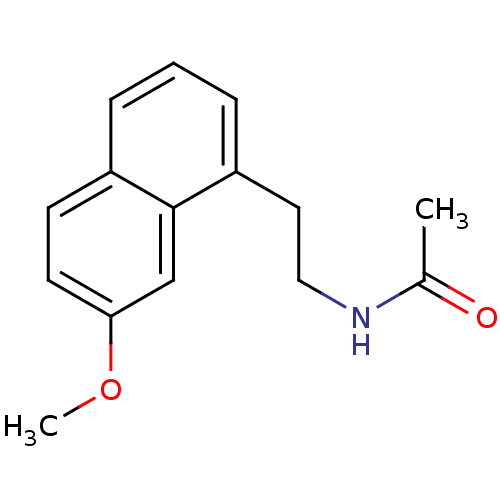
(Agomelatine | CHEMBL10878 | N-[2-(7-Methoxy-naphth...)Show InChI InChI=1S/C15H17NO2/c1-11(17)16-9-8-13-5-3-4-12-6-7-14(18-2)10-15(12)13/h3-7,10H,8-9H2,1-2H3,(H,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 92: 8734-8 (1995)
Article DOI: 10.1073/pnas.92.19.8734
BindingDB Entry DOI: 10.7270/Q2GH9GGM |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50210860
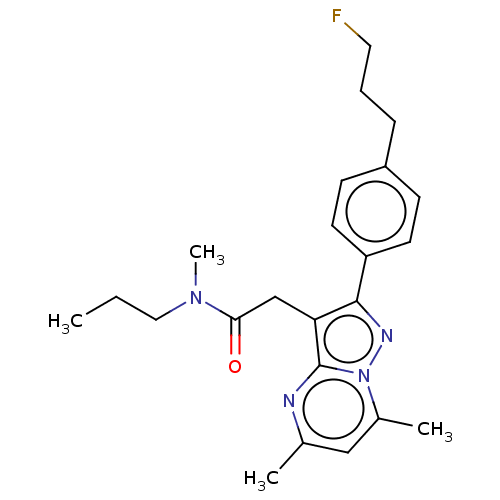
(CHEMBL3936390)Show SMILES CCCN(C)C(=O)Cc1c(nn2c(C)cc(C)nc12)-c1ccc(CCCF)cc1 Show InChI InChI=1S/C23H29FN4O/c1-5-13-27(4)21(29)15-20-22(19-10-8-18(9-11-19)7-6-12-24)26-28-17(3)14-16(2)25-23(20)28/h8-11,14H,5-7,12-13,15H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
France; Inserm/CEA/Universit£ Paris Sud
Curated by ChEMBL
| Assay Description
Displacement of [3H]PK11195 from TSPO in rat heart membranes after 15 mins by scintillation counting method |
Eur J Med Chem 125: 346-359 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.025
BindingDB Entry DOI: 10.7270/Q2TH8PVR |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50342713

(3,4-Dihydro-2-[4-(4-(2-methoxyphenyl)piperazin-1-y...)Show SMILES COc1ccccc1N1CCN(CCCCN2CCc3cc(ccc3C2=O)-c2ccccc2)CC1 Show InChI InChI=1S/C30H35N3O2/c1-35-29-12-6-5-11-28(29)32-21-19-31(20-22-32)16-7-8-17-33-18-15-26-23-25(13-14-27(26)30(33)34)24-9-3-2-4-10-24/h2-6,9-14,23H,7-8,15-22H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Santiago de Compostela
Curated by ChEMBL
| Assay Description
Displacement of [3H]Spiperone from human dopamine D3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 2670-4 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.083
BindingDB Entry DOI: 10.7270/Q2GF0TT4 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50342719

(2,4-dimethyl-5-(4-methyl-5-(3-(5-(4-(trifluorometh...)Show SMILES Cc1nc(C)c(s1)-c1nnc(SCCCN2CCC3(CC23)c2ccc(cc2)C(F)(F)F)n1C Show InChI InChI=1S/C23H26F3N5S2/c1-14-19(33-15(2)27-14)20-28-29-21(30(20)3)32-12-4-10-31-11-9-22(13-18(22)31)16-5-7-17(8-6-16)23(24,25)26/h5-8,18H,4,9-13H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Santiago de Compostela
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D3 receptor |
Bioorg Med Chem Lett 21: 2670-4 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.083
