 Found 528 hits with Last Name = 'gudi' and Initial = 'gs'
Found 528 hits with Last Name = 'gudi' and Initial = 'gs' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50130865

(CHEMBL3634853)Show SMILES O=c1ccc(-c2ccc(OCc3ccc4[nH]ccc4n3)cc2)c([nH]1)-c1ccccc1 Show InChI InChI=1S/C25H19N3O2/c29-24-13-11-21(25(28-24)18-4-2-1-3-5-18)17-6-9-20(10-7-17)30-16-19-8-12-22-23(27-19)14-15-26-22/h1-15,26H,16H2,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ... |
J Med Chem 58: 8292-308 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01240
BindingDB Entry DOI: 10.7270/Q2833TVD |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50130760
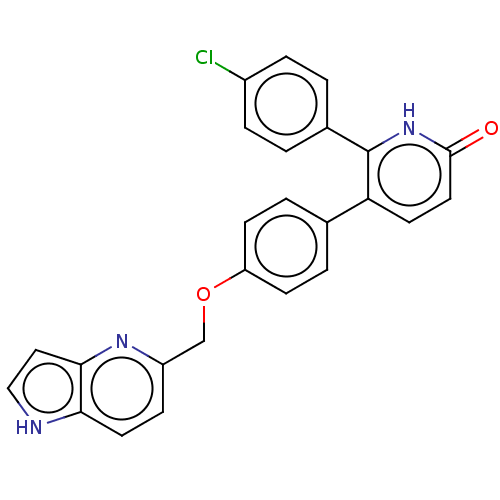
(CHEMBL3634855)Show SMILES Clc1ccc(cc1)-c1[nH]c(=O)ccc1-c1ccc(OCc2ccc3[nH]ccc3n2)cc1 Show InChI InChI=1S/C25H18ClN3O2/c26-18-5-1-17(2-6-18)25-21(10-12-24(30)29-25)16-3-8-20(9-4-16)31-15-19-7-11-22-23(28-19)13-14-27-22/h1-14,27H,15H2,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ... |
J Med Chem 58: 8292-308 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01240
BindingDB Entry DOI: 10.7270/Q2833TVD |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50048260
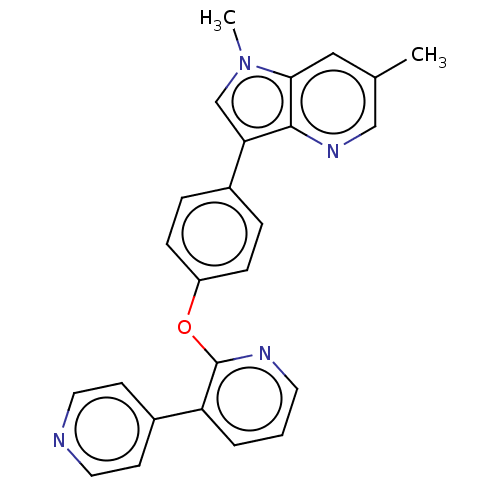
(CHEMBL3315045)Show SMILES Cc1cnc2c(cn(C)c2c1)-c1ccc(Oc2ncccc2-c2ccncc2)cc1 Show InChI InChI=1S/C25H20N4O/c1-17-14-23-24(28-15-17)22(16-29(23)2)18-5-7-20(8-6-18)30-25-21(4-3-11-27-25)19-9-12-26-13-10-19/h3-16H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged PDE10A using [3H]-cAMP as substrate after 30 mins by scintillation proximity assay |
Bioorg Med Chem Lett 24: 3238-42 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.028
BindingDB Entry DOI: 10.7270/Q25D8TGJ |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50130759

(CHEMBL3634854)Show SMILES Fc1ccc(cc1)-c1[nH]c(=O)ccc1-c1ccc(OCc2ccc3[nH]ccc3n2)cc1 Show InChI InChI=1S/C25H18FN3O2/c26-18-5-1-17(2-6-18)25-21(10-12-24(30)29-25)16-3-8-20(9-4-16)31-15-19-7-11-22-23(28-19)13-14-27-22/h1-14,27H,15H2,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ... |
J Med Chem 58: 8292-308 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01240
BindingDB Entry DOI: 10.7270/Q2833TVD |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM31592
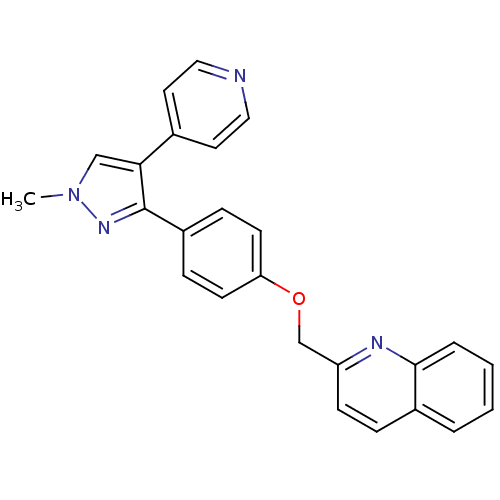
(PF-2545920 | US9138494, MP-10 | substituted pyraz...)Show SMILES Cn1cc(c(n1)-c1ccc(OCc2ccc3ccccc3n2)cc1)-c1ccncc1 Show InChI InChI=1S/C25H20N4O/c1-29-16-23(18-12-14-26-15-13-18)25(28-29)20-7-10-22(11-8-20)30-17-21-9-6-19-4-2-3-5-24(19)27-21/h2-16H,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged human recombinant PDE10A assessed as substrate hydrolysis using [3H]cAMP as substrate after 30 mins by two-step r... |
Bioorg Med Chem Lett 24: 2073-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.054
BindingDB Entry DOI: 10.7270/Q22V2HNB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50130761
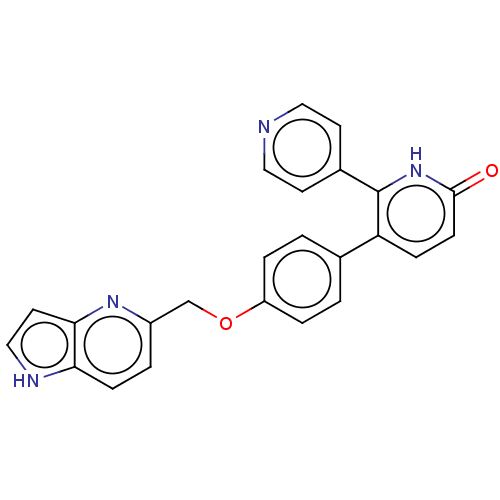
(CHEMBL3634856)Show SMILES O=c1ccc(-c2ccc(OCc3ccc4[nH]ccc4n3)cc2)c([nH]1)-c1ccncc1 Show InChI InChI=1S/C24H18N4O2/c29-23-8-6-20(24(28-23)17-9-12-25-13-10-17)16-1-4-19(5-2-16)30-15-18-3-7-21-22(27-18)11-14-26-21/h1-14,26H,15H2,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ... |
J Med Chem 58: 8292-308 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01240
BindingDB Entry DOI: 10.7270/Q2833TVD |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50130763
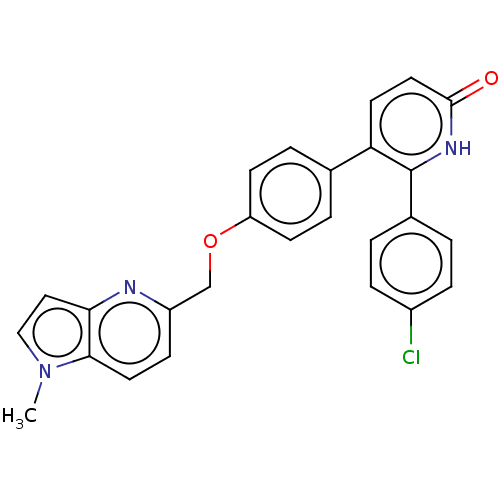
(CHEMBL3634858)Show SMILES Cn1ccc2nc(COc3ccc(cc3)-c3ccc(=O)[nH]c3-c3ccc(Cl)cc3)ccc12 Show InChI InChI=1S/C26H20ClN3O2/c1-30-15-14-23-24(30)12-8-20(28-23)16-32-21-9-4-17(5-10-21)22-11-13-25(31)29-26(22)18-2-6-19(27)7-3-18/h2-15H,16H2,1H3,(H,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ... |
J Med Chem 58: 8292-308 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01240
