

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50039111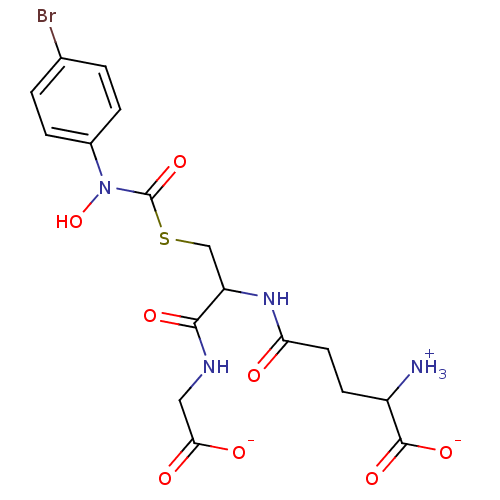 (S-(N-Hydroxy-N-(4-bromophenyl)carbamoyl)glutathion...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Tested for inhibitory activity against human erythrocyte glyoxalase I | J Med Chem 37: 2161-6 (1994) BindingDB Entry DOI: 10.7270/Q2TT4RKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50092826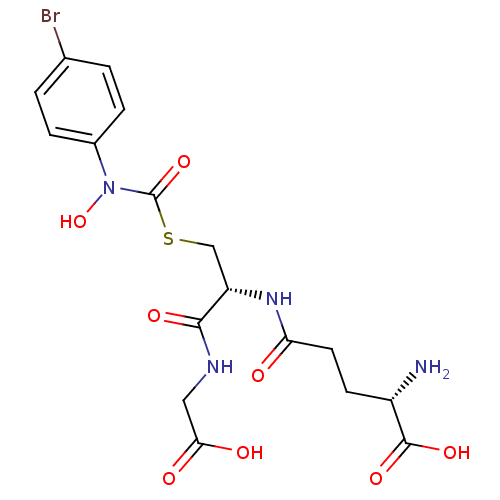 ((2S)-2-amino-5-{[(1R)-1-[({[(4-bromophenyl)(hydrox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid UniChem Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Binding affinity for Glyoxalase I | J Med Chem 43: 3981-6 (2000) BindingDB Entry DOI: 10.7270/Q2BC3XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50092827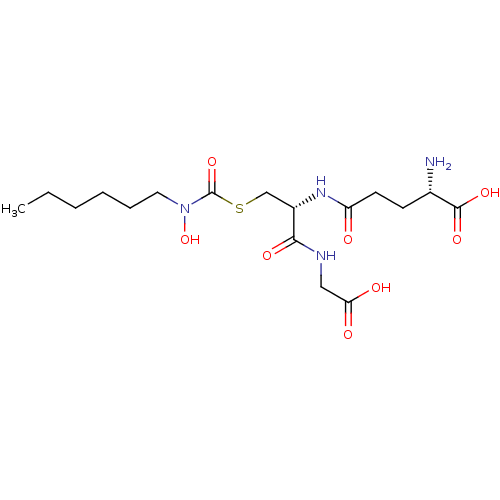 (CHEMBL127840 | S-(N-hexyl-N-hydroxycarbamoyl)gluta...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Binding affinity for Glyoxalase I | J Med Chem 43: 3981-6 (2000) BindingDB Entry DOI: 10.7270/Q2BC3XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50092831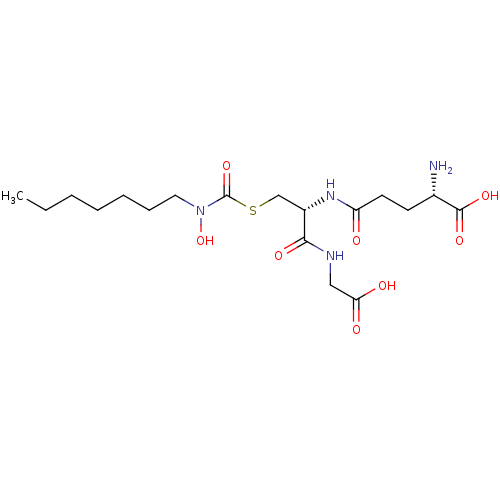 (CHEMBL128836 | S-(N-heptyl-N-hydroxycarbamoyl)glut...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Binding affinity for Glyoxalase I | J Med Chem 43: 3981-6 (2000) BindingDB Entry DOI: 10.7270/Q2BC3XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50092824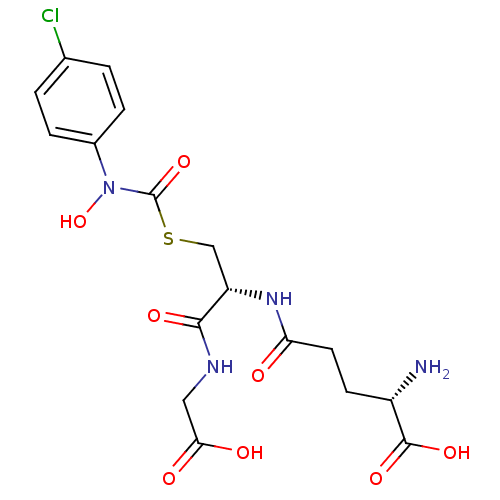 (CHEMBL131578 | S-(N-4chlorophenyl-N-hydroxycarbamo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Binding affinity for Glyoxalase I | J Med Chem 43: 3981-6 (2000) BindingDB Entry DOI: 10.7270/Q2BC3XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50039108 (S-(N-Hydroxy-N-(4-chlorophenyl)carbamoyl)glutathio...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Tested for inhibitory activity against human erythrocyte glyoxalase I | J Med Chem 37: 2161-6 (1994) BindingDB Entry DOI: 10.7270/Q2TT4RKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50039113 (S-(N-Hydroxy-N-phenylcarbamoyl)glutathione) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Tested for inhibitory activity against human erythrocyte glyoxalase I | J Med Chem 37: 2161-6 (1994) BindingDB Entry DOI: 10.7270/Q2TT4RKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50092825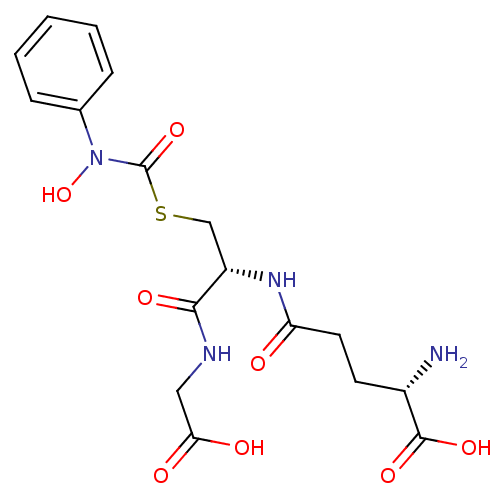 (CHEMBL128935 | S-(N-phenyl-N-hydroxycarbamoyl)glut...