 Found 1253 hits with Last Name = 'hara' and Initial = 'r'
Found 1253 hits with Last Name = 'hara' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(RAT) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
Dopamine receptor D4
(CANINE) | BDBM50005120
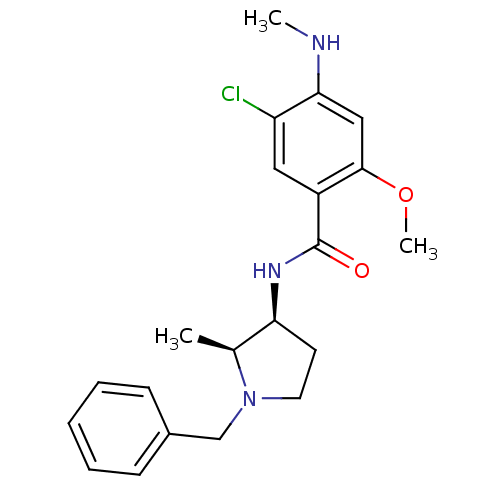
(CHEMBL274491 | N-((2S,3S)-1-Benzyl-2-methyl-pyrrol...)Show SMILES CNc1cc(OC)c(cc1Cl)C(=O)N[C@H]1CCN(Cc2ccccc2)[C@H]1C Show InChI InChI=1S/C21H26ClN3O2/c1-14-18(9-10-25(14)13-15-7-5-4-6-8-15)24-21(26)16-11-17(22)19(23-2)12-20(16)27-3/h4-8,11-12,14,18,23H,9-10,13H2,1-3H3,(H,24,26)/t14-,18-/m0/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50005120

(CHEMBL274491 | N-((2S,3S)-1-Benzyl-2-methyl-pyrrol...)Show SMILES CNc1cc(OC)c(cc1Cl)C(=O)N[C@H]1CCN(Cc2ccccc2)[C@H]1C Show InChI InChI=1S/C21H26ClN3O2/c1-14-18(9-10-25(14)13-15-7-5-4-6-8-15)24-21(26)16-11-17(22)19(23-2)12-20(16)27-3/h4-8,11-12,14,18,23H,9-10,13H2,1-3H3,(H,24,26)/t14-,18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
Dopamine receptor D4
(CANINE) | BDBM50007518

((S)-3-chloro-5-ethyl-N-((1-ethylpyrrolidin-2-yl)me...)Show InChI InChI=1S/C17H25ClN2O3/c1-4-11-9-13(18)16(23-3)14(15(11)21)17(22)19-10-12-7-6-8-20(12)5-2/h9,12,21H,4-8,10H2,1-3H3,(H,19,22)/t12-/m0/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
Dopamine receptor D4
(CANINE) | BDBM50005120

(CHEMBL274491 | N-((2S,3S)-1-Benzyl-2-methyl-pyrrol...)Show SMILES CNc1cc(OC)c(cc1Cl)C(=O)N[C@H]1CCN(Cc2ccccc2)[C@H]1C Show InChI InChI=1S/C21H26ClN3O2/c1-14-18(9-10-25(14)13-15-7-5-4-6-8-15)24-21(26)16-11-17(22)19(23-2)12-20(16)27-3/h4-8,11-12,14,18,23H,9-10,13H2,1-3H3,(H,24,26)/t14-,18-/m0/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM81793
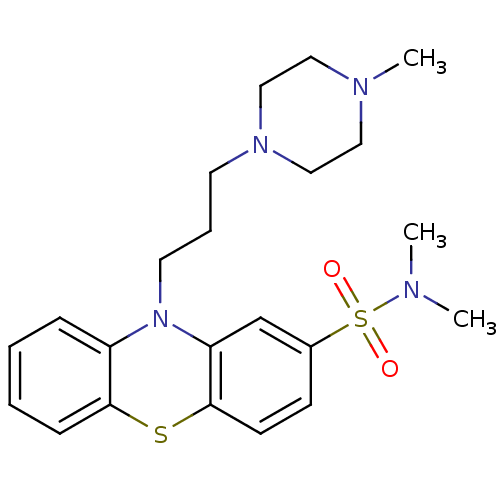
(CAS_316-81-4 | NSC_92178 | Thioproperazine)Show SMILES CN(C)S(=O)(=O)c1ccc2Sc3ccccc3N(CCCN3CCN(C)CC3)c2c1 Show InChI InChI=1S/C22H30N4O2S2/c1-23(2)30(27,28)18-9-10-22-20(17-18)26(19-7-4-5-8-21(19)29-22)12-6-11-25-15-13-24(3)14-16-25/h4-5,7-10,17H,6,11-16H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
D(1B) dopamine receptor
(Homo sapiens (Human)) | BDBM82247
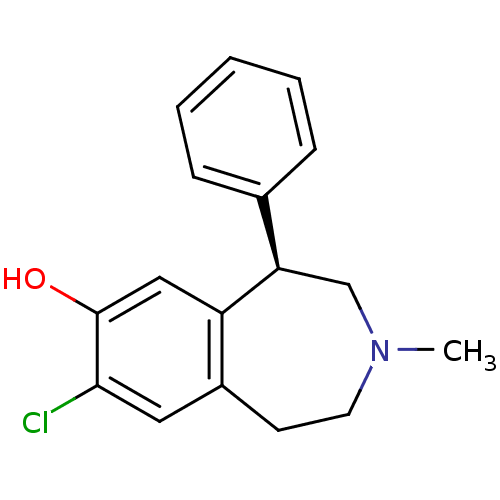
(8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...)Show InChI InChI=1S/C17H18ClNO/c1-19-8-7-13-9-16(18)17(20)10-14(13)15(11-19)12-5-3-2-4-6-12/h2-6,9-10,15,20H,7-8,11H2,1H3/t15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 614-9 (1991)
Article DOI: 10.1038/350614a0
BindingDB Entry DOI: 10.7270/Q2NV9GQR |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50010301
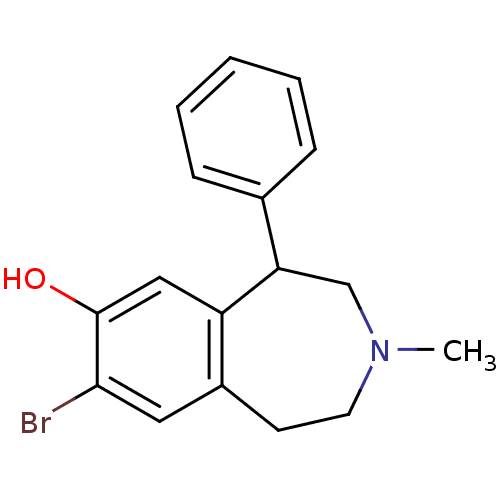
(8-Bromo-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-be...)Show InChI InChI=1S/C17H18BrNO/c1-19-8-7-13-9-16(18)17(20)10-14(13)15(11-19)12-5-3-2-4-6-12/h2-6,9-10,15,20H,7-8,11H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 614-9 (1991)
Article DOI: 10.1038/350614a0
BindingDB Entry DOI: 10.7270/Q2NV9GQR |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM82547
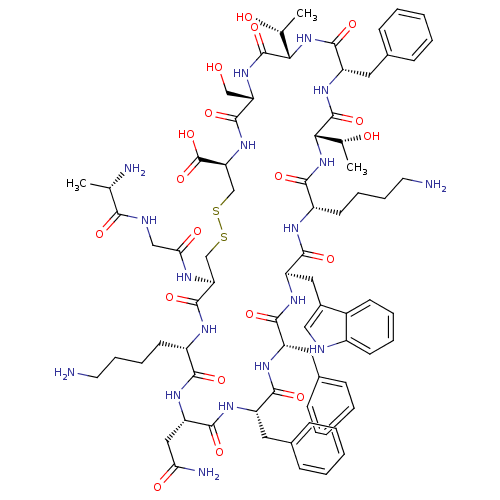
(SRIF-D-Trp8)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O |r| Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50-,51-,52-,53-,54-,55+,56-,57-,58-,59-,62-,63-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Mol Pharmacol 43: 894-901 (1993)
BindingDB Entry DOI: 10.7270/Q29G5K96 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM82247

