

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gamma-aminobutyric acid receptor subunit alpha-5 (Homo sapiens (Human)) | BDBM403740 (6-Chloro-7-((3-(cyclopropylmethyl)-7-methyl-3H-imi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd US Patent | Assay Description The GABAAα5/β3/γ2 protein used for the Scintillation Proximity Assay was derived from membranes produced from HEK293 GABAA α5/ ... | US Patent US10016439 (2018) BindingDB Entry DOI: 10.7270/Q2NC63J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-5 (Homo sapiens (Human)) | BDBM400239 (7-((1-(2-Cyclopropylethyl)-5-fluoro-1H-benzo[d]imi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd US Patent | Assay Description The GABAAα5/β3/γ2 protein used for the Scintillation Proximity Assay was derived from membranes produced from HEK293 GABAA α5/ ... | US Patent US10016439 (2018) BindingDB Entry DOI: 10.7270/Q2NC63J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-5 (Homo sapiens (Human)) | BDBM400243 (6-((3-(Cyclopropylmethyl)-7-methyl-3H-imidazo[4,5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd US Patent | Assay Description The GABAAα5/β3/γ2 protein used for the Scintillation Proximity Assay was derived from membranes produced from HEK293 GABAA α5/ ... | US Patent US10016439 (2018) BindingDB Entry DOI: 10.7270/Q2NC63J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-5 (Homo sapiens (Human)) | BDBM400238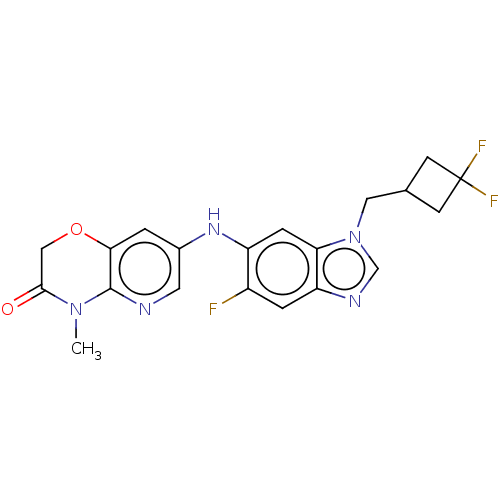 (7-((1-((3,3-Difluorocyclobutyl)methyl)-5-fluoro-1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd US Patent | Assay Description The GABAAα5/β3/γ2 protein used for the Scintillation Proximity Assay was derived from membranes produced from HEK293 GABAA α5/ ... | US Patent US10016439 (2018) BindingDB Entry DOI: 10.7270/Q2NC63J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-5 (Homo sapiens (Human)) | BDBM400240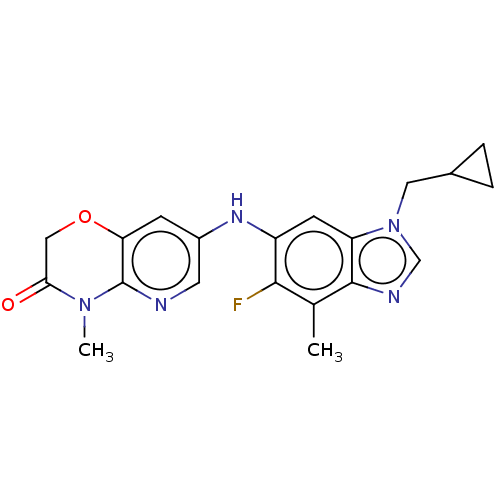 (7-((1-(Cyclopropylmethyl)-5-fluoro-4-methyl-1H-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 11.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd US Patent | Assay Description The GABAAα5/β3/γ2 protein used for the Scintillation Proximity Assay was derived from membranes produced from HEK293 GABAA α5/ ... | US Patent US10016439 (2018) BindingDB Entry DOI: 10.7270/Q2NC63J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-5 (Homo sapiens (Human)) | BDBM400242 (N-(6-(1H-Imidazol-1-yl)pyridin-3-yl)-3-(cyclopropy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 18.