 Found 2416 hits with Last Name = 'heinrich' and Initial = 't'
Found 2416 hits with Last Name = 'heinrich' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50524862
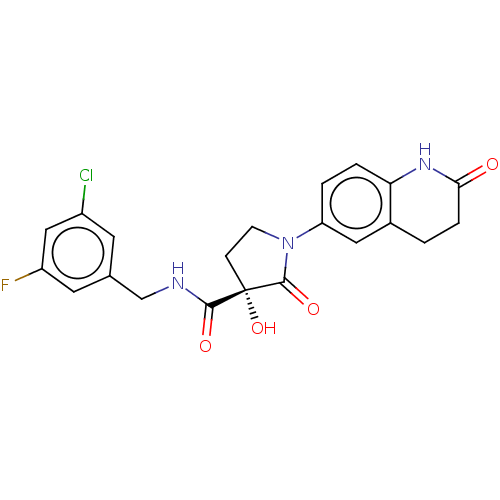
(CHEMBL4475680)Show SMILES O[C@@]1(CCN(C1=O)c1ccc2NC(=O)CCc2c1)C(=O)NCc1cc(F)cc(Cl)c1 |r| Show InChI InChI=1S/C21H19ClFN3O4/c22-14-7-12(8-15(23)10-14)11-24-19(28)21(30)5-6-26(20(21)29)16-2-3-17-13(9-16)1-4-18(27)25-17/h2-3,7-10,30H,1,4-6,11H2,(H,24,28)(H,25,27)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare
Curated by ChEMBL
| Assay Description
Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b... |
J Med Chem 62: 11119-11134 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01070
BindingDB Entry DOI: 10.7270/Q26H4MXQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50524862

(CHEMBL4475680)Show SMILES O[C@@]1(CCN(C1=O)c1ccc2NC(=O)CCc2c1)C(=O)NCc1cc(F)cc(Cl)c1 |r| Show InChI InChI=1S/C21H19ClFN3O4/c22-14-7-12(8-15(23)10-14)11-24-19(28)21(30)5-6-26(20(21)29)16-2-3-17-13(9-16)1-4-18(27)25-17/h2-3,7-10,30H,1,4-6,11H2,(H,24,28)(H,25,27)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare
Curated by ChEMBL
| Assay Description
Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b... |
J Med Chem 62: 11119-11134 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01070
BindingDB Entry DOI: 10.7270/Q26H4MXQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM401307

(US10005756, Compound A78)Show SMILES O[C@@]1(CCN(C1=O)c1cnc2[nH]ccc2c1)C(=O)NCc1cc(F)cc(F)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare
Curated by ChEMBL
| Assay Description
Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b... |
J Med Chem 62: 11119-11134 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01070
BindingDB Entry DOI: 10.7270/Q26H4MXQ |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM401307

(US10005756, Compound A78)Show SMILES O[C@@]1(CCN(C1=O)c1cnc2[nH]ccc2c1)C(=O)NCc1cc(F)cc(F)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare
Curated by ChEMBL
| Assay Description
Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b... |
J Med Chem 62: 11119-11134 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01070
BindingDB Entry DOI: 10.7270/Q26H4MXQ |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50531161

(CHEMBL4448724)Show SMILES O[C@@]1(CCN(C1=O)c1cnc2[nH]ccc2c1)C(=O)NCc1cc(F)cc(Cl)c1 |r| Show InChI InChI=1S/C19H16ClFN4O3/c20-13-5-11(6-14(21)8-13)9-24-17(26)19(28)2-4-25(18(19)27)15-7-12-1-3-22-16(12)23-10-15/h1,3,5-8,10,28H,2,4,9H2,(H,22,23)(H,24,26)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare
Curated by ChEMBL
| Assay Description
Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b... |
J Med Chem 62: 11119-11134 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01070
BindingDB Entry DOI: 10.7270/Q26H4MXQ |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50531161

(CHEMBL4448724)Show SMILES O[C@@]1(CCN(C1=O)c1cnc2[nH]ccc2c1)C(=O)NCc1cc(F)cc(Cl)c1 |r| Show InChI InChI=1S/C19H16ClFN4O3/c20-13-5-11(6-14(21)8-13)9-24-17(26)19(28)2-4-25(18(19)27)15-7-12-1-3-22-16(12)23-10-15/h1,3,5-8,10,28H,2,4,9H2,(H,22,23)(H,24,26)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare
Curated by ChEMBL
| Assay Description
Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b... |
J Med Chem 62: 11119-11134 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01070
BindingDB Entry DOI: 10.7270/Q26H4MXQ |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50531163
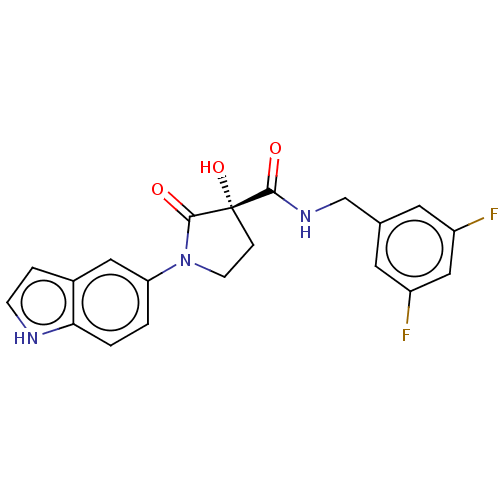
(CHEMBL4464946)Show SMILES O[C@@]1(CCN(C1=O)c1ccc2[nH]ccc2c1)C(=O)NCc1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C20H17F2N3O3/c21-14-7-12(8-15(22)10-14)11-24-18(26)20(28)4-6-25(19(20)27)16-1-2-17-13(9-16)3-5-23-17/h1-3,5,7-10,23,28H,4,6,11H2,(H,24,26)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare
Curated by ChEMBL
| Assay Description
Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b... |
J Med Chem 62: 11119-11134 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01070
BindingDB Entry DOI: 10.7270/Q26H4MXQ |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50531163

(CHEMBL4464946)Show SMILES O[C@@]1(CCN(C1=O)c1ccc2[nH]ccc2c1)C(=O)NCc1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C20H17F2N3O3/c21-14-7-12(8-15(22)10-14)11-24-18(26)20(28)4-6-25(19(20)27)16-1-2-17-13(9-16)3-5-23-17/h1-3,5,7-10,23,28H,4,6,11H2,(H,24,26)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare
Curated by ChEMBL
| Assay Description
Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b... |
J Med Chem 62: 11119-11134 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01070
BindingDB Entry DOI: 10.7270/Q26H4MXQ |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610836