(8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...)Show InChI InChI=1S/C17H18ClNO/c1-19-8-7-13-9-16(18)17(20)10-14(13)15(11-19)12-5-3-2-4-6-12/h2-6,9-10,15,20H,7-8,11H2,1H3/t15-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 614-9 (1991)
Article DOI: 10.1038/350614a0
BindingDB Entry DOI: 10.7270/Q2NV9GQR |
More data for this
Ligand-Target Pair | |
Dopamine receptor D4
(CANINE) | BDBM81777
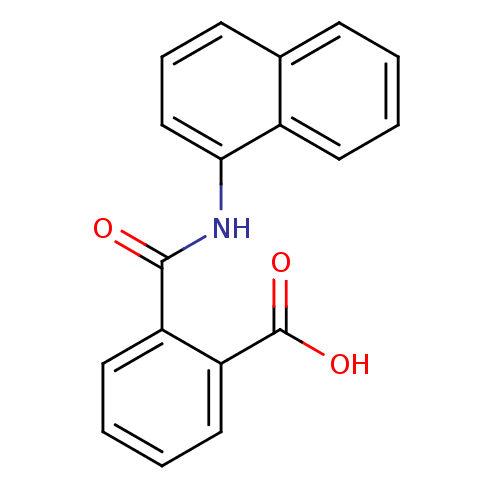
(CAS_132-66-1 | NPA | NPA,(+) | NPA,(-) | NSC_8594)Show InChI InChI=1S/C18H13NO3/c20-17(14-9-3-4-10-15(14)18(21)22)19-16-11-5-7-12-6-1-2-8-13(12)16/h1-11H,(H,19,20)(H,21,22) | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
Dopamine receptor D4
(CANINE) | BDBM81872
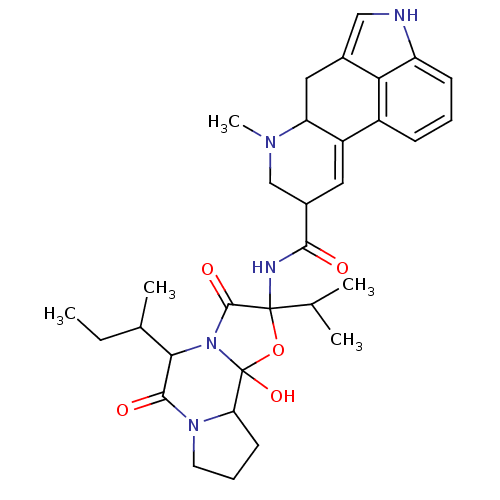
(CAS_161309 | Ergocryptine, beta | NSC_161309)Show SMILES CCC(C)C1N2C(=O)C(NC(=O)C3CN(C)C4Cc5c[nH]c6cccc(C4=C3)c56)(OC2(O)C2CCCN2C1=O)C(C)C |c:27| Show InChI InChI=1S/C32H41N5O5/c1-6-18(4)27-29(39)36-12-8-11-25(36)32(41)37(27)30(40)31(42-32,17(2)3)34-28(38)20-13-22-21-9-7-10-23-26(21)19(15-33-23)14-24(22)35(5)16-20/h7,9-10,13,15,17-18,20,24-25,27,33,41H,6,8,11-12,14,16H2,1-5H3,(H,34,38) | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
D(1B) dopamine receptor
(Homo sapiens (Human)) | BDBM50010301

