 Found 149 hits with Last Name = 'hightower' and Initial = 'ke'
Found 149 hits with Last Name = 'hightower' and Initial = 'ke' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50195876
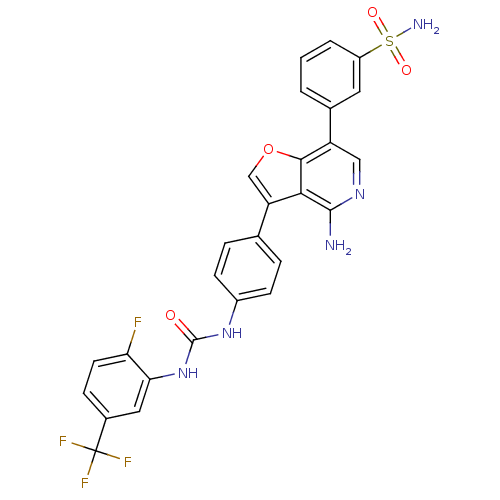
(3-(4-amino-3-{4-[3-(2-fluoro-5-trifluoromethyl-phe...)Show SMILES Nc1ncc(-c2cccc(c2)S(N)(=O)=O)c2occ(-c3ccc(NC(=O)Nc4cc(ccc4F)C(F)(F)F)cc3)c12 Show InChI InChI=1S/C27H19F4N5O4S/c28-21-9-6-16(27(29,30)31)11-22(21)36-26(37)35-17-7-4-14(5-8-17)20-13-40-24-19(12-34-25(32)23(20)24)15-2-1-3-18(10-15)41(33,38)39/h1-13H,(H2,32,34)(H2,33,38,39)(H2,35,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline K.K.
Curated by ChEMBL
| Assay Description
Inhibition of human EphB4 by scintillation proximity method |
Bioorg Med Chem Lett 17: 250-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.050
BindingDB Entry DOI: 10.7270/Q2SB45D5 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50195876

(3-(4-amino-3-{4-[3-(2-fluoro-5-trifluoromethyl-phe...)Show SMILES Nc1ncc(-c2cccc(c2)S(N)(=O)=O)c2occ(-c3ccc(NC(=O)Nc4cc(ccc4F)C(F)(F)F)cc3)c12 Show InChI InChI=1S/C27H19F4N5O4S/c28-21-9-6-16(27(29,30)31)11-22(21)36-26(37)35-17-7-4-14(5-8-17)20-13-40-24-19(12-34-25(32)23(20)24)15-2-1-3-18(10-15)41(33,38)39/h1-13H,(H2,32,34)(H2,33,38,39)(H2,35,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline K.K.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-6XHis-VEGFR2 by HTRF method |
Bioorg Med Chem Lett 17: 250-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.050
BindingDB Entry DOI: 10.7270/Q2SB45D5 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50195878
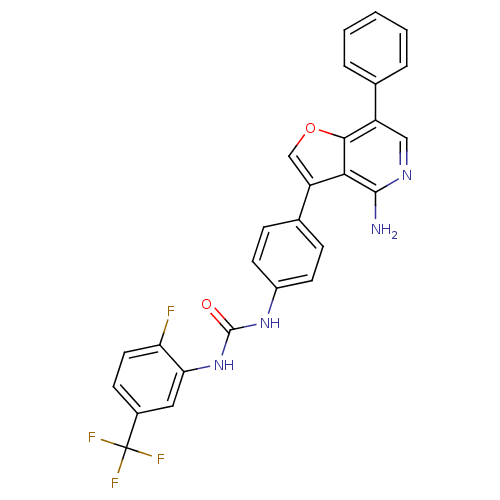
(1-(4-(4-amino-7-phenylfuro[3,2-c]pyridin-3-yl)phen...)Show SMILES Nc1ncc(-c2ccccc2)c2occ(-c3ccc(NC(=O)Nc4cc(ccc4F)C(F)(F)F)cc3)c12 Show InChI InChI=1S/C27H18F4N4O2/c28-21-11-8-17(27(29,30)31)12-22(21)35-26(36)34-18-9-6-16(7-10-18)20-14-37-24-19(13-33-25(32)23(20)24)15-4-2-1-3-5-15/h1-14H,(H2,32,33)(H2,34,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline K.K.