(MSC-4381) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610833

(CHEMBL5276884) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610834
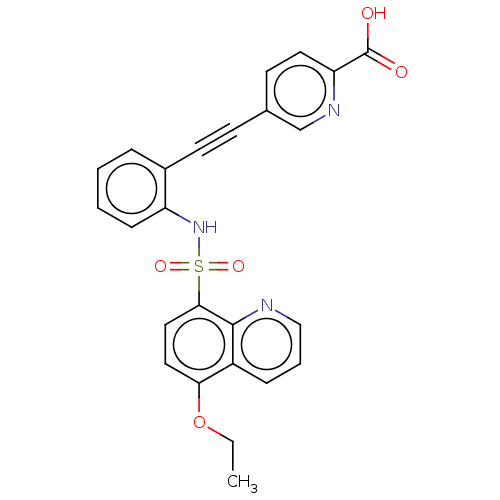
(CHEMBL5279064) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610837
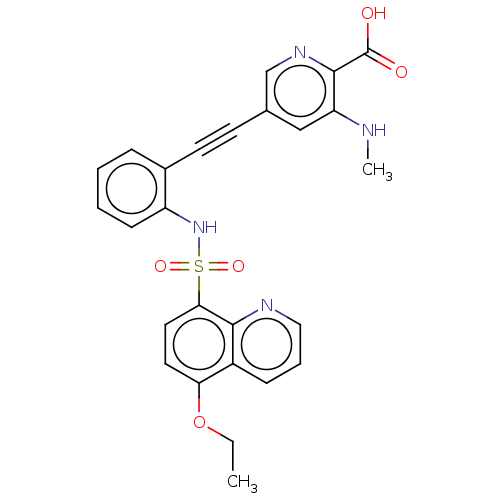
(CHEMBL5281492) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610835

(CHEMBL5267349) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610830
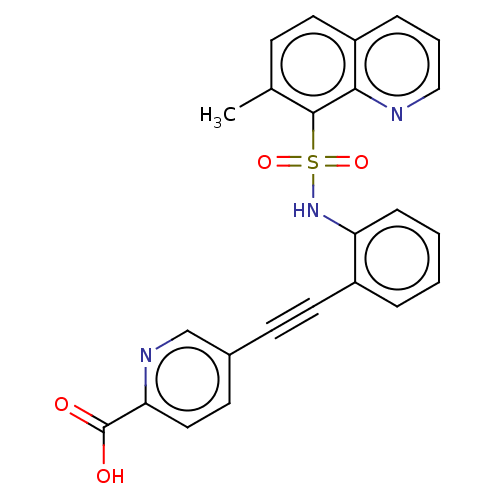
(CHEMBL5267752) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610826

(CHEMBL5287351) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610827
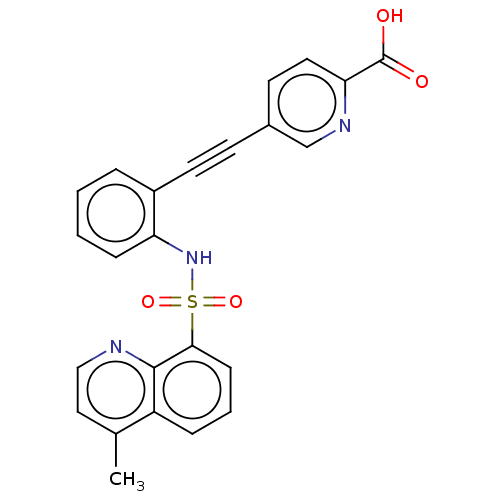
(CHEMBL5287780) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610824

(CHEMBL5268966) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610829
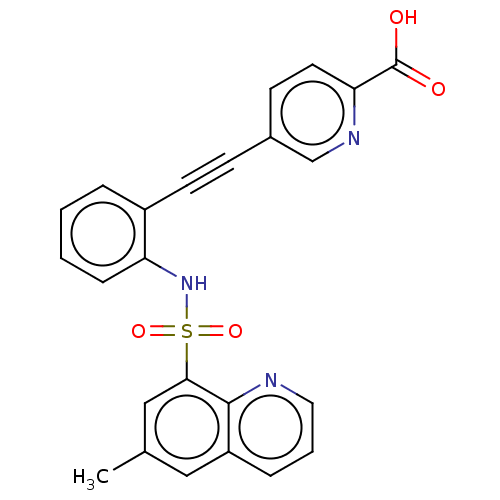
(CHEMBL5288904) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610831
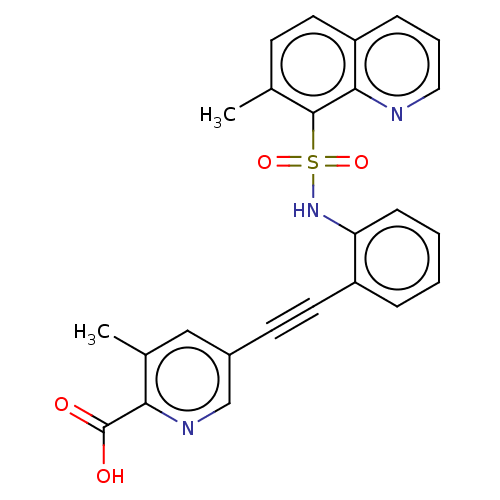
(CHEMBL5265956) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610825