BindingDB Entry DOI: 10.7270/Q2GF0TT4 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM50034110

(CHEMBL15060 | Melatonin,2-Phenyl | N-[2-(5-Methoxy...)Show InChI InChI=1S/C19H20N2O2/c1-13(22)20-11-10-16-17-12-15(23-2)8-9-18(17)21-19(16)14-6-4-3-5-7-14/h3-9,12,21H,10-11H2,1-2H3,(H,20,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 92: 8734-8 (1995)
Article DOI: 10.1073/pnas.92.19.8734
BindingDB Entry DOI: 10.7270/Q2GH9GGM |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50210866

(CHEMBL3979767)Show SMILES CCCN(CCC)C(=O)Cc1c(nn2c(C)cc(C)nc12)-c1ccc(CCCF)cc1 Show InChI InChI=1S/C25H33FN4O/c1-5-14-29(15-6-2)23(31)17-22-24(21-11-9-20(10-12-21)8-7-13-26)28-30-19(4)16-18(3)27-25(22)30/h9-12,16H,5-8,13-15,17H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
France; Inserm/CEA/Universit£ Paris Sud
Curated by ChEMBL
| Assay Description
Displacement of [3H]PK11195 from TSPO in rat heart membranes after 15 mins by scintillation counting method |
Eur J Med Chem 125: 346-359 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.025
BindingDB Entry DOI: 10.7270/Q2TH8PVR |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50342710
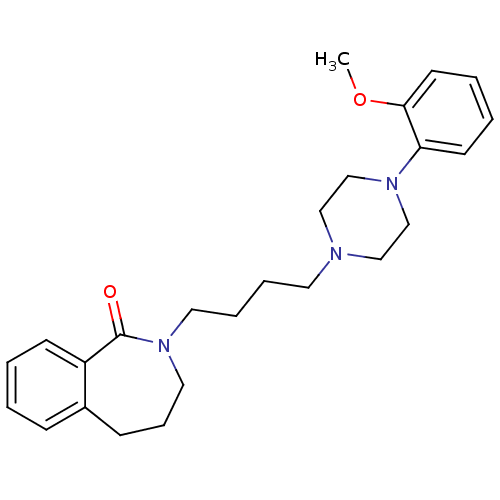
(2-(4-(4-(2-methoxyphenyl)piperazin-1-yl)butyl)-2,3...)Show InChI InChI=1S/C25H33N3O2/c1-30-24-13-5-4-12-23(24)27-19-17-26(18-20-27)14-6-7-15-28-16-8-10-21-9-2-3-11-22(21)25(28)29/h2-5,9,11-13H,6-8,10,14-20H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Santiago de Compostela
Curated by ChEMBL
| Assay Description
Displacement of [3H]Spiperone from human dopamine D3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 2670-4 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.083
BindingDB Entry DOI: 10.7270/Q2GF0TT4 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50082556

((S)-2,2-Dimethyl-4-[4-(pyridin-4-yloxy)-benzenesul...)Show SMILES CC1(C)SCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C18H21N3O5S2/c1-18(2)16(17(22)20-23)21(11-12-27-18)28(24,25)15-5-3-13(4-6-15)26-14-7-9-19-10-8-14/h3-10,16,23H,11-12H2,1-2H3,(H,20,22)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloprotease-3 |
J Med Chem 42: 4547-62 (1999)
BindingDB Entry DOI: 10.7270/Q2D79C32 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50342711
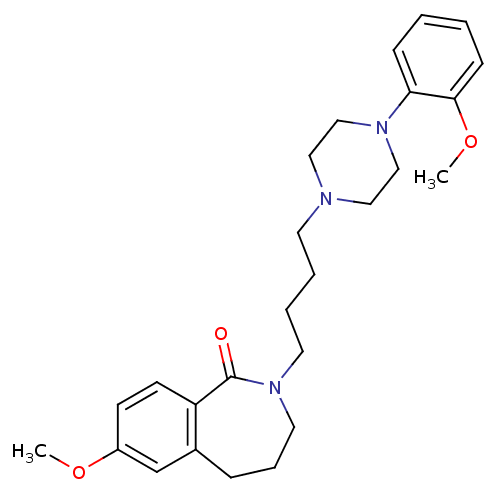
