

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3-oxoacyl-[acyl-carrier-protein] synthase 3 (Escherichia coli) | BDBM50349524 (CHEMBL1808849) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00000103 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli FabH expressed in Escherichia coli DH10B cells assessed as incorporation of 3H signal in the product after 25 mins by ... | Bioorg Med Chem 19: 4413-20 (2011) Article DOI: 10.1016/j.bmc.2011.06.049 BindingDB Entry DOI: 10.7270/Q2HD7W18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 3 (Escherichia coli) | BDBM50349326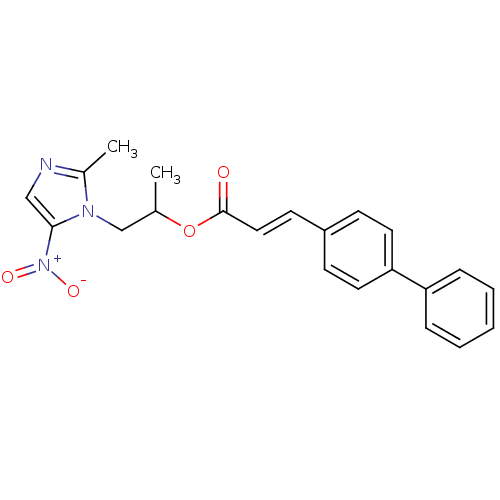 (CHEMBL1807657) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00000156 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged Escherichia coli FabH expressed in Escherichia coli BL21 (DE3) using [3H]acetyl-coA after 25 mins by liquid scint... | Bioorg Med Chem 19: 4513-9 (2011) Article DOI: 10.1016/j.bmc.2011.06.021 BindingDB Entry DOI: 10.7270/Q2XK8FWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 3 (Escherichia coli) | BDBM50349522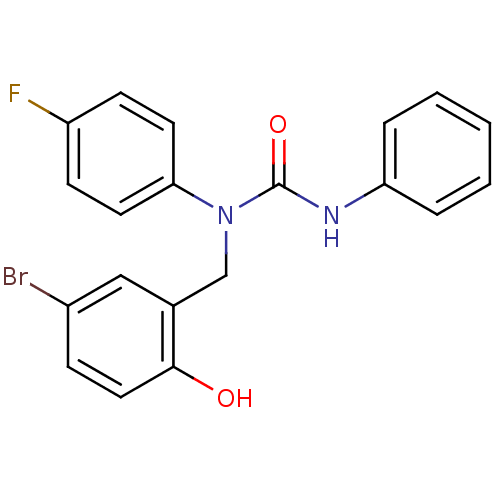 (CHEMBL1808847) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00000161 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli FabH expressed in Escherichia coli DH10B cells assessed as incorporation of 3H signal in the product after 25 mins by ... | Bioorg Med Chem 19: 4413-20 (2011) Article DOI: 10.1016/j.bmc.2011.06.049 BindingDB Entry DOI: 10.7270/Q2HD7W18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 3 (Escherichia coli) | BDBM50349526 (CHEMBL1808851) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00000548 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli FabH expressed in Escherichia coli DH10B cells assessed as incorporation of 3H signal in the product after 25 mins by ... | Bioorg Med Chem 19: 4413-20 (2011) Article DOI: 10.1016/j.bmc.2011.06.049 BindingDB Entry DOI: 10.7270/Q2HD7W18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 3 (Escherichia coli) | BDBM50349525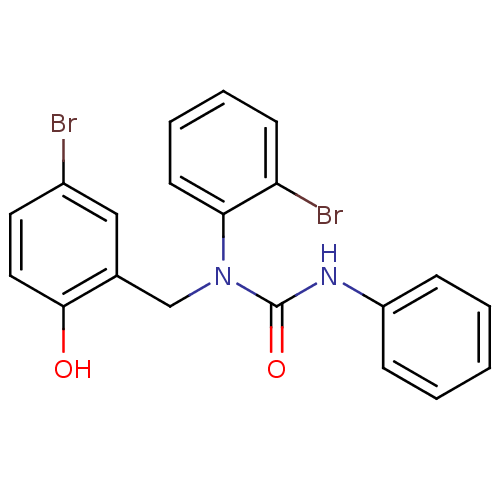 (CHEMBL1808850) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00000706 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli FabH expressed in Escherichia coli DH10B cells assessed as incorporation of 3H signal in the product after 25 mins by ... | Bioorg Med Chem 19: 4413-20 (2011) Article DOI: 10.1016/j.bmc.2011.06.049 BindingDB Entry DOI: 10.7270/Q2HD7W18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 3 (Escherichia coli) | BDBM50349530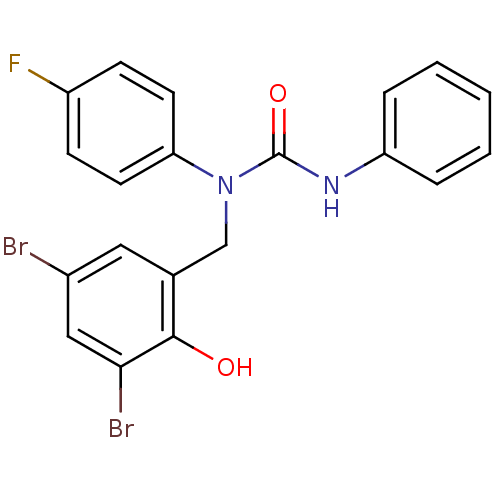 (CHEMBL1808855) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00000713 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli FabH expressed in Escherichia coli DH10B cells assessed as incorporation of 3H signal in the product