(CHEMBL5266104) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610828
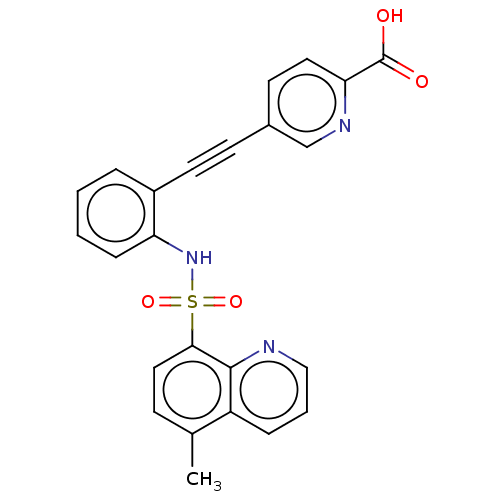
(CHEMBL5271816) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 222 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Mus musculus) | BDBM50610868
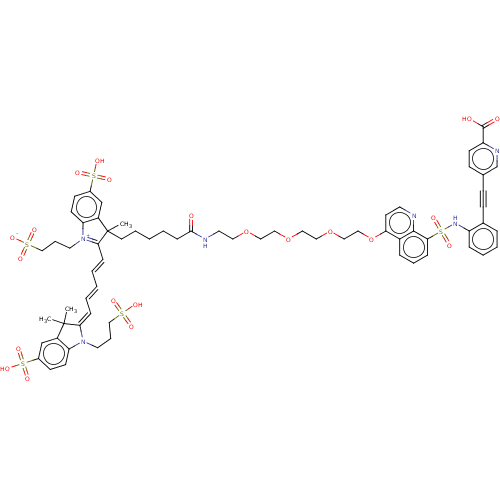
(CHEMBL5282978) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| UniChem
| | 301 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50151969
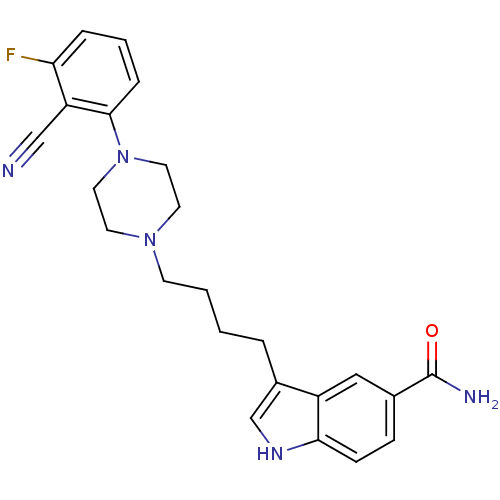
(3-{4-[4-(2-Cyano-3-fluoro-phenyl)-piperazin-1-yl]-...)Show SMILES NC(=O)c1ccc2[nH]cc(CCCCN3CCN(CC3)c3cccc(F)c3C#N)c2c1 Show InChI InChI=1S/C24H26FN5O/c25-21-5-3-6-23(20(21)15-26)30-12-10-29(11-13-30)9-2-1-4-18-16-28-22-8-7-17(24(27)31)14-19(18)22/h3,5-8,14,16,28H,1-2,4,9-13H2,(H2,27,31) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Inhibition of 8-OH DPAT bindign to rat hydroxytryptamine 1A receptor expressed in CHO cells |
J Med Chem 47: 4677-83 (2004)
Article DOI: 10.1021/jm040792y
BindingDB Entry DOI: 10.7270/Q28S4PCR |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50151976
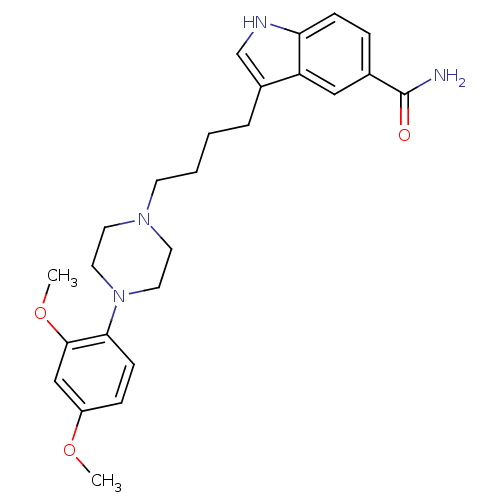
(3-{4-[4-(2,4-Dimethoxy-phenyl)-piperazin-1-yl]-but...)Show SMILES COc1ccc(N2CCN(CCCCc3c[nH]c4ccc(cc34)C(N)=O)CC2)c(OC)c1 Show InChI InChI=1S/C25H32N4O3/c1-31-20-7-9-23(24(16-20)32-2)29-13-11-28(12-14-29)10-4-3-5-19-17-27-22-8-6-18(25(26)30)15-21(19)22/h6-9,15-17,27H,3-5,10-14H2,1-2H3,(H2,26,30) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Inhibition of 8-OH DPAT bindign to rat hydroxytryptamine 1A receptor expressed in CHO cells |
J Med Chem 47: 4677-83 (2004)
Article DOI: 10.1021/jm040792y
BindingDB Entry DOI: 10.7270/Q28S4PCR |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50151946