Curated by ChEMBL
| Assay Description
Inhibition of human EphB4 by scintillation proximity method |
Bioorg Med Chem Lett 17: 250-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.050
BindingDB Entry DOI: 10.7270/Q2SB45D5 |
More data for this
Ligand-Target Pair | |
Angiopoietin-1 receptor
(Homo sapiens (Human)) | BDBM50195876

(3-(4-amino-3-{4-[3-(2-fluoro-5-trifluoromethyl-phe...)Show SMILES Nc1ncc(-c2cccc(c2)S(N)(=O)=O)c2occ(-c3ccc(NC(=O)Nc4cc(ccc4F)C(F)(F)F)cc3)c12 Show InChI InChI=1S/C27H19F4N5O4S/c28-21-9-6-16(27(29,30)31)11-22(21)36-26(37)35-17-7-4-14(5-8-17)20-13-40-24-19(12-34-25(32)23(20)24)15-2-1-3-18(10-15)41(33,38)39/h1-13H,(H2,32,34)(H2,33,38,39)(H2,35,36,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline K.K.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-Tie2 by HTRF method |
Bioorg Med Chem Lett 17: 250-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.050
BindingDB Entry DOI: 10.7270/Q2SB45D5 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50195879
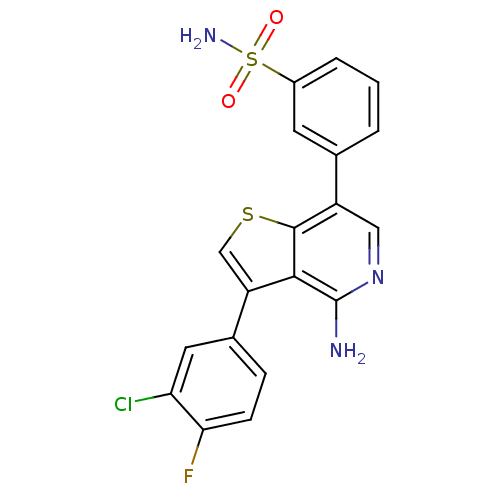
(3-(4-amino-3-(3-chloro-4-fluorophenyl)thieno[3,2-c...)Show SMILES Nc1ncc(-c2cccc(c2)S(N)(=O)=O)c2scc(-c3ccc(F)c(Cl)c3)c12 Show InChI InChI=1S/C19H13ClFN3O2S2/c20-15-7-11(4-5-16(15)21)14-9-27-18-13(8-24-19(22)17(14)18)10-2-1-3-12(6-10)28(23,25)26/h1-9H,(H2,22,24)(H2,23,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline K.K.
Curated by ChEMBL
| Assay Description
Inhibition of human EphB4 by scintillation proximity method |
Bioorg Med Chem Lett 17: 250-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.050
BindingDB Entry DOI: 10.7270/Q2SB45D5 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50195878

(1-(4-(4-amino-7-phenylfuro[3,2-c]pyridin-3-yl)phen...)Show SMILES Nc1ncc(-c2ccccc2)c2occ(-c3ccc(NC(=O)Nc4cc(ccc4F)C(F)(F)F)cc3)c12 Show InChI InChI=1S/C27H18F4N4O2/c28-21-11-8-17(27(29,30)31)12-22(21)35-26(36)34-18-9-6-16(7-10-18)20-14-37-24-19(13-33-25(32)23(20)24)15-4-2-1-3-5-15/h1-14H,(H2,32,33)(H2,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline K.K.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-6XHis-VEGFR2 by HTRF method |
Bioorg Med Chem Lett 17: 250-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.050
BindingDB Entry DOI: 10.7270/Q2SB45D5 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50195888
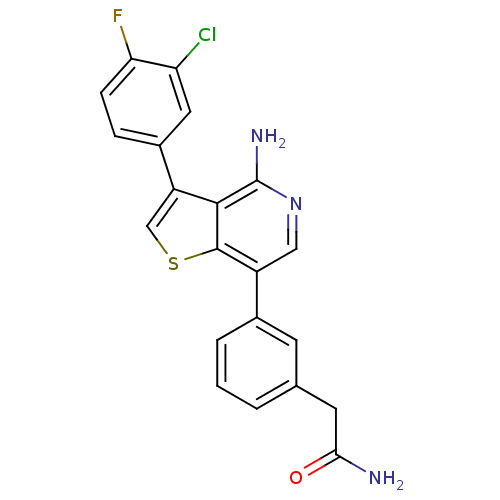
(2-(3-(4-amino-3-(3-chloro-4-fluorophenyl)thieno[3,...)Show SMILES NC(=O)Cc1cccc(c1)-c1cnc(N)c2c(csc12)-c1ccc(F)c(Cl)c1 Show InChI InChI=1S/C21H15ClFN3OS/c22-16-8-13(4-5-17(16)23)15-10-28-20-14(9-26-21(25)19(15)20)12-3-1-2-11(6-12)7-18(24)27/h1-6,8-10H,7H2,(H2,24,27)(H2,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline K.K.