after 25 mins by ... | Bioorg Med Chem 19: 4413-20 (2011) Article DOI: 10.1016/j.bmc.2011.06.049 BindingDB Entry DOI: 10.7270/Q2HD7W18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 3 (Escherichia coli) | BDBM50349529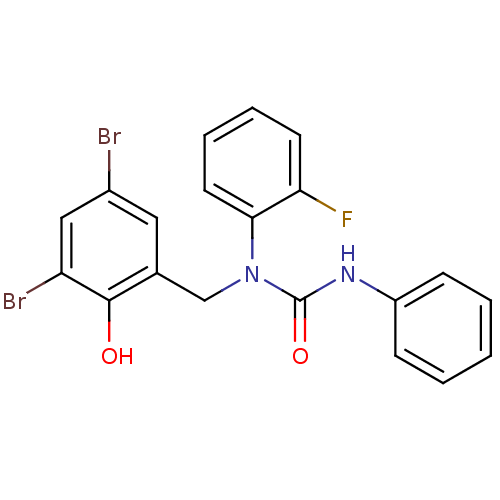 (CHEMBL1808854) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00000800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli FabH expressed in Escherichia coli DH10B cells assessed as incorporation of 3H signal in the product after 25 mins by ... | Bioorg Med Chem 19: 4413-20 (2011) Article DOI: 10.1016/j.bmc.2011.06.049 BindingDB Entry DOI: 10.7270/Q2HD7W18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 3 (Escherichia coli) | BDBM50349531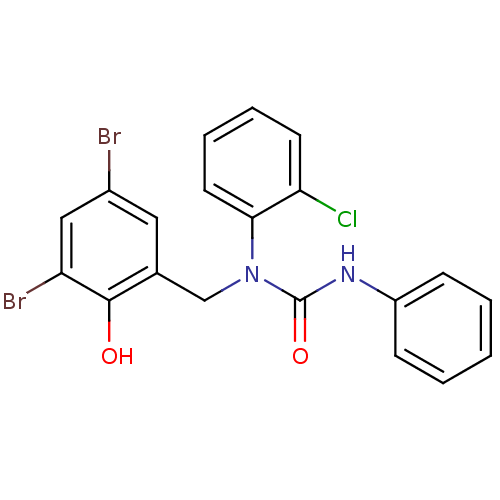 (CHEMBL1808856) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00000843 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli FabH expressed in Escherichia coli DH10B cells assessed as incorporation of 3H signal in the product after 25 mins by ... | Bioorg Med Chem 19: 4413-20 (2011) Article DOI: 10.1016/j.bmc.2011.06.049 BindingDB Entry DOI: 10.7270/Q2HD7W18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 3 (Escherichia coli) | BDBM50349532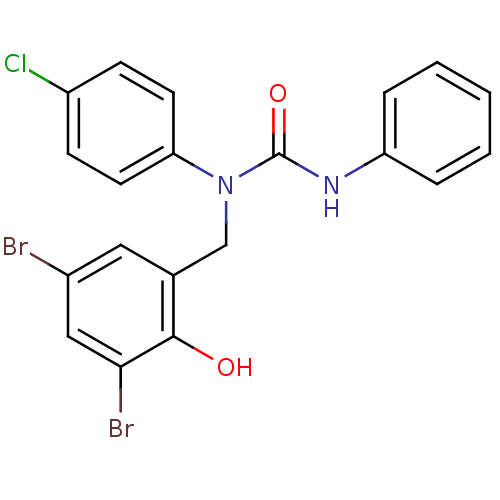 (CHEMBL1808857) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00000911 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli FabH expressed in Escherichia coli DH10B cells assessed as incorporation of 3H signal in the product after 25 mins by ... | Bioorg Med Chem 19: 4413-20 (2011) Article DOI: 10.1016/j.bmc.2011.06.049 BindingDB Entry DOI: 10.7270/Q2HD7W18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 3 (Escherichia coli) | BDBM50349327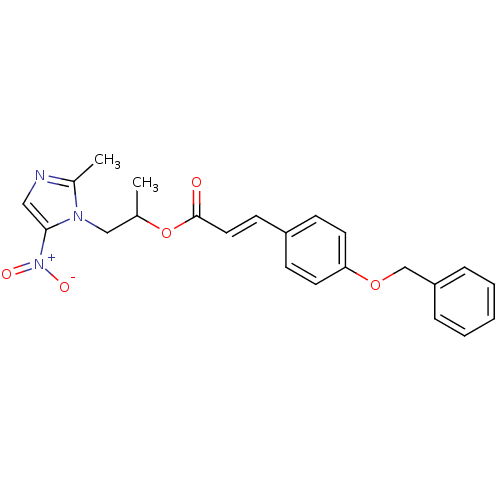 (CHEMBL1807658) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged Escherichia coli FabH expressed in Escherichia coli BL21 (DE3) using [3H]acetyl-coA after 25 mins by liquid scint... | Bioorg Med Chem 19: 4513-9 (2011) Article DOI: 10.1016/j.bmc.2011.06.021 BindingDB Entry DOI: 10.7270/Q2XK8FWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 3 (Escherichia coli) | BDBM50349523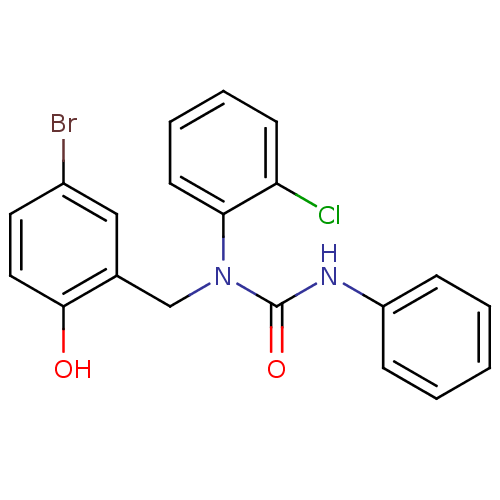 (CHEMBL1808848) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0000124 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli FabH expressed in Escherichia coli DH10B cells assessed as incorporation of 3H signal in the product after 25 mins by ... | Bioorg Med Chem 19: 4413-20 (2011) Article DOI: 10.1016/j.bmc.2011.06.049 BindingDB Entry DOI: 10.7270/Q2HD7W18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 3 (Escherichia coli) | BDBM50349534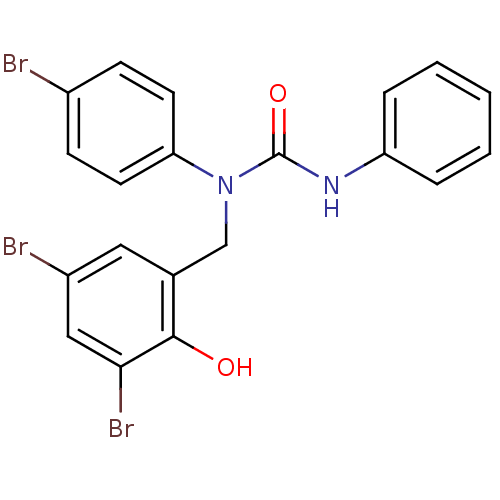 (CHEMBL1808859) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0000172 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli FabH expressed in Escherichia coli DH10B cells assessed as incorporation of 3H signal in the product after 25 mins by ... | Bioorg Med Chem 19: 4413-20 (2011) Article DOI: 10.1016/j.bmc.2011.06.049 BindingDB Entry DOI: 10.7270/Q2HD7W18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 3 (Escherichia coli) | BDBM50349535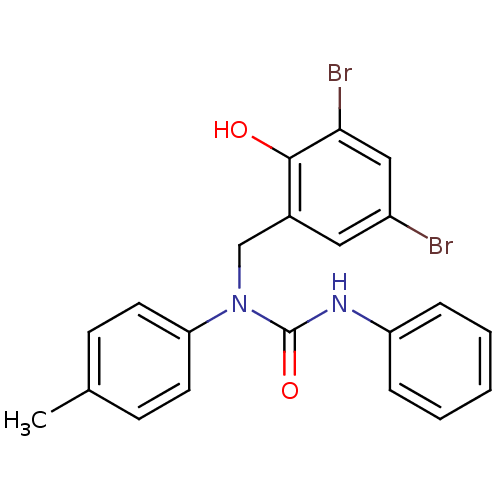 (CHEMBL1808860) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0000246 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli FabH expressed in Escherichia coli DH10B cells assessed as incorporation of 3H signal in the product after 25 mins by ... | Bioorg Med Chem 19: 4413-20 (2011) Article DOI: 10.1016/j.bmc.2011.06.049 BindingDB Entry DOI: 10.7270/Q2HD7W18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 3 (Escherichia coli) | BDBM50349331 (CHEMBL1807660) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0000261 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged Escherichia coli FabH expressed in Escherichia coli BL21 (DE3) using [3H]acetyl-coA after 25 mins by liquid scint... | Bioorg Med Chem 19: 4513-9 (2011) Article DOI: 10.1016/j.bmc.2011.06.021 BindingDB Entry DOI: 10.7270/Q2XK8FWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 3 (Escherichia coli) | BDBM50349332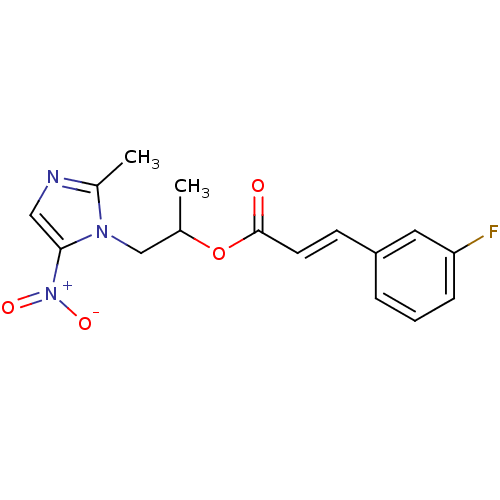 (CHEMBL1807926) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0000336 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged Escherichia coli FabH expressed in Escherichia coli BL21 (DE3) using [3H]acetyl-coA after 25 mins by liquid scint... | Bioorg Med Chem 19: 4513-9 (2011) Article DOI: 10.1016/j.bmc.2011.06.021 BindingDB Entry DOI: 10.7270/Q2XK8FWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 3 (Escherichia coli) | BDBM50349528 (CHEMBL1808853) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0000356 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli FabH expressed in Escherichia coli DH10B cells assessed as incorporation of 3H signal in the product after 25 mins by ... | Bioorg Med Chem 19: 4413-20 (2011) Article DOI: 10.1016/j.bmc.2011.06.049 BindingDB Entry DOI: 10.7270/Q2HD7W18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 3 (Escherichia coli) | BDBM50349521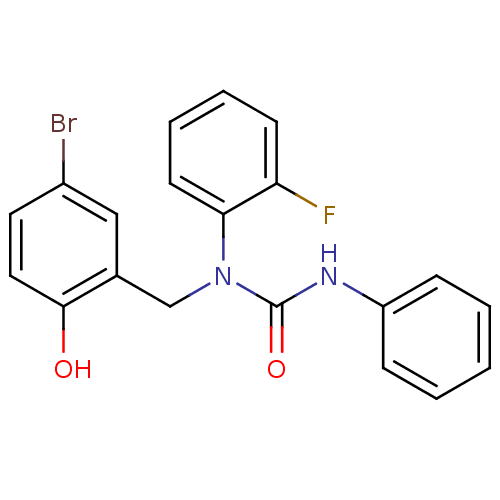 (CHEMBL1808846) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0000384 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli FabH expressed in Escherichia coli DH10B cells assessed as incorporation of 3H signal in the product after 25 mins by ... | Bioorg Med Chem 19: 4413-20 (2011) Article DOI: 10.1016/j.bmc.2011.06.049 BindingDB Entry DOI: 10.7270/Q2HD7W18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 3 (Escherichia coli) | BDBM50349527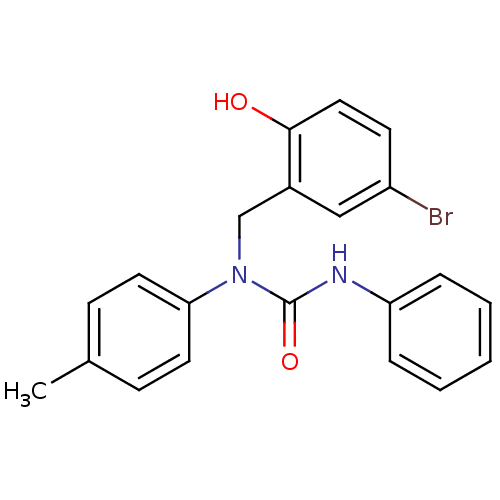 (CHEMBL1808852) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0000578 