(7-methoxy-2-(4-(4-(2-methoxyphenyl)piperazin-1-yl)...)Show SMILES COc1ccc2c(CCCN(CCCCN3CCN(CC3)c3ccccc3OC)C2=O)c1 Show InChI InChI=1S/C26H35N3O3/c1-31-22-11-12-23-21(20-22)8-7-15-29(26(23)30)14-6-5-13-27-16-18-28(19-17-27)24-9-3-4-10-25(24)32-2/h3-4,9-12,20H,5-8,13-19H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Santiago de Compostela
Curated by ChEMBL
| Assay Description
Displacement of [3H]Spiperone from human dopamine D3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 2670-4 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.083
BindingDB Entry DOI: 10.7270/Q2GF0TT4 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(RAT) | BDBM81767

(15-28-Somatostatin-28 | CAS_38916-34-6 | CB6417646...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O |r| Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,62-,63-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tulane University
Curated by PDSP Ki Database
| |
Peptides 15: 1421-4 (1994)
Article DOI: 10.1016/0196-9781(94)90118-x
BindingDB Entry DOI: 10.7270/Q24748C1 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50342705
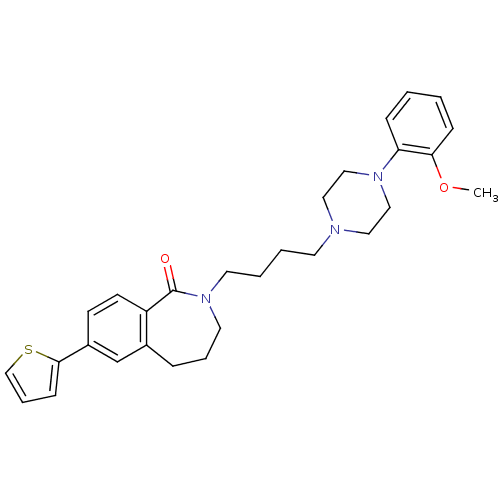
(2-[4-(4-(2-Methoxyphenyl)piperazin-1-yl)butyl]-2,3...)Show SMILES COc1ccccc1N1CCN(CCCCN2CCCc3cc(ccc3C2=O)-c2cccs2)CC1 Show InChI InChI=1S/C29H35N3O2S/c1-34-27-10-3-2-9-26(27)31-19-17-30(18-20-31)14-4-5-15-32-16-6-8-23-22-24(28-11-7-21-35-28)12-13-25(23)29(32)33/h2-3,7,9-13,21-22H,4-6,8,14-20H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Santiago de Compostela
Curated by ChEMBL
| Assay Description
Displacement of [3H]Spiperone from human dopamine D3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 2670-4 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.083
BindingDB Entry DOI: 10.7270/Q2GF0TT4 |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50117259
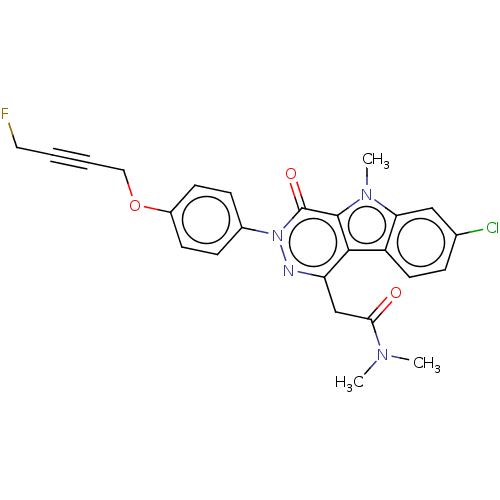
(CHEMBL3613363)Show SMILES CN(C)C(=O)Cc1nn(-c2ccc(OCC#CCF)cc2)c(=O)c2n(C)c3cc(Cl)ccc3c12 Show InChI InChI=1S/C25H22ClFN4O3/c1-29(2)22(32)15-20-23-19-11-6-16(26)14-21(19)30(3)24(23)25(33)31(28-20)17-7-9-18(10-8-17)34-13-5-4-12-27/h6-11,14H,12-13,15H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
France; Inserm / CEA / Universit£ Paris Sud
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PK11195 from rat heart membrane TSPO |
Eur J Med Chem 101: 736-45 (2015)