(8-Bromo-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-be...)Show InChI InChI=1S/C17H18BrNO/c1-19-8-7-13-9-16(18)17(20)10-14(13)15(11-19)12-5-3-2-4-6-12/h2-6,9-10,15,20H,7-8,11H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 614-9 (1991)
Article DOI: 10.1038/350614a0
BindingDB Entry DOI: 10.7270/Q2NV9GQR |
More data for this
Ligand-Target Pair | |
Dopamine receptor D4
(CANINE) | BDBM78433
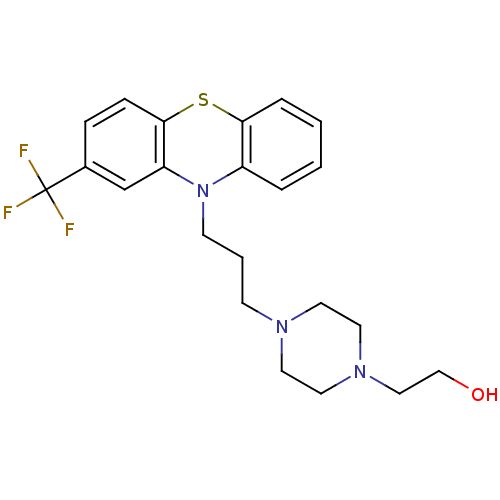
(2-[4-[3-[2-(trifluoromethyl)-10-phenothiazinyl]pro...)Show SMILES OCCN1CCN(CCCN2c3ccccc3Sc3ccc(cc23)C(F)(F)F)CC1 Show InChI InChI=1S/C22H26F3N3OS/c23-22(24,25)17-6-7-21-19(16-17)28(18-4-1-2-5-20(18)30-21)9-3-8-26-10-12-27(13-11-26)14-15-29/h1-2,4-7,16,29H,3,8-15H2 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
Dopamine receptor D4
(CANINE) | BDBM50007568
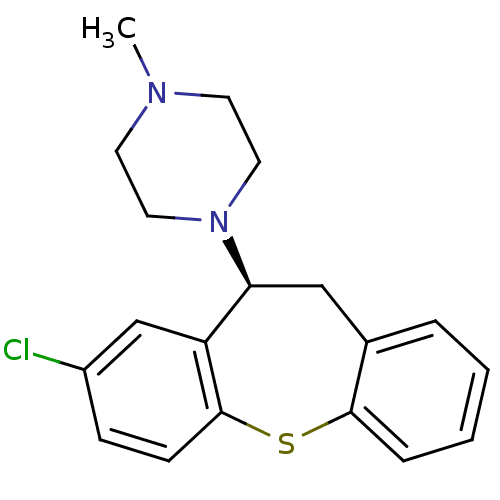
(1-((S)-8-Chloro-10,11-dihydro-dibenzo[b,f]thiepin-...)Show InChI InChI=1S/C19H21ClN2S/c1-21-8-10-22(11-9-21)17-12-14-4-2-3-5-18(14)23-19-7-6-15(20)13-16(17)19/h2-7,13,17H,8-12H2,1H3/t17-/m0/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
Dopamine receptor D4
(CANINE) | BDBM50054062
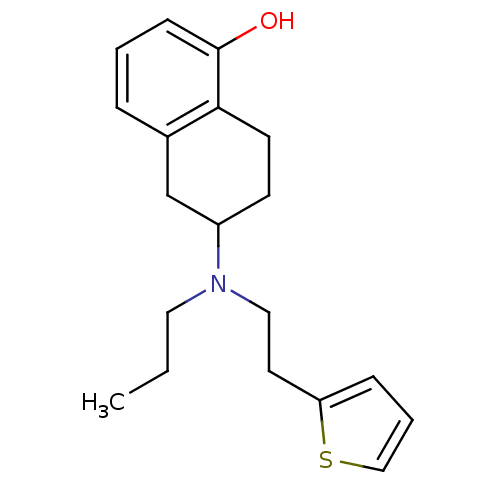
(6-[Propyl-(2-thiophen-2-yl-ethyl)-amino]-5,6,7,8-t...)Show InChI InChI=1S/C19H25NOS/c1-2-11-20(12-10-17-6-4-13-22-17)16-8-9-18-15(14-16)5-3-7-19(18)21/h3-7,13,16,21H,2,8-12,14H2,1H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50008735
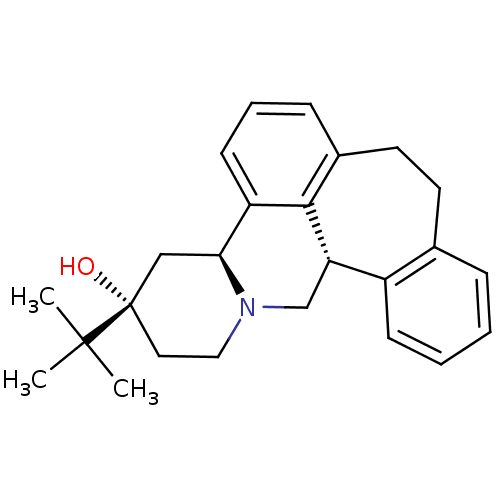
((+)-3-(tert-butyl)-(3S,4aS,13bS)-2,3,4,4a,8,9,13b,...)Show SMILES CC(C)(C)[C@]1(O)CCN2C[C@H]3c4ccccc4CCc4cccc([C@@H]2C1)c34 Show InChI InChI=1S/C25H31NO/c1-24(2,3)25(27)13-14-26-16-21-19-9-5-4-7-17(19)11-12-18-8-6-10-20(23(18)21)22(26)15-25/h4-10,21-22,27H,11-16H2,1-3H3/t21-,22-,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50019568
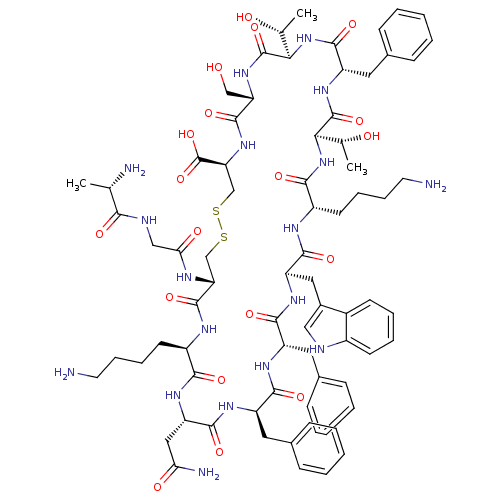
(Ala-Gly-c(Cys-Lys-Asn-Phe-Phe-Trp-Lys-Thr-Phe-Thr-...)Show SMILES C[C@@H](O)[C@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](CCCCN)NC(=O)[C@@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50+,51-,52+,53-,54-,55+,56-,57-,58+,59-,62+,63+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.09 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Mol Pharmacol 43: 894-901 (1993)
BindingDB Entry DOI: 10.7270/Q29G5K96 |
More data for this
Ligand-Target Pair | |
Dopamine receptor D4
(CANINE) | BDBM50020217
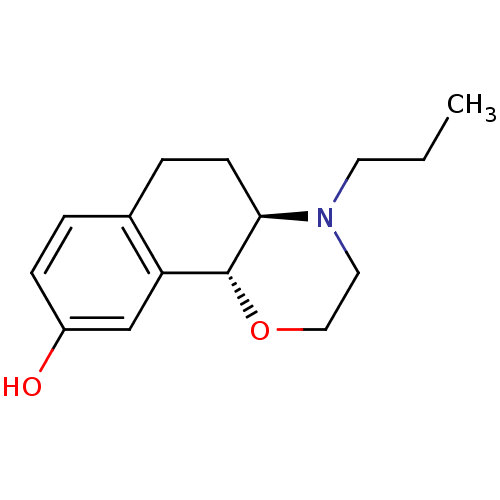
((4aR,10bR)-4-propyl-3,4,4a,5,6,10b-hexahydro-2H-na...)Show InChI InChI=1S/C15H21NO2/c1-2-7-16-8-9-18-15-13-10-12(17)5-3-11(13)4-6-14(15)16/h3,5,10,14-15,17H,2,4,6-9H2,1H3/t14-,15-/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
Dopamine receptor D4
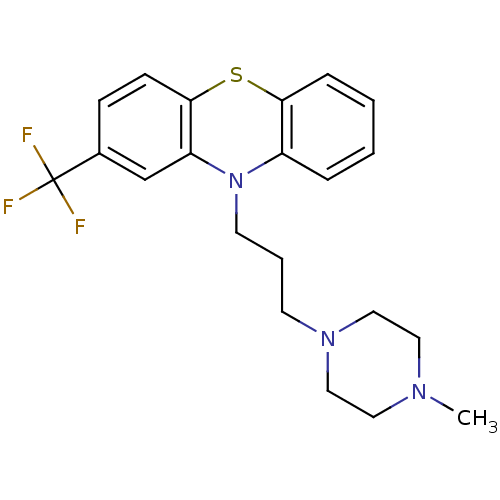
(CANINE) | BDBM79181
(10-[3-(4-methyl-1-piperazinyl)propyl]-2-(trifluoro...)Show SMILES CN1CCN(CCCN2c3ccccc3Sc3ccc(cc23)C(F)(F)F)CC1 Show InChI InChI=1S/C21H24F3N3S/c1-25-11-13-26(14-12-25)9-4-10-27-17-5-2-3-6-19(17)28-20-8-7-16(15-18(20)27)21(22,23)24/h2-3,5-8,15H,4,9-14H2,1H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50059090
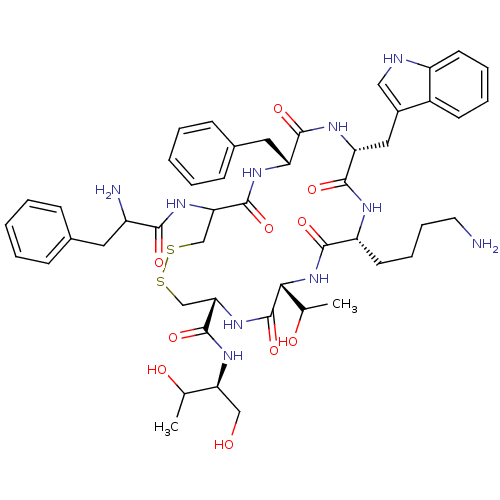
(10-(4-Amino-butyl)-19-(2-amino-3-phenyl-propionyla...)Show SMILES CC(O)[C@H](CO)NC(=O)[C@@H]1CSSCC(NC(=O)C(N)Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@H](CCCCN)C(=O)N[C@H](C(C)O)C(=O)N1 Show InChI InChI=1S/C49H66N10O10S2/c1-28(61)39(25-60)56-48(68)41-27-71-70-26-40(57-43(63)34(51)21-30-13-5-3-6-14-30)47(67)54-37(22-31-15-7-4-8-16-31)45(65)55-38(23-32-24-52-35-18-10-9-17-33(32)35)46(66)53-36(19-11-12-20-50)44(64)59-42(29(2)62)49(69)58-41/h3-10,13-18,24,28-29,34,36-42,52,60-62H,11-12,19-23,25-27,50-51H2,1-2H3,(H,53,66)(H,54,67)(H,55,65)(H,56,68)(H,57,63)(H,58,69)(H,59,64)/t28?,29?,34?,36-,37-,38-,39+,40?,41+,42-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Mol Pharmacol 43: 894-901 (1993)
BindingDB Entry DOI: 10.7270/Q29G5K96 |
More data for this
Ligand-Target Pair | |
Dopamine receptor D4
(CANINE) | BDBM50007568