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Binding affinity for Glyoxalase I | J Med Chem 43: 3981-6 (2000) BindingDB Entry DOI: 10.7270/Q2BC3XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50092830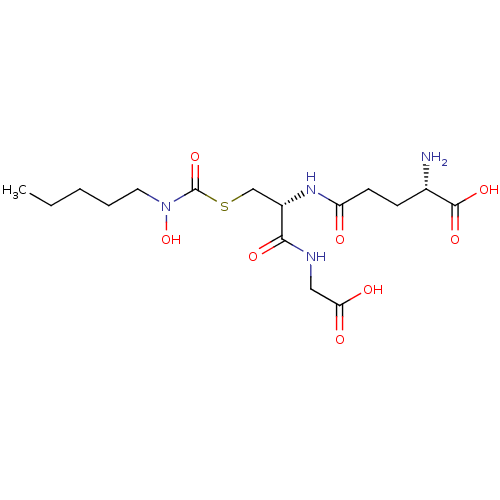 (CHEMBL129965 | S-(N-pentyl-N-hydroxycarbamoyl)glut...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Binding affinity for Glyoxalase I | J Med Chem 43: 3981-6 (2000) BindingDB Entry DOI: 10.7270/Q2BC3XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50092822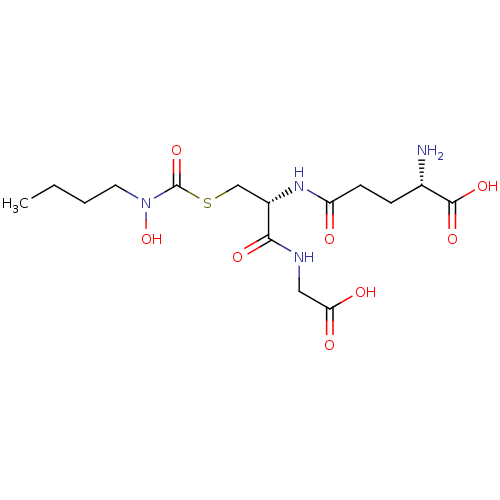 (CHEMBL128447 | S-(N-butyl-N-hydroxycarbamoyl)gluta...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Binding affinity for Glyoxalase I | J Med Chem 43: 3981-6 (2000) BindingDB Entry DOI: 10.7270/Q2BC3XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50092829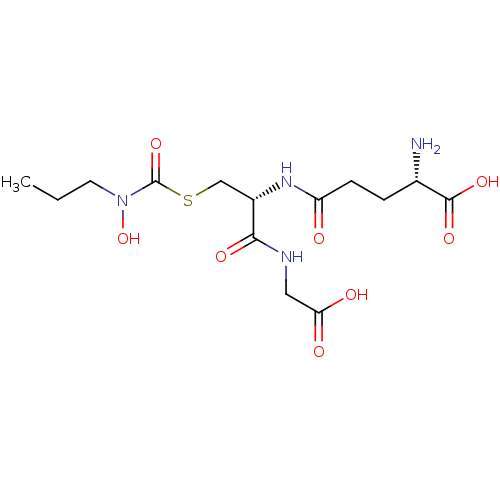 (CHEMBL129597 | S-(N-propyl-N-hydroxycarbamoyl)glut...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Binding affinity for Glyoxalase I | J Med Chem 43: 3981-6 (2000) BindingDB Entry DOI: 10.7270/Q2BC3XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50092832 (CHEMBL128867 | S-(N-ethyl-N-hydroxycarbamoyl)gluta...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Binding affinity for Glyoxalase I | J Med Chem 43: 3981-6 (2000) BindingDB Entry DOI: 10.7270/Q2BC3XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50092826 ((2S)-2-amino-5-{[(1R)-1-[({[(4-bromophenyl)(hydrox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid UniChem Similars | PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Binding affinity of the compound on yeast glyoxalase I (GlxI) was determined | J Med Chem 43: 3981-6 (2000) BindingDB Entry DOI: 10.7270/Q2BC3XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxyacylglutathione hydrolase, mitochondrial (Homo sapiens (Human)) | BDBM50039111 (S-(N-Hydroxy-N-(4-bromophenyl)carbamoyl)glutathion...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Inhibition constants obtained from using the enediol analogs as competitive inhibitors for the inhibition of thehydrolysis of S-D-lactoylglutathione ... | J Med Chem 37: 2161-6 (1994) BindingDB Entry DOI: 10.7270/Q2TT4RKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50039111 (S-(N-Hydroxy-N-(4-bromophenyl)carbamoyl)glutathion...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Tested for inhibitory activity against yeast glyoxalase I | J Med Chem 37: 2161-6 (1994) BindingDB Entry DOI: 10.7270/Q2TT4RKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50092823 (CHEMBL129435 | S-(N-methyl-N-hydroxycarbamoyl)glut...