BindingDB Entry DOI: 10.7270/Q2833TVD |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM50048260

(CHEMBL3315045)Show SMILES Cc1cnc2c(cn(C)c2c1)-c1ccc(Oc2ncccc2-c2ccncc2)cc1 Show InChI InChI=1S/C25H20N4O/c1-17-14-23-24(28-15-17)22(16-29(23)2)18-5-7-20(8-6-18)30-25-21(4-3-11-27-25)19-9-12-26-13-10-19/h3-16H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant GST-tagged PDE10A using [3H]-cAMP as substrate by scintillation proximity assay |
Bioorg Med Chem Lett 24: 3238-42 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.028
BindingDB Entry DOI: 10.7270/Q25D8TGJ |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50494753
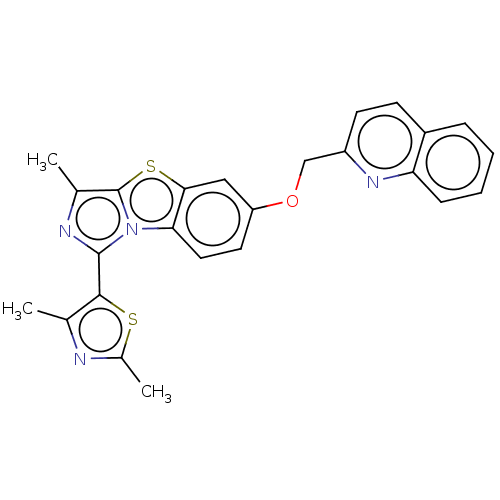
(CHEMBL3094144)Show SMILES Cc1nc(C)c(s1)-c1nc(C)c2sc3cc(OCc4ccc5ccccc5n4)ccc3n12 Show InChI InChI=1S/C25H20N4OS2/c1-14-23(31-16(3)26-14)24-27-15(2)25-29(24)21-11-10-19(12-22(21)32-25)30-13-18-9-8-17-6-4-5-7-20(17)28-18/h4-12H,13H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE10A using [3H]-cAMP/[3H]-cGMP as substrate after 30 mins by radiometric assay |
Bioorg Med Chem Lett 23: 6747-54 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.027
BindingDB Entry DOI: 10.7270/Q2RV0RP4 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50130849
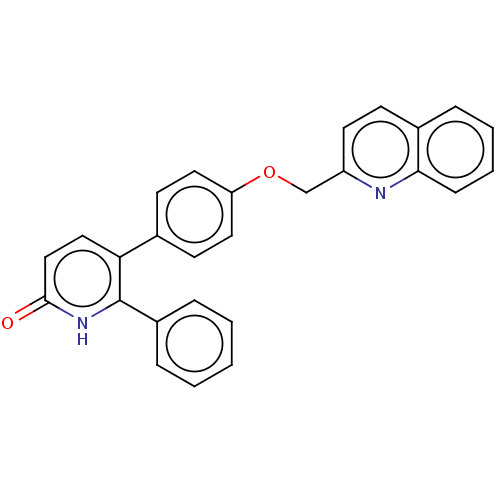
(CHEMBL3634744)Show SMILES O=c1ccc(-c2ccc(OCc3ccc4ccccc4n3)cc2)c([nH]1)-c1ccccc1 Show InChI InChI=1S/C27H20N2O2/c30-26-17-16-24(27(29-26)21-7-2-1-3-8-21)19-11-14-23(15-12-19)31-18-22-13-10-20-6-4-5-9-25(20)28-22/h1-17H,18H2,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ... |
J Med Chem 58: 8292-308 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01240
BindingDB Entry DOI: 10.7270/Q2833TVD |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50130850
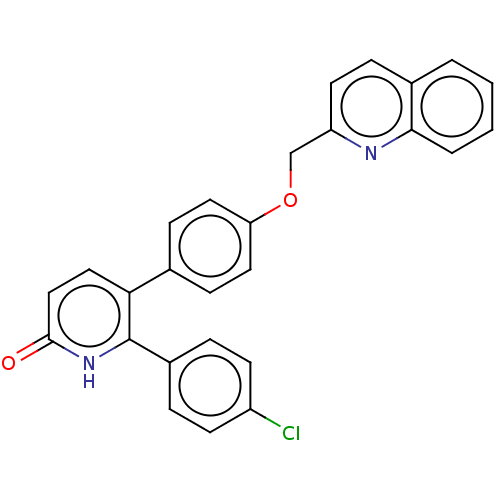
(CHEMBL3634746)Show SMILES Clc1ccc(cc1)-c1[nH]c(=O)ccc1-c1ccc(OCc2ccc3ccccc3n2)cc1 Show InChI InChI=1S/C27H19ClN2O2/c28-21-10-5-20(6-11-21)27-24(15-16-26(31)30-27)18-8-13-23(14-9-18)32-17-22-12-7-19-3-1-2-4-25(19)29-22/h1-16H,17H2,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ... |
J Med Chem 58: 8292-308 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01240
BindingDB Entry DOI: 10.7270/Q2833TVD |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50130860
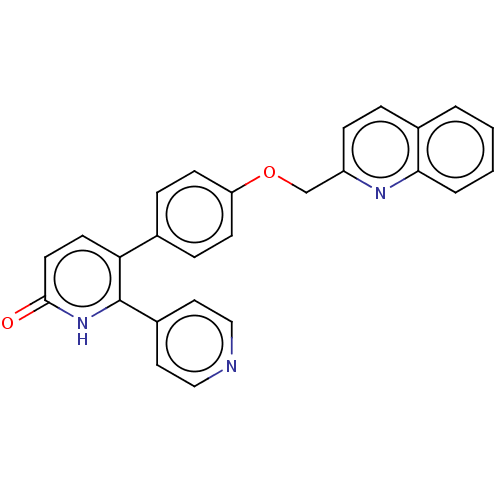
(CHEMBL3634847)Show SMILES O=c1ccc(-c2ccc(OCc3ccc4ccccc4n3)cc2)c([nH]1)-c1ccncc1 Show InChI InChI=1S/C26H19N3O2/c30-25-12-11-23(26(29-25)20-13-15-27-16-14-20)18-6-9-22(10-7-18)31-17-21-8-5-19-3-1-2-4-24(19)28-21/h1-16H,17H2,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ... |
J Med Chem 58: 8292-308 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01240
BindingDB Entry DOI: 10.7270/Q2833TVD |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50130853

(CHEMBL3634748)Show SMILES Fc1cccc(c1)-c1[nH]c(=O)ccc1-c1ccc(OCc2ccc3ccccc3n2)cc1 Show InChI InChI=1S/C27H19FN2O2/c28-21-6-3-5-20(16-21)27-24(14-15-26(31)30-27)18-9-12-23(13-10-18)32-17-22-11-8-19-4-1-2-7-25(19)29-22/h1-16H,17H2,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ... |
J Med Chem 58: 8292-308 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01240
BindingDB Entry DOI: 10.7270/Q2833TVD |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Mus musculus) | BDBM50048260

(CHEMBL3315045)Show SMILES Cc1cnc2c(cn(C)c2c1)-c1ccc(Oc2ncccc2-c2ccncc2)cc1 Show InChI InChI=1S/C25H20N4O/c1-17-14-23-24(28-15-17)22(16-29(23)2)18-5-7-20(8-6-18)30-25-21(4-3-11-27-25)19-9-12-26-13-10-19/h3-16H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant GST-tagged PDE10A using [3H]-cAMP as substrate after 30 mins by scintillation proximity assay |
Bioorg Med Chem Lett 24: 3238-42 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.028
BindingDB Entry DOI: 10.7270/Q25D8TGJ |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50381287