(1-((S)-8-Chloro-10,11-dihydro-dibenzo[b,f]thiepin-...)Show InChI InChI=1S/C19H21ClN2S/c1-21-8-10-22(11-9-21)17-12-14-4-2-3-5-18(14)23-19-7-6-15(20)13-16(17)19/h2-7,13,17H,8-12H2,1H3/t17-/m0/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM82070
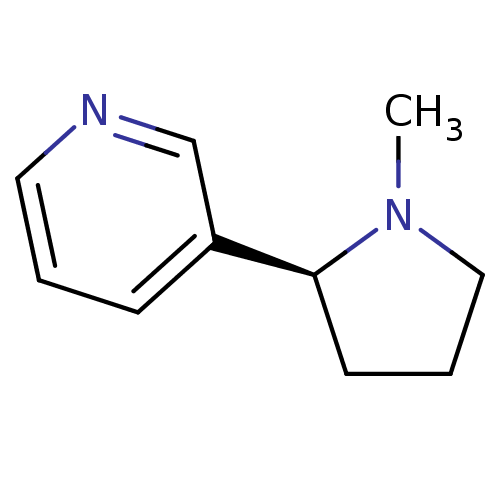
(CAS_29790-52-1 | NICOTINE-L (BASE) | Nicotine-D sa...)Show InChI InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Displacement of [3H]cytisine from alpha4beta2 nAChR expressed in human recombinant SH-SY5Y cell membranes after 120 mins |
J Med Chem 60: 9239-9250 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01113
BindingDB Entry DOI: 10.7270/Q2445PW2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50001888