Curated by ChEMBL
| Assay Description
Inhibition of human EphB4 by scintillation proximity method |
Bioorg Med Chem Lett 17: 250-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.050
BindingDB Entry DOI: 10.7270/Q2SB45D5 |
More data for this
Ligand-Target Pair | |
Angiopoietin-1 receptor
(Homo sapiens (Human)) | BDBM50195878

(1-(4-(4-amino-7-phenylfuro[3,2-c]pyridin-3-yl)phen...)Show SMILES Nc1ncc(-c2ccccc2)c2occ(-c3ccc(NC(=O)Nc4cc(ccc4F)C(F)(F)F)cc3)c12 Show InChI InChI=1S/C27H18F4N4O2/c28-21-11-8-17(27(29,30)31)12-22(21)35-26(36)34-18-9-6-16(7-10-18)20-14-37-24-19(13-33-25(32)23(20)24)15-4-2-1-3-5-15/h1-14H,(H2,32,33)(H2,34,35,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline K.K.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-Tie2 by HTRF method |
Bioorg Med Chem Lett 17: 250-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.050
BindingDB Entry DOI: 10.7270/Q2SB45D5 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50195877
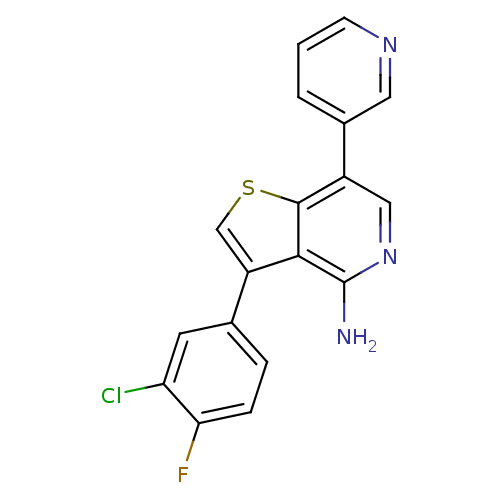
(3-(3-chloro-4-fluorophenyl)-7-(pyridin-3-yl)thieno...)Show SMILES Nc1ncc(-c2cccnc2)c2scc(-c3ccc(F)c(Cl)c3)c12 Show InChI InChI=1S/C18H11ClFN3S/c19-14-6-10(3-4-15(14)20)13-9-24-17-12(8-23-18(21)16(13)17)11-2-1-5-22-7-11/h1-9H,(H2,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline K.K.
Curated by ChEMBL
| Assay Description
Inhibition of human EphB4 by scintillation proximity method |
Bioorg Med Chem Lett 17: 250-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.050
BindingDB Entry DOI: 10.7270/Q2SB45D5 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50195892
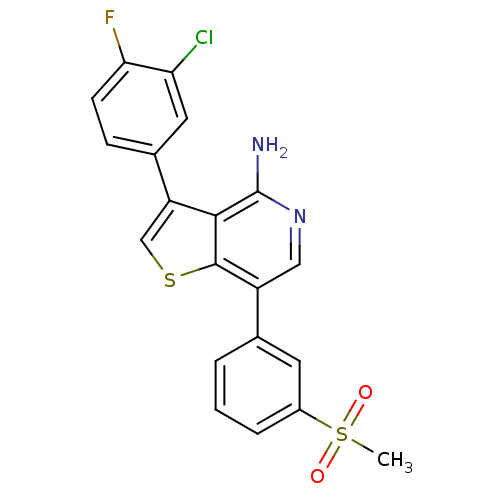
(3-(3-chloro-4-fluorophenyl)-7-(3-(methylsulfonyl)p...)Show SMILES CS(=O)(=O)c1cccc(c1)-c1cnc(N)c2c(csc12)-c1ccc(F)c(Cl)c1 Show InChI InChI=1S/C20H14ClFN2O2S2/c1-28(25,26)13-4-2-3-11(7-13)14-9-24-20(23)18-15(10-27-19(14)18)12-5-6-17(22)16(21)8-12/h2-10H,1H3,(H2,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline K.K.
Curated by ChEMBL
| Assay Description
Inhibition of human EphB4 by scintillation proximity method |
Bioorg Med Chem Lett 17: 250-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.050
BindingDB Entry DOI: 10.7270/Q2SB45D5 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50195884
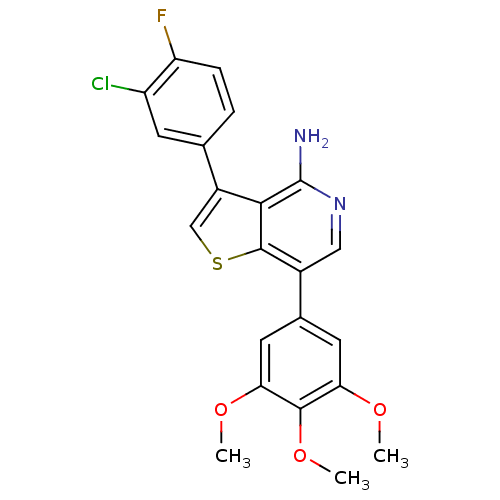
(3-(3-chloro-4-fluorophenyl)-7-(3,4,5-trimethoxyphe...)Show SMILES COc1cc(cc(OC)c1OC)-c1cnc(N)c2c(csc12)-c1ccc(F)c(Cl)c1 Show InChI InChI=1S/C22H18ClFN2O3S/c1-27-17-7-12(8-18(28-2)20(17)29-3)13-9-26-22(25)19-14(10-30-21(13)19)11-4-5-16(24)15(23)6-11/h4-10H,1-3H3,(H2,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline K.K.
