

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50063698 (4-Guanidino-benzoic acid 6-carbamimidoyl-naphthale...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of matriptase (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50063698 (4-Guanidino-benzoic acid 6-carbamimidoyl-naphthale...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of matripase (unknown origin) using Boc-QAR-AMC as substrate incubated for 30 mins prior to substrate addition by fluorescence assay | Bioorg Med Chem 23: 2328-43 (2015) Article DOI: 10.1016/j.bmc.2015.03.072 BindingDB Entry DOI: 10.7270/Q23F4RB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor corepressor 1 (Homo sapiens (Human)) | BDBM50354086 (FK-228 | Istodax | ROMIDEPSIN) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant human HDAC3/NcoR1 enzyme using flurogenic Ac-LeuGlyLys (Ac)-AMC as substrate after 15 to 30 mins | J Med Chem 56: 6512-20 (2013) Article DOI: 10.1021/jm4008449 BindingDB Entry DOI: 10.7270/Q27S7Q6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123658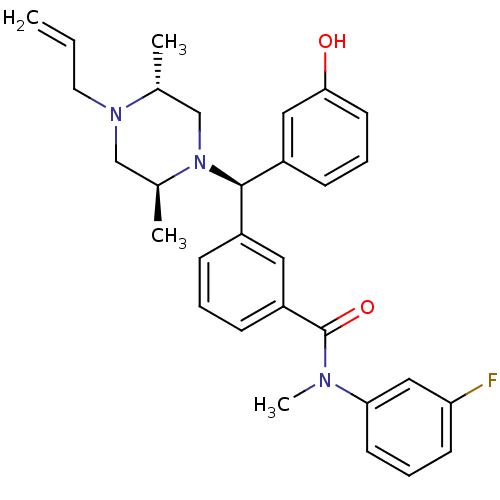 (3-[(R)-((2S,5R)-4-Allyl-2,5-dimethyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound evaluated for Opioid receptor delta 1 isolated from rat brain | J Med Chem 46: 623-33 (2003) Article DOI: 10.1021/jm020395s BindingDB Entry DOI: 10.7270/Q2DZ0915 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123658 (3-[(R)-((2S,5R)-4-Allyl-2,5-dimethyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound evaluated for Opioid receptor delta 1 isolated from rat brain | J Med Chem 46: 623-33 (2003) Article DOI: 10.1021/jm020395s BindingDB Entry DOI: 10.7270/Q2DZ0915 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50032999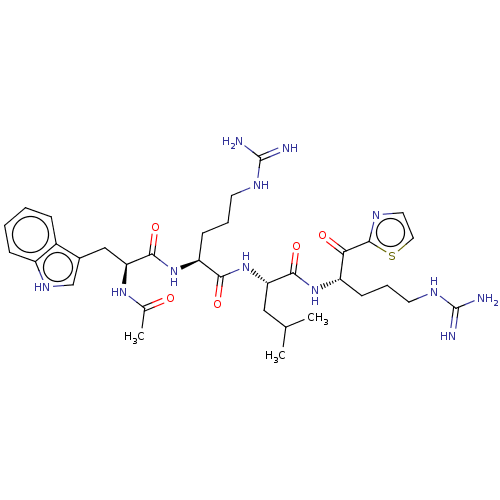 (CHEMBL3356597) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant hepsin (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50032934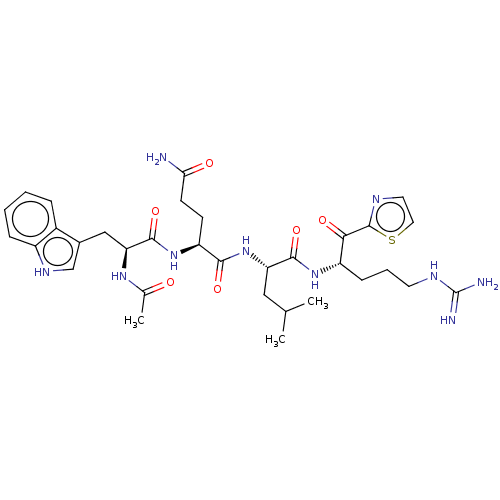 (CHEMBL3356591) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant hepsin (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50032936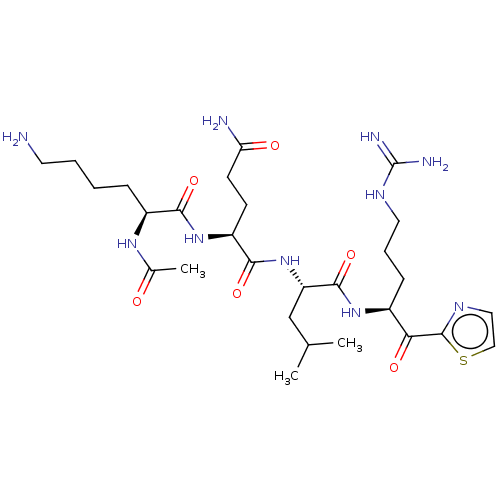 (CHEMBL3356589) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant hepsin (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50032931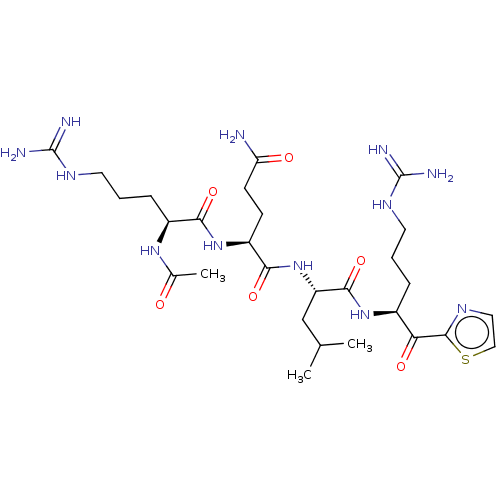 (CHEMBL3356594) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant hepsin (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50032931 (CHEMBL3356594) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of matriptase (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50032930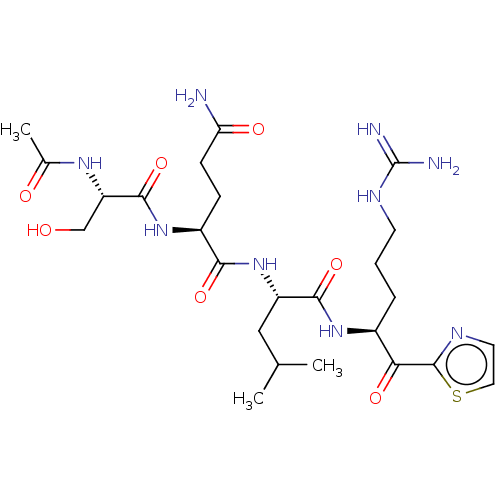 (CHEMBL3356595) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant hepsin (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50032935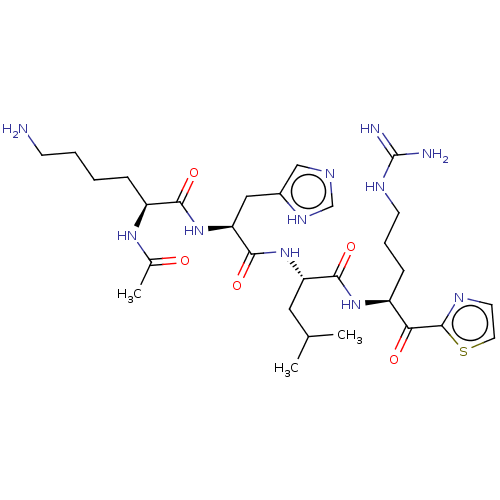 (CHEMBL3356590) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant hepsin (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123658 (3-[(R)-((2S,5R)-4-Allyl-2,5-dimethyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound evaluated for Opioid receptor mu 1 isolated from rat brain | J Med Chem 46: 623-33 (2003) Article DOI: 10.1021/jm020395s BindingDB Entry DOI: 10.7270/Q2DZ0915 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123658 (3-[(R)-((2S,5R)-4-Allyl-2,5-dimethyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound evaluated for Opioid receptor mu 1 isolated from rat brain | J Med Chem 46: 623-33 (2003) Article DOI: 10.1021/jm020395s BindingDB Entry DOI: 10.7270/Q2DZ0915 