Article DOI: 10.1016/j.ejmech.2015.07.033
BindingDB Entry DOI: 10.7270/Q26H4K6W |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50210858

(CHEMBL3906952)Show SMILES CN(Cc1ccccc1)C(=O)Cc1c(nn2c(C)cc(C)nc12)-c1ccc(CCCF)cc1 Show InChI InChI=1S/C27H29FN4O/c1-19-16-20(2)32-27(29-19)24(17-25(33)31(3)18-22-8-5-4-6-9-22)26(30-32)23-13-11-21(12-14-23)10-7-15-28/h4-6,8-9,11-14,16H,7,10,15,17-18H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
France; Inserm/CEA/Universit£ Paris Sud
Curated by ChEMBL
| Assay Description
Displacement of [3H]PK11195 from TSPO in rat heart membranes after 15 mins by scintillation counting method |
Eur J Med Chem 125: 346-359 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.025
BindingDB Entry DOI: 10.7270/Q2TH8PVR |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50342717
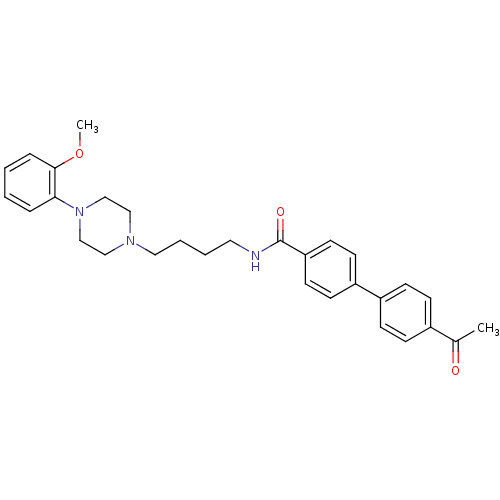
(4'-Acetyl-biphenyl-4-carboxylic acid {4-[4-(2-meth...)Show SMILES COc1ccccc1N1CCN(CCCCNC(=O)c2ccc(cc2)-c2ccc(cc2)C(C)=O)CC1 Show InChI InChI=1S/C30H35N3O3/c1-23(34)24-9-11-25(12-10-24)26-13-15-27(16-14-26)30(35)31-17-5-6-18-32-19-21-33(22-20-32)28-7-3-4-8-29(28)36-2/h3-4,7-16H,5-6,17-22H2,1-2H3,(H,31,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Santiago de Compostela
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D3 receptor |
Bioorg Med Chem Lett 21: 2670-4 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.083
BindingDB Entry DOI: 10.7270/Q2GF0TT4 |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50005293
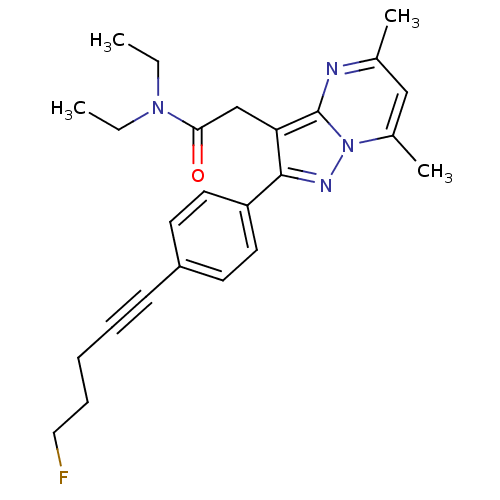
(CHEMBL3125363)Show SMILES CCN(CC)C(=O)Cc1c(nn2c(C)cc(C)nc12)-c1ccc(cc1)C#CCCCF Show InChI InChI=1S/C25H29FN4O/c1-5-29(6-2)23(31)17-22-24(28-30-19(4)16-18(3)27-25(22)30)21-13-11-20(12-14-21)10-8-7-9-15-26/h11-14,16H,5-7,9,15,17H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA
Curated by ChEMBL
| Assay Description
Displacement of [3H]PK11195 from TSPO in rat heart membrane homogenates |
Bioorg Med Chem Lett 24: 1550-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.01.080
BindingDB Entry DOI: 10.7270/Q29S1SJD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50420890