Curated by ChEMBL
| Assay Description
Inhibition of human EphB4 by scintillation proximity method |
Bioorg Med Chem Lett 17: 250-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.050
BindingDB Entry DOI: 10.7270/Q2SB45D5 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50195890
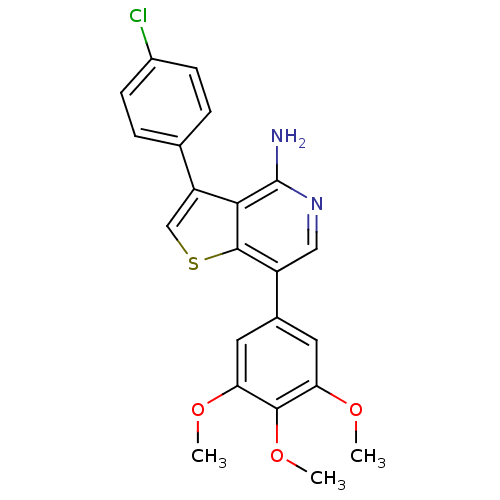
(3-(4-chlorophenyl)-7-(3,4,5-trimethoxyphenyl)thien...)Show SMILES COc1cc(cc(OC)c1OC)-c1cnc(N)c2c(csc12)-c1ccc(Cl)cc1 Show InChI InChI=1S/C22H19ClN2O3S/c1-26-17-8-13(9-18(27-2)20(17)28-3)15-10-25-22(24)19-16(11-29-21(15)19)12-4-6-14(23)7-5-12/h4-11H,1-3H3,(H2,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline K.K.
Curated by ChEMBL
| Assay Description
Inhibition of human EphB4 by scintillation proximity method |
Bioorg Med Chem Lett 17: 250-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.050
BindingDB Entry DOI: 10.7270/Q2SB45D5 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM86638
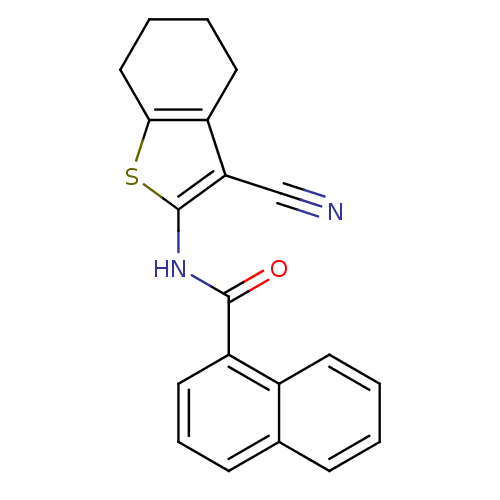
Show InChI InChI=1S/C20H16N2OS/c21-12-17-15-9-3-4-11-18(15)24-20(17)22-19(23)16-10-5-7-13-6-1-2-8-14(13)16/h1-2,5-8,10H,3-4,9,11H2,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of human truncated JNK3 |
Bioorg Med Chem Lett 17: 1296-301 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.003
BindingDB Entry DOI: 10.7270/Q2TB1840 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50195893
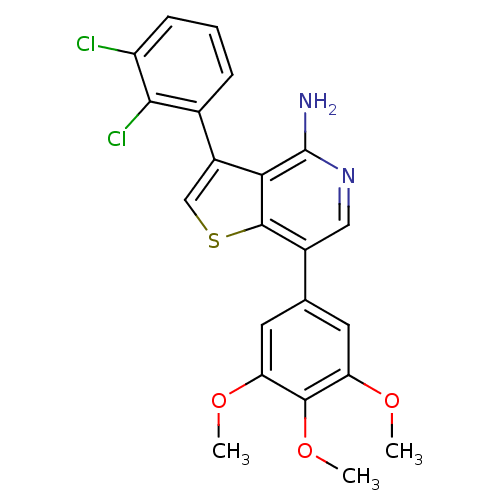
(3-(2,3-dichlorophenyl)-7-(3,4,5-trimethoxyphenyl)t...)Show SMILES COc1cc(cc(OC)c1OC)-c1cnc(N)c2c(csc12)-c1cccc(Cl)c1Cl Show InChI InChI=1S/C22H18Cl2N2O3S/c1-27-16-7-11(8-17(28-2)20(16)29-3)13-9-26-22(25)18-14(10-30-21(13)18)12-5-4-6-15(23)19(12)24/h4-10H,1-3H3,(H2,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline K.K.