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Binding affinity for Glyoxalase I | J Med Chem 43: 3981-6 (2000) BindingDB Entry DOI: 10.7270/Q2BC3XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50039110 (S-(N-Methyl-N-hydroxycarbomyl)ethylglutathione) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Tested for inhibitory activity against human erythrocyte glyoxalase I | J Med Chem 37: 2161-6 (1994) BindingDB Entry DOI: 10.7270/Q2TT4RKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxyacylglutathione hydrolase, mitochondrial (Homo sapiens (Human)) | BDBM50039108 (S-(N-Hydroxy-N-(4-chlorophenyl)carbamoyl)glutathio...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Inhibition constants obtained from using the enediol analogs as competitive inhibitors for the inhibition of thehydrolysis of S-D-lactoylglutathione ... | J Med Chem 37: 2161-6 (1994) BindingDB Entry DOI: 10.7270/Q2TT4RKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50092824 (CHEMBL131578 | S-(N-4chlorophenyl-N-hydroxycarbamo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Binding affinity of the compound on yeast glyoxalase I (GlxI) was determined | J Med Chem 43: 3981-6 (2000) BindingDB Entry DOI: 10.7270/Q2BC3XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50039108 (S-(N-Hydroxy-N-(4-chlorophenyl)carbamoyl)glutathio...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Tested for inhibitory activity against yeast glyoxalase I | J Med Chem 37: 2161-6 (1994) BindingDB Entry DOI: 10.7270/Q2TT4RKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50092826 ((2S)-2-amino-5-{[(1R)-1-[({[(4-bromophenyl)(hydrox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid UniChem Similars | PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Binding affinity of the compound on Pseudomonas putida glyoxalase I (GlxI) was determined | J Med Chem 43: 3981-6 (2000) BindingDB Entry DOI: 10.7270/Q2BC3XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxyacylglutathione hydrolase, mitochondrial (Homo sapiens (Human)) | BDBM50039113 (S-(N-Hydroxy-N-phenylcarbamoyl)glutathione) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Inhibition constant for the inhibition of the hydrolysis of S-D-lactoylglutathione by glyoxalase II | J Med Chem 37: 2161-6 (1994) BindingDB Entry DOI: 10.7270/Q2TT4RKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50092825 (CHEMBL128935 | S-(N-phenyl-N-hydroxycarbamoyl)glut...