(CHEMBL2087099)Show SMILES CCCCCn1cc(C(=O)C(=O)NC23CC4CC(CC(C4)C2)C3)c2cc(ccc12)-c1ccco1 |TLB:12:13:16:20.19.18,THB:14:15:18:22.13.21,14:13:16.15.20:18,21:13:16:20.19.18,21:19:16:22.14.13| Show InChI InChI=1S/C29H34N2O3/c1-2-3-4-9-31-18-24(23-14-22(7-8-25(23)31)26-6-5-10-34-26)27(32)28(33)30-29-15-19-11-20(16-29)13-21(12-19)17-29/h5-8,10,14,18-21H,2-4,9,11-13,15-17H2,1H3,(H,30,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from human recombinant CB2 receptor transfected in HEK cells |
J Med Chem 55: 5391-402 (2012)
Article DOI: 10.1021/jm3003334
BindingDB Entry DOI: 10.7270/Q2K64KCF |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50243008
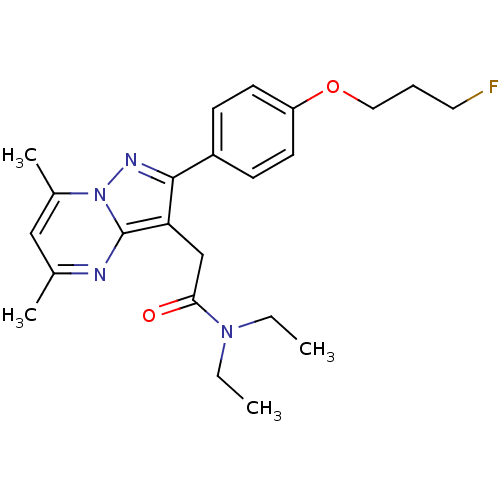
(CHEMBL469682 | N,N-Diethyl-2-(2-(4-(3-fluoropropox...)Show SMILES CCN(CC)C(=O)Cc1c(nn2c(C)cc(C)nc12)-c1ccc(OCCCF)cc1 Show InChI InChI=1S/C23H29FN4O2/c1-5-27(6-2)21(29)15-20-22(26-28-17(4)14-16(3)25-23(20)28)18-8-10-19(11-9-18)30-13-7-12-24/h8-11,14H,5-7,12-13,15H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
France; Inserm/CEA/Universit£ Paris Sud
Curated by ChEMBL
| Assay Description
Displacement of [3H]PK11195 from TSPO in rat heart membranes after 15 mins by scintillation counting method |
Eur J Med Chem 125: 346-359 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.025
BindingDB Entry DOI: 10.7270/Q2TH8PVR |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50117212
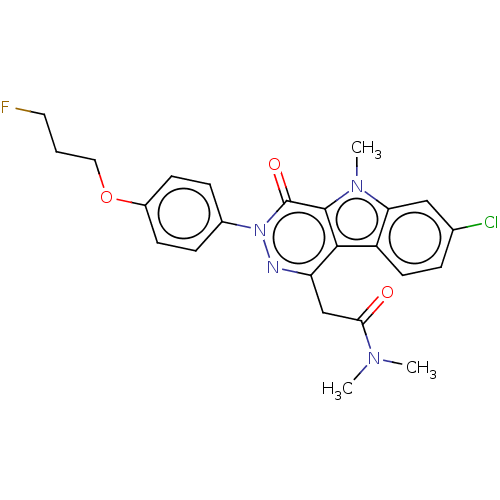
(CHEMBL3613360)Show SMILES CN(C)C(=O)Cc1nn(-c2ccc(OCCCF)cc2)c(=O)c2n(C)c3cc(Cl)ccc3c12 Show InChI InChI=1S/C24H24ClFN4O3/c1-28(2)21(31)14-19-22-18-10-5-15(25)13-20(18)29(3)23(22)24(32)30(27-19)16-6-8-17(9-7-16)33-12-4-11-26/h5-10,13H,4,11-12,14H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
France; Inserm / CEA / Universit£ Paris Sud
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PK11195 from rat heart membrane TSPO |
Eur J Med Chem 101: 736-45 (2015)
Article DOI: 10.1016/j.ejmech.2015.07.033
BindingDB Entry DOI: 10.7270/Q26H4K6W |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50331916
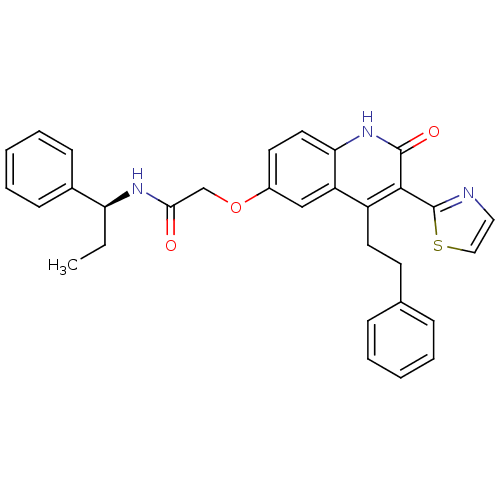
((S)-2-(2-oxo-4-phenethyl-3-(thiazol-2-yl)-1,2-dihy...)Show SMILES CC[C@H](NC(=O)COc1ccc2[nH]c(=O)c(-c3nccs3)c(CCc3ccccc3)c2c1)c1ccccc1 |r| Show InChI InChI=1S/C31H29N3O3S/c1-2-26(22-11-7-4-8-12-22)33-28(35)20-37-23-14-16-27-25(19-23)24(15-13-21-9-5-3-6-10-21)29(30(36)34-27)31-32-17-18-38-31/h3-12,14,16-19,26H,2,13,15,20H2,1H3,(H,33,35)(H,34,36)/t26-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at adenosien A2B receptor in human HMC-1 cells assessed as inhibition of NECA-induced IL-8 release after 6 hr by ELISA |