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd US Patent | Assay Description The GABAAα5/β3/γ2 protein used for the Scintillation Proximity Assay was derived from membranes produced from HEK293 GABAA α5/ ... | US Patent US10016439 (2018) BindingDB Entry DOI: 10.7270/Q2NC63J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-5 (Homo sapiens (Human)) | BDBM400234 (7-((1-(Cyclopropylmethyl)-1H-imidazo[4,5-b]pyrazin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd US Patent | Assay Description The GABAAα5/β3/γ2 protein used for the Scintillation Proximity Assay was derived from membranes produced from HEK293 GABAA α5/ ... | US Patent US10016439 (2018) BindingDB Entry DOI: 10.7270/Q2NC63J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-5 (Homo sapiens (Human)) | BDBM400241 (7-((3-(Cyclopropylmethyl)-3H-imidazo[4,5-b]pyridin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 34.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd US Patent | Assay Description The GABAAα5/β3/γ2 protein used for the Scintillation Proximity Assay was derived from membranes produced from HEK293 GABAA α5/ ... | US Patent US10016439 (2018) BindingDB Entry DOI: 10.7270/Q2NC63J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-5 (Homo sapiens (Human)) | BDBM400236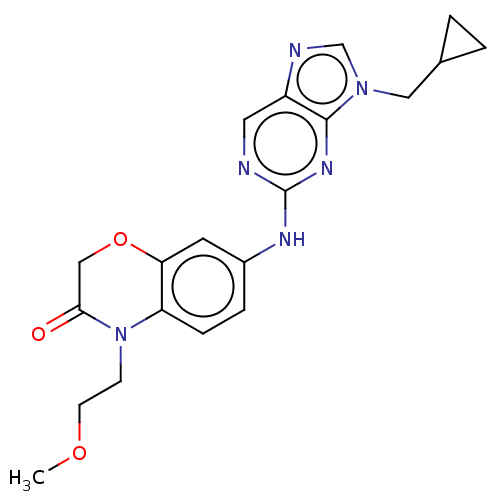 (7-((9-(Cyclopropylmethyl)-9H-purin-2-yl)amino)-4-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd US Patent | Assay Description The GABAAα5/β3/γ2 protein used for the Scintillation Proximity Assay was derived from membranes produced from HEK293 GABAA α5/ ... | US Patent US10016439 (2018) BindingDB Entry DOI: 10.7270/Q2NC63J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-5 (Homo sapiens (Human)) | BDBM400233 (4-Cyclopropyl-7-((1-(cyclopropylmethyl)-1H-imidazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 38.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd US Patent | Assay Description The GABAAα5/β3/γ2 protein used for the Scintillation Proximity Assay was derived from membranes produced from HEK293 GABAA α5/ ... | US Patent US10016439 (2018) BindingDB Entry DOI: 10.7270/Q2NC63J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-5 (Homo sapiens (Human)) | BDBM400235 (6-((1-(Cyclopropylmethyl)-1H-imidazo[4,5-b]pyrazin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd US Patent | Assay Description The GABAAα5/β3/γ2 protein used for the Scintillation Proximity Assay was derived from membranes produced from HEK293 GABAA α5/ ... | US Patent US10016439 (2018) BindingDB Entry DOI: 10.7270/Q2NC63J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50134782 (6-amino-9-[(Z)-(2-{[(1,2,3,3-tetrahydroxy-1,2,3-tr...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University School of Medicine Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Reverse transcriptase wild-type (RT wt) | J Med Chem 46: 4799-802 (2003) Article DOI: 10.1021/jm030048y BindingDB Entry DOI: 10.7270/Q2D50NQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50211727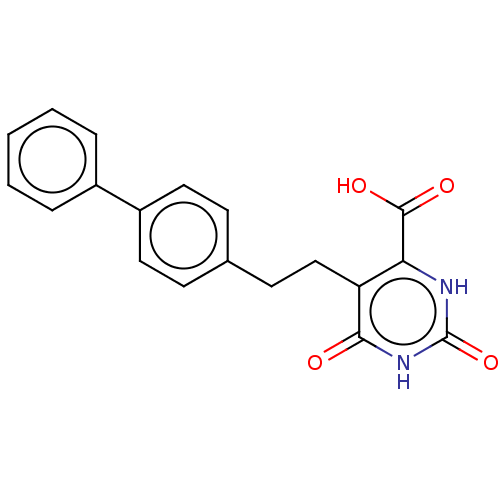 (CHEMBL3974614) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 485 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University Curated by ChEMBL | Assay Description Inhibition of human dihydroorotate dehydrogenase assessed as