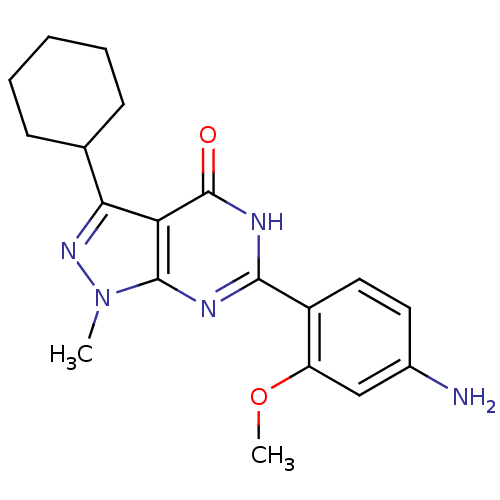
(CHEMBL2019020)Show SMILES COc1cc(N)ccc1-c1nc2n(C)nc(C3CCCCC3)c2c(=O)[nH]1 Show InChI InChI=1S/C19H23N5O2/c1-24-18-15(16(23-24)11-6-4-3-5-7-11)19(25)22-17(21-18)13-9-8-12(20)10-14(13)26-2/h8-11H,3-7,20H2,1-2H3,(H,21,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Displacement of [3H]-cAMP from human recombinant PDE7A |
Bioorg Med Chem Lett 22: 3223-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.025
BindingDB Entry DOI: 10.7270/Q2NP25F2 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50048261
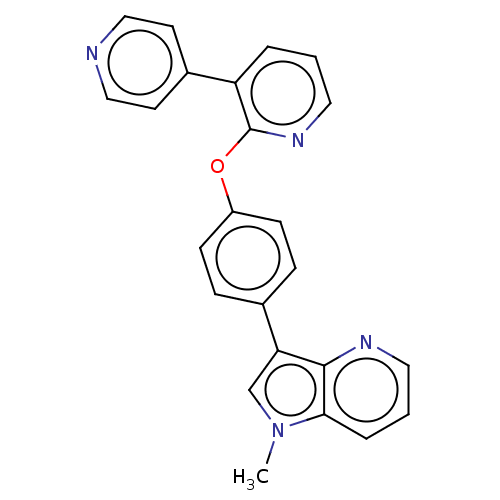
(CHEMBL3315044)Show SMILES Cn1cc(-c2ccc(Oc3ncccc3-c3ccncc3)cc2)c2ncccc12 Show InChI InChI=1S/C24H18N4O/c1-28-16-21(23-22(28)5-3-12-26-23)17-6-8-19(9-7-17)29-24-20(4-2-13-27-24)18-10-14-25-15-11-18/h2-16H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged PDE10A using [3H]-cAMP as substrate after 30 mins by scintillation proximity assay |
Bioorg Med Chem Lett 24: 3238-42 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.028
BindingDB Entry DOI: 10.7270/Q25D8TGJ |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50130854
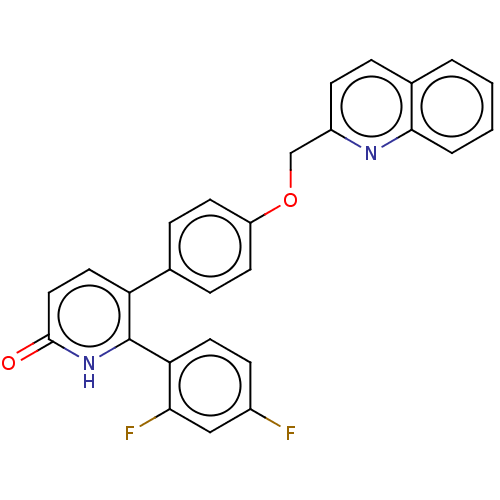
(CHEMBL3634749)Show SMILES Fc1ccc(c(F)c1)-c1[nH]c(=O)ccc1-c1ccc(OCc2ccc3ccccc3n2)cc1 Show InChI InChI=1S/C27H18F2N2O2/c28-19-8-12-23(24(29)15-19)27-22(13-14-26(32)31-27)17-6-10-21(11-7-17)33-16-20-9-5-18-3-1-2-4-25(18)30-20/h1-15H,16H2,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ... |
J Med Chem 58: 8292-308 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01240
BindingDB Entry DOI: 10.7270/Q2833TVD |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM31596
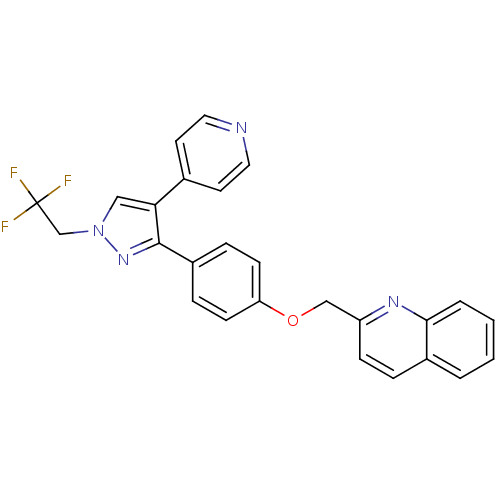
(substituted pyrazole, 13)Show SMILES FC(F)(F)Cn1cc(c(n1)-c1ccc(OCc2ccc3ccccc3n2)cc1)-c1ccncc1 Show InChI InChI=1S/C26H19F3N4O/c27-26(28,29)17-33-15-23(18-11-13-30-14-12-18)25(32-33)20-6-9-22(10-7-20)34-16-21-8-5-19-3-1-2-4-24(19)31-21/h1-15H,16-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged human recombinant PDE10A assessed as substrate hydrolysis using [3H]cAMP as substrate after 30 mins by two-step r... |
Bioorg Med Chem Lett 24: 2073-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.054
BindingDB Entry DOI: 10.7270/Q22V2HNB |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50130841
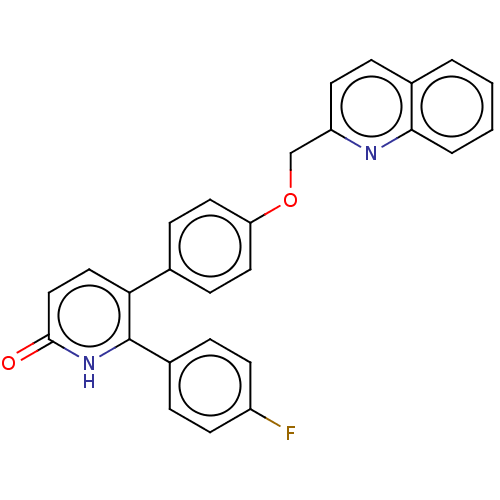
(CHEMBL3634745)Show SMILES Fc1ccc(cc1)-c1[nH]c(=O)ccc1-c1ccc(OCc2ccc3ccccc3n2)cc1 Show InChI InChI=1S/C27H19FN2O2/c28-21-10-5-20(6-11-21)27-24(15-16-26(31)30-27)18-8-13-23(14-9-18)32-17-22-12-7-19-3-1-2-4-25(19)29-22/h1-16H,17H2,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ... |
J Med Chem 58: 8292-308 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01240
BindingDB Entry DOI: 10.7270/Q2833TVD |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50130762
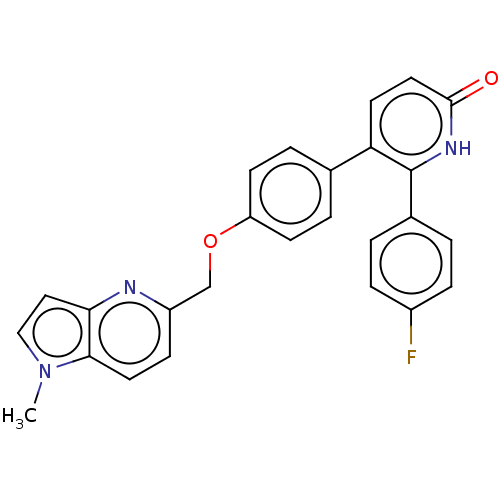
(CHEMBL3634857)Show SMILES Cn1ccc2nc(COc3ccc(cc3)-c3ccc(=O)[nH]c3-c3ccc(F)cc3)ccc12 Show InChI InChI=1S/C26H20FN3O2/c1-30-15-14-23-24(30)12-8-20(28-23)16-32-21-9-4-17(5-10-21)22-11-13-25(31)29-26(22)18-2-6-19(27)7-3-18/h2-15H,16H2,1H3,(H,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ... |
J Med Chem 58: 8292-308 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01240
BindingDB Entry DOI: 10.7270/Q2833TVD |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50130858

(CHEMBL3634845)Show SMILES COc1ccc(cc1)-c1[nH]c(=O)ccc1-c1ccc(OCc2ccc3ccccc3n2)cc1 Show InChI InChI=1S/C28H22N2O3/c1-32-23-12-9-21(10-13-23)28-25(16-17-27(31)30-28)19-7-14-24(15-8-19)33-18-22-11-6-20-4-2-3-5-26(20)29-22/h2-17H,18H2,1H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ... |
J Med Chem 58: 8292-308 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01240
BindingDB Entry DOI: 10.7270/Q2833TVD |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50227631