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli FabH expressed in Escherichia coli DH10B cells assessed as incorporation of 3H signal in the product after 25 mins by ... | Bioorg Med Chem 19: 4413-20 (2011) Article DOI: 10.1016/j.bmc.2011.06.049 BindingDB Entry DOI: 10.7270/Q2HD7W18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 3 (Escherichia coli) | BDBM50349324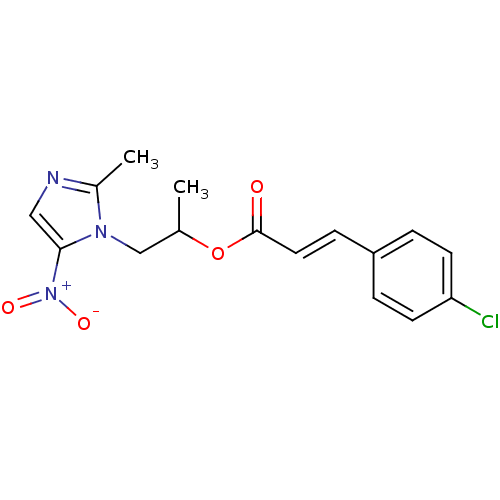 (CHEMBL1807653) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0000640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged Escherichia coli FabH expressed in Escherichia coli BL21 (DE3) using [3H]acetyl-coA after 25 mins by liquid scint... | Bioorg Med Chem 19: 4513-9 (2011) Article DOI: 10.1016/j.bmc.2011.06.021 BindingDB Entry DOI: 10.7270/Q2XK8FWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 3 (Escherichia coli) | BDBM50349314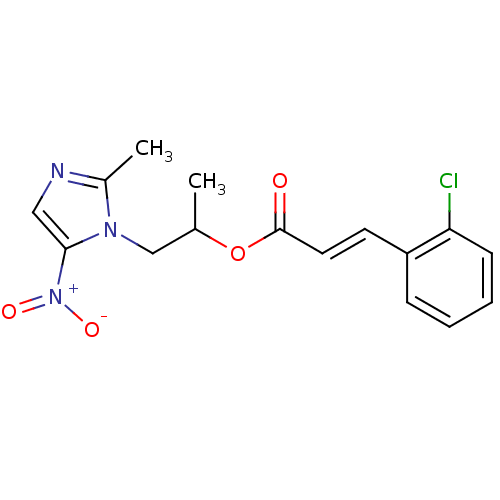 (CHEMBL1807923) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0000652 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged Escherichia coli FabH expressed in Escherichia coli BL21 (DE3) using [3H]acetyl-coA after 25 mins by liquid scint... | Bioorg Med Chem 19: 4513-9 (2011) Article DOI: 10.1016/j.bmc.2011.06.021 BindingDB Entry DOI: 10.7270/Q2XK8FWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 3 (Escherichia coli) | BDBM50349325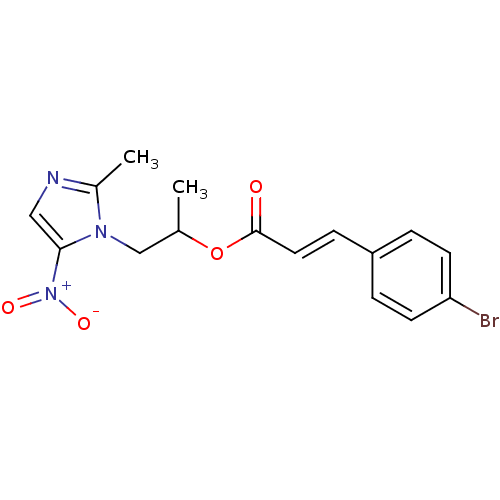 (CHEMBL1807654) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0000799 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged Escherichia coli FabH expressed in Escherichia coli BL21 (DE3) using [3H]acetyl-coA after 25 mins by liquid scint... | Bioorg Med Chem 19: 4513-9 (2011) Article DOI: 10.1016/j.bmc.2011.06.021 BindingDB Entry DOI: 10.7270/Q2XK8FWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 3 (Escherichia coli) | BDBM50349328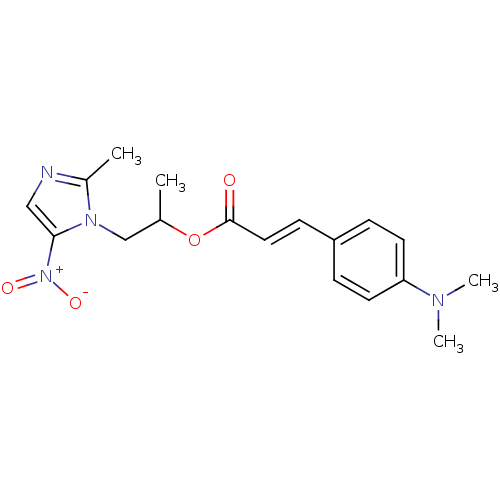 (CHEMBL1807659) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.000111 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged Escherichia coli FabH expressed in Escherichia coli BL21 (DE3) using [3H]acetyl-coA after 25 mins by liquid scint... | Bioorg Med Chem 19: 4513-9 (2011) Article DOI: 10.1016/j.bmc.2011.06.021 BindingDB Entry DOI: 10.7270/Q2XK8FWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 3 (Escherichia coli) | BDBM50349317 (CHEMBL1807929) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.000169 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged Escherichia coli FabH expressed in Escherichia coli BL21 (DE3) using [3H]acetyl-coA after 25 mins by liquid scint... | Bioorg Med Chem 19: 4513-9 (2011) Article DOI: 10.1016/j.bmc.2011.06.021 BindingDB Entry DOI: 10.7270/Q2XK8FWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 3 (Escherichia