(3-{4-[4-(4-Methoxy-phenyl)-piperazin-1-yl]-butyl}-...)Show SMILES COc1ccc(cc1)N1CCN(CCCCc2c[nH]c3ccc(cc23)C(N)=O)CC1 Show InChI InChI=1S/C24H30N4O2/c1-30-21-8-6-20(7-9-21)28-14-12-27(13-15-28)11-3-2-4-19-17-26-23-10-5-18(24(25)29)16-22(19)23/h5-10,16-17,26H,2-4,11-15H2,1H3,(H2,25,29) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Inhibition of 8-OH DPAT bindign to rat hydroxytryptamine 1A receptor expressed in CHO cells |
J Med Chem 47: 4677-83 (2004)
Article DOI: 10.1021/jm040792y
BindingDB Entry DOI: 10.7270/Q28S4PCR |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50151978
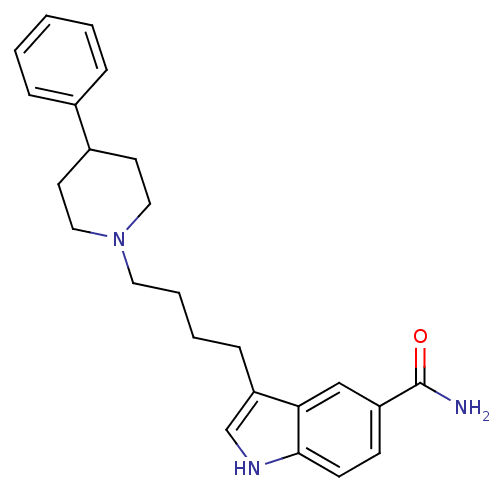
(3-[4-(4-Phenyl-piperidin-1-yl)-butyl]-1H-indole-5-...)Show SMILES NC(=O)c1ccc2[nH]cc(CCCCN3CCC(CC3)c3ccccc3)c2c1 Show InChI InChI=1S/C24H29N3O/c25-24(28)20-9-10-23-22(16-20)21(17-26-23)8-4-5-13-27-14-11-19(12-15-27)18-6-2-1-3-7-18/h1-3,6-7,9-10,16-17,19,26H,4-5,8,11-15H2,(H2,25,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Inhibition of 8-OH DPAT bindign to rat hydroxytryptamine 1A receptor expressed in CHO cells |
J Med Chem 47: 4677-83 (2004)
Article DOI: 10.1021/jm040792y
BindingDB Entry DOI: 10.7270/Q28S4PCR |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50151954
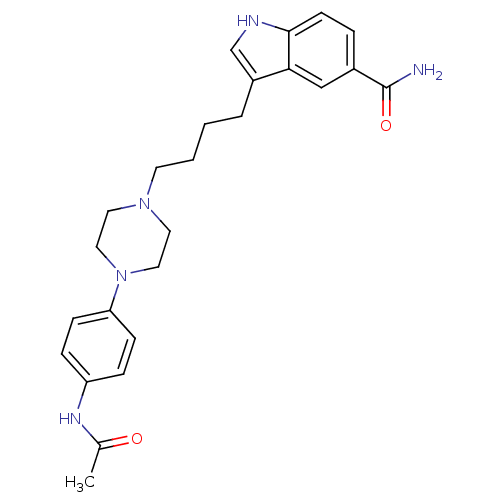
(3-{4-[4-(4-Acetylamino-phenyl)-piperazin-1-yl]-but...)Show SMILES CC(=O)Nc1ccc(cc1)N1CCN(CCCCc2c[nH]c3ccc(cc23)C(N)=O)CC1 Show InChI InChI=1S/C25H31N5O2/c1-18(31)28-21-6-8-22(9-7-21)30-14-12-29(13-15-30)11-3-2-4-20-17-27-24-10-5-19(25(26)32)16-23(20)24/h5-10,16-17,27H,2-4,11-15H2,1H3,(H2,26,32)(H,28,31) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Inhibition of 8-OH DPAT bindign to rat hydroxytryptamine 1A receptor expressed in CHO cells |
J Med Chem 47: 4677-83 (2004)
Article DOI: 10.1021/jm040792y
BindingDB Entry DOI: 10.7270/Q28S4PCR |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50151983
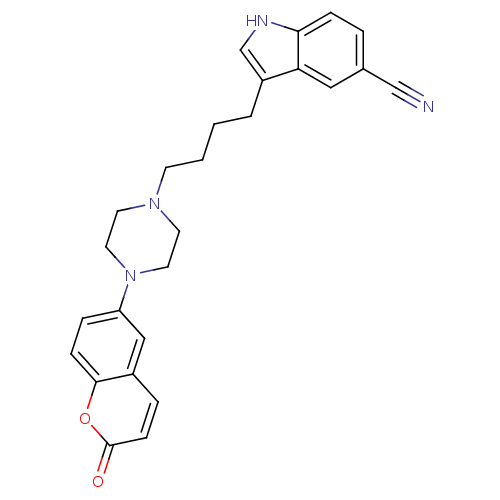
(3-{4-[4-(2-Oxo-2H-chromen-6-yl)-piperazin-1-yl]-bu...)Show SMILES O=c1ccc2cc(ccc2o1)N1CCN(CCCCc2c[nH]c3ccc(cc23)C#N)CC1 Show InChI InChI=1S/C26H26N4O2/c27-17-19-4-7-24-23(15-19)21(18-28-24)3-1-2-10-29-11-13-30(14-12-29)22-6-8-25-20(16-22)5-9-26(31)32-25/h4-9,15-16,18,28H,1-3,10-14H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Inhibition of 8-OH DPAT binding to rat hydroxytryptamine 1A receptor expresssed in CHO cells |
J Med Chem 47: 4684-92 (2004)
Article DOI: 10.1021/jm040793q
BindingDB Entry DOI: 10.7270/Q2513XPS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50151932
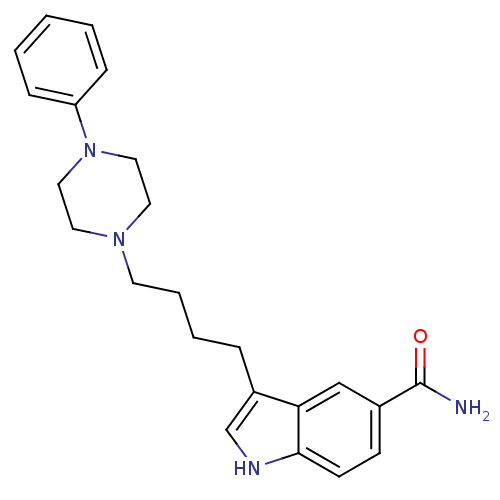
(3-[4-(4-Phenyl-piperazin-1-yl)-butyl]-1H-indole-5-...)Show SMILES NC(=O)c1ccc2[nH]cc(CCCCN3CCN(CC3)c3ccccc3)c2c1 Show InChI InChI=1S/C23H28N4O/c24-23(28)18-9-10-22-21(16-18)19(17-25-22)6-4-5-11-26-12-14-27(15-13-26)20-7-2-1-3-8-20/h1-3,7-10,16-17,25H,4-6,11-15H2,(H2,24,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Inhibition of 8-OH DPAT bindign to rat hydroxytryptamine 1A receptor expressed in CHO cells |
J Med Chem 47: 4677-83 (2004)
Article DOI: 10.1021/jm040792y
BindingDB Entry DOI: 10.7270/Q28S4PCR |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50151937

(3-{4-[4-(2-Methoxy-phenyl)-piperazin-1-yl]-butyl}-...)Show SMILES COc1ccccc1N1CCN(CCCCc2c[nH]c3ccc(cc23)C(N)=O)CC1 Show InChI InChI=1S/C24H30N4O2/c1-30-23-8-3-2-7-22(23)28-14-12-27(13-15-28)11-5-4-6-19-17-26-21-10-9-18(24(25)29)16-20(19)21/h2-3,7-10,16-17,26H,4-6,11-15H2,1H3,(H2,25,29) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Inhibition of 8-OH DPAT bindign to rat hydroxytryptamine 1A receptor expressed in CHO cells |
J Med Chem 47: 4677-83 (2004)
Article DOI: 10.1021/jm040792y
BindingDB Entry DOI: 10.7270/Q28S4PCR |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50151933
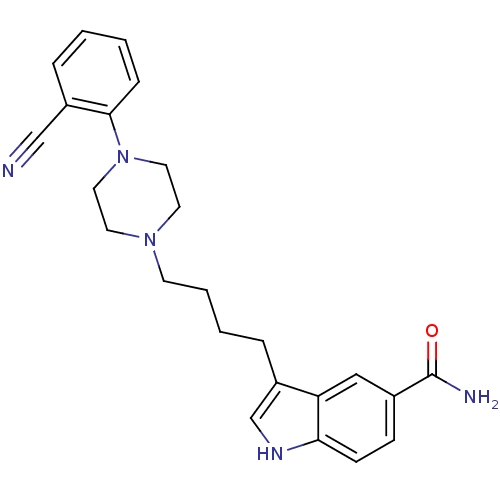
(3-{4-[4-(2-Cyano-phenyl)-piperazin-1-yl]-butyl}-1H...)Show SMILES NC(=O)c1ccc2[nH]cc(CCCCN3CCN(CC3)c3ccccc3C#N)c2c1 Show InChI InChI=1S/C24H27N5O/c25-16-19-5-1-2-7-23(19)29-13-11-28(12-14-29)10-4-3-6-20-17-27-22-9-8-18(24(26)30)15-21(20)22/h1-2,5,7-9,15,17,27H,3-4,6,10-14H2,(H2,26,30) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Inhibition of 8-OH DPAT bindign to rat hydroxytryptamine 1A receptor expressed in CHO cells |
J Med Chem 47: 4677-83 (2004)
Article DOI: 10.1021/jm040792y
BindingDB Entry DOI: 10.7270/Q28S4PCR |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50151948