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50032947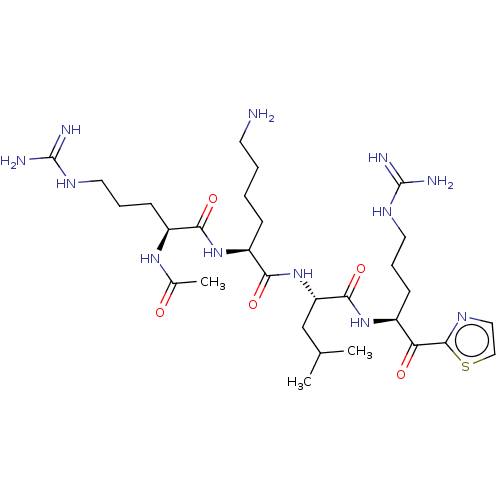 (CHEMBL3356606) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant hepsin (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50063698 (4-Guanidino-benzoic acid 6-carbamimidoyl-naphthale...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant hepsin (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50063698 (4-Guanidino-benzoic acid 6-carbamimidoyl-naphthale...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of hepsin (unknown origin) using Boc-QAR-AMC as substrate after 30 mins prior to substrate addition by fluorescence assay | Bioorg Med Chem 23: 2328-43 (2015) Article DOI: 10.1016/j.bmc.2015.03.072 BindingDB Entry DOI: 10.7270/Q23F4RB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50032941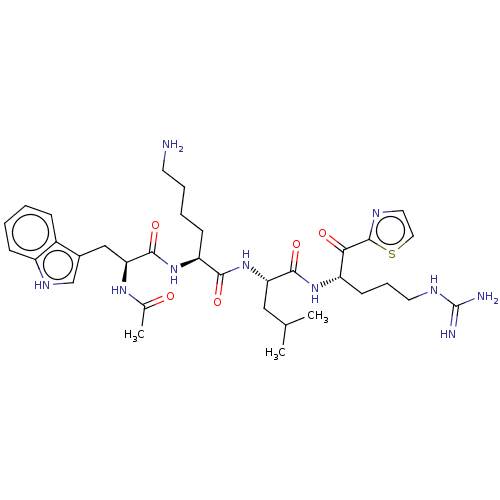 (CHEMBL3356600) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant hepsin (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50032991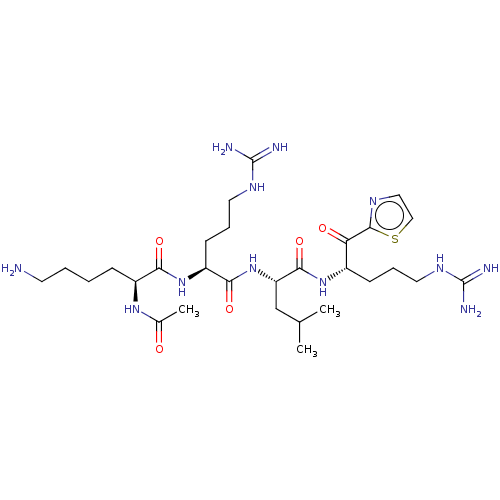 (CHEMBL3356596) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant hepsin (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50306993 ((S)-4-(4-(4-(ethylcarbamoyl)piperidin-1-yl)-6-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Inhibition of human P2Y12 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 1388-94 (2010) Article DOI: 10.1016/j.bmcl.2009.12.110 BindingDB Entry DOI: 10.7270/Q2ZP467B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50032933 (CHEMBL3356592) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant hepsin (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50032940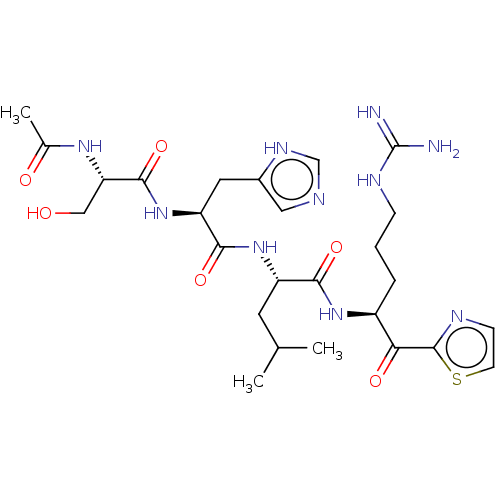 (CHEMBL3356599) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant hepsin (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50032945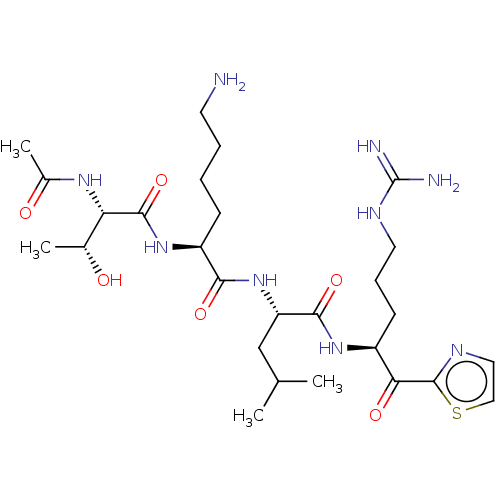 (CHEMBL3356604) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant hepsin (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50306935 ((S)-4-(4-(3-carbamoylazetidin-1-yl)-6-phenylpicoli...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Inhibition of human P2Y12 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 1388-94 (2010) Article DOI: 10.1016/j.bmcl.2009.12.110 BindingDB Entry DOI: 10.7270/Q2ZP467B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50032944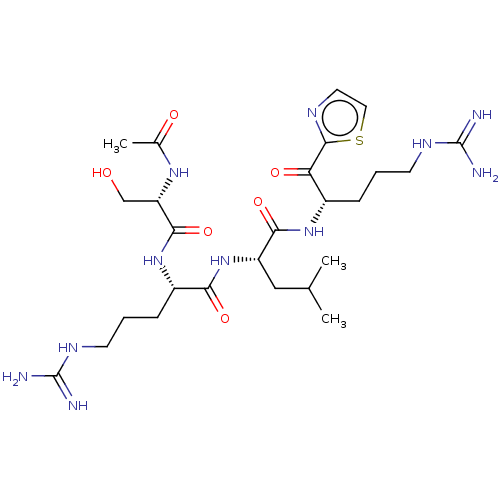 (CHEMBL3356603) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant hepsin (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50302697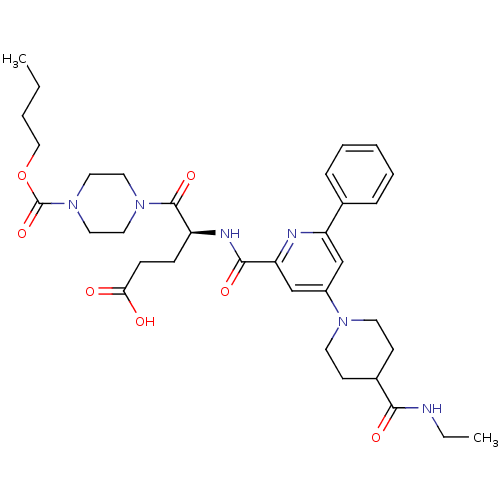 ((S)-5-(4-(butoxycarbonyl)piperazin-1-yl)-4-(4-(4-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Inhibition of human P2Y12 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 1388-94 (2010) Article DOI: 10.1016/j.bmcl.2009.12.110 BindingDB Entry DOI: 10.7270/Q2ZP467B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50032933 (CHEMBL3356592) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of matriptase (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50306996 ((S)-5-(4-(butoxycarbonyl)piperazin-1-yl)-5-oxo-4-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Inhibition of human P2Y12 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 1388-94 (2010) Article DOI: 10.1016/j.bmcl.2009.12.110 BindingDB Entry DOI: 10.7270/Q2ZP467B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50159507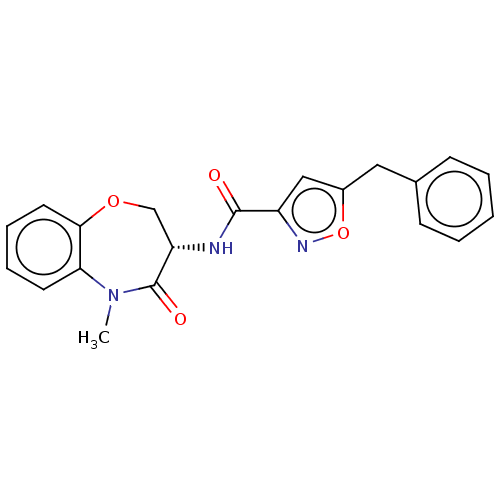 (CHEMBL3785703) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Competitive