Bioorg Med Chem Lett 20: 7414-20 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.030
BindingDB Entry DOI: 10.7270/Q2WH2Q60 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(RAT) | BDBM82253

(BIM 23052 | CAS_133073-82-2)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](N)Cc1ccccc1)[C@@H](C)O)C(N)=O |r| Show InChI InChI=1S/C61H75N11O10/c1-37(73)52(54(64)75)71-60(81)50(34-42-25-13-6-14-26-42)70-61(82)53(38(2)74)72-56(77)47(29-17-18-30-62)66-59(80)51(35-43-36-65-46-28-16-15-27-44(43)46)69-58(79)49(33-41-23-11-5-12-24-41)68-57(78)48(32-40-21-9-4-10-22-40)67-55(76)45(63)31-39-19-7-3-8-20-39/h3-16,19-28,36-38,45,47-53,65,73-74H,17-18,29-35,62-63H2,1-2H3,(H2,64,75)(H,66,80)(H,67,76)(H,68,78)(H,69,79)(H,70,82)(H,71,81)(H,72,77)/t37-,38-,45-,47+,48+,49+,50+,51-,52+,53+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tulane University
Curated by PDSP Ki Database
| |
Peptides 15: 1421-4 (1994)
Article DOI: 10.1016/0196-9781(94)90118-x
BindingDB Entry DOI: 10.7270/Q24748C1 |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50117211
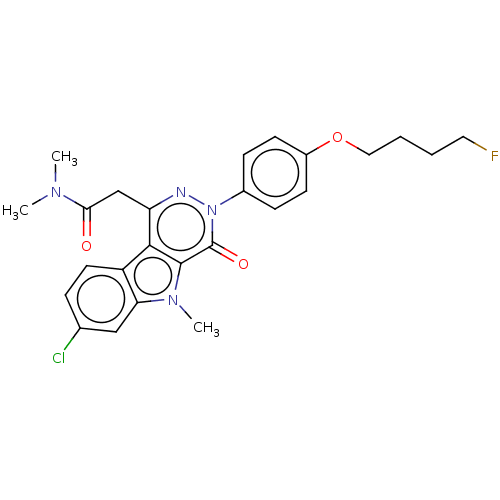
(CHEMBL3613361)Show SMILES CN(C)C(=O)Cc1nn(-c2ccc(OCCCCF)cc2)c(=O)c2n(C)c3cc(Cl)ccc3c12 Show InChI InChI=1S/C25H26ClFN4O3/c1-29(2)22(32)15-20-23-19-11-6-16(26)14-21(19)30(3)24(23)25(33)31(28-20)17-7-9-18(10-8-17)34-13-5-4-12-27/h6-11,14H,4-5,12-13,15H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
France; Inserm / CEA / Universit£ Paris Sud
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PK11195 from rat heart membrane TSPO |
Eur J Med Chem 101: 736-45 (2015)
Article DOI: 10.1016/j.ejmech.2015.07.033
BindingDB Entry DOI: 10.7270/Q26H4K6W |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50331917
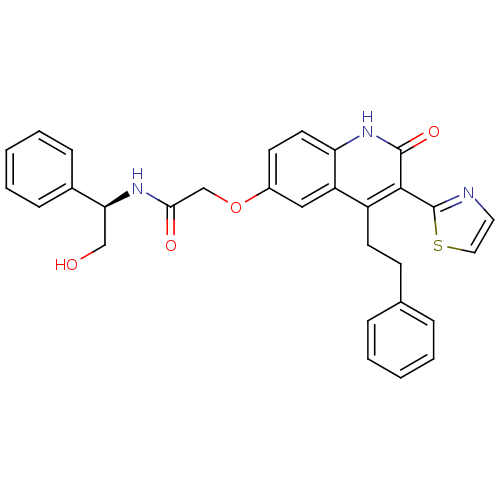
((R)-N-(2-hydroxy-1-phenylethyl)-2-(2-oxo-4-pheneth...)Show SMILES OC[C@H](NC(=O)COc1ccc2[nH]c(=O)c(-c3nccs3)c(CCc3ccccc3)c2c1)c1ccccc1 |r| Show InChI InChI=1S/C30H27N3O4S/c34-18-26(21-9-5-2-6-10-21)32-27(35)19-37-22-12-14-25-24(17-22)23(13-11-20-7-3-1-4-8-20)28(29(36)33-25)30-31-15-16-38-30/h1-10,12,14-17,26,34H,11,13,18-19H2,(H,32,35)(H,33,36)/t26-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at adenosien A2B receptor in human HMC-1 cells assessed as inhibition of NECA-induced IL-8 release after 6 hr by ELISA |