(3-{4-[4-(2,3-Dihydro-benzo[1,4]dioxin-6-yl)-pipera...)Show SMILES NC(=O)c1ccc2[nH]cc(CCCCN3CCN(CC3)c3ccc4OCCOc4c3)c2c1 Show InChI InChI=1S/C25H30N4O3/c26-25(30)18-4-6-22-21(15-18)19(17-27-22)3-1-2-8-28-9-11-29(12-10-28)20-5-7-23-24(16-20)32-14-13-31-23/h4-7,15-17,27H,1-3,8-14H2,(H2,26,30) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Inhibition of 8-OH DPAT bindign to rat hydroxytryptamine 1A receptor expressed in CHO cells |
J Med Chem 47: 4677-83 (2004)
Article DOI: 10.1021/jm040792y
BindingDB Entry DOI: 10.7270/Q28S4PCR |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50151994
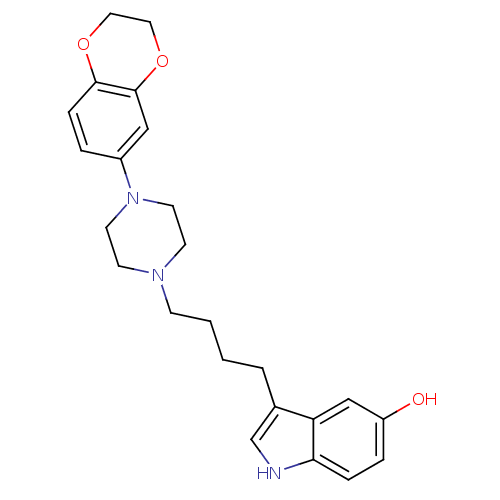
(3-{4-[4-(2,3-Dihydro-benzo[1,4]dioxin-6-yl)-pipera...)Show SMILES Oc1ccc2[nH]cc(CCCCN3CCN(CC3)c3ccc4OCCOc4c3)c2c1 Show InChI InChI=1S/C24H29N3O3/c28-20-5-6-22-21(16-20)18(17-25-22)3-1-2-8-26-9-11-27(12-10-26)19-4-7-23-24(15-19)30-14-13-29-23/h4-7,15-17,25,28H,1-3,8-14H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Inhibition of 8-OH DPAT binding to rat hydroxytryptamine 1A receptor expresssed in CHO cells |
J Med Chem 47: 4684-92 (2004)
Article DOI: 10.1021/jm040793q
BindingDB Entry DOI: 10.7270/Q2513XPS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
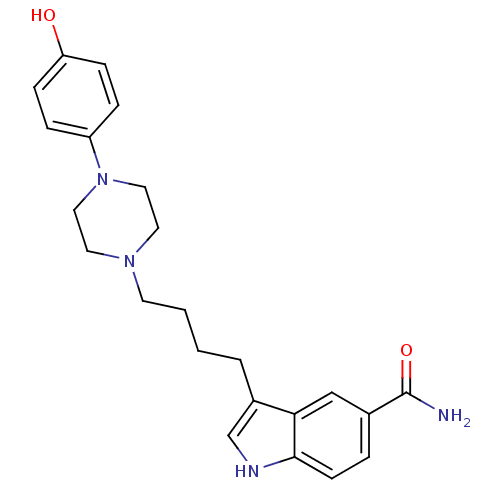
(Rattus norvegicus (rat)) | BDBM50151966
(3-{4-[4-(4-Hydroxy-phenyl)-piperazin-1-yl]-butyl}-...)Show SMILES NC(=O)c1ccc2[nH]cc(CCCCN3CCN(CC3)c3ccc(O)cc3)c2c1 Show InChI InChI=1S/C23H28N4O2/c24-23(29)17-4-9-22-21(15-17)18(16-25-22)3-1-2-10-26-11-13-27(14-12-26)19-5-7-20(28)8-6-19/h4-9,15-16,25,28H,1-3,10-14H2,(H2,24,29) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Inhibition of 8-OH DPAT bindign to rat hydroxytryptamine 1A receptor expressed in CHO cells |
J Med Chem 47: 4677-83 (2004)
Article DOI: 10.1021/jm040792y
BindingDB Entry DOI: 10.7270/Q28S4PCR |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50151948

(3-{4-[4-(2,3-Dihydro-benzo[1,4]dioxin-6-yl)-pipera...)Show SMILES NC(=O)c1ccc2[nH]cc(CCCCN3CCN(CC3)c3ccc4OCCOc4c3)c2c1 Show InChI InChI=1S/C25H30N4O3/c26-25(30)18-4-6-22-21(15-18)19(17-27-22)3-1-2-8-28-9-11-29(12-10-28)20-5-7-23-24(16-20)32-14-13-31-23/h4-7,15-17,27H,1-3,8-14H2,(H2,26,30) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Inhibition of 8-OH DPAT binding to rat hydroxytryptamine 1A receptor expresssed in CHO cells |
J Med Chem 47: 4684-92 (2004)
Article DOI: 10.1021/jm040793q
BindingDB Entry DOI: 10.7270/Q2513XPS |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50520160
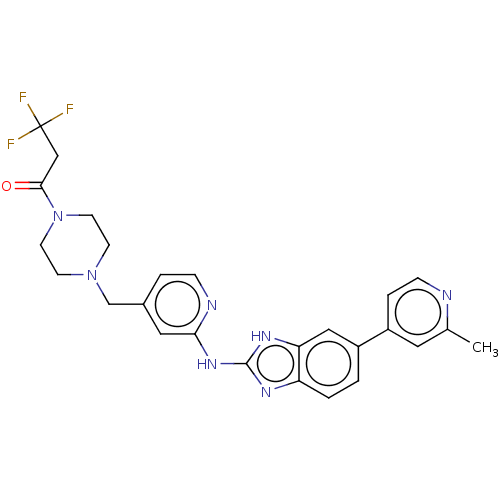
(CHEMBL4515413 | US10894784, Example 01.03)Show SMILES Cc1cc(ccn1)-c1ccc2nc(Nc3cc(CN4CCN(CC4)C(=O)CC(F)(F)F)ccn3)[nH]c2c1 Show InChI InChI=1S/C26H26F3N7O/c1-17-12-20(5-7-30-17)19-2-3-21-22(14-19)33-25(32-21)34-23-13-18(4-6-31-23)16-35-8-10-36(11-9-35)24(37)15-26(27,28)29/h2-7,12-14H,8-11,15-16H2,1H3,(H2,31,32,33,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal GST-tagged FLT3 (564 to end residues) expressed in sf21 cells using biotin labelled Ahx-GGEEEEYFELVKKKK pe... |
J Med Chem 63: 601-612 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01460
BindingDB Entry DOI: 10.7270/Q2CN7795 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50151982