reduction in orotate production using L-dihydroorotate as substrate measured after 20 mi... | Bioorg Med Chem 25: 1465-1470 (2017) Article DOI: 10.1016/j.bmc.2017.01.009 BindingDB Entry DOI: 10.7270/Q2CC12TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50164644 (2',3'-Dideoxyadenosine Triphosphate (Ddatp) | 2',3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University School of Medicine Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase (RT M184V) | J Med Chem 46: 4799-802 (2003) Article DOI: 10.1021/jm030048y BindingDB Entry DOI: 10.7270/Q2D50NQB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50164644 (2',3'-Dideoxyadenosine Triphosphate (Ddatp) | 2',3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University School of Medicine Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Reverse transcriptase wild-type (RT wt) | J Med Chem 46: 4799-802 (2003) Article DOI: 10.1021/jm030048y BindingDB Entry DOI: 10.7270/Q2D50NQB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50164644 (2',3'-Dideoxyadenosine Triphosphate (Ddatp) | 2',3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University School of Medicine Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase (RT M184I) | J Med Chem 46: 4799-802 (2003) Article DOI: 10.1021/jm030048y BindingDB Entry DOI: 10.7270/Q2D50NQB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50134782 (6-amino-9-[(Z)-(2-{[(1,2,3,3-tetrahydroxy-1,2,3-tr...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University School of Medicine Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase (RT M184V) | J Med Chem 46: 4799-802 (2003) Article DOI: 10.1021/jm030048y BindingDB Entry DOI: 10.7270/Q2D50NQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50211940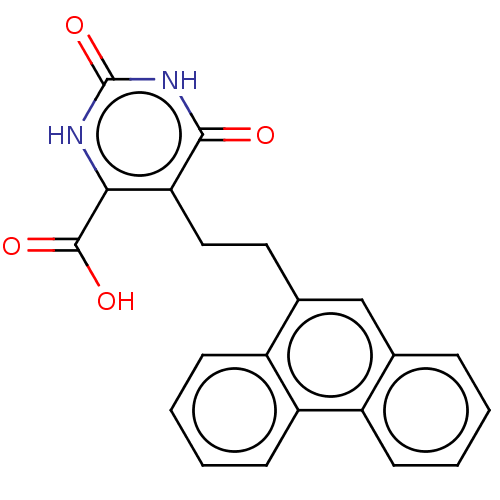 (CHEMBL3966747) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University Curated by ChEMBL | Assay Description Inhibition of human dihydroorotate dehydrogenase assessed as reduction in orotate production using L-dihydroorotate as substrate measured after 20 mi... | Bioorg Med Chem 25: 1465-1470 (2017) Article DOI: 10.1016/j.bmc.2017.01.009 BindingDB Entry DOI: 10.7270/Q2CC12TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50134782 (6-amino-9-[(Z)-(2-{[(1,2,3,3-tetrahydroxy-1,2,3-tr...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University School of Medicine Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase (RT M184I) | J Med Chem 46: 4799-802 (2003) Article DOI: 10.1021/jm030048y BindingDB Entry DOI: 10.7270/Q2D50NQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50211954 (CHEMBL3901542) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University Curated by ChEMBL | Assay Description Inhibition of human dihydroorotate dehydrogenase assessed as reduction in orotate production using L-dihydroorotate as substrate measured after 20 mi... | Bioorg Med Chem 25: 