Curated by ChEMBL
| Assay Description
Inhibition of human EphB4 by scintillation proximity method |
Bioorg Med Chem Lett 17: 250-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.050
BindingDB Entry DOI: 10.7270/Q2SB45D5 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50411407
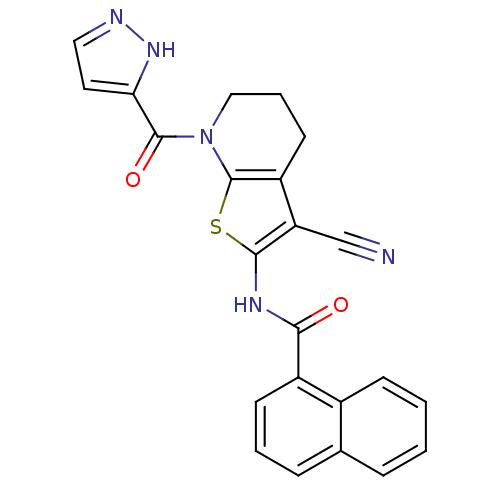
(CHEMBL232610)Show SMILES O=C(Nc1sc2N(CCCc2c1C#N)C(=O)c1ccn[nH]1)c1cccc2ccccc12 Show InChI InChI=1S/C23H17N5O2S/c24-13-18-17-9-4-12-28(22(30)19-10-11-25-27-19)23(17)31-21(18)26-20(29)16-8-3-6-14-5-1-2-7-15(14)16/h1-3,5-8,10-11H,4,9,12H2,(H,25,27)(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 251 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of human truncated JNK3 |
Bioorg Med Chem Lett 17: 1296-301 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.003
BindingDB Entry DOI: 10.7270/Q2TB1840 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50411406
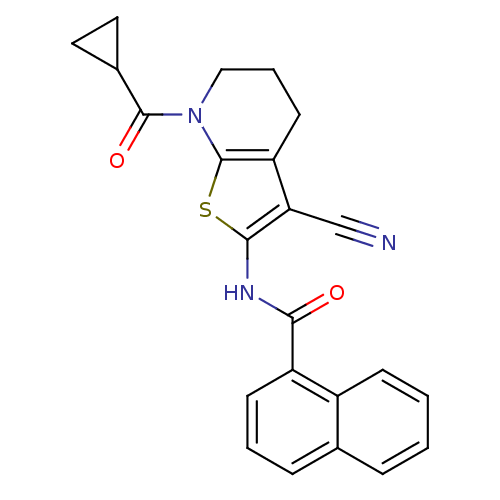
(CHEMBL232601)Show SMILES O=C(Nc1sc2N(CCCc2c1C#N)C(=O)C1CC1)c1cccc2ccccc12 Show InChI InChI=1S/C23H19N3O2S/c24-13-19-18-9-4-12-26(22(28)15-10-11-15)23(18)29-21(19)25-20(27)17-8-3-6-14-5-1-2-7-16(14)17/h1-3,5-8,15H,4,9-12H2,(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 251 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of human truncated JNK3 |
Bioorg Med Chem Lett 17: 1296-301 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.003
BindingDB Entry DOI: 10.7270/Q2TB1840 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50411406

(CHEMBL232601)Show SMILES O=C(Nc1sc2N(CCCc2c1C#N)C(=O)C1CC1)c1cccc2ccccc12 Show InChI InChI=1S/C23H19N3O2S/c24-13-19-18-9-4-12-26(22(28)15-10-11-15)23(18)29-21(19)25-20(27)17-8-3-6-14-5-1-2-7-16(14)17/h1-3,5-8,15H,4,9-12H2,(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of human JNK2alpha2 |
Bioorg Med Chem Lett 17: 1296-301 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.003
BindingDB Entry DOI: 10.7270/Q2TB1840 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM86638

Show InChI InChI=1S/C20H16N2OS/c21-12-17-15-9-3-4-11-18(15)24-20(17)22-19(23)16-10-5-7-13-6-1-2-8-14(13)16/h1-2,5-8,10H,3-4,9,11H2,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of human JNK2alpha2 |
Bioorg Med Chem Lett 17: 1296-301 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.003
BindingDB Entry DOI: 10.7270/Q2TB1840 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50195885
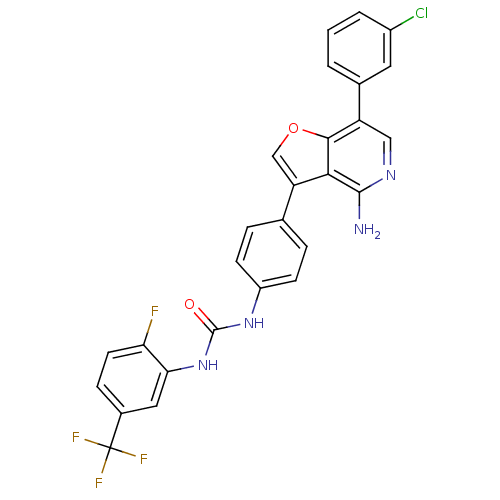
(1-(4-(4-amino-7-(3-chlorophenyl)furo[3,2-c]pyridin...)Show SMILES Nc1ncc(-c2cccc(Cl)c2)c2occ(-c3ccc(NC(=O)Nc4cc(ccc4F)C(F)(F)F)cc3)c12 Show InChI InChI=1S/C27H17ClF4N4O2/c28-17-3-1-2-15(10-17)19-12-34-25(33)23-20(13-38-24(19)23)14-4-7-18(8-5-14)35-26(37)36-22-11-16(27(30,31)32)6-9-21(22)29/h1-13H,(H2,33,34)(H2,35,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 389 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline K.K.