(5-{4-[4-(5-Cyano-1H-indol-3-yl)-butyl]-piperazin-1...)Show SMILES NC(=O)c1cc2cc(ccc2o1)N1CCN(CCCCc2c[nH]c3ccc(cc23)C#N)CC1 Show InChI InChI=1S/C26H27N5O2/c27-16-18-4-6-23-22(13-18)19(17-29-23)3-1-2-8-30-9-11-31(12-10-30)21-5-7-24-20(14-21)15-25(33-24)26(28)32/h4-7,13-15,17,29H,1-3,8-12H2,(H2,28,32) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Inhibition of 8-OH DPAT binding to rat hydroxytryptamine 1A receptor expresssed in CHO cells |
J Med Chem 47: 4684-92 (2004)
Article DOI: 10.1021/jm040793q
BindingDB Entry DOI: 10.7270/Q2513XPS |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50152027
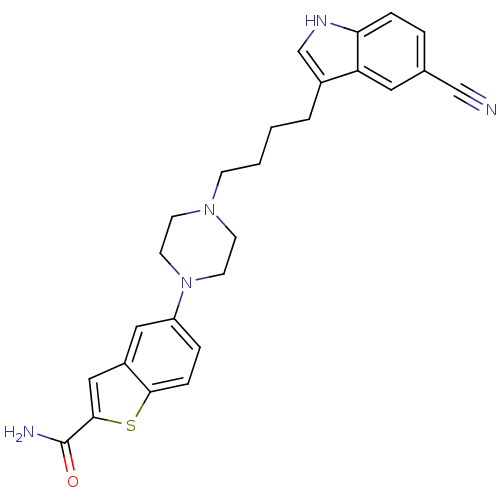
(5-{4-[4-(5-Cyano-1H-indol-3-yl)-butyl]-piperazin-1...)Show SMILES NC(=O)c1cc2cc(ccc2s1)N1CCN(CCCCc2c[nH]c3ccc(cc23)C#N)CC1 Show InChI InChI=1S/C26H27N5OS/c27-16-18-4-6-23-22(13-18)19(17-29-23)3-1-2-8-30-9-11-31(12-10-30)21-5-7-24-20(14-21)15-25(33-24)26(28)32/h4-7,13-15,17,29H,1-3,8-12H2,(H2,28,32) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-5-HT re-uptake in rat synaptosomes |
J Med Chem 47: 4684-92 (2004)
Article DOI: 10.1021/jm040793q
BindingDB Entry DOI: 10.7270/Q2513XPS |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50151999
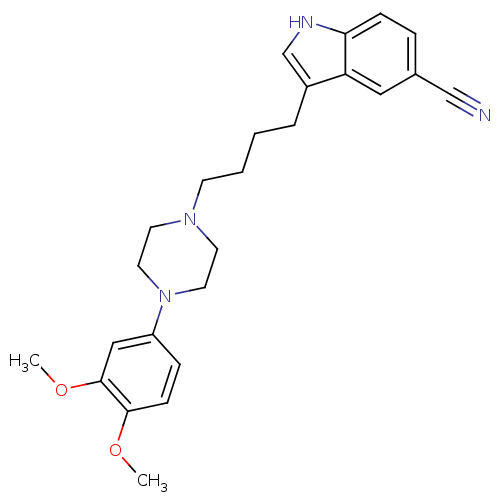
(3-{4-[4-(3,4-Dimethoxy-phenyl)-piperazin-1-yl]-but...)Show SMILES COc1ccc(cc1OC)N1CCN(CCCCc2c[nH]c3ccc(cc23)C#N)CC1 Show InChI InChI=1S/C25H30N4O2/c1-30-24-9-7-21(16-25(24)31-2)29-13-11-28(12-14-29)10-4-3-5-20-18-27-23-8-6-19(17-26)15-22(20)23/h6-9,15-16,18,27H,3-5,10-14H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-5-HT re-uptake in rat synaptosomes |
J Med Chem 47: 4684-92 (2004)
Article DOI: 10.1021/jm040793q
BindingDB Entry DOI: 10.7270/Q2513XPS |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50152012
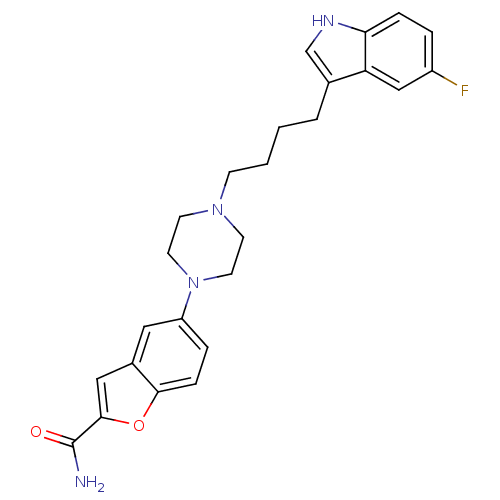
(5-{4-[4-(5-Fluoro-1H-indol-3-yl)-butyl]-piperazin-...)Show SMILES NC(=O)c1cc2cc(ccc2o1)N1CCN(CCCCc2c[nH]c3ccc(F)cc23)CC1 Show InChI InChI=1S/C25H27FN4O2/c26-19-4-6-22-21(15-19)17(16-28-22)3-1-2-8-29-9-11-30(12-10-29)20-5-7-23-18(13-20)14-24(32-23)25(27)31/h4-7,13-16,28H,1-3,8-12H2,(H2,27,31) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-5-HT re-uptake in rat synaptosomes |
J Med Chem 47: 4684-92 (2004)
Article DOI: 10.1021/jm040793q
BindingDB Entry DOI: 10.7270/Q2513XPS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50151950
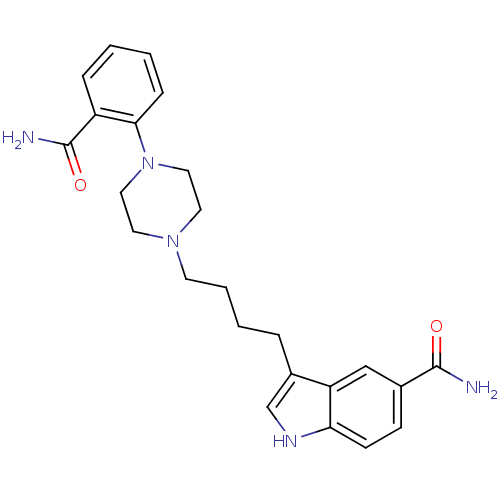
(3-{4-[4-(2-Carbamoyl-phenyl)-piperazin-1-yl]-butyl...)Show SMILES NC(=O)c1ccc2[nH]cc(CCCCN3CCN(CC3)c3ccccc3C(N)=O)c2c1 Show InChI InChI=1S/C24H29N5O2/c25-23(30)17-8-9-21-20(15-17)18(16-27-21)5-3-4-10-28-11-13-29(14-12-28)22-7-2-1-6-19(22)24(26)31/h1-2,6-9,15-16,27H,3-5,10-14H2,(H2,25,30)(H2,26,31) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Inhibition of 8-OH DPAT bindign to rat hydroxytryptamine 1A receptor expressed in CHO cells |
J Med Chem 47: 4677-83 (2004)
Article DOI: 10.1021/jm040792y
BindingDB Entry DOI: 10.7270/Q28S4PCR |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TBK1
(Homo sapiens (Human)) | BDBM478724
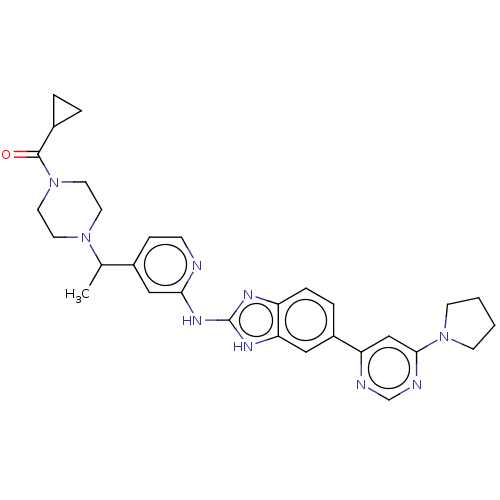
(US10894784, Example 117.02 | cyclopropyl(4-{(1R or...)Show SMILES CC(N1CCN(CC1)C(=O)C1CC1)c1ccnc(Nc2nc3ccc(cc3[nH]2)-c2cc(ncn2)N2CCCC2)c1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT
US Patent
| Assay Description
Recombinant full-length N-terminally His-tagged human TBK1, expressed in insect cells and purified by Ni-NTA affinity chromatography, was purchased f... |
US Patent US10894784 (2021)
BindingDB Entry DOI: 10.7270/Q2PV6PFT |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50151962
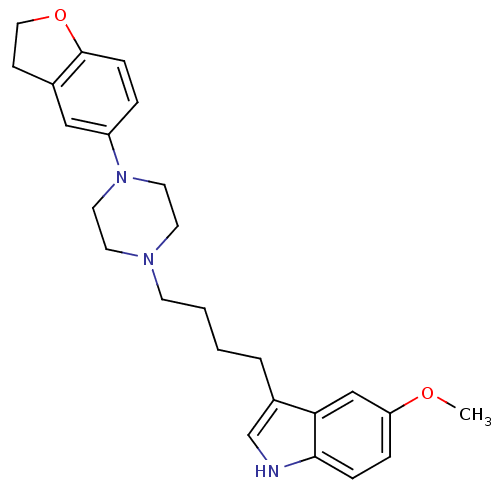
(3-{4-[4-(2,3-Dihydro-benzofuran-5-yl)-piperazin-1-...)Show SMILES COc1ccc2[nH]cc(CCCCN3CCN(CC3)c3ccc4OCCc4c3)c2c1 Show InChI InChI=1S/C25H31N3O2/c1-29-22-6-7-24-23(17-22)20(18-26-24)4-2-3-10-27-11-13-28(14-12-27)21-5-8-25-19(16-21)9-15-30-25/h5-8,16-18,26H,2-4,9-15H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Inhibition of 8-OH DPAT bindign to rat hydroxytryptamine 1A receptor expressed in CHO cells |
J Med Chem 47: 4677-83 (2004)
Article DOI: 10.1021/jm040792y
BindingDB Entry DOI: 10.7270/Q28S4PCR |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50151988
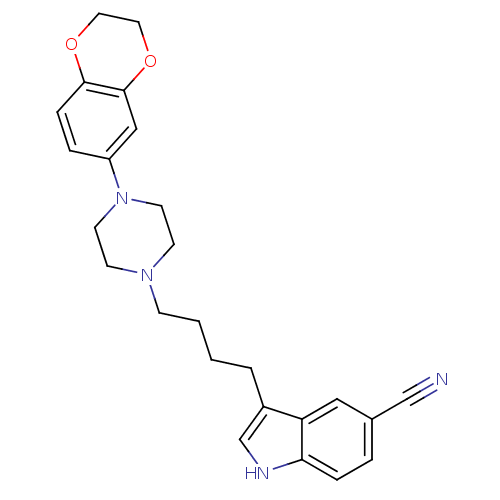
(3-{4-[4-(2,3-Dihydro-benzo[1,4]dioxin-6-yl)-pipera...)Show SMILES N#Cc1ccc2[nH]cc(CCCCN3CCN(CC3)c3ccc4OCCOc4c3)c2c1 Show InChI InChI=1S/C25H28N4O2/c26-17-19-4-6-23-22(15-19)20(18-27-23)3-1-2-8-28-9-11-29(12-10-28)21-5-7-24-25(16-21)31-14-13-30-24/h4-7,15-16,18,27H,1-3,8-14H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-5-HT re-uptake in rat synaptosomes |
J Med Chem 47: 4684-92 (2004)
Article DOI: 10.1021/jm040793q
BindingDB Entry DOI: 10.7270/Q2513XPS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50151957