Bioorg Med Chem Lett 20: 7414-20 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.030
BindingDB Entry DOI: 10.7270/Q2WH2Q60 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM294039
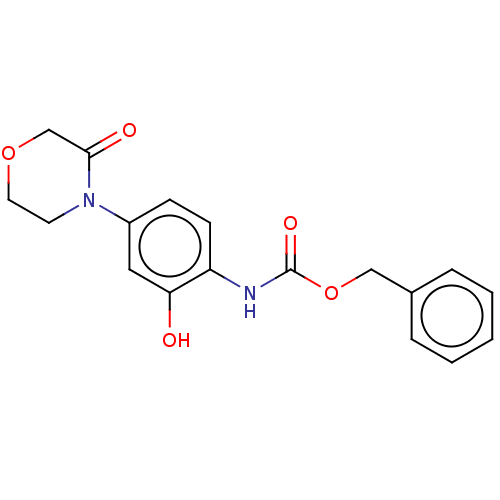
(Process 1 | US10106557, Compound 1)Show InChI InChI=1S/C18H18N2O5/c21-16-10-14(20-8-9-24-12-17(20)22)6-7-15(16)19-18(23)25-11-13-4-2-1-3-5-13/h1-7,10,21H,8-9,11-12H2,(H,19,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
NORTH CHINA PHARMACEUTICAL NEW DRUG R&D CO., LTD.
US Patent
| Assay Description
The activity of the compound to be tested against prothrombinase was determined by the production of thrombin. In summary, 12.5 μL human factor ... |
US Patent US10106557 (2018)
BindingDB Entry DOI: 10.7270/Q2X63Q0W |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50119390
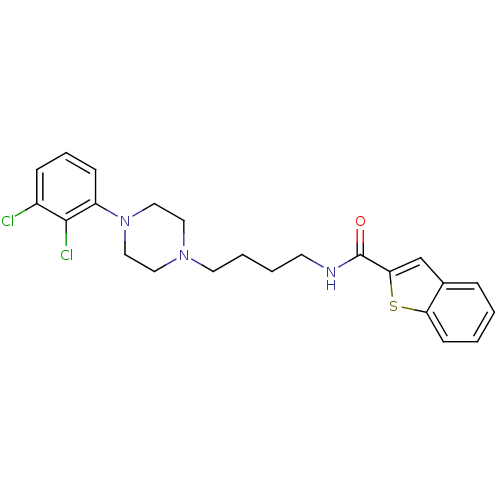
(Benzo[b]thiophene-2-carboxylic acid {4-[4-(2,3-dic...)Show SMILES Clc1cccc(N2CCN(CCCCNC(=O)c3cc4ccccc4s3)CC2)c1Cl Show InChI InChI=1S/C23H25Cl2N3OS/c24-18-7-5-8-19(22(18)25)28-14-12-27(13-15-28)11-4-3-10-26-23(29)21-16-17-6-1-2-9-20(17)30-21/h1-2,5-9,16H,3-4,10-15H2,(H,26,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Santiago de Compostela
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D3 receptor |
Bioorg Med Chem Lett 21: 2670-4 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.083
BindingDB Entry DOI: 10.7270/Q2GF0TT4 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
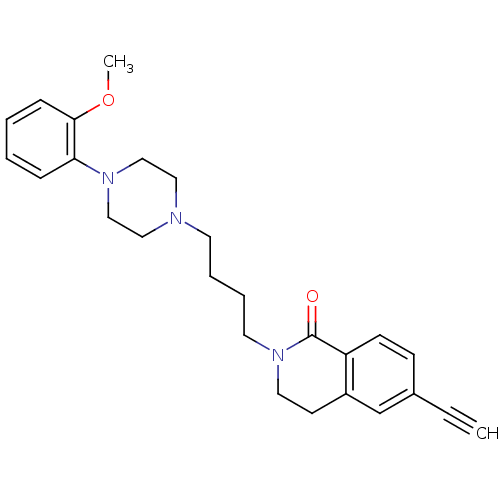
(Homo sapiens (Human)) | BDBM50342708
(3,4-Dihydro-6-ethynyl-2-[4-(4-(2-methoxyphenyl)pip...)Show SMILES COc1ccccc1N1CCN(CCCCN2CCc3cc(ccc3C2=O)C#C)CC1 Show InChI InChI=1S/C26H31N3O2/c1-3-21-10-11-23-22(20-21)12-15-29(26(23)30)14-7-6-13-27-16-18-28(19-17-27)24-8-4-5-9-25(24)31-2/h1,4-5,8-11,20H,6-7,12-19H2,2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Santiago de Compostela
Curated by ChEMBL
| Assay Description
Displacement of [3H]Spiperone from human dopamine D3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 2670-4 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.083
BindingDB Entry DOI: 10.7270/Q2GF0TT4 |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