coli) | BDBM50349536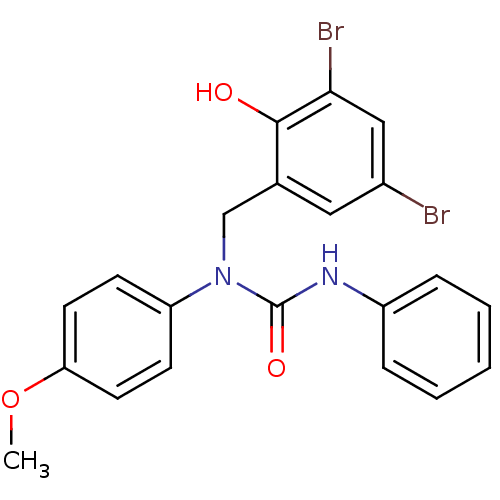 (CHEMBL1808861) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.000192 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli FabH expressed in Escherichia coli DH10B cells assessed as incorporation of 3H signal in the product after 25 mins by ... | Bioorg Med Chem 19: 4413-20 (2011) Article DOI: 10.1016/j.bmc.2011.06.049 BindingDB Entry DOI: 10.7270/Q2HD7W18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 3 (Escherichia coli) | BDBM50349323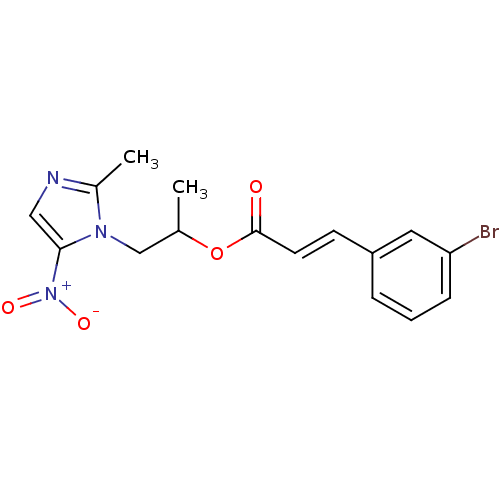 (CHEMBL1807927) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.000214 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged Escherichia coli FabH expressed in Escherichia coli BL21 (DE3) using [3H]acetyl-coA after 25 mins by liquid scint... | Bioorg Med Chem 19: 4513-9 (2011) Article DOI: 10.1016/j.bmc.2011.06.021 BindingDB Entry DOI: 10.7270/Q2XK8FWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 3 (Escherichia coli) | BDBM50349329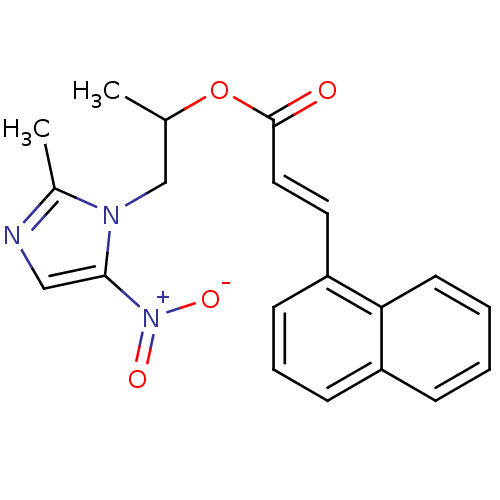 (CHEMBL1807661) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.000443 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged Escherichia coli FabH expressed in Escherichia coli BL21 (DE3) using [3H]acetyl-coA after 25 mins by liquid scint... | Bioorg Med Chem 19: 4513-9 (2011) Article DOI: 10.1016/j.bmc.2011.06.021 BindingDB Entry DOI: 10.7270/Q2XK8FWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 3 (Escherichia coli) | BDBM50349320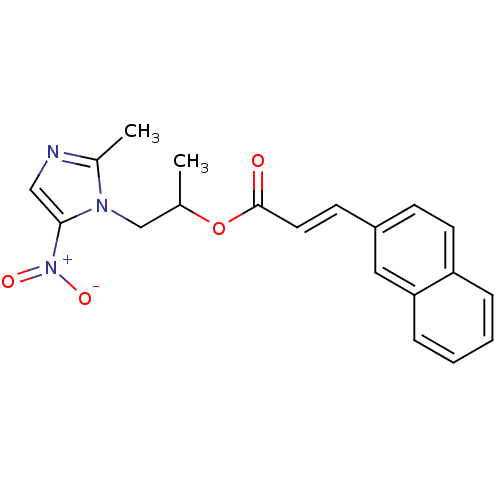 (CHEMBL1807662) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.000526 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged Escherichia coli FabH expressed in Escherichia coli BL21 (DE3) using [3H]acetyl-coA after 25 mins by liquid scint... | Bioorg Med Chem 19: 4513-9 (2011) Article DOI: 10.1016/j.bmc.2011.06.021 BindingDB Entry DOI: 10.7270/Q2XK8FWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 3 (Escherichia coli) | BDBM50349322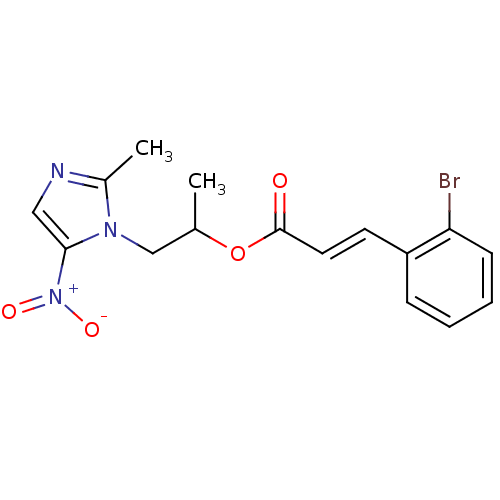 (CHEMBL1807924) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.000529 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged Escherichia coli FabH expressed in Escherichia coli BL21 (DE3) using [3H]acetyl-coA after 25 mins by liquid scint... | Bioorg Med Chem 19: 4513-9 (2011) Article DOI: 10.1016/j.bmc.2011.06.021 BindingDB Entry DOI: 10.7270/Q2XK8FWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 3 (Escherichia coli) | BDBM50349321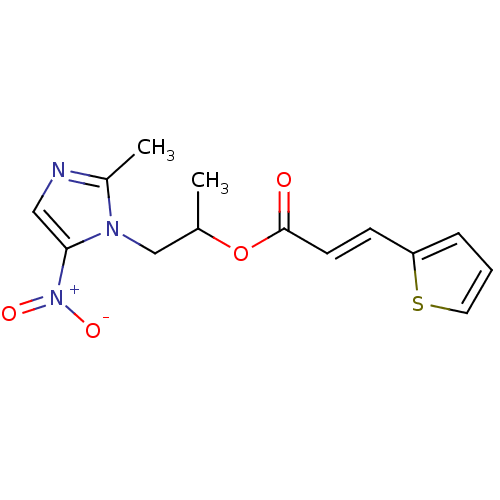 (CHEMBL1807663) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.000555 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged Escherichia coli FabH expressed in Escherichia coli BL21 (DE3) using [3H]acetyl-coA after 25 mins by liquid scint... | Bioorg Med Chem 19: 4513-9 (2011) Article DOI: 