(3-[4-(4-Benzo[1,3]dioxol-5-yl-3,6-dihydro-2H-pyrid...)Show SMILES COc1ccc2[nH]cc(CCCCN3CCC(=CC3)c3ccc4OCOc4c3)c2c1 |c:16| Show InChI InChI=1S/C25H28N2O3/c1-28-21-6-7-23-22(15-21)20(16-26-23)4-2-3-11-27-12-9-18(10-13-27)19-5-8-24-25(14-19)30-17-29-24/h5-9,14-16,26H,2-4,10-13,17H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Inhibition of 8-OH DPAT bindign to rat hydroxytryptamine 1A receptor expressed in CHO cells |
J Med Chem 47: 4677-83 (2004)
Article DOI: 10.1021/jm040792y
BindingDB Entry DOI: 10.7270/Q28S4PCR |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50152009
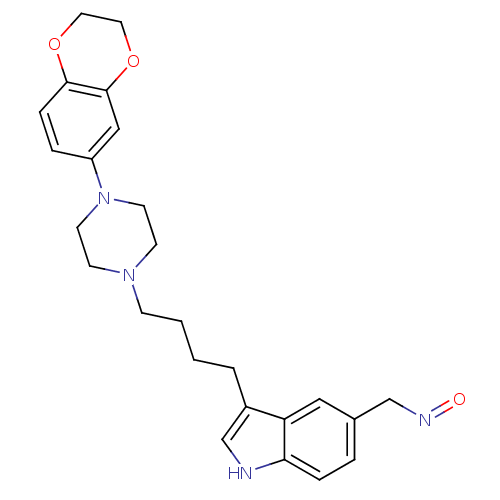
(3-{4-[4-(2,3-Dihydro-benzo[1,4]dioxin-6-yl)-pipera...)Show SMILES O=NCc1ccc2[nH]cc(CCCCN3CCN(CC3)c3ccc4OCCOc4c3)c2c1 Show InChI InChI=1S/C25H30N4O3/c30-27-17-19-4-6-23-22(15-19)20(18-26-23)3-1-2-8-28-9-11-29(12-10-28)21-5-7-24-25(16-21)32-14-13-31-24/h4-7,15-16,18,26H,1-3,8-14,17H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Inhibition of 8-OH DPAT binding to rat hydroxytryptamine 1A receptor expresssed in CHO cells |
J Med Chem 47: 4684-92 (2004)
Article DOI: 10.1021/jm040793q
BindingDB Entry DOI: 10.7270/Q2513XPS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50151930
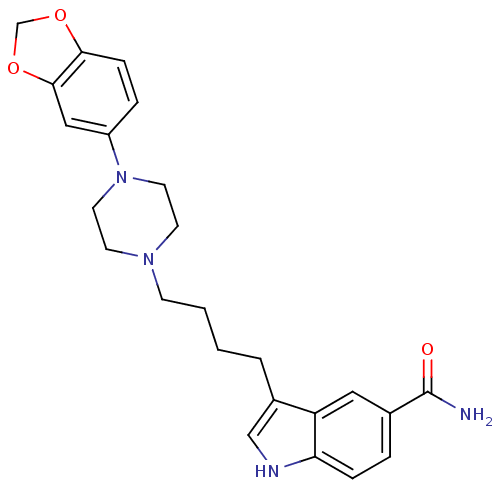
(3-[4-(4-Benzo[1,3]dioxol-5-yl-piperazin-1-yl)-buty...)Show SMILES NC(=O)c1ccc2[nH]cc(CCCCN3CCN(CC3)c3ccc4OCOc4c3)c2c1 Show InChI InChI=1S/C24H28N4O3/c25-24(29)17-4-6-21-20(13-17)18(15-26-21)3-1-2-8-27-9-11-28(12-10-27)19-5-7-22-23(14-19)31-16-30-22/h4-7,13-15,26H,1-3,8-12,16H2,(H2,25,29) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Inhibition of 8-OH DPAT bindign to rat hydroxytryptamine 1A receptor expressed in CHO cells |
J Med Chem 47: 4677-83 (2004)
Article DOI: 10.1021/jm040792y
BindingDB Entry DOI: 10.7270/Q28S4PCR |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50151938