Curated by ChEMBL
| Assay Description
Inhibition of human EphB4 by scintillation proximity method |
Bioorg Med Chem Lett 17: 250-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.050
BindingDB Entry DOI: 10.7270/Q2SB45D5 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50411413
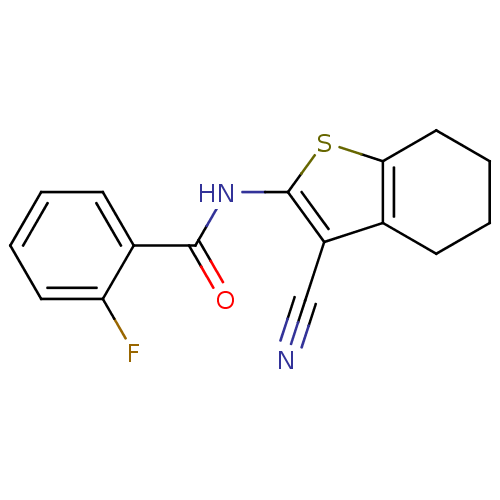
(CHEMBL234838)Show InChI InChI=1S/C16H13FN2OS/c17-13-7-3-1-6-11(13)15(20)19-16-12(9-18)10-5-2-4-8-14(10)21-16/h1,3,6-7H,2,4-5,8H2,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 398 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of human truncated JNK3 |
Bioorg Med Chem Lett 17: 1296-301 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.003
BindingDB Entry DOI: 10.7270/Q2TB1840 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Integrase
(Human immunodeficiency virus 1) | BDBM50021589
(CHEMBL3290944)Show InChI InChI=1S/C17H17N5O3/c23-17-20-15(14-2-1-7-18-16(14)22(17)24)19-12-3-5-13(6-4-12)21-8-10-25-11-9-21/h1-7,24H,8-11H2,(H,19,20,23) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of recombinant HIV1 integrase bound with donor DNA-SPA beads using [3H] target DNA as substrate assessed as strand transfer activity prein... |
Eur J Med Chem 83: 609-16 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.061
BindingDB Entry DOI: 10.7270/Q2MG7R34 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50411417
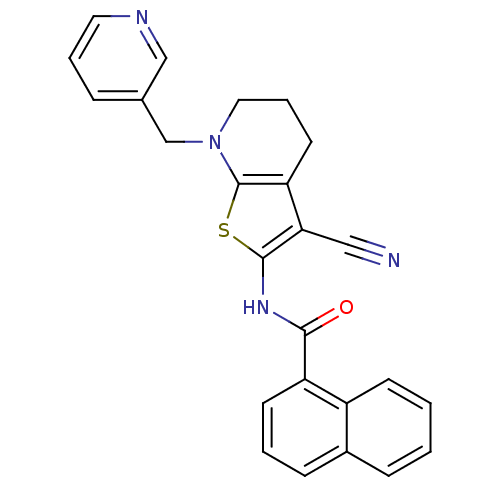
(CHEMBL391656)Show SMILES O=C(Nc1sc2N(Cc3cccnc3)CCCc2c1C#N)c1cccc2ccccc12 Show InChI InChI=1S/C25H20N4OS/c26-14-22-21-11-5-13-29(16-17-6-4-12-27-15-17)25(21)31-24(22)28-23(30)20-10-3-8-18-7-1-2-9-19(18)20/h1-4,6-10,12,15H,5,11,13,16H2,(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 501 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of human truncated JNK3 |
Bioorg Med Chem Lett 17: 1296-301 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.003
BindingDB Entry DOI: 10.7270/Q2TB1840 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50195883
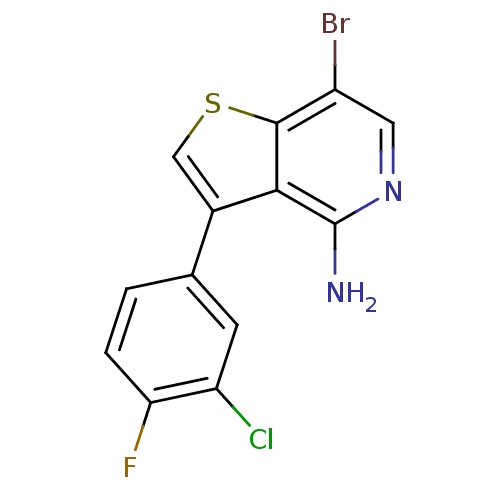
(7-bromo-3-(3-chloro-4-fluorophenyl)thieno[3,2-c]py...)Show InChI InChI=1S/C13H7BrClFN2S/c14-8-4-18-13(17)11-7(5-19-12(8)11)6-1-2-10(16)9(15)3-6/h1-5H,(H2,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 575 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline K.K.