1465-1470 (2017) Article DOI: 10.1016/j.bmc.2017.01.009 BindingDB Entry DOI: 10.7270/Q2CC12TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alternative oxidase, mitochondrial (Trypanosoma brucei brucei) | BDBM50459787 (CHEMBL4206503) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Binding affinity to recombinant Trypanosoma brucei brucei 427 alternative oxidase expressed in Escherichia coli BL21 cells using ubiquinol-1 as subst... | ACS Med Chem Lett 9: 923-928 (2018) Article DOI: 10.1021/acsmedchemlett.8b00282 BindingDB Entry DOI: 10.7270/Q21J9DD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50211709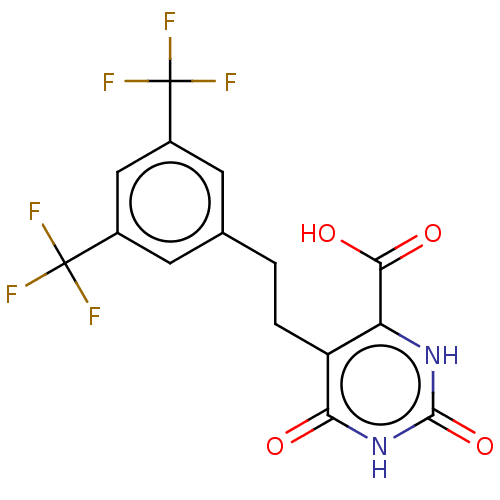 (CHEMBL3926030) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University Curated by ChEMBL | Assay Description Inhibition of human dihydroorotate dehydrogenase assessed as reduction in orotate production using L-dihydroorotate as substrate measured after 20 mi... | Bioorg Med Chem 25: 1465-1470 (2017) Article DOI: 10.1016/j.bmc.2017.01.009 BindingDB Entry DOI: 10.7270/Q2CC12TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50211702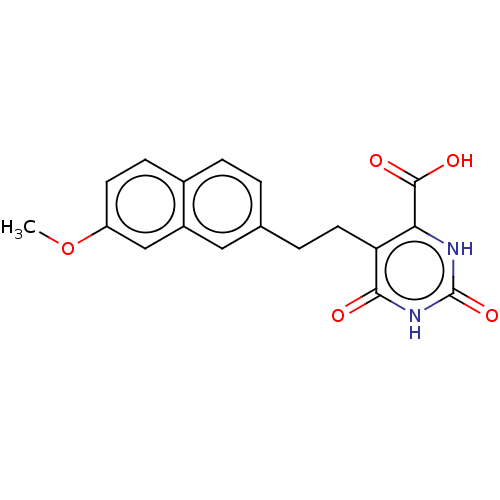 (CHEMBL3962745) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 6.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University Curated by ChEMBL | Assay Description Inhibition of human dihydroorotate dehydrogenase assessed as reduction in orotate production using L-dihydroorotate as substrate measured after 20 mi... | Bioorg Med Chem 25: 1465-1470 (2017) Article DOI: 10.1016/j.bmc.2017.01.009 BindingDB Entry DOI: 10.7270/Q2CC12TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50211957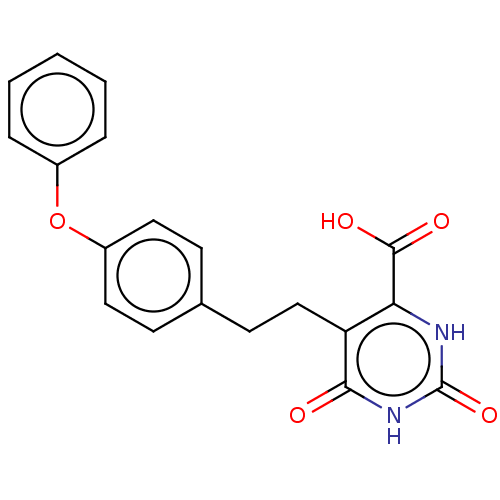 (CHEMBL3943890) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University Curated by ChEMBL | Assay Description Inhibition of human dihydroorotate dehydrogenase assessed as reduction in orotate production using L-dihydroorotate as substrate measured after 20 mi... | Bioorg Med Chem 25: 1465-1470 (2017) Article DOI: 10.1016/j.bmc.2017.01.009 BindingDB Entry DOI: 10.7270/Q2CC12TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50211731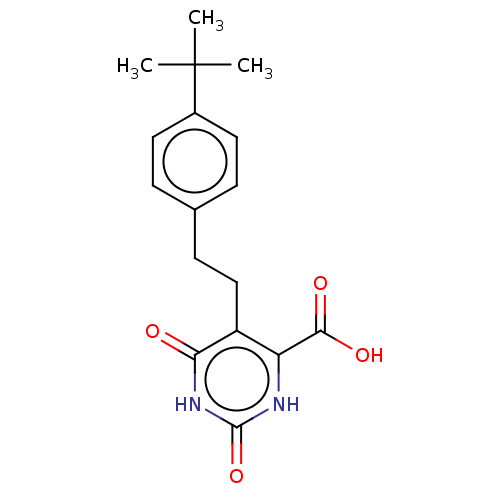 (CHEMBL3892598) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University Curated by ChEMBL | Assay Description Inhibition of human dihydroorotate dehydrogenase assessed as reduction in orotate production using L-dihydroorotate as substrate measured after 20 mi... | Bioorg Med Chem 25: 1465-1470 (2017) Article DOI: 10.1016/j.bmc.2017.01.009 BindingDB Entry DOI: 10.7270/Q2CC12TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50134783 (6-amino-9-[(E)-(2-{[(1,2,3,3-tetrahydroxy-1,2,3-tr...