((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...)Show InChI InChI=1S/C17H19ClN2S/c1-19(2)10-5-11-20-14-6-3-4-7-16(14)21-17-9-8-13(18)12-15(17)20/h3-4,6-9,12H,5,10-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50246607

(CHEMBL4083241)Show InChI InChI=1S/C11H16N2O/c1-3-10(7-12-5-1)9-14-11-4-2-6-13-8-11/h1,3,5,7,11,13H,2,4,6,8-9H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Displacement of [3H]cytisine from alpha4beta2 nAChR expressed in human recombinant SH-SY5Y cell membranes after 120 mins |
J Med Chem 60: 9239-9250 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01113
BindingDB Entry DOI: 10.7270/Q2445PW2 |
More data for this
Ligand-Target Pair | |
Dopamine receptor D4
(CANINE) | BDBM50007567
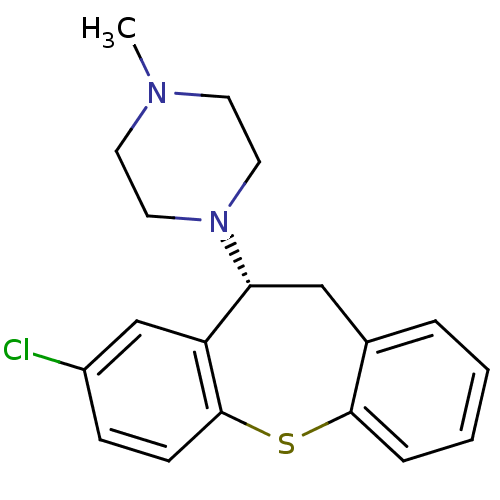
(1-(8-Chloro-10,11-dihydro-dibenzo[b,f]thiepin-10-y...)Show InChI InChI=1S/C19H21ClN2S/c1-21-8-10-22(11-9-21)17-12-14-4-2-3-5-18(14)23-19-7-6-15(20)13-16(17)19/h2-7,13,17H,8-12H2,1H3/t17-/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
Dopamine receptor D4
(CANINE) | BDBM81195
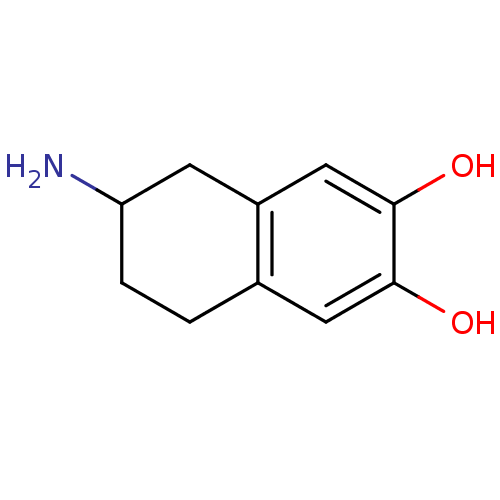
((+/-)-2-Amino-6,7-dihydroxy-1,2,3,4-tetrahydronaph...)Show InChI InChI=1S/C10H13NO2/c11-8-2-1-6-4-9(12)10(13)5-7(6)3-8/h4-5,8,12-13H,1-3,11H2 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50005118
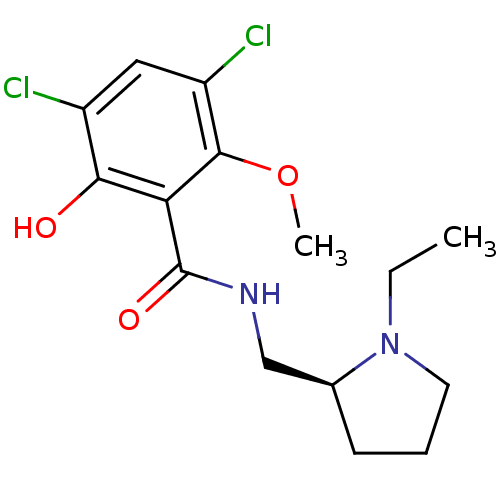
((S)-3,5-Dichloro-N-(1-ethyl-pyrrolidin-2-ylmethyl)...)Show InChI InChI=1S/C15H20Cl2N2O3/c1-3-19-6-4-5-9(19)8-18-15(21)12-13(20)10(16)7-11(17)14(12)22-2/h7,9,20H,3-6,8H2,1-2H3,(H,18,21)/t9-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
Dopamine receptor D4
(CANINE) | BDBM50001955

((-)6-Methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]qu...)Show InChI InChI=1S/C17H17NO2/c1-18-8-7-10-3-2-4-12-15(10)13(18)9-11-5-6-14(19)17(20)16(11)12/h2-6,13,19-20H,7-9H2,1H3/t13-/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
Dopamine receptor D4
(CANINE) | BDBM50007518