Curated by ChEMBL
| Assay Description
Inhibition of human EphB4 by scintillation proximity method |
Bioorg Med Chem Lett 17: 250-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.050
BindingDB Entry DOI: 10.7270/Q2SB45D5 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50021586
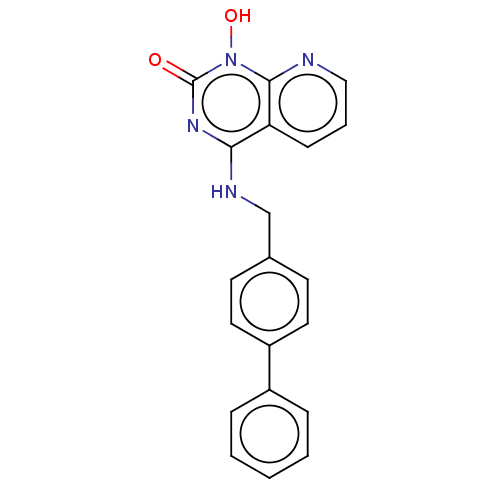
(CHEMBL3290941)Show InChI InChI=1S/C20H16N4O2/c25-20-23-18(17-7-4-12-21-19(17)24(20)26)22-13-14-8-10-16(11-9-14)15-5-2-1-3-6-15/h1-12,26H,13H2,(H,22,23,25) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of recombinant HIV1 integrase bound with donor DNA-SPA beads using [3H] target DNA as substrate assessed as strand transfer activity prein... |
Eur J Med Chem 83: 609-16 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.061
BindingDB Entry DOI: 10.7270/Q2MG7R34 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50411411
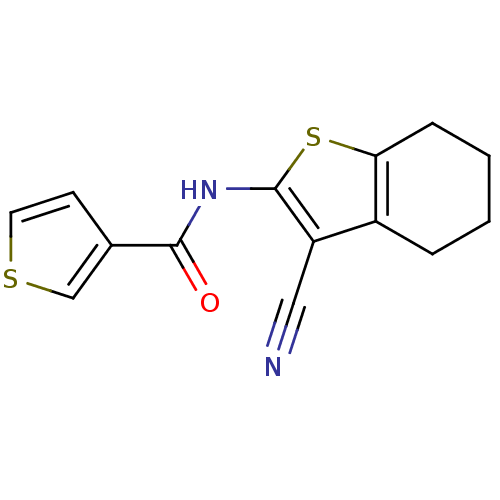
(CHEMBL397356)Show InChI InChI=1S/C14H12N2OS2/c15-7-11-10-3-1-2-4-12(10)19-14(11)16-13(17)9-5-6-18-8-9/h5-6,8H,1-4H2,(H,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 631 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of human truncated JNK3 |
Bioorg Med Chem Lett 17: 1296-301 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.003
BindingDB Entry DOI: 10.7270/Q2TB1840 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50021585
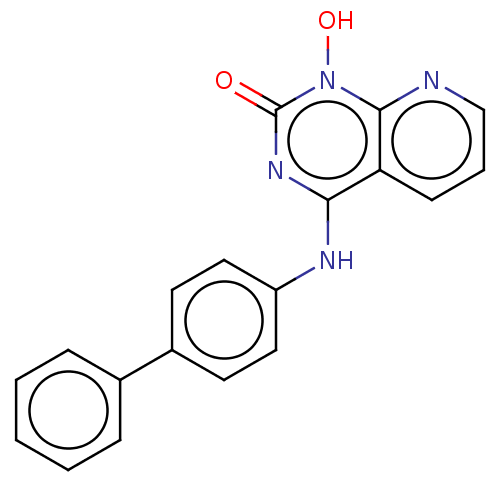
(CHEMBL3290940)Show InChI InChI=1S/C19H14N4O2/c24-19-22-17(16-7-4-12-20-18(16)23(19)25)21-15-10-8-14(9-11-15)13-5-2-1-3-6-13/h1-12,25H,(H,21,22,24) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of recombinant HIV1 integrase bound with donor DNA-SPA beads using [3H] target DNA as substrate assessed as strand transfer activity prein... |
Eur J Med Chem 83: 609-16 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.061
BindingDB Entry DOI: 10.7270/Q2MG7R34 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50195885

(1-(4-(4-amino-7-(3-chlorophenyl)furo[3,2-c]pyridin...)Show SMILES Nc1ncc(-c2cccc(Cl)c2)c2occ(-c3ccc(NC(=O)Nc4cc(ccc4F)C(F)(F)F)cc3)c12 Show InChI InChI=1S/C27H17ClF4N4O2/c28-17-3-1-2-15(10-17)19-12-34-25(33)23-20(13-38-24(19)23)14-4-7-18(8-5-14)35-26(37)36-22-11-16(27(30,31)32)6-9-21(22)29/h1-13H,(H2,33,34)(H2,35,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 933 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline K.K.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-6XHis-VEGFR2 by HTRF method |
Bioorg Med Chem Lett 17: 250-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.050