(4-{1-[4-(5-Methoxy-1H-indol-3-yl)-butyl]-1,2,3,6-t...)Show SMILES COc1ccc2[nH]cc(CCCCN3CCC(=CC3)c3ccc(O)cc3)c2c1 |c:16| Show InChI InChI=1S/C24H28N2O2/c1-28-22-9-10-24-23(16-22)20(17-25-24)4-2-3-13-26-14-11-19(12-15-26)18-5-7-21(27)8-6-18/h5-11,16-17,25,27H,2-4,12-15H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Inhibition of 8-OH DPAT bindign to rat hydroxytryptamine 1A receptor expressed in CHO cells |
J Med Chem 47: 4677-83 (2004)
Article DOI: 10.1021/jm040792y
BindingDB Entry DOI: 10.7270/Q28S4PCR |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50152011

(3-{4-[4-(2-Cyano-benzofuran-5-yl)-piperazin-1-yl]-...)Show SMILES N#Cc1cc2cc(ccc2o1)N1CCN(CCCCc2c[nH]c3ccc(cc23)C#N)CC1 Show InChI InChI=1S/C26H25N5O/c27-16-19-4-6-25-24(13-19)20(18-29-25)3-1-2-8-30-9-11-31(12-10-30)22-5-7-26-21(14-22)15-23(17-28)32-26/h4-7,13-15,18,29H,1-3,8-12H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Inhibition of 8-OH DPAT binding to rat hydroxytryptamine 1A receptor expresssed in CHO cells |
J Med Chem 47: 4684-92 (2004)
Article DOI: 10.1021/jm040793q
BindingDB Entry DOI: 10.7270/Q2513XPS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TBK1
(Homo sapiens (Human)) | BDBM478844
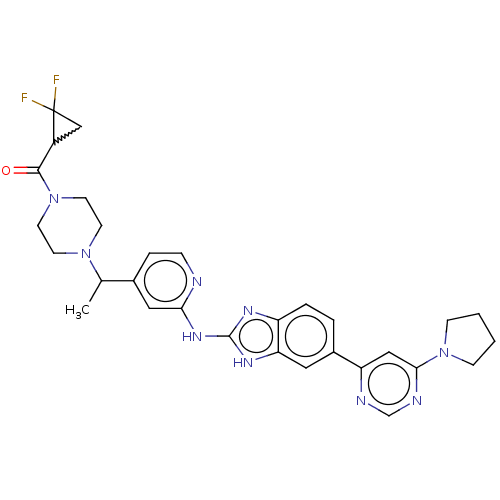
(US10894784, Example 117.05)Show SMILES CC(N1CCN(CC1)C(=O)C1CC1(F)F)c1ccnc(Nc2nc3ccc(cc3[nH]2)-c2cc(ncn2)N2CCCC2)c1 |w:11.11| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.473 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT
US Patent
| Assay Description
Recombinant full-length N-terminally His-tagged human TBK1, expressed in insect cells and purified by Ni-NTA affinity chromatography, was purchased f... |
US Patent US10894784 (2021)
BindingDB Entry DOI: 10.7270/Q2PV6PFT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data