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University School of Medicine Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Reverse transcriptase wild-type (RT wt) | J Med Chem 46: 4799-802 (2003) Article DOI: 10.1021/jm030048y BindingDB Entry DOI: 10.7270/Q2D50NQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50211716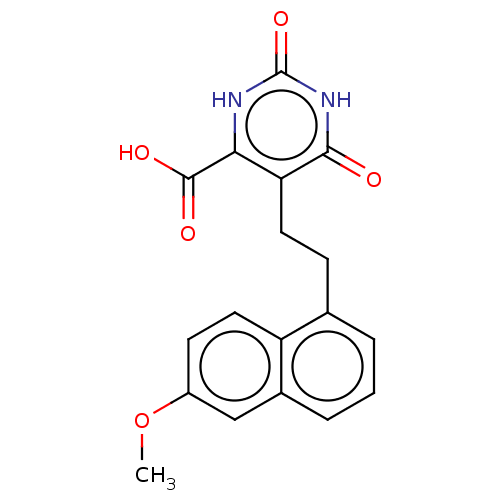 (CHEMBL3973700) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University Curated by ChEMBL | Assay Description Inhibition of human dihydroorotate dehydrogenase assessed as reduction in orotate production using L-dihydroorotate as substrate measured after 20 mi... | Bioorg Med Chem 25: 1465-1470 (2017) Article DOI: 10.1016/j.bmc.2017.01.009 BindingDB Entry DOI: 10.7270/Q2CC12TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50211943 (CHEMBL3914213) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University Curated by ChEMBL | Assay Description Inhibition of human dihydroorotate dehydrogenase assessed as reduction in orotate production using L-dihydroorotate as substrate measured after 20 mi... | Bioorg Med Chem 25: 1465-1470 (2017) Article DOI: 10.1016/j.bmc.2017.01.009 BindingDB Entry DOI: 10.7270/Q2CC12TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50211705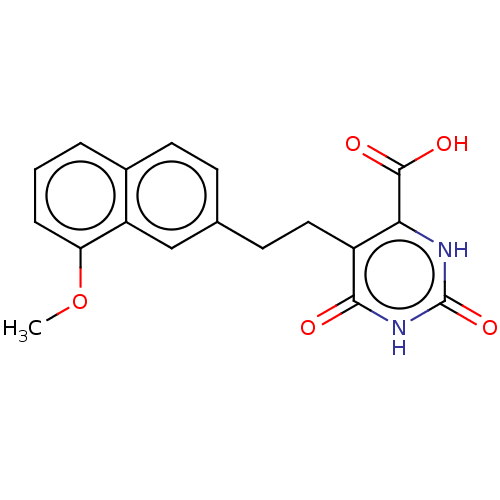 (CHEMBL3964957) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University Curated by ChEMBL | Assay Description Inhibition of human dihydroorotate dehydrogenase assessed as reduction in orotate production using L-dihydroorotate as substrate measured after 20 mi... | Bioorg Med Chem 25: 1465-1470 (2017) Article DOI: 10.1016/j.bmc.2017.01.009 BindingDB Entry DOI: 10.7270/Q2CC12TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50211959 (CHEMBL3955099) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University Curated by ChEMBL | Assay Description Inhibition of human dihydroorotate dehydrogenase assessed as reduction in orotate production using L-dihydroorotate as substrate measured after 20 mi... | Bioorg Med Chem 25: 1465-1470 (2017) Article DOI: 10.1016/j.bmc.2017.01.009 BindingDB Entry DOI: 10.7270/Q2CC12TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50211710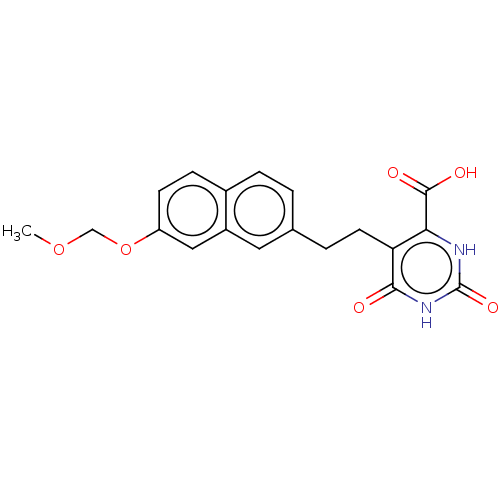 (CHEMBL3936361) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University Curated by ChEMBL | Assay Description Inhibition of human dihydroorotate dehydrogenase assessed as reduction in orotate production using L-dihydroorotate as substrate measured after 20 mi... | Bioorg Med Chem 25: 1465-1470 (2017) Article DOI: 10.1016/j.bmc.2017.01.009 BindingDB Entry DOI: 10.7270/Q2CC12TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50211942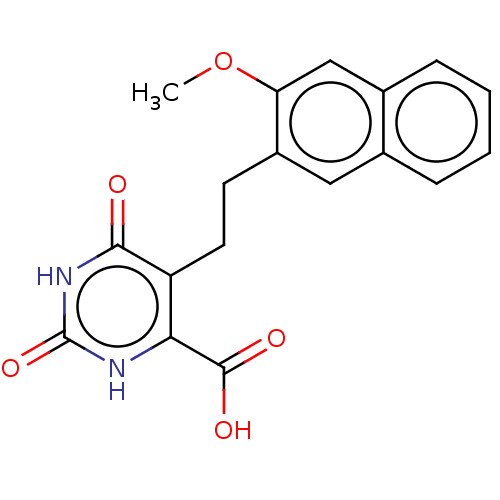 (CHEMBL3935254) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University Curated by ChEMBL | Assay Description Inhibition of human dihydroorotate dehydrogenase assessed as reduction in orotate production using L-dihydroorotate as substrate measured after 20 mi... | Bioorg Med Chem 25: 1465-1470 (2017) Article DOI: 10.1016/j.bmc.2017.01.009 BindingDB Entry DOI: 