inhibition of human RIP1 (1 to 375 residues) in presence of increasing ATP by ADP-Glo reagent based assay | J Med Chem 59: 2163-78 (2016) Article DOI: 10.1021/acs.jmedchem.5b01898 BindingDB Entry DOI: 10.7270/Q26H4K97 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50032947 (CHEMBL3356606) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of matriptase (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50032936 (CHEMBL3356589) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of matriptase (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50306989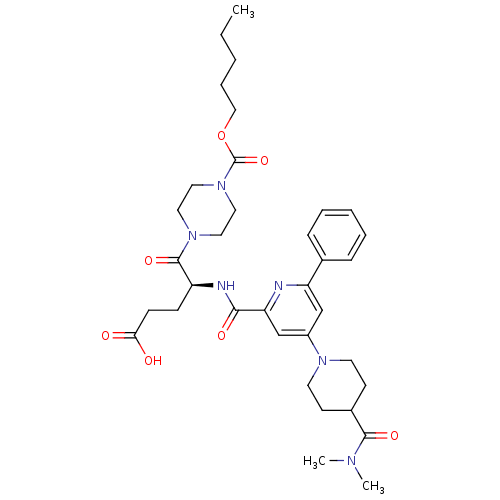 ((S)-4-(4-(4-(dimethylcarbamoyl)piperidin-1-yl)-6-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Inhibition of human P2Y12 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 1388-94 (2010) Article DOI: 10.1016/j.bmcl.2009.12.110 BindingDB Entry DOI: 10.7270/Q2ZP467B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50000092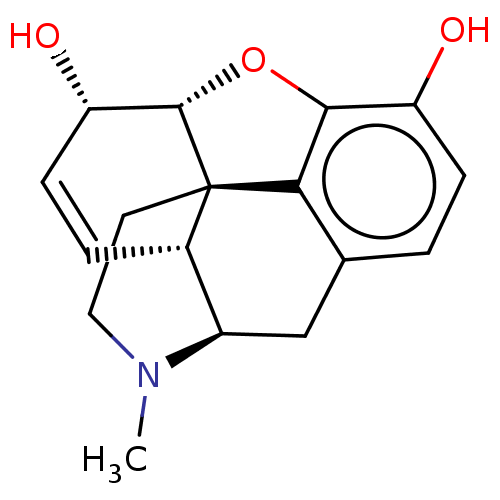 ((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound evaluated for Opioid receptor mu 1 isolated from rat brain | J Med Chem 46: 623-33 (2003) Article DOI: 10.1021/jm020395s BindingDB Entry DOI: 10.7270/Q2DZ0915 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50307602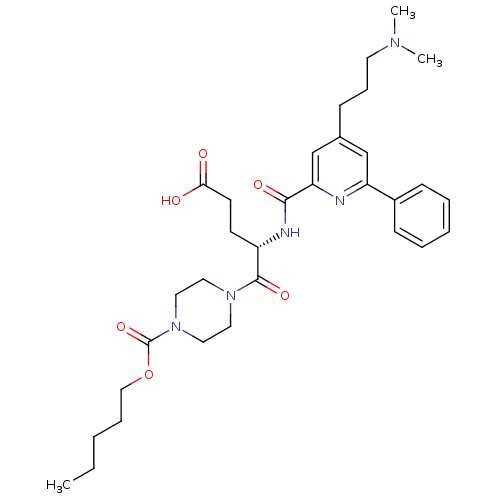 ((4S)-4-[({4-[3-(Dimethylamino)propyl]-6-phenylpyri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Displacement of [33P]ADP from human recombinant P2Y12 receptor expressed in CHO cells | J Med Chem 53: 2010-37 (2010) Article DOI: 10.1021/jm901518t BindingDB Entry DOI: 10.7270/Q2HH6K50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123655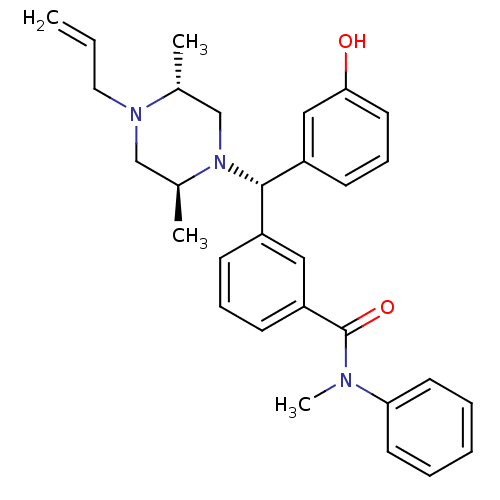 (3-[(S)-((2S,5R)-4-Allyl-2,5-dimethyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.06 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound evaluated for Opioid receptor delta 1 isolated from rat brain | J Med Chem 46: 623-33 (2003) Article DOI: 10.1021/jm020395s BindingDB Entry DOI: 10.7270/Q2DZ0915 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123647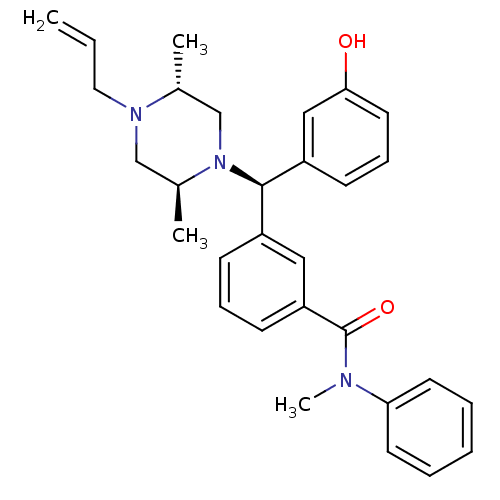 (3-[(R)-((2S,5R)-4-Allyl-2,5-dimethyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.06 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound evaluated for Opioid receptor mu 1 isolated from rat brain | J Med Chem 46: 623-33 (2003) Article DOI: 10.1021/jm020395s BindingDB Entry DOI: 10.7270/Q2DZ0915 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50307002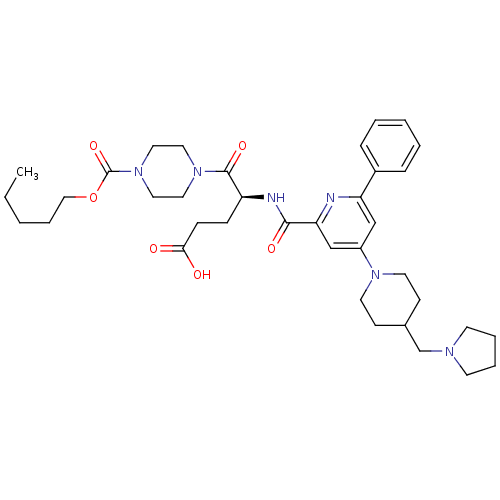 ((4S)-5-Oxo-5-{4-[(pentyloxy)carbonyl]piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Displacement of [33P]ADP from human recombinant P2Y12 receptor expressed in CHO cells | J Med Chem 53: 2010-37 (2010) Article DOI: 10.1021/jm901518t BindingDB Entry DOI: 10.7270/Q2HH6K50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50032991 (CHEMBL3356596) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of matriptase (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50307002 ((4S)-5-Oxo-5-{4-[(pentyloxy)carbonyl]piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Inhibition of human P2Y12 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 1388-94 (2010) Article DOI: 10.1016/j.bmcl.2009.12.110 BindingDB Entry DOI: 10.7270/Q2ZP467B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50306987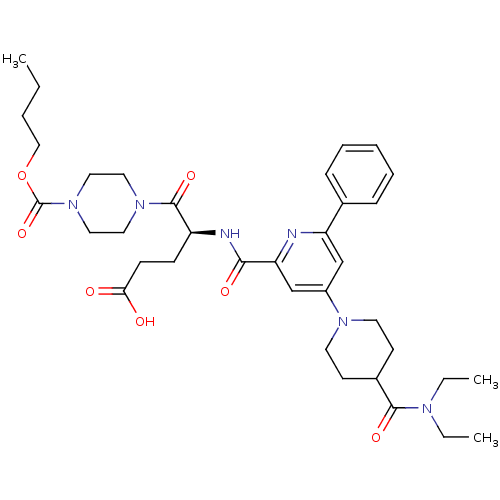 ((S)-5-(4-(butoxycarbonyl)piperazin-1-yl)-4-(4-(4-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Inhibition of human