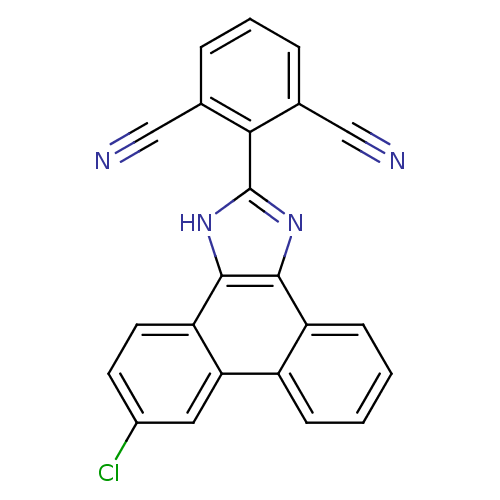
(2-(6-chloro-1H-phenanthro[9,10-d]imidazol-2-yl)iso...)Show SMILES Clc1ccc2c3[nH]c(nc3c3ccccc3c2c1)-c1c(cccc1C#N)C#N Show InChI InChI=1S/C23H11ClN4/c24-15-8-9-18-19(10-15)16-6-1-2-7-17(16)21-22(18)28-23(27-21)20-13(11-25)4-3-5-14(20)12-26/h1-10H,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mPGES-1 expressed in CHO cells assessed as reduction in conversion of PGH2 to PGE2 incubated for 10 mins followed by ... |
Bioorg Med Chem Lett 26: 5977-5984 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.079
BindingDB Entry DOI: 10.7270/Q2XK8HJ8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50227631

(2-(6-chloro-1H-phenanthro[9,10-d]imidazol-2-yl)iso...)Show SMILES Clc1ccc2c3[nH]c(nc3c3ccccc3c2c1)-c1c(cccc1C#N)C#N Show InChI InChI=1S/C23H11ClN4/c24-15-8-9-18-19(10-15)16-6-1-2-7-17(16)21-22(18)28-23(27-21)20-13(11-25)4-3-5-14(20)12-26/h1-10H,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human mPGES-1 expressed in CHO cells assessed as reduction in PGE2 formation using PGH2 a substrate preincubated for 10 min... |
Bioorg Med Chem Lett 27: 2594-2601 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.068
BindingDB Entry DOI: 10.7270/Q24B33Q3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50130856
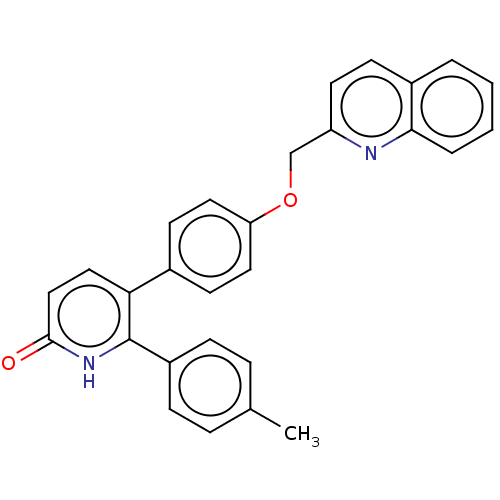
(CHEMBL3634751)Show SMILES Cc1ccc(cc1)-c1[nH]c(=O)ccc1-c1ccc(OCc2ccc3ccccc3n2)cc1 Show InChI InChI=1S/C28H22N2O2/c1-19-6-8-22(9-7-19)28-25(16-17-27(31)30-28)20-11-14-24(15-12-20)32-18-23-13-10-21-4-2-3-5-26(21)29-23/h2-17H,18H2,1H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ... |
J Med Chem 58: 8292-308 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01240
BindingDB Entry DOI: 10.7270/Q2833TVD |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50452066
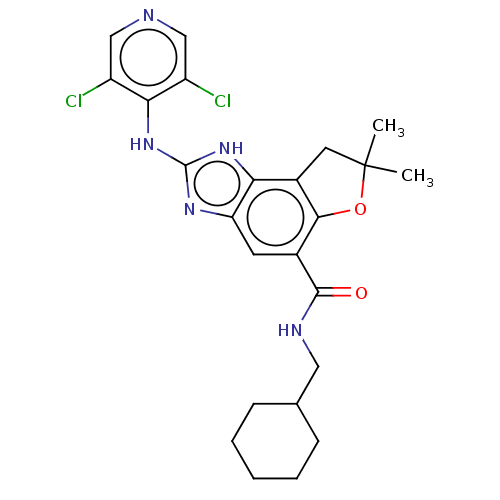
(CHEMBL4206145)Show SMILES CC1(C)Cc2c(O1)c(cc1nc(Nc3c(Cl)cncc3Cl)[nH]c21)C(=O)NCC1CCCCC1 Show InChI InChI=1S/C24H27Cl2N5O2/c1-24(2)9-15-19-18(29-23(30-19)31-20-16(25)11-27-12-17(20)26)8-14(21(15)33-24)22(32)28-10-13-6-4-3-5-7-13/h8,11-13H,3-7,9-10H2,1-2H3,(H,28,32)(H2,27,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human mPGES1 expressed in CHO cells using PGH2 as substrate pretreated for 10 mins followed by substrate addition measured ... |
Bioorg Med Chem Lett 27: 5131-5138 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.062
BindingDB Entry DOI: 10.7270/Q2JS9T0V |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50452084
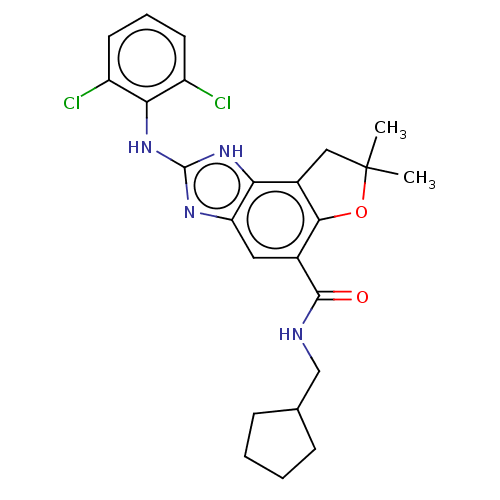
(CHEMBL4207228)Show SMILES CC1(C)Cc2c(O1)c(cc1nc(Nc3c(Cl)cccc3Cl)[nH]c21)C(=O)NCC1CCCC1 Show InChI InChI=1S/C24H26Cl2N4O2/c1-24(2)11-15-19-18(28-23(29-19)30-20-16(25)8-5-9-17(20)26)10-14(21(15)32-24)22(31)27-12-13-6-3-4-7-13/h5,8-10,13H,3-4,6-7,11-12H2,1-2H3,(H,27,31)(H2,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human mPGES1 expressed in CHO cells using PGH2 as substrate pretreated for 10 mins followed by substrate addition measured ... |
Bioorg Med Chem Lett 27: 5131-5138 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.062
BindingDB Entry DOI: 10.7270/Q2JS9T0V |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50207335

(CHEMBL3961834)Show SMILES Cn1c(Nc2c(F)cccc2Cl)nc2cc(C(=O)Nc3ccc(cc3)C(F)(F)F)c3OC(C)(C)Cc3c12 Show InChI InChI=1S/C26H21ClF4N4O2/c1-25(2)12-16-21-19(33-24(35(21)3)34-20-17(27)5-4-6-18(20)28)11-15(22(16)37-25)23(36)32-14-9-7-13(8-10-14)26(29,30)31/h4-11H,12H2,1-3H3,(H,32,36)(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of mPGES1 in human A549 cells assessed as reduction in IL-1beta-induced PGE2 production preincubated for 30 mins followed by incubation wi... |
Bioorg Med Chem Lett 28: 1211-1218 (2018)
Article DOI: 10.1016/j.bmcl.2018.02.048