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Binding affinity of the compound on yeast glyoxalase I (GlxI) was determined | J Med Chem 43: 3981-6 (2000) BindingDB Entry DOI: 10.7270/Q2BC3XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50039113 (S-(N-Hydroxy-N-phenylcarbamoyl)glutathione) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Tested for inhibitory activity against yeast glyoxalase I | J Med Chem 37: 2161-6 (1994) BindingDB Entry DOI: 10.7270/Q2TT4RKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50092824 (CHEMBL131578 | S-(N-4chlorophenyl-N-hydroxycarbamo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Binding affinity of the compound on Pseudomonas putida glyoxalase I (GlxI) was determined | J Med Chem 43: 3981-6 (2000) BindingDB Entry DOI: 10.7270/Q2BC3XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50092825 (CHEMBL128935 | S-(N-phenyl-N-hydroxycarbamoyl)glut...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Binding affinity of the compound on Pseudomonas putida glyoxalase I (GlxI) was determined | J Med Chem 43: 3981-6 (2000) BindingDB Entry DOI: 10.7270/Q2BC3XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50092823 (CHEMBL129435 | S-(N-methyl-N-hydroxycarbamoyl)glut...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 6.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Binding affinity of the compound on yeast glyoxalase I (GlxI) was determined | J Med Chem 43: 3981-6 (2000) BindingDB Entry DOI: 10.7270/Q2BC3XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50039110 (S-(N-Methyl-N-hydroxycarbomyl)ethylglutathione) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 6.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Tested for inhibitory activity against yeast glyoxalase I | J Med Chem 37: 2161-6 (1994) BindingDB Entry DOI: 10.7270/Q2TT4RKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50092823 (CHEMBL129435 | S-(N-methyl-N-hydroxycarbamoyl)glut...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 8.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Binding affinity of the compound on Pseudomonas putida glyoxalase I (GlxI) was determined | J Med Chem 43: 3981-6 (2000) BindingDB Entry DOI: 10.7270/Q2BC3XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50092828 (CHEMBL419969 | S-(N-hydroxycarbamoyl)glutathione) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.83E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Binding affinity for Glyoxalase