P2Y12 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 1388-94 (2010) Article DOI: 10.1016/j.bmcl.2009.12.110 BindingDB Entry DOI: 10.7270/Q2ZP467B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123654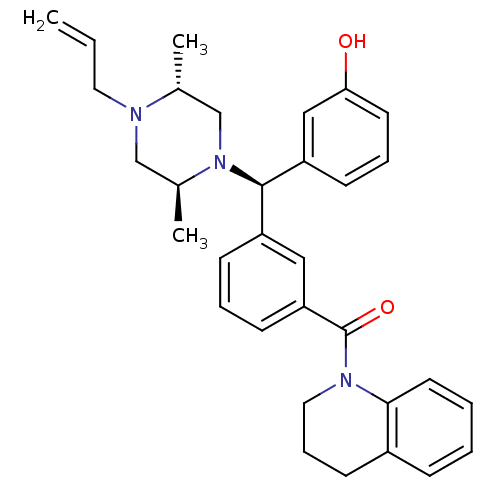 (CHEMBL157660 | {3-[(R)-((2S,5R)-4-Allyl-2,5-dimeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound evaluated for Opioid receptor delta 1 isolated from rat brain | J Med Chem 46: 623-33 (2003) Article DOI: 10.1021/jm020395s BindingDB Entry DOI: 10.7270/Q2DZ0915 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50306984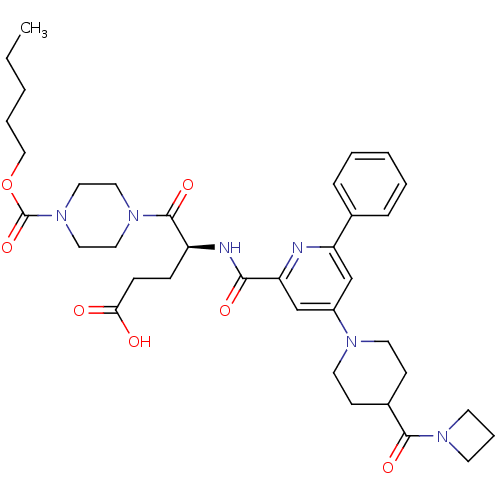 ((S)-4-(4-(4-(azetidine-1-carbonyl)piperidin-1-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Inhibition of human P2Y12 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 1388-94 (2010) Article DOI: 10.1016/j.bmcl.2009.12.110 BindingDB Entry DOI: 10.7270/Q2ZP467B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50032946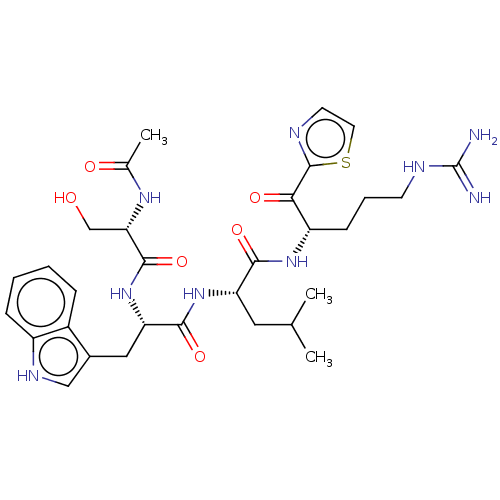 (CHEMBL3356605) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant hepsin (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50032939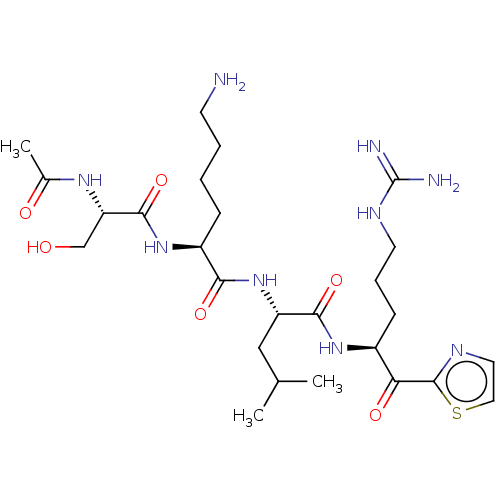 (CHEMBL3356598) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant hepsin (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123664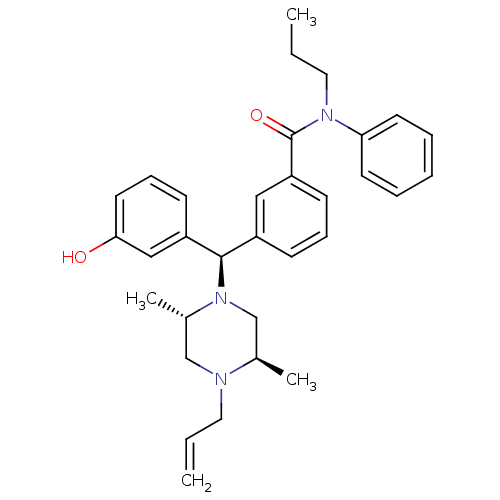 (3-[(R)-((2S,5R)-4-Allyl-2,5-dimethyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound evaluated for Opioid receptor delta 1 isolated from rat brain | J Med Chem 46: 623-33 (2003) Article DOI: 10.1021/jm020395s BindingDB Entry DOI: 10.7270/Q2DZ0915 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50307604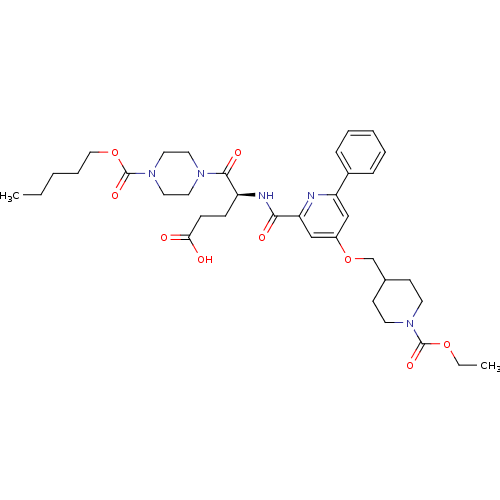 ((4S)4-{[(4-{[1-(Ethoxycarbonyl)piperidin-4-yl]meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Displacement of [33P]ADP from human recombinant P2Y12 receptor expressed in CHO cells | J Med Chem 53: 2010-37 (2010) Article DOI: 10.1021/jm901518t BindingDB Entry DOI: 10.7270/Q2HH6K50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50307603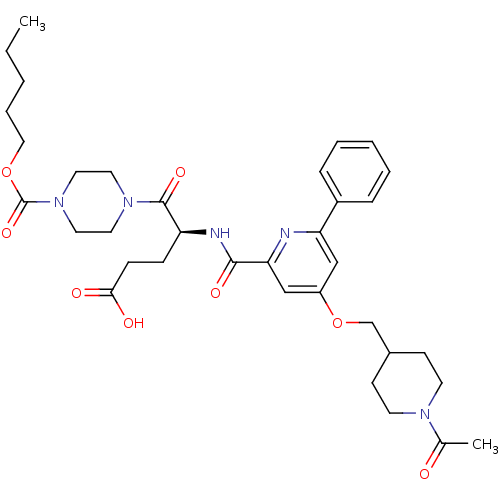 ((4S)4-[({4-[(1-Acetylpiperidin-4-yl)ethoxy]-6-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Displacement of [33P]ADP from human recombinant P2Y12 receptor expressed in CHO cells | J Med Chem 53: 2010-37 (2010) Article DOI: 10.1021/jm901518t BindingDB Entry DOI: 10.7270/Q2HH6K50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123647 (3-[(R)-((2S,5R)-4-Allyl-2,5-dimethyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound evaluated for Opioid receptor delta 1 isolated from rat brain | J Med Chem 46: 623-33 (2003) Article DOI: 10.1021/jm020395s BindingDB Entry DOI: 10.7270/Q2DZ0915 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50306941 ((S)-5-oxo-5-(4-(pentyloxycarbonyl)piperazin-1-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Inhibition of human P2Y12 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 1388-94 (2010) Article DOI: 10.1016/j.bmcl.2009.12.110 BindingDB Entry DOI: 10.7270/Q2ZP467B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50307557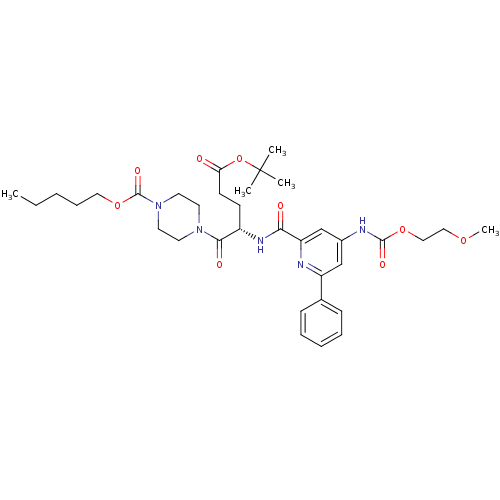 ((4S)4-{[(4-{[(2-Methoxyethoxy)carbonyl]amino}-6-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Displacement of [33P]ADP from human recombinant P2Y12 receptor expressed in CHO cells | J Med Chem 53: 2010-37 (2010) Article DOI: 10.1021/jm901518t BindingDB Entry DOI: 10.7270/Q2HH6K50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1626 total ) | Next | Last >> |