BindingDB Entry DOI: 10.7270/Q2N300K5 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Mus musculus) | BDBM50130841

(CHEMBL3634745)Show SMILES Fc1ccc(cc1)-c1[nH]c(=O)ccc1-c1ccc(OCc2ccc3ccccc3n2)cc1 Show InChI InChI=1S/C27H19FN2O2/c28-21-10-5-20(6-11-21)27-24(15-16-26(31)30-27)18-8-13-23(14-9-18)32-17-22-12-7-19-3-1-2-4-25(19)29-22/h1-16H,17H2,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of mouse PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 mins by scintillation proximity as... |
J Med Chem 58: 8292-308 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01240
BindingDB Entry DOI: 10.7270/Q2833TVD |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50048258
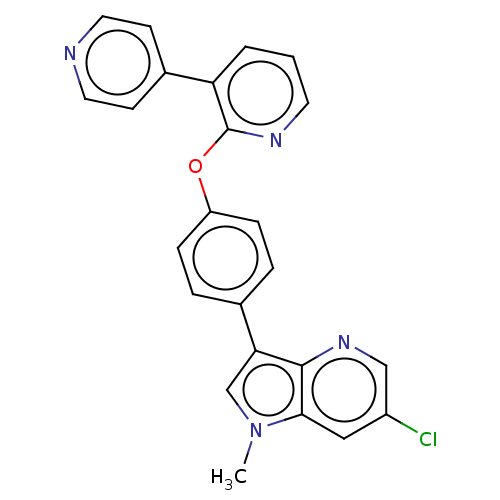
(CHEMBL3315046)Show SMILES Cn1cc(-c2ccc(Oc3ncccc3-c3ccncc3)cc2)c2ncc(Cl)cc12 Show InChI InChI=1S/C24H17ClN4O/c1-29-15-21(23-22(29)13-18(25)14-28-23)16-4-6-19(7-5-16)30-24-20(3-2-10-27-24)17-8-11-26-12-9-17/h2-15H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged PDE10A using [3H]-cAMP as substrate after 30 mins by scintillation proximity assay |
Bioorg Med Chem Lett 24: 3238-42 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.028
BindingDB Entry DOI: 10.7270/Q25D8TGJ |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50130851
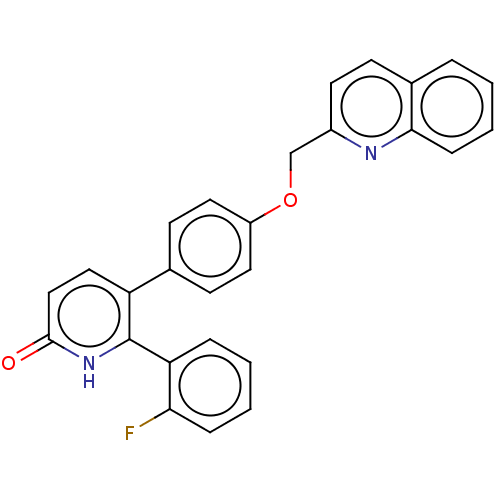
(CHEMBL3634747)Show SMILES Fc1ccccc1-c1[nH]c(=O)ccc1-c1ccc(OCc2ccc3ccccc3n2)cc1 Show InChI InChI=1S/C27H19FN2O2/c28-24-7-3-2-6-23(24)27-22(15-16-26(31)30-27)18-10-13-21(14-11-18)32-17-20-12-9-19-5-1-4-8-25(19)29-20/h1-16H,17H2,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ... |
J Med Chem 58: 8292-308 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01240
BindingDB Entry DOI: 10.7270/Q2833TVD |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50452071
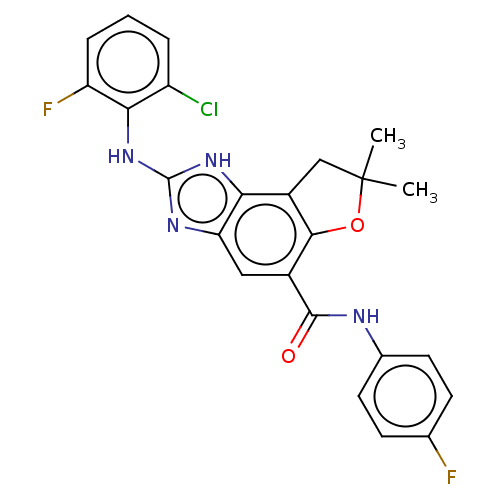
(CHEMBL4208718)Show SMILES CC1(C)Cc2c(O1)c(cc1nc(Nc3c(F)cccc3Cl)[nH]c21)C(=O)Nc1ccc(F)cc1 Show InChI InChI=1S/C24H19ClF2N4O2/c1-24(2)11-15-19-18(29-23(30-19)31-20-16(25)4-3-5-17(20)27)10-14(21(15)33-24)22(32)28-13-8-6-12(26)7-9-13/h3-10H,11H2,1-2H3,(H,28,32)(H2,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human mPGES1 expressed in CHO cells using PGH2 as substrate pretreated for 10 mins followed by substrate addition measured ... |
Bioorg Med Chem Lett 27: 5131-5138 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.062
BindingDB Entry DOI: 10.7270/Q2JS9T0V |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50460529
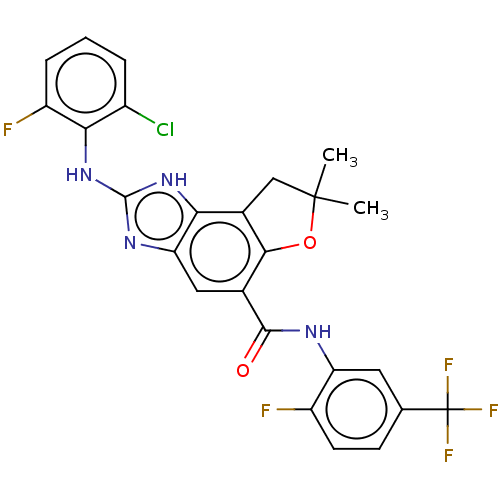
(CHEMBL4228804)Show SMILES CC1(C)Cc2c(O1)c(cc1nc(Nc3c(F)cccc3Cl)[nH]c21)C(=O)Nc1cc(ccc1F)C(F)(F)F Show InChI InChI=1S/C25H18ClF5N4O2/c1-24(2)10-13-19-18(33-23(34-19)35-20-14(26)4-3-5-16(20)28)9-12(21(13)37-24)22(36)32-17-8-11(25(29,30)31)6-7-15(17)27/h3-9H,10H2,1-2H3,(H,32,36)(H2,33,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human mPGES1 expressed in CHO cells assessed as reduction in PGE2 production using PGH2 as substrate incubated for 10 mins ... |
Bioorg Med Chem Lett 28: 1211-1218 (2018)
Article DOI: 10.1016/j.bmcl.2018.02.048
BindingDB Entry DOI: 10.7270/Q2N300K5 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50397056
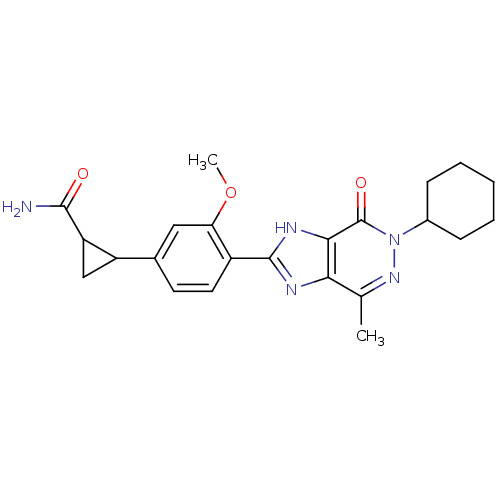
(CHEMBL2171440)Show SMILES COc1cc(ccc1-c1nc2c(C)nn(C3CCCCC3)c(=O)c2[nH]1)C1CC1C(N)=O Show InChI InChI=1S/C23H27N5O3/c1-12-19-20(23(30)28(27-12)14-6-4-3-5-7-14)26-22(25-19)15-9-8-13(10-18(15)31-2)16-11-17(16)21(24)29/h8-10,14,16-17H,3-7,11H2,1-2H3,(H2,24,29)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of cloned human recombinant PDE7A assessed as [3H]cAMP hydrolysis by radiometric assay |
Bioorg Med Chem Lett 22: 6286-91 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.077
BindingDB Entry DOI: 10.7270/Q21Z45JB |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50460527
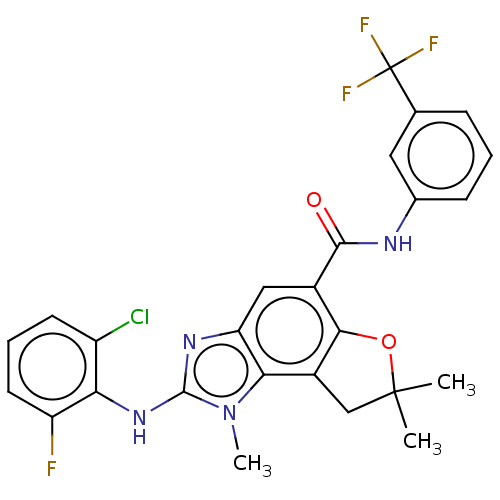