10.1016/j.bmc.2011.06.021 BindingDB Entry DOI: 10.7270/Q2XK8FWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 3 (Escherichia coli) | BDBM50349319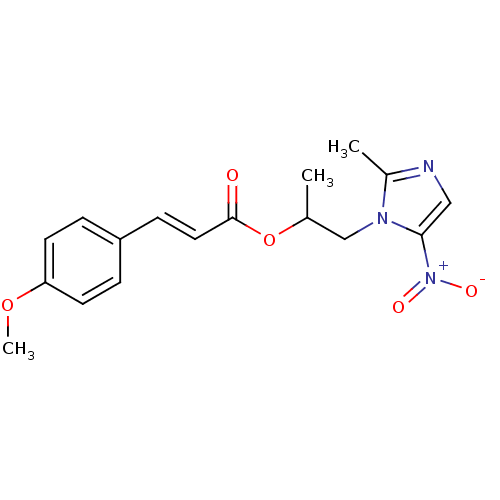 (CHEMBL1807656) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.000610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged Escherichia coli FabH expressed in Escherichia coli BL21 (DE3) using [3H]acetyl-coA after 25 mins by liquid scint... | Bioorg Med Chem 19: 4513-9 (2011) Article DOI: 10.1016/j.bmc.2011.06.021 BindingDB Entry DOI: 10.7270/Q2XK8FWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 3 (Escherichia coli) | BDBM50349533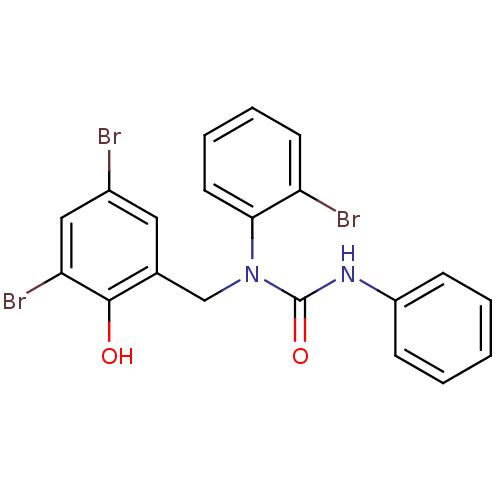 (CHEMBL1808858) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.000759 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli FabH expressed in Escherichia coli DH10B cells assessed as incorporation of 3H signal in the product after 25 mins by ... | Bioorg Med Chem 19: 4413-20 (2011) Article DOI: 10.1016/j.bmc.2011.06.049 BindingDB Entry DOI: 10.7270/Q2HD7W18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 3 (Escherichia coli) | BDBM50349318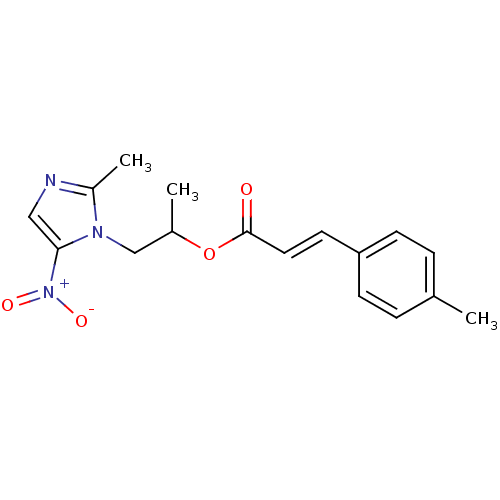 (CHEMBL1807655) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.000896 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged Escherichia coli FabH expressed in Escherichia coli BL21 (DE3) using [3H]acetyl-coA after 25 mins by liquid scint... | Bioorg Med Chem 19: 4513-9 (2011) Article DOI: 10.1016/j.bmc.2011.06.021 BindingDB Entry DOI: 10.7270/Q2XK8FWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 3 (Escherichia coli) | BDBM50349313 (CHEMBL1807922) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.000975 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged Escherichia coli FabH expressed in Escherichia coli BL21 (DE3) using [3H]acetyl-coA after 25 mins by liquid scint... | Bioorg Med Chem 19: 4513-9 (2011) Article DOI: 10.1016/j.bmc.2011.06.021 BindingDB Entry DOI: 10.7270/Q2XK8FWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 3 (Escherichia coli) | BDBM50349312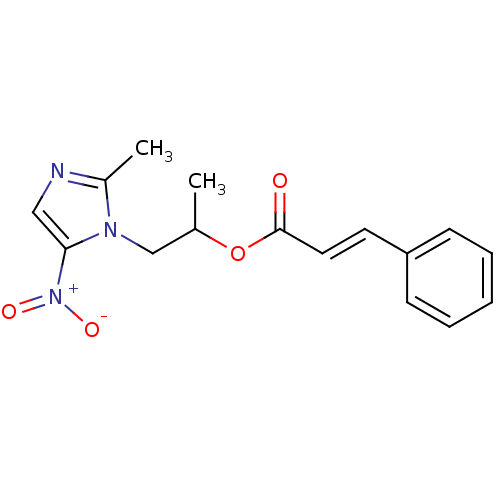 (CHEMBL1807921) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.00132 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged Escherichia coli FabH expressed in Escherichia coli BL21 (DE3) using [3H]acetyl-coA after 25 mins by liquid scint... | Bioorg Med Chem 19: 4513-9 (2011) Article DOI: 10.1016/j.bmc.2011.06.021 BindingDB Entry DOI: 10.7270/Q2XK8FWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 3 (Escherichia coli) | BDBM50349315 (CHEMBL1807925) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00179 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged Escherichia coli FabH expressed in Escherichia coli BL21 (DE3) using [3H]acetyl-coA after 25 mins by liquid