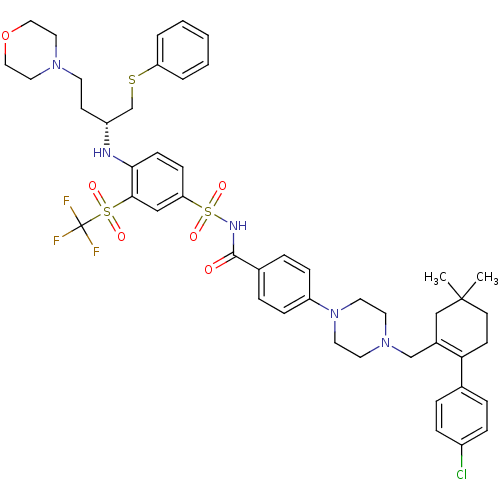
(Homo sapiens (Human)) | BDBM50270877
((R)-4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex...)Show SMILES CC1(C)CCC(=C(CN2CCN(CC2)c2ccc(cc2)C(=O)NS(=O)(=O)c2ccc(N[C@H](CCN3CCOCC3)CSc3ccccc3)c(c2)S(=O)(=O)C(F)(F)F)C1)c1ccc(Cl)cc1 |r,t:5| Show InChI InChI=1S/C47H55ClF3N5O6S3/c1-46(2)20-18-42(34-8-12-37(48)13-9-34)36(31-46)32-55-22-24-56(25-23-55)39-14-10-35(11-15-39)45(57)53-65(60,61)41-16-17-43(44(30-41)64(58,59)47(49,50)51)52-38(19-21-54-26-28-62-29-27-54)33-63-40-6-4-3-5-7-40/h3-17,30,38,52H,18-29,31-33H2,1-2H3,(H,53,57)/t38-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| <0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BCL2 (unknown origin) by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00242
BindingDB Entry DOI: 10.7270/Q2KD22HB |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2b
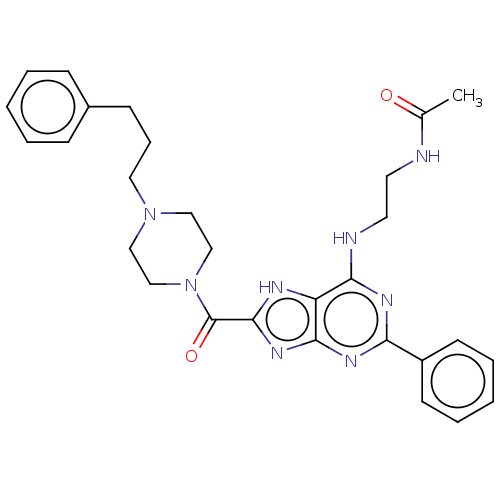
(Homo sapiens (Human)) | BDBM50159502
(CHEMBL3786849)Show SMILES CC(=O)NCCNc1nc(nc2nc([nH]c12)C(=O)N1CCN(CCCc2ccccc2)CC1)-c1ccccc1 Show InChI InChI=1S/C29H34N8O2/c1-21(38)30-14-15-31-26-24-27(34-25(33-26)23-12-6-3-7-13-23)35-28(32-24)29(39)37-19-17-36(18-20-37)16-8-11-22-9-4-2-5-10-22/h2-7,9-10,12-13H,8,11,14-20H2,1H3,(H,30,38)(H2,31,32,33,34,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [3H]OSIP339391 from human recombinant adenosine A2B receptor expressed in HEK293 cell membranes after 60 mins by liquid scintillation... |
J Med Chem 60: 3372-3382 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00138
BindingDB Entry DOI: 10.7270/Q2TQ6407 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human cloned 5HT2A receptor |
Bioorg Med Chem Lett 19: 6059-62 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.041
BindingDB Entry DOI: 10.7270/Q2M046P5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin human cloned serotonin 5HT2A receptor |
Bioorg Med Chem Lett 17: 4873-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.045
BindingDB Entry DOI: 10.7270/Q2J38WCB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela
Curated by ChEMBL
| Assay Description
Binding affinity towards serotonin 5-hydroxytryptamine 2A receptor |
Bioorg Med Chem Lett 14: 585-9 (2004)
BindingDB Entry DOI: 10.7270/Q2VT1V9D |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data