10.7270/Q2CC12TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50212058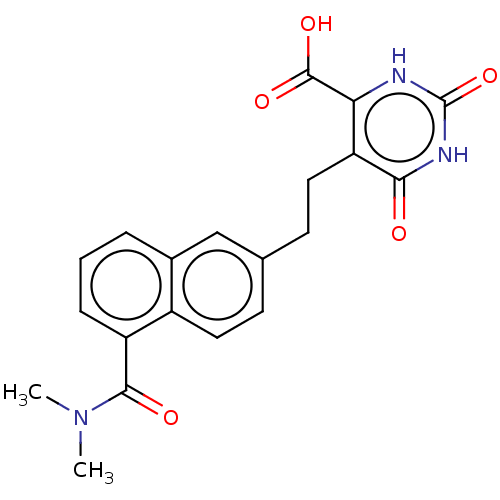 (CHEMBL3928909) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University Curated by ChEMBL | Assay Description Inhibition of human dihydroorotate dehydrogenase assessed as reduction in orotate production using L-dihydroorotate as substrate measured after 20 mi... | Bioorg Med Chem 25: 1465-1470 (2017) Article DOI: 10.1016/j.bmc.2017.01.009 BindingDB Entry DOI: 10.7270/Q2CC12TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50211712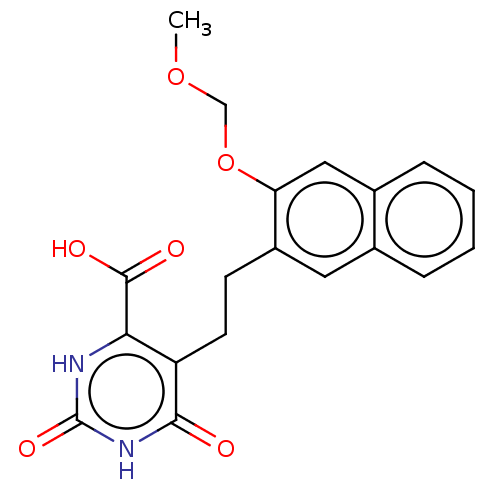 (CHEMBL3905364) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University Curated by ChEMBL | Assay Description Inhibition of human dihydroorotate dehydrogenase assessed as reduction in orotate production using L-dihydroorotate as substrate measured after 20 mi... | Bioorg Med Chem 25: 1465-1470 (2017) Article DOI: 10.1016/j.bmc.2017.01.009 BindingDB Entry DOI: 10.7270/Q2CC12TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50211695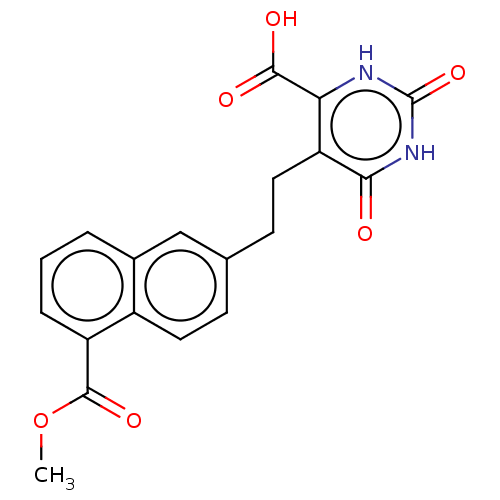 (CHEMBL3933268) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University Curated by ChEMBL | Assay Description Inhibition of human dihydroorotate dehydrogenase assessed as reduction in orotate production using L-dihydroorotate as substrate measured after 20 mi... | Bioorg Med Chem 25: 1465-1470 (2017) Article DOI: 10.1016/j.bmc.2017.01.009 BindingDB Entry DOI: 10.7270/Q2CC12TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50211715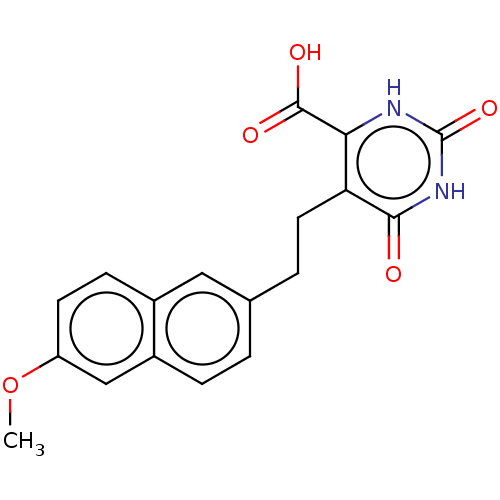 (CHEMBL3925700) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University Curated by ChEMBL | Assay Description Inhibition of human dihydroorotate dehydrogenase assessed as reduction in orotate production using L-dihydroorotate as substrate measured after 20 mi... | Bioorg Med Chem 25: 1465-1470 (2017) Article DOI: 10.1016/j.bmc.2017.01.009 BindingDB Entry DOI: 10.7270/Q2CC12TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50211939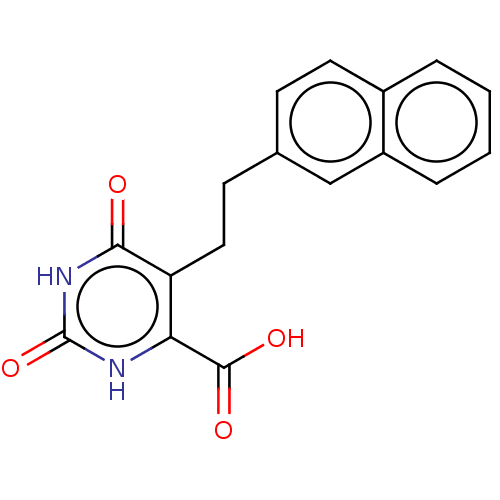 (CHEMBL3984245) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University Curated by ChEMBL | Assay Description Inhibition of human dihydroorotate dehydrogenase assessed as reduction in orotate production using L-dihydroorotate as substrate measured after 20 mi... | Bioorg Med Chem 25: 1465-1470 (2017) Article DOI: 10.1016/j.bmc.2017.01.009 BindingDB Entry DOI: 10.7270/Q2CC12TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50212059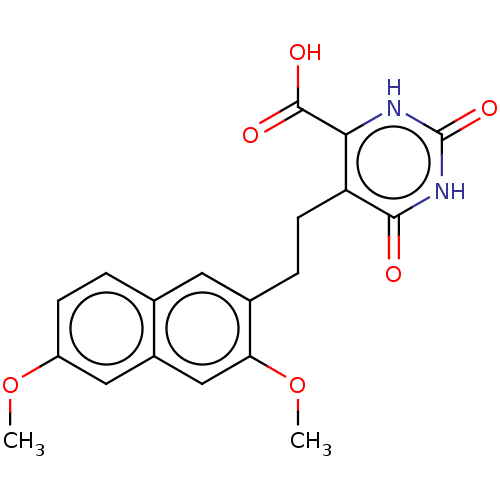 (CHEMBL3945267) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University Curated by ChEMBL | Assay Description Inhibition of human dihydroorotate dehydrogenase assessed as reduction in orotate production using L-dihydroorotate as substrate measured after 20 mi... | Bioorg Med Chem 25: 1465-1470 (2017) Article DOI: 10.1016/j.bmc.2017.01.009 BindingDB Entry DOI: 