scint... | Bioorg Med Chem 19: 4513-9 (2011) Article DOI: 10.1016/j.bmc.2011.06.021 BindingDB Entry DOI: 10.7270/Q2XK8FWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 3 (Escherichia coli) | BDBM50349316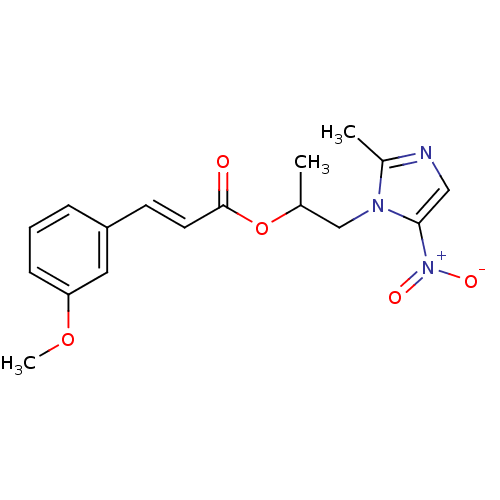 (CHEMBL1807928) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00273 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged Escherichia coli FabH expressed in Escherichia coli BL21 (DE3) using [3H]acetyl-coA after 25 mins by liquid scint... | Bioorg Med Chem 19: 4513-9 (2011) Article DOI: 10.1016/j.bmc.2011.06.021 BindingDB Entry DOI: 10.7270/Q2XK8FWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM50324510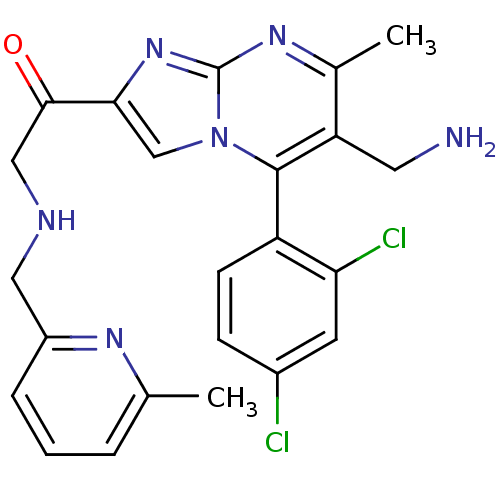 (1-(6-(aminomethyl)-5-(2,4-dichlorophenyl)-7-methyl...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human DPP8 | J Med Chem 53: 5620-8 (2010) Article DOI: 10.1021/jm100634a BindingDB Entry DOI: 10.7270/Q2S182PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50052442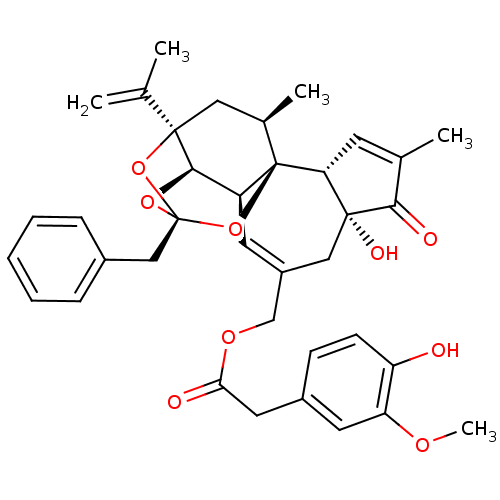 ((4-Hydroxy-3-methoxy-phenyl)-acetic acid (2R,3S,3a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description In vitro binding to Rat Vanilloid receptor 1 (VR1) expressing CHO cells compared to capsacin | J Med Chem 46: 3116-26 (2003) Article DOI: 10.1021/jm030089u BindingDB Entry DOI: 10.7270/Q2SB4551 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 9 (Homo sapiens (Human)) | BDBM50324512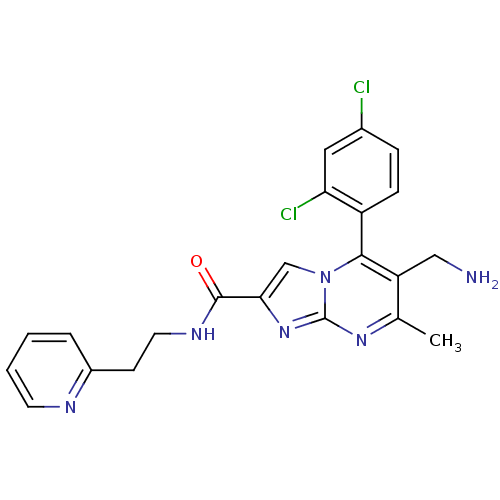 (6-(aminomethyl)-5-(2,4-dichlorophenyl)-7-methyl-N-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human DPP9 | J Med Chem 53: 5620-8 (2010) Article DOI: 10.1021/jm100634a BindingDB Entry DOI: 10.7270/Q2S182PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50324523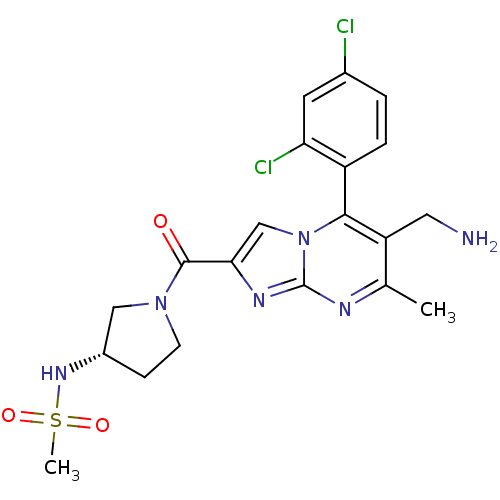 (CHEMBL1215018 | N-((3S)-1-(6-(aminomethyl)-5-(2,4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP4 assessed as Gly-Pro-pNA cleavage | J Med Chem 53: 5620-8 (2010) Article DOI: 10.1021/jm100634a BindingDB Entry DOI: 10.7270/Q2S182PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM50324512 (6-(aminomethyl)-5-(2,4-dichlorophenyl)-7-methyl-N-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human DPP8 | J Med Chem 53: 5620-8 (2010) Article DOI: 10.1021/jm100634a BindingDB Entry DOI: 10.7270/Q2S182PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 9 (Homo sapiens (Human)) | BDBM50324510 (1-(6-(aminomethyl)-5-(2,4-dichlorophenyl)-7-methyl...