((S)-3-chloro-5-ethyl-N-((1-ethylpyrrolidin-2-yl)me...)Show InChI InChI=1S/C17H25ClN2O3/c1-4-11-9-13(18)16(23-3)14(15(11)21)17(22)19-10-12-7-6-8-20(12)5-2/h9,12,21H,4-8,10H2,1-3H3,(H,19,22)/t12-/m0/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50254239
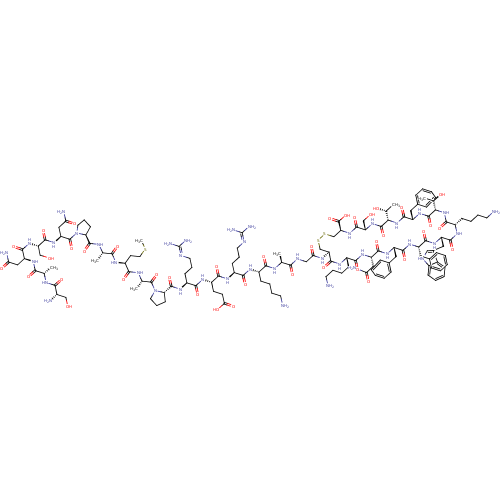
(somatostatin-28)Show SMILES CSCC[C@H](NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CO)C(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)NCC(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC1=O)[C@@H](C)O)[C@@H](C)O)C(O)=O |r,wU:42.43,28.31,13.12,4.4,66.67,86.87,106.108,115.116,194.201,172.177,149.152,134.136,124.126,120.224,wD:47.48,34.39,20.25,8.8,54.55,62.64,77.78,97.98,202.209,183.189,158.161,145.218,211.220,130.221,214.223,(-22.23,-7.83,;-23.64,-7.23,;-24.87,-8.16,;-26.29,-7.56,;-27.51,-8.49,;-28.93,-7.9,;-29.12,-6.37,;-27.9,-5.44,;-30.54,-5.78,;-31.77,-6.71,;-30.73,-4.25,;-32.15,-3.66,;-33.4,-4.59,;-32.34,-2.13,;-31.06,-1.26,;-31.8,.25,;-33.45,.03,;-33.78,-1.63,;-35.09,-2.39,;-35.19,-3.93,;-36.39,-1.55,;-37.77,-2.23,;-37.83,-3.76,;-39.2,-4.44,;-36.56,-4.61,;-36.28,-.01,;-37.58,.85,;-38.94,.16,;-37.46,2.37,;-36.09,3.05,;-34.83,2.21,;-38.75,3.22,;-38.65,4.76,;-37.27,5.45,;-39.93,5.6,;-41.31,4.92,;-41.42,3.4,;-40.12,2.54,;-42.8,2.71,;-39.84,7.14,;-41.12,8,;-42.5,7.31,;-41.02,9.53,;-39.65,10.21,;-42.31,10.39,;-42.21,11.93,;-40.83,12.61,;-43.49,12.76,;-43.38,14.3,;-44.87,12.08,;-44.96,10.56,;-27.32,-10.02,;-28.56,-10.95,;-25.9,-10.61,;-25.71,-12.14,;-26.93,-13.07,;-24.29,-12.75,;-23.09,-11.81,;-24.1,-14.26,;-25.25,-15.51,;-24.43,-16.97,;-22.76,-16.63,;-22.84,-15.08,;-21.53,-14.32,;-21.53,-12.78,;-20.18,-15.08,;-18.84,-14.32,;-18.84,-12.78,;-17.53,-12.02,;-17.53,-10.47,;-16.2,-9.71,;-16.2,-8.17,;-17.52,-7.41,;-14.86,-7.41,;-17.53,-15.08,;-17.53,-16.63,;-16.18,-14.32,;-14.85,-15.09,;-14.85,-16.63,;-13.54,-17.39,;-13.54,-18.94,;-14.87,-19.72,;-12.18,-19.71,;-13.54,-14.32,;-13.54,-12.78,;-12.19,-15.09,;-10.86,-14.33,;-10.86,-12.79,;-9.51,-12.02,;-9.51,-10.48,;-8.18,-9.72,;-8.18,-8.18,;-9.52,-7.42,;-6.84,-7.41,;-9.51,-15.11,;-9.51,-16.63,;-8.2,-14.33,;-6.85,-15.11,;-6.85,-16.64,;-5.52,-17.42,;-5.52,-18.94,;-4.2,-19.72,;-4.2,-21.27,;-5.52,-14.33,;-5.52,-12.79,;-4.19,-15.11,;-2.85,-14.33,;-2.85,-12.81,;-1.53,-15.11,;-1.53,-16.64,;-.2,-14.34,;1.14,-15.12,;2.46,-14.34,;2.46,-12.82,;3.81,-15.12,;5.13,-14.34,;5.14,-3.92,;6.49,-3.13,;50.46,-3.19,;50.41,-14.4,;49.07,-15.18,;47.74,-14.4,;46.4,-15.18,;46.4,-16.7,;45.07,-14.39,;45.07,-12.87,;46.4,-12.09,;43.75,-15.17,;42.41,-14.39,;42.41,-12.87,;41.08,-15.17,;39.75,-14.39,;38.42,-15.15,;38.42,-16.7,;37.09,-14.39,;37.09,-12.85,;38.42,-12.08,;39.76,-12.87,;41.08,-12.09,;41.09,-10.55,;39.76,-9.78,;38.42,-10.54,;35.75,-15.15,;34.42,-14.38,;34.42,-12.84,;33.1,-15.15,;31.76,-14.38,;30.44,-15.14,;30.44,-16.69,;29.09,-14.38,;29.09,-12.84,;30.44,-12.08,;30.44,-10.54,;31.76,-9.77,;31.76,-8.23,;27.77,-15.14,;26.44,-14.38,;26.44,-12.84,;25.1,-15.14,;25.1,-16.68,;26.44,-17.45,;27.84,-16.82,;28.87,-17.98,;28.09,-19.3,;28.57,-20.76,;27.53,-21.91,;26.03,-21.59,;25.57,-20.12,;26.59,-18.98,;23.78,-14.38,;22.44,-15.14,;22.44,-16.68,;21.11,-14.37,;21.11,-12.83,;22.44,-12.07,;23.78,-12.83,;25.1,-12.07,;25.1,-10.53,;23.78,-9.77,;22.44,-10.53,;19.79,-15.13,;18.44,-14.37,;18.44,-12.83,;17.12,-15.13,;17.12,-16.68,;18.44,-17.44,;19.76,-16.68,;21.1,-17.45,;21.09,-18.99,;19.75,-19.75,;18.44,-18.98,;15.78,-14.37,;14.45,-15.13,;14.45,-16.67,;13.11,-14.37,;13.11,-12.83,;14.45,-12.04,;15.78,-12.83,;14.45,-10.52,;11.79,-15.13,;10.46,-14.36,;10.46,-12.82,;9.12,-15.12,;9.12,-16.67,;10.46,-17.43,;10.46,-18.98,;11.78,-19.74,;11.78,-21.28,;7.79,-14.36,;6.47,-15.12,;6.47,-16.67,;33.1,-16.69,;31.76,-17.46,;34.42,-17.45,;41.08,-16.7,;39.75,-17.48,;42.41,-17.48,;49.07,-16.71,;47.74,-17.49,;50.41,-17.49,)| Show InChI InChI=1S/C137H207N41O39S3/c1-69(154-113(194)82(37-19-22-47-138)159-115(196)85(40-25-50-149-136(145)146)160-118(199)87(44-45-106(188)189)163-116(197)86(41-26-51-150-137(147)148)164-130(211)101-43-27-52-177(101)133(214)72(4)156-114(195)88(46-54-218-7)158-110(191)71(3)155-129(210)100-42-28-53-178(100)134(215)95(61-104(144)186)171-126(207)96(65-180)172-124(205)93(59-102(142)184)165-111(192)70(2)153-112(193)80(141)64-179)109(190)152-63-105(187)157-98-67-219-220-68-99(135(216)217)174-127(208)97(66-181)173-132(213)108(74(6)183)176-125(206)91(57-77-33-15-10-16-34-77)170-131(212)107(73(5)182)175-119(200)84(39-21-24-49-140)161-122(203)92(58-78-62-151-81-36-18-17-35-79(78)81)168-121(202)90(56-76-31-13-9-14-32-76)166-120(201)89(55-75-29-11-8-12-30-75)167-123(204)94(60-103(143)185)169-117(198)83(162-128(98)209)38-20-23-48-139/h8-18,29-36,62,69-74,80,82-101,107-108,151,179-183H,19-28,37-61,63-68,138-141H2,1-7H3,(H2,142,184)(H2,143,185)(H2,144,186)(H,152,190)(H,153,193)(H,154,194)(H,155,210)(H,156,195)(H,157,187)(H,158,191)(H,159,196)(H,160,199)(H,161,203)(H,162,209)(H,163,197)(H,164,211)(H,165,192)(H,166,201)(H,167,204)(H,168,202)(H,169,198)(H,170,212)(H,171,207)(H,172,205)(H,173,213)(H,174,208)(H,175,200)(H,176,206)(H,188,189)(H,216,217)(H4,145,146,149)(H4,147,148,150)/t69-,70-,71-,72-,73+,74+,80-,82-,83-,84-,85-,86-,87-,88-,89-,90-,91-,92-,93-,94-,95-,96-,97-,98-,99-,100-,101-,107-,108-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Mol Pharmacol 43: 894-901 (1993)
BindingDB Entry DOI: 10.7270/Q29G5K96 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50334150
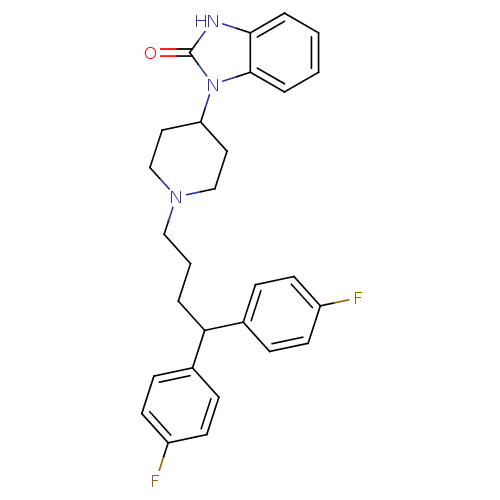
(1-(1-(4,4-bis(4-fluorophenyl)butyl)piperidin-4-yl)...)Show SMILES Fc1ccc(cc1)C(CCCN1CCC(CC1)n1c2ccccc2[nH]c1=O)c1ccc(F)cc1 Show InChI InChI=1S/C28H29F2N3O/c29-22-11-7-20(8-12-22)25(21-9-13-23(30)14-10-21)4-3-17-32-18-15-24(16-19-32)33-27-6-2-1-5-26(27)31-28(33)34/h1-2,5-14,24-25H,3-4,15-19H2,(H,31,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
Dopamine receptor D4
(CANINE) | BDBM60917