(CHEMBL4228430)Show SMILES Cn1c(Nc2c(F)cccc2Cl)nc2cc(C(=O)Nc3cccc(c3)C(F)(F)F)c3OC(C)(C)Cc3c12 Show InChI InChI=1S/C26H21ClF4N4O2/c1-25(2)12-16-21-19(33-24(35(21)3)34-20-17(27)8-5-9-18(20)28)11-15(22(16)37-25)23(36)32-14-7-4-6-13(10-14)26(29,30)31/h4-11H,12H2,1-3H3,(H,32,36)(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of mPGES1 in human A549 cells assessed as reduction in IL-1beta-induced PGE2 production preincubated for 30 mins followed by incubation wi... |
Bioorg Med Chem Lett 28: 1211-1218 (2018)
Article DOI: 10.1016/j.bmcl.2018.02.048
BindingDB Entry DOI: 10.7270/Q2N300K5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM124296
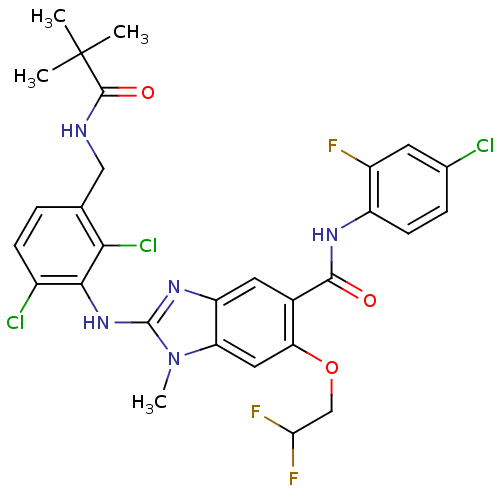
(US8759537, 33)Show SMILES Cn1c(Nc2c(Cl)ccc(CNC(=O)C(C)(C)C)c2Cl)nc2cc(C(=O)Nc3ccc(Cl)cc3F)c(OCC(F)F)cc12 Show InChI InChI=1S/C29H27Cl3F3N5O3/c1-29(2,3)27(42)36-12-14-5-7-17(31)25(24(14)32)39-28-38-20-10-16(22(43-13-23(34)35)11-21(20)40(28)4)26(41)37-19-8-6-15(30)9-18(19)33/h5-11,23H,12-13H2,1-4H3,(H,36,42)(H,37,41)(H,38,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human mPGES1 using PGH2 as substrate pretreated for 25 mins followed by substrate addition measured after 60 secs by HTRF a... |
Bioorg Med Chem Lett 27: 5131-5138 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.062
BindingDB Entry DOI: 10.7270/Q2JS9T0V |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50460542
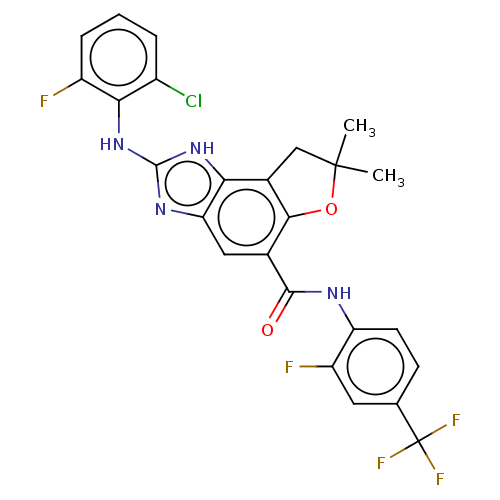
(CHEMBL4227545)Show SMILES CC1(C)Cc2c(O1)c(cc1nc(Nc3c(F)cccc3Cl)[nH]c21)C(=O)Nc1ccc(cc1F)C(F)(F)F Show InChI InChI=1S/C25H18ClF5N4O2/c1-24(2)10-13-19-18(33-23(34-19)35-20-14(26)4-3-5-15(20)27)9-12(21(13)37-24)22(36)32-17-7-6-11(8-16(17)28)25(29,30)31/h3-9H,10H2,1-2H3,(H,32,36)(H2,33,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human mPGES1 expressed in CHO cells assessed as reduction in PGE2 production using PGH2 as substrate incubated for 10 mins ... |
Bioorg Med Chem Lett 28: 1211-1218 (2018)
Article DOI: 10.1016/j.bmcl.2018.02.048
BindingDB Entry DOI: 10.7270/Q2N300K5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50460540
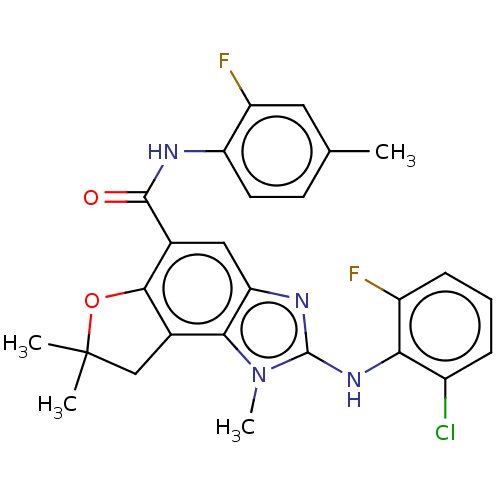
(CHEMBL4227388)Show SMILES Cc1ccc(NC(=O)c2cc3nc(Nc4c(F)cccc4Cl)n(C)c3c3CC(C)(C)Oc23)c(F)c1 Show InChI InChI=1S/C26H23ClF2N4O2/c1-13-8-9-19(18(29)10-13)30-24(34)14-11-20-22(15-12-26(2,3)35-23(14)15)33(4)25(31-20)32-21-16(27)6-5-7-17(21)28/h5-11H,12H2,1-4H3,(H,30,34)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human mPGES1 expressed in CHO cells assessed as reduction in PGE2 production using PGH2 as substrate incubated for 10 mins ... |
Bioorg Med Chem Lett 28: 1211-1218 (2018)
Article DOI: 10.1016/j.bmcl.2018.02.048
BindingDB Entry DOI: 10.7270/Q2N300K5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50460537
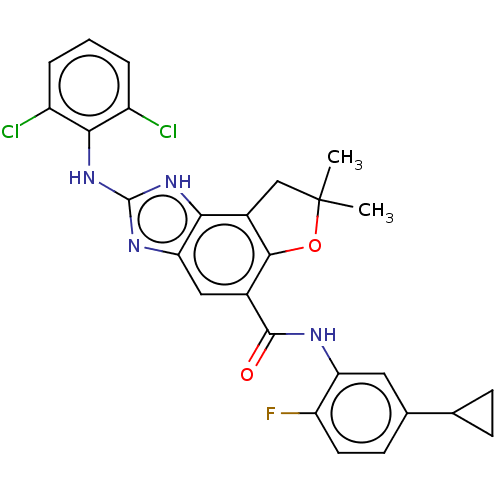
(CHEMBL4226536)Show SMILES CC1(C)Cc2c(O1)c(cc1nc(Nc3c(Cl)cccc3Cl)[nH]c21)C(=O)Nc1cc(ccc1F)C1CC1 Show InChI InChI=1S/C27H23Cl2FN4O2/c1-27(2)12-16-22-21(32-26(33-22)34-23-17(28)4-3-5-18(23)29)11-15(24(16)36-27)25(35)31-20-10-14(13-6-7-13)8-9-19(20)30/h3-5,8-11,13H,6-7,12H2,1-2H3,(H,31,35)(H2,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human mPGES1 expressed in CHO cells assessed as reduction in PGE2 production using PGH2 as substrate incubated for 10 mins ... |
Bioorg Med Chem Lett 28: 1211-1218 (2018)
Article DOI: 10.1016/j.bmcl.2018.02.048
BindingDB Entry DOI: 10.7270/Q2N300K5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50207335

(CHEMBL3961834)Show SMILES Cn1c(Nc2c(F)cccc2Cl)nc2cc(C(=O)Nc3ccc(cc3)C(F)(F)F)c3OC(C)(C)Cc3c12 Show InChI InChI=1S/C26H21ClF4N4O2/c1-25(2)12-16-21-19(33-24(35(21)3)34-20-17(27)5-4-6-18(20)28)11-15(22(16)37-25)23(36)32-14-9-7-13(8-10-14)26(29,30)31/h4-11H,12H2,1-3H3,(H,32,36)(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mPGES-1 expressed in CHO cells assessed as reduction in conversion of PGH2 to PGE2 incubated for 10 mins followed by ... |