I | J Med Chem 43: 3981-6 (2000) BindingDB Entry DOI: 10.7270/Q2BC3XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxyacylglutathione hydrolase, mitochondrial (Homo sapiens (Human)) | BDBM50039110 (S-(N-Methyl-N-hydroxycarbomyl)ethylglutathione) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 4.26E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Inhibition constants obtained from using the enediol analogs as competitive inhibitors for the inhibition of thehydrolysis of S-D-lactoylglutathione ... | J Med Chem 37: 2161-6 (1994) BindingDB Entry DOI: 10.7270/Q2TT4RKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50039115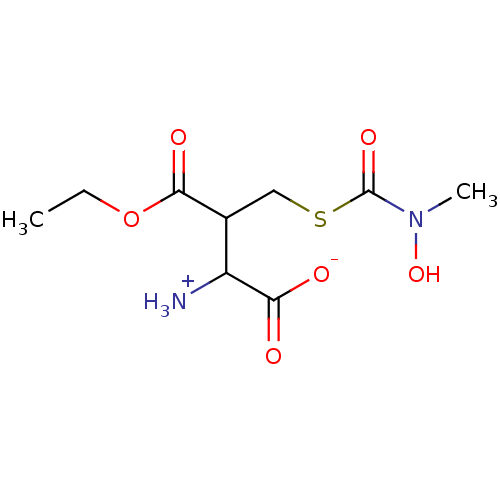 (CHEMBL68824 | S-(N-Methyl-N-hydroxycarbomyl)glutat...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Tested for inhibitory activity against yeast glyoxalase I | J Med Chem 37: 2161-6 (1994) BindingDB Entry DOI: 10.7270/Q2TT4RKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50039114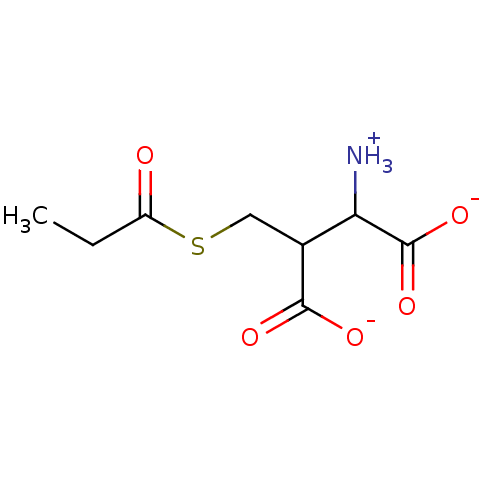 (1,2-Dicarboxy-3-propionylsulfanyl-propyl-ammonium) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.98E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Tested for inhibitory activity against yeast glyoxalase I | J Med Chem 37: 2161-6 (1994) BindingDB Entry DOI: 10.7270/Q2TT4RKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50039109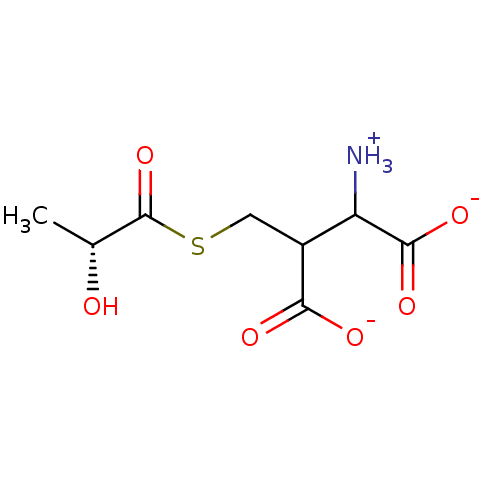 (S-D-Lactoylglutathione) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 2.23E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Tested for inhibitory activity against yeast glyoxalase I | J Med Chem 37: 2161-6 (1994) BindingDB Entry DOI: 10.7270/Q2TT4RKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50039116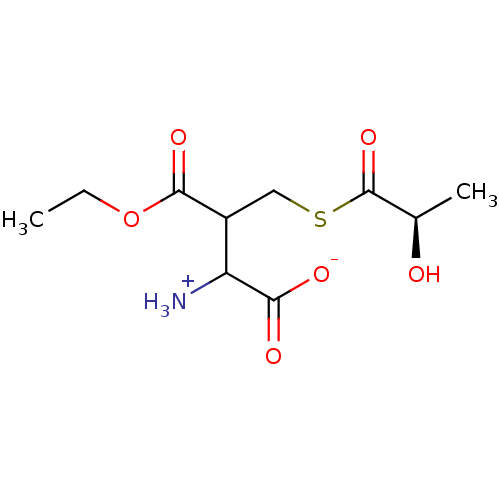 (CHEMBL68031 | S-D-Lactoylethylglutathione) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.16E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Tested for inhibitory activity against yeast glyoxalase I | J Med Chem 37: 2161-6 (1994) BindingDB Entry DOI: 10.7270/Q2TT4RKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50039112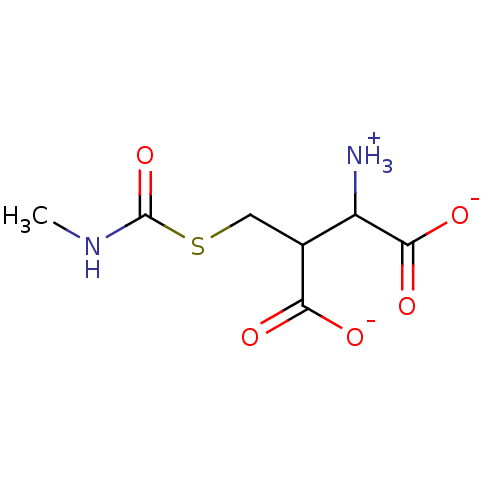 (S-(N-Methyl-N-carbomyl)glutathione) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 5.67E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Tested for inhibitory activity against yeast glyoxalase I | J Med Chem 37: 2161-6 (1994) BindingDB Entry DOI: 10.7270/Q2TT4RKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||