10.7270/Q2CC12TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50211714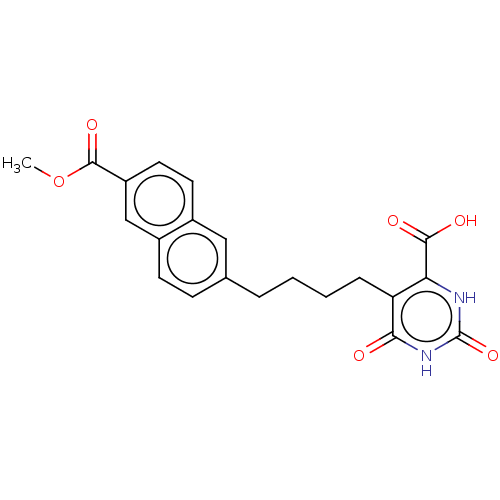 (CHEMBL3938624) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University Curated by ChEMBL | Assay Description Inhibition of human dihydroorotate dehydrogenase assessed as reduction in orotate production using L-dihydroorotate as substrate measured after 20 mi... | Bioorg Med Chem 25: 1465-1470 (2017) Article DOI: 10.1016/j.bmc.2017.01.009 BindingDB Entry DOI: 10.7270/Q2CC12TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50211941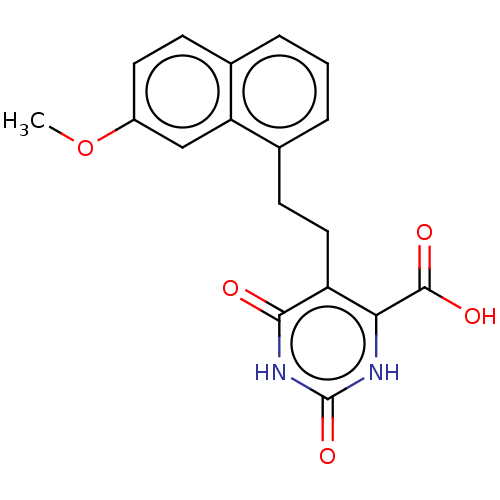 (CHEMBL3904389) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University Curated by ChEMBL | Assay Description Inhibition of human dihydroorotate dehydrogenase assessed as reduction in orotate production using L-dihydroorotate as substrate measured after 20 mi... | Bioorg Med Chem 25: 1465-1470 (2017) Article DOI: 10.1016/j.bmc.2017.01.009 BindingDB Entry DOI: 10.7270/Q2CC12TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50212056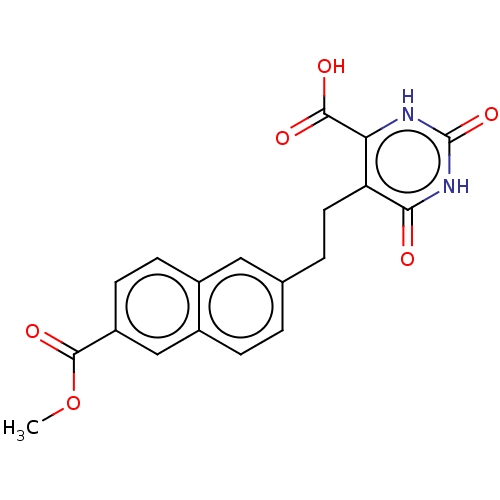 (CHEMBL3960727) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University Curated by ChEMBL | Assay Description Inhibition of human dihydroorotate dehydrogenase assessed as reduction in orotate production using L-dihydroorotate as substrate measured after 20 mi... | Bioorg Med Chem 25: 1465-1470 (2017) Article DOI: 10.1016/j.bmc.2017.01.009 BindingDB Entry DOI: 10.7270/Q2CC12TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50211787 (CHEMBL3986776) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University Curated by ChEMBL | Assay Description Inhibition of human dihydroorotate dehydrogenase assessed as reduction in orotate production using L-dihydroorotate as substrate measured after 20 mi... | Bioorg Med Chem 25: 1465-1470 (2017) Article DOI: 10.1016/j.bmc.2017.01.009 BindingDB Entry DOI: 10.7270/Q2CC12TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50134783 (6-amino-9-[(E)-(2-{[(1,2,3,3-tetrahydroxy-1,2,3-tr...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University School of Medicine Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase (RT M184V) | J Med Chem 46: 4799-802 (2003) Article DOI: 10.1021/jm030048y BindingDB Entry DOI: 10.7270/Q2D50NQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50134783 (6-amino-9-[(E)-(2-{[(1,2,3,3-tetrahydroxy-1,2,3-tr...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University School of Medicine Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase (RT M184I) | J Med Chem 46: 4799-802 (2003) Article DOI: 10.1021/jm030048y BindingDB Entry DOI: 10.7270/Q2D50NQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50211947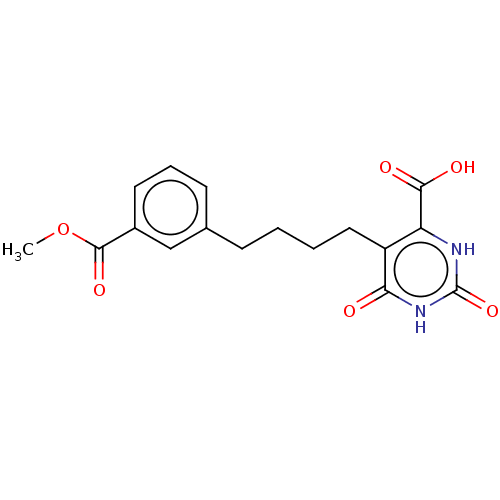 (CHEMBL3901641) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University Curated by ChEMBL | Assay Description Inhibition of human dihydroorotate