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human DPP9 | J Med Chem 53: 5620-8 (2010) Article DOI: 10.1021/jm100634a BindingDB Entry DOI: 10.7270/Q2S182PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 9 (Homo sapiens (Human)) | BDBM50324511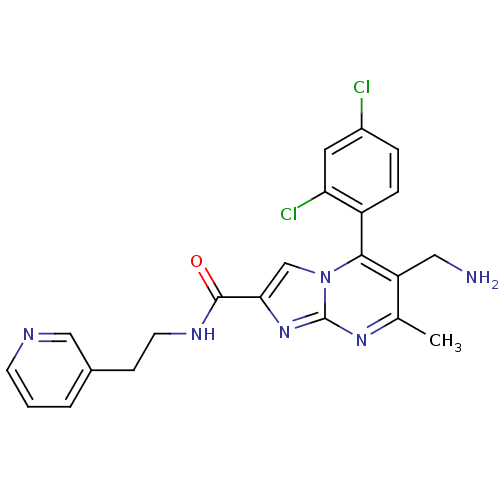 (6-(aminomethyl)-5-(2,4-dichlorophenyl)-7-methyl-N-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human DPP9 | J Med Chem 53: 5620-8 (2010) Article DOI: 10.1021/jm100634a BindingDB Entry DOI: 10.7270/Q2S182PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Mus musculus) | BDBM50303803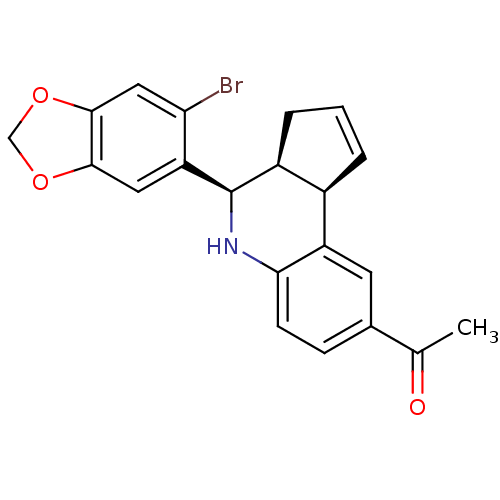 (1-((3aS,4R,9bR)-4-(6-bromobenzo[d][1,3]dioxol-5-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico Health Sciences Center Curated by ChEMBL | Assay Description Binding affinity to ERalpha receptor in mouse COS7 cells by competitive binding assay | Nat Chem Biol 5: 421-7 (2009) Article DOI: 10.1038/nchembio.168 BindingDB Entry DOI: 10.7270/Q2RB74TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Mus musculus) | BDBM50303804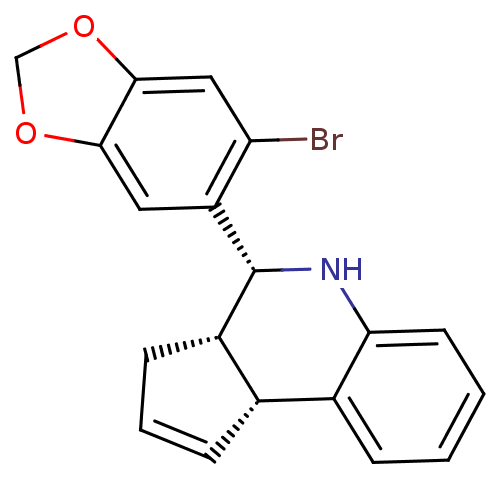 ((3aS,4R,9bR)-4-(6-bromobenzo[d][1,3]dioxol-5-yl)-3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico Health Sciences Center Curated by ChEMBL | Assay Description Binding affinity to ERbeta receptor in mouse COS7 cells by competitive binding assay | Nat Chem Biol 5: 421-7 (2009) Article DOI: 10.1038/nchembio.168 BindingDB Entry DOI: 10.7270/Q2RB74TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50324525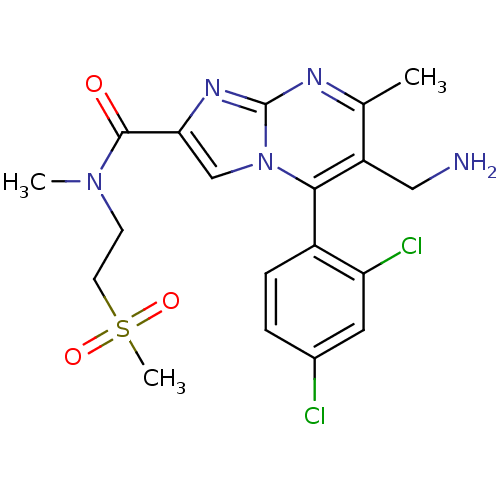 (6-(aminomethyl)-5-(2,4-dichlorophenyl)-N,7-dimethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP4 assessed as Gly-Pro-pNA cleavage | J Med Chem 53: 5620-8 (2010) Article DOI: 10.1021/jm100634a BindingDB Entry DOI: 10.7270/Q2S182PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM50324500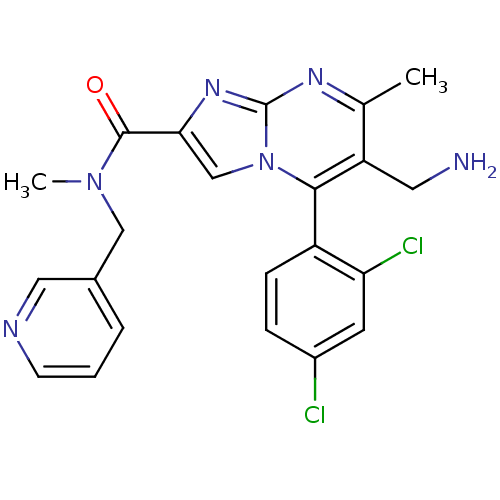 (1-(6-(aminomethyl)-5-(2,4-dichlorophenyl)-7-methyl...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human DPP8 | J Med Chem 53: 5620-8 (2010) Article DOI: 10.1021/jm100634a BindingDB Entry DOI: 10.7270/Q2S182PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM50324524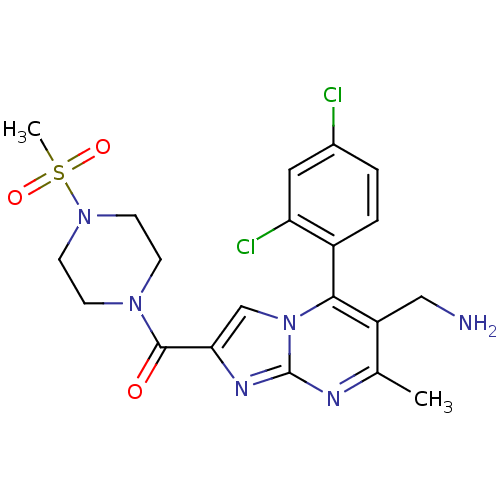 ((6-(aminomethyl)-5-(2,4-dichlorophenyl)-7-methylim...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human DPP8 | J Med Chem 53: 5620-8 (2010) Article DOI: 10.1021/jm100634a BindingDB Entry DOI: 10.7270/Q2S182PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50324524 ((6-(aminomethyl)-5-(2,4-dichlorophenyl)-7-methylim...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP4 assessed as Gly-Pro-pNA cleavage | J Med Chem 53: 5620-8 (2010) Article DOI: 10.1021/jm100634a BindingDB Entry DOI: 10.7270/Q2S182PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 9 (Homo sapiens (Human)) | BDBM50324504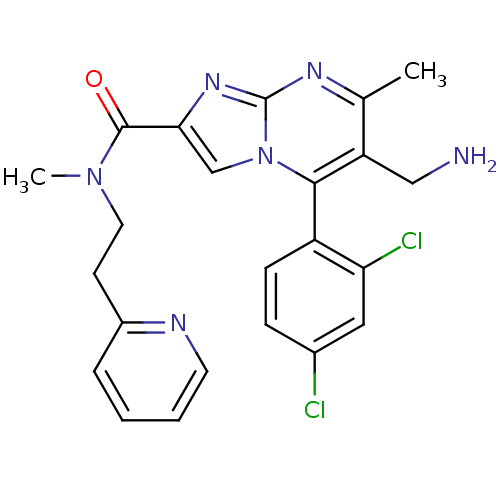 (6-(aminomethyl)-5-(2,4-dichlorophenyl)-N,7-dimethy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human DPP9 | J Med Chem 53: 5620-8 (2010) Article DOI: 10.1021/jm100634a BindingDB Entry DOI: 10.7270/Q2S182PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2959 total ) | Next | Last >> |