(9-chloranyl-5-(4-hydroxyphenyl)-2,3,4,5-tetrahydro...)Show InChI InChI=1S/C16H16ClNO3/c17-15-11-5-6-18-8-13(9-1-3-10(19)4-2-9)12(11)7-14(20)16(15)21/h1-4,7,13,18-21H,5-6,8H2 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50001888

((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...)Show InChI InChI=1S/C17H19ClN2S/c1-19(2)10-5-11-20-14-6-3-4-7-16(14)21-17-9-8-13(18)12-15(17)20/h3-4,6-9,12H,5,10-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM55121
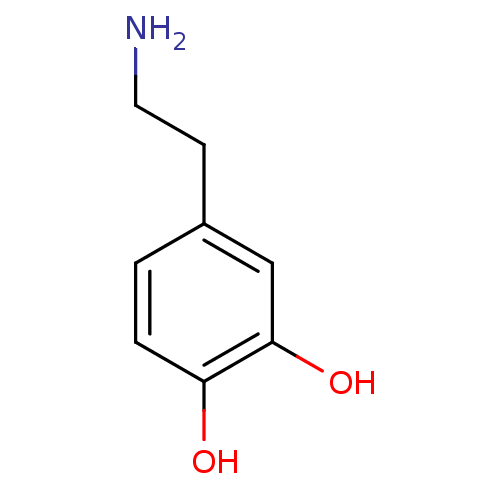
(3-HYDROXYTYRAMINE HYDROCHLORIDE | 4-(2-aminoethyl)...)Show InChI InChI=1S/C8H11NO2/c9-4-3-6-1-2-7(10)8(11)5-6/h1-2,5,10-11H,3-4,9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
Dopamine receptor D4
(CANINE) | BDBM79181