dehydrogenase assessed as reduction in orotate production using L-dihydroorotate as substrate measured after 20 mi... | Bioorg Med Chem 25: 1465-1470 (2017) Article DOI: 10.1016/j.bmc.2017.01.009 BindingDB Entry DOI: 10.7270/Q2CC12TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50211697 (CHEMBL3981004) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University Curated by ChEMBL | Assay Description Inhibition of human dihydroorotate dehydrogenase assessed as reduction in orotate production using L-dihydroorotate as substrate measured after 20 mi... | Bioorg Med Chem 25: 1465-1470 (2017) Article DOI: 10.1016/j.bmc.2017.01.009 BindingDB Entry DOI: 10.7270/Q2CC12TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50211696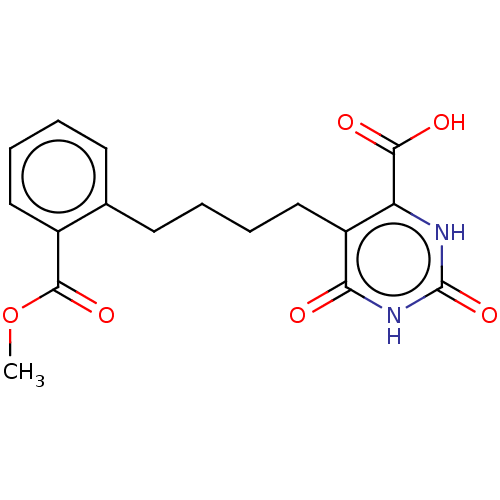 (CHEMBL3956130) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.02E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University Curated by ChEMBL | Assay Description Inhibition of human dihydroorotate dehydrogenase assessed as reduction in orotate production using L-dihydroorotate as substrate measured after 20 mi... | Bioorg Med Chem 25: 1465-1470 (2017) Article DOI: 10.1016/j.bmc.2017.01.009 BindingDB Entry DOI: 10.7270/Q2CC12TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50211692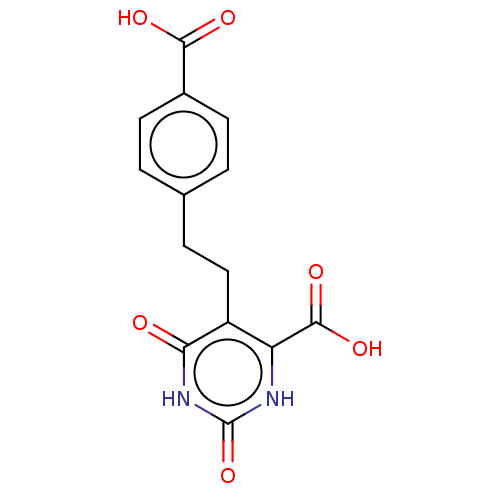 (CHEMBL3944370) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | >1.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University Curated by ChEMBL | Assay Description Inhibition of human dihydroorotate dehydrogenase assessed as reduction in orotate production using L-dihydroorotate as substrate measured after 20 mi... | Bioorg Med Chem 25: 1465-1470 (2017) Article DOI: 10.1016/j.bmc.2017.01.009 BindingDB Entry DOI: 10.7270/Q2CC12TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50211694 (CHEMBL3917382) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.39E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University Curated by ChEMBL | Assay Description Inhibition of human dihydroorotate dehydrogenase assessed as reduction in orotate production using L-dihydroorotate as substrate measured after 20 mi... | Bioorg Med Chem 25: 1465-1470 (2017) Article DOI: 10.1016/j.bmc.2017.01.009 BindingDB Entry DOI: 10.7270/Q2CC12TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50211745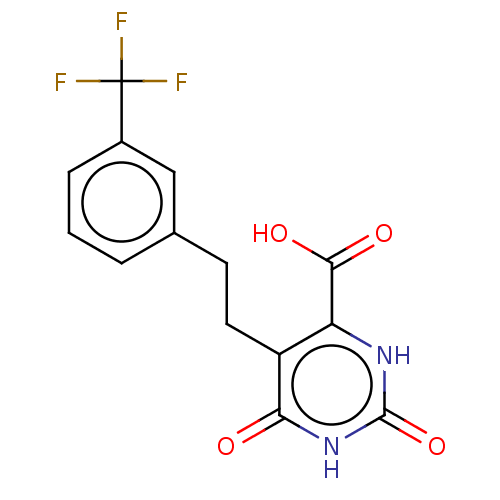 (CHEMBL3899009) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.42E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University Curated by ChEMBL | Assay Description Inhibition of human dihydroorotate dehydrogenase assessed as reduction in orotate production using L-dihydroorotate as substrate measured after 20 mi... | Bioorg Med Chem 25: 1465-1470 (2017) Article DOI: 10.1016/j.bmc.2017.01.009 BindingDB Entry DOI: 10.7270/Q2CC12TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 404 total ) | Next | Last >> |