Bioorg Med Chem Lett 26: 5977-5984 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.079
BindingDB Entry DOI: 10.7270/Q2XK8HJ8 |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50460531
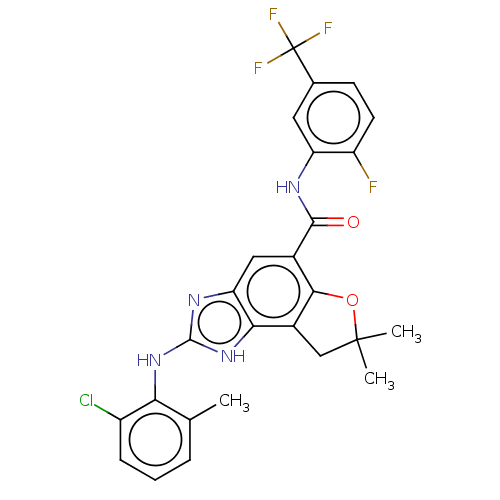
(CHEMBL4226545)Show SMILES Cc1cccc(Cl)c1Nc1nc2cc(C(=O)Nc3cc(ccc3F)C(F)(F)F)c3OC(C)(C)Cc3c2[nH]1 Show InChI InChI=1S/C26H21ClF4N4O2/c1-12-5-4-6-16(27)20(12)34-24-33-19-10-14(22-15(21(19)35-24)11-25(2,3)37-22)23(36)32-18-9-13(26(29,30)31)7-8-17(18)28/h4-10H,11H2,1-3H3,(H,32,36)(H2,33,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human mPGES1 expressed in CHO cells assessed as reduction in PGE2 production using PGH2 as substrate incubated for 10 mins ... |
Bioorg Med Chem Lett 28: 1211-1218 (2018)
Article DOI: 10.1016/j.bmcl.2018.02.048
BindingDB Entry DOI: 10.7270/Q2N300K5 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50130840

(CHEMBL3634865)Show SMILES O=c1ccc(-c2ccncc2)c([nH]1)-c1ccc(OCc2ccc3ccccc3n2)cc1 Show InChI InChI=1S/C26H19N3O2/c30-25-12-11-23(18-13-15-27-16-14-18)26(29-25)20-6-9-22(10-7-20)31-17-21-8-5-19-3-1-2-4-24(19)28-21/h1-16H,17H2,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ... |
J Med Chem 58: 8292-308 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01240
BindingDB Entry DOI: 10.7270/Q2833TVD |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50207335

(CHEMBL3961834)Show SMILES Cn1c(Nc2c(F)cccc2Cl)nc2cc(C(=O)Nc3ccc(cc3)C(F)(F)F)c3OC(C)(C)Cc3c12 Show InChI InChI=1S/C26H21ClF4N4O2/c1-25(2)12-16-21-19(33-24(35(21)3)34-20-17(27)5-4-6-18(20)28)11-15(22(16)37-25)23(36)32-14-9-7-13(8-10-14)26(29,30)31/h4-11H,12H2,1-3H3,(H,32,36)(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human mPGES1 expressed in CHO cells assessed as reduction in PGE2 production using PGH2 as substrate incubated for 10 mins ... |
Bioorg Med Chem Lett 28: 1211-1218 (2018)
Article DOI: 10.1016/j.bmcl.2018.02.048
BindingDB Entry DOI: 10.7270/Q2N300K5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50452078
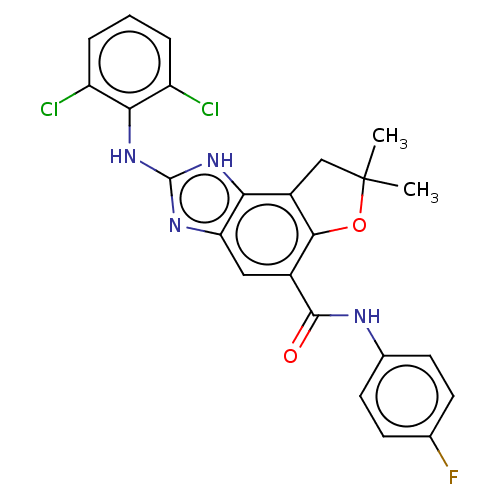
(CHEMBL4203842)Show SMILES CC1(C)Cc2c(O1)c(cc1nc(Nc3c(Cl)cccc3Cl)[nH]c21)C(=O)Nc1ccc(F)cc1 Show InChI InChI=1S/C24H19Cl2FN4O2/c1-24(2)11-15-19-18(29-23(30-19)31-20-16(25)4-3-5-17(20)26)10-14(21(15)33-24)22(32)28-13-8-6-12(27)7-9-13/h3-10H,11H2,1-2H3,(H,28,32)(H2,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human mPGES1 expressed in CHO cells using PGH2 as substrate pretreated for 10 mins followed by substrate addition measured ... |
Bioorg Med Chem Lett 27: 5131-5138 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.062
BindingDB Entry DOI: 10.7270/Q2JS9T0V |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50460536
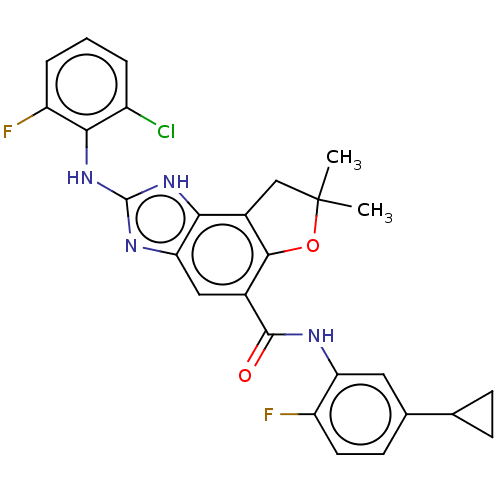
(CHEMBL4225183)Show SMILES CC1(C)Cc2c(O1)c(cc1nc(Nc3c(F)cccc3Cl)[nH]c21)C(=O)Nc1cc(ccc1F)C1CC1 Show InChI InChI=1S/C27H23ClF2N4O2/c1-27(2)12-16-22-21(32-26(33-22)34-23-17(28)4-3-5-19(23)30)11-15(24(16)36-27)25(35)31-20-10-14(13-6-7-13)8-9-18(20)29/h3-5,8-11,13H,6-7,12H2,1-2H3,(H,31,35)(H2,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human mPGES1 expressed in CHO cells assessed as reduction in PGE2 production using PGH2 as substrate incubated for 10 mins ... |
Bioorg Med Chem Lett 28: 1211-1218 (2018)
Article DOI: 10.1016/j.bmcl.2018.02.048
BindingDB Entry DOI: 10.7270/Q2N300K5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50207322
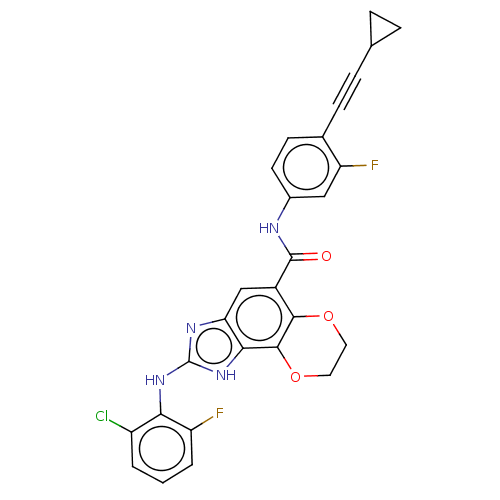
(CHEMBL3916119)Show SMILES Fc1cccc(Cl)c1Nc1nc2cc(C(=O)Nc3ccc(C#CC4CC4)c(F)c3)c3OCCOc3c2[nH]1 Show InChI InChI=1S/C27H19ClF2N4O3/c28-18-2-1-3-19(29)22(18)33-27-32-21-13-17(24-25(23(21)34-27)37-11-10-36-24)26(35)31-16-9-8-15(20(30)12-16)7-6-14-4-5-14/h1-3,8-9,12-14H,4-5,10-11H2,(H,31,35)(H2,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mPGES-1 expressed in CHO cells assessed as reduction in conversion of PGH2 to PGE2 incubated for 10 mins followed by ... |