(10-[3-(4-methyl-1-piperazinyl)propyl]-2-(trifluoro...)Show SMILES CN1CCN(CCCN2c3ccccc3Sc3ccc(cc23)C(F)(F)F)CC1 Show InChI InChI=1S/C21H24F3N3S/c1-25-11-13-26(14-12-25)9-4-10-27-17-5-2-3-6-19(17)28-20-8-7-16(15-18(20)27)21(22,23)24/h2-3,5-8,15H,4,9-14H2,1H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50008735

((+)-3-(tert-butyl)-(3S,4aS,13bS)-2,3,4,4a,8,9,13b,...)Show SMILES CC(C)(C)[C@]1(O)CCN2C[C@H]3c4ccccc4CCc4cccc([C@@H]2C1)c34 Show InChI InChI=1S/C25H31NO/c1-24(2,3)25(27)13-14-26-16-21-19-9-5-4-7-17(19)11-12-18-8-6-10-20(23(18)21)22(26)15-25/h4-10,21-22,27H,11-16H2,1-3H3/t21-,22-,25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 614-9 (1991)
Article DOI: 10.1038/350614a0
BindingDB Entry DOI: 10.7270/Q2NV9GQR |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50005118

((S)-3,5-Dichloro-N-(1-ethyl-pyrrolidin-2-ylmethyl)...)Show InChI InChI=1S/C15H20Cl2N2O3/c1-3-19-6-4-5-9(19)8-18-15(21)12-13(20)10(16)7-11(17)14(12)22-2/h7,9,20H,3-6,8H2,1-2H3,(H,18,21)/t9-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50002338
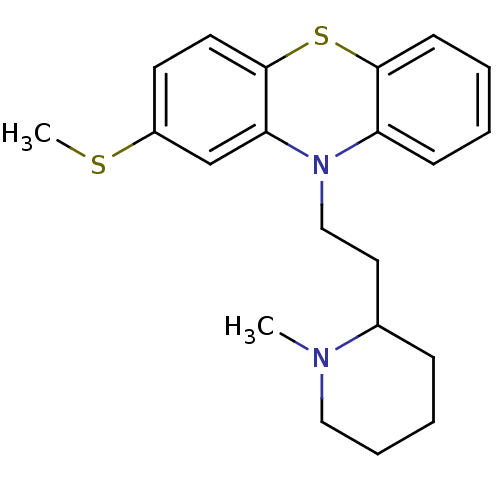
((Thioridazine)10-[2-(1-Methyl-piperidin-2-yl)-ethy...)Show InChI InChI=1S/C21H26N2S2/c1-22-13-6-5-7-16(22)12-14-23-18-8-3-4-9-20(18)25-21-11-10-17(24-2)15-19(21)23/h3-4,8-11,15-16H,5-7,12-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50026957
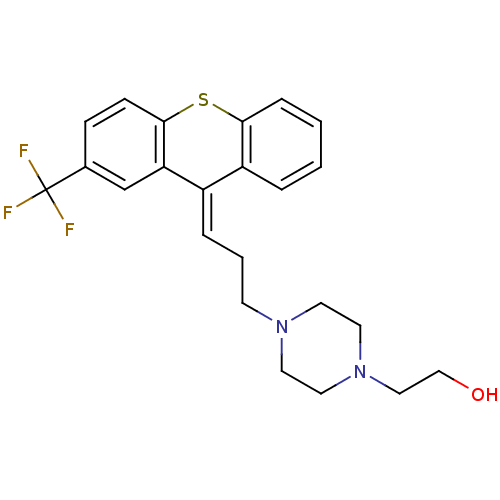
((cis) 2-{4-[3-(2-Trifluoromethyl-thioxanthen-9-yli...)Show SMILES OCCN1CCN(CC\C=C2/c3ccccc3Sc3ccc(cc23)C(F)(F)F)CC1 Show InChI InChI=1S/C23H25F3N2OS/c24-23(25,26)17-7-8-22-20(16-17)18(19-4-1-2-6-21(19)30-22)5-3-9-27-10-12-28(13-11-27)14-15-29/h1-2,4-8,16,29H,3,9-15H2/b18-5+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 614-9 (1991)
Article DOI: 10.1038/350614a0
BindingDB Entry DOI: 10.7270/Q2NV9GQR |
More data for this
Ligand-Target Pair | |
Dopamine receptor D4
(CANINE) | BDBM50001955

((-)6-Methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]qu...)Show InChI InChI=1S/C17H17NO2/c1-18-8-7-10-3-2-4-12-15(10)13(18)9-11-5-6-14(19)17(20)16(11)12/h2-6,13,19-20H,7-9H2,1H3/t13-/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM81774
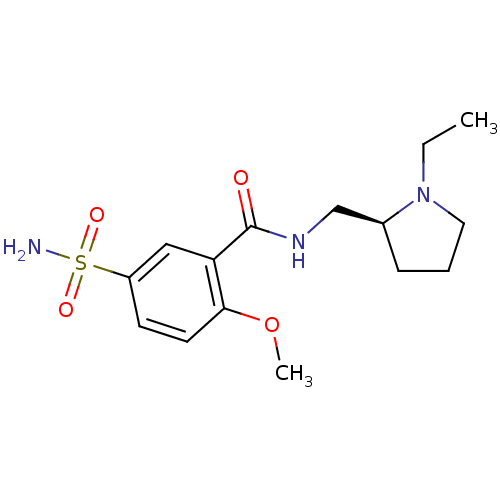
(CAS_15676-16-1 | SULPIRIDE,(+) | Sulpiride-S | Sul...)Show SMILES CCN1CCC[C@H]1CNC(=O)c1cc(ccc1OC)S(N)(=O)=O |r| Show InChI InChI=1S/C15H23N3O4S/c1-3-18-8-4-5-11(18)10-17-15(19)13-9-12(23(16,20)21)6-7-14(13)22-2/h6-7,9,11H,3-5,8,10H2,1-2H3,(H,17,19)(H2,16,20,21)/t11-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data