Bioorg Med Chem Lett 26: 5977-5984 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.079
BindingDB Entry DOI: 10.7270/Q2XK8HJ8 |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50452079
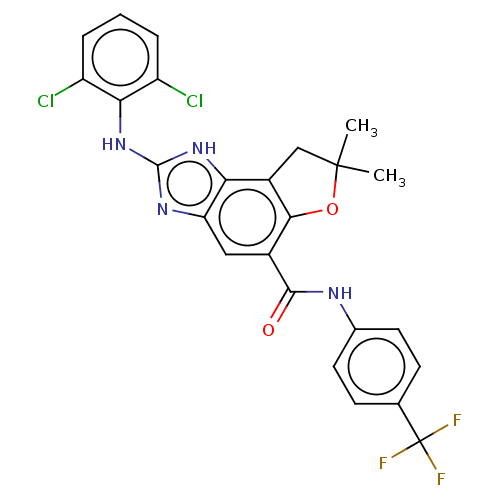
(CHEMBL4206581)Show SMILES CC1(C)Cc2c(O1)c(cc1nc(Nc3c(Cl)cccc3Cl)[nH]c21)C(=O)Nc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C25H19Cl2F3N4O2/c1-24(2)11-15-19-18(32-23(33-19)34-20-16(26)4-3-5-17(20)27)10-14(21(15)36-24)22(35)31-13-8-6-12(7-9-13)25(28,29)30/h3-10H,11H2,1-2H3,(H,31,35)(H2,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human mPGES1 expressed in CHO cells using PGH2 as substrate pretreated for 10 mins followed by substrate addition measured ... |
Bioorg Med Chem Lett 27: 5131-5138 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.062
BindingDB Entry DOI: 10.7270/Q2JS9T0V |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50452073
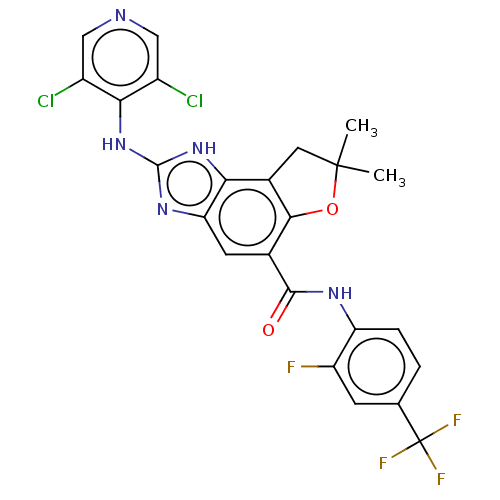
(CHEMBL4210425)Show SMILES CC1(C)Cc2c(O1)c(cc1nc(Nc3c(Cl)cncc3Cl)[nH]c21)C(=O)Nc1ccc(cc1F)C(F)(F)F Show InChI InChI=1S/C24H17Cl2F4N5O2/c1-23(2)7-12-18-17(33-22(34-18)35-19-13(25)8-31-9-14(19)26)6-11(20(12)37-23)21(36)32-16-4-3-10(5-15(16)27)24(28,29)30/h3-6,8-9H,7H2,1-2H3,(H,32,36)(H2,31,33,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human mPGES1 expressed in CHO cells using PGH2 as substrate pretreated for 10 mins followed by substrate addition measured ... |
Bioorg Med Chem Lett 27: 5131-5138 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.062
BindingDB Entry DOI: 10.7270/Q2JS9T0V |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50207343
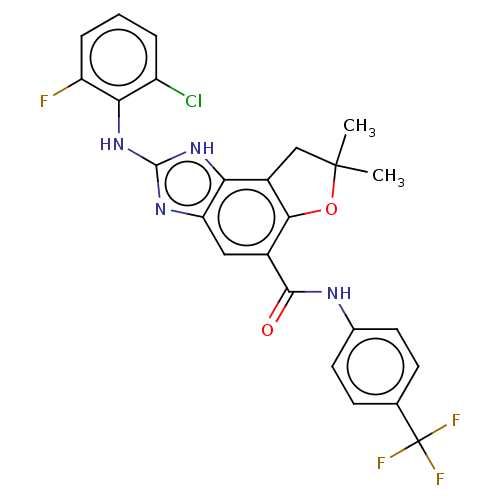
(CHEMBL3931344)Show SMILES CC1(C)Cc2c(O1)c(cc1nc(Nc3c(F)cccc3Cl)[nH]c21)C(=O)Nc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C25H19ClF4N4O2/c1-24(2)11-15-19-18(32-23(33-19)34-20-16(26)4-3-5-17(20)27)10-14(21(15)36-24)22(35)31-13-8-6-12(7-9-13)25(28,29)30/h3-10H,11H2,1-2H3,(H,31,35)(H2,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mPGES-1 expressed in CHO cells assessed as reduction in conversion of PGH2 to PGE2 incubated for 10 mins followed by ... |
Bioorg Med Chem Lett 26: 5977-5984 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.079
BindingDB Entry DOI: 10.7270/Q2XK8HJ8 |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50248978
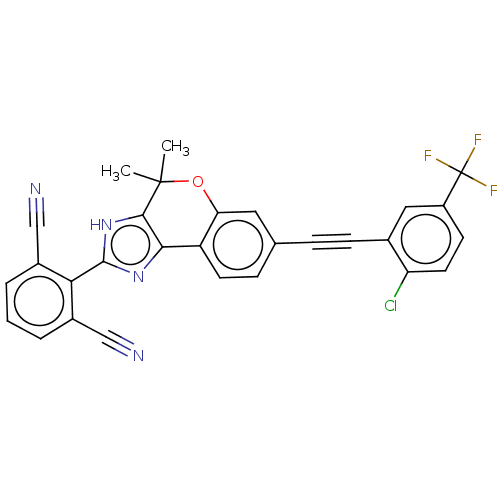
(CHEMBL4076884)Show SMILES CC1(C)Oc2cc(ccc2-c2nc([nH]c12)-c1c(cccc1C#N)C#N)C#Cc1cc(ccc1Cl)C(F)(F)F Show InChI InChI=1S/C29H16ClF3N4O/c1-28(2)26-25(36-27(37-26)24-18(14-34)4-3-5-19(24)15-35)21-10-7-16(12-23(21)38-28)6-8-17-13-20(29(31,32)33)9-11-22(17)30/h3-5,7,9-13H,1-2H3,(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human mPGES-1 expressed in CHO cells assessed as reduction in PGE2 formation using PGH2 a substrate preincubated for 10 min... |
Bioorg Med Chem Lett 27: 2594-2601 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.068
BindingDB Entry DOI: 10.7270/Q24B33Q3 |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50207343

(CHEMBL3931344)Show SMILES CC1(C)Cc2c(O1)c(cc1nc(Nc3c(F)cccc3Cl)[nH]c21)C(=O)Nc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C25H19ClF4N4O2/c1-24(2)11-15-19-18(32-23(33-19)34-20-16(26)4-3-5-17(20)27)10-14(21(15)36-24)22(35)31-13-8-6-12(7-9-13)25(28,29)30/h3-10H,11H2,1-2H3,(H,31,35)(H2,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human mPGES1 expressed in CHO cells using PGH2 as substrate pretreated for 10 mins followed by substrate addition measured ... |
Bioorg Med Chem Lett 27: 5131-5138 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.062
BindingDB Entry DOI: 10.7270/Q2JS9T0V |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data