BindingDB Entry DOI: 10.7270/Q2SB45D5 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50411408

(CHEMBL391655)Show SMILES CS(=O)(=O)c1ccc(CN2CCCc3c2sc(NC(=O)c2cccc4ccccc24)c3C#N)cc1 Show InChI InChI=1S/C27H23N3O3S2/c1-35(32,33)20-13-11-18(12-14-20)17-30-15-5-10-23-24(16-28)26(34-27(23)30)29-25(31)22-9-4-7-19-6-2-3-8-21(19)22/h2-4,6-9,11-14H,5,10,15,17H2,1H3,(H,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of human truncated JNK3 |
Bioorg Med Chem Lett 17: 1296-301 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.003
BindingDB Entry DOI: 10.7270/Q2TB1840 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50411419
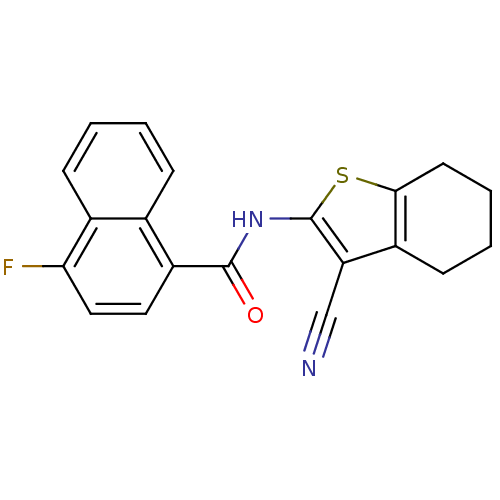
(CHEMBL233002 | SB-814597)Show InChI InChI=1S/C20H15FN2OS/c21-17-10-9-15(12-5-1-2-6-13(12)17)19(24)23-20-16(11-22)14-7-3-4-8-18(14)25-20/h1-2,5-6,9-10H,3-4,7-8H2,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of human truncated JNK3 |
Bioorg Med Chem Lett 17: 1296-301 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.003
BindingDB Entry DOI: 10.7270/Q2TB1840 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50021588
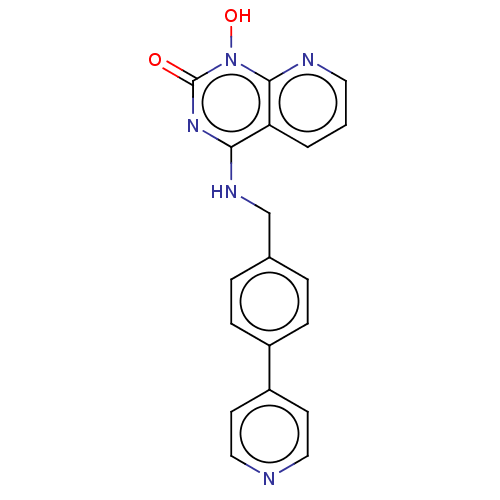
(CHEMBL3290943)Show InChI InChI=1S/C19H15N5O2/c25-19-23-17(16-2-1-9-21-18(16)24(19)26)22-12-13-3-5-14(6-4-13)15-7-10-20-11-8-15/h1-11,26H,12H2,(H,22,23,25) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of recombinant HIV1 integrase bound with donor DNA-SPA beads using [3H] target DNA as substrate assessed as strand transfer activity prein... |
Eur J Med Chem 83: 609-16 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.061
BindingDB Entry DOI: 10.7270/Q2MG7R34 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50021591
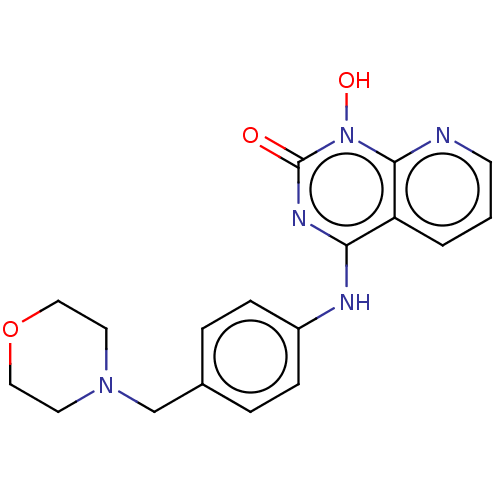
(CHEMBL3290946)Show InChI InChI=1S/C18H19N5O3/c24-18-21-16(15-2-1-7-19-17(15)23(18)25)20-14-5-3-13(4-6-14)12-22-8-10-26-11-9-22/h1-7,25H,8-12H2,(H,20,21,24) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of recombinant HIV1 integrase bound with donor DNA-SPA beads using [3H] target DNA as substrate assessed as strand transfer activity prein... |
Eur J Med Chem 83: 609-16 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.061
BindingDB Entry DOI: 10.7270/Q2MG7R34 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50195880
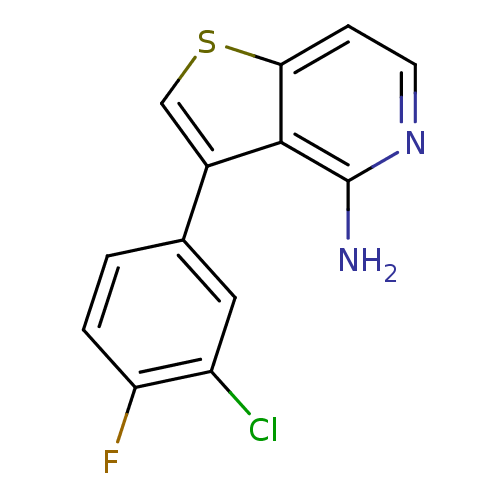
(3-(3-chloro-4-fluorophenyl)thieno[3,2-c]pyridin-4-...)Show InChI InChI=1S/C13H8ClFN2S/c14-9-5-7(1-2-10(9)15)8-6-18-11-3-4-17-13(16)12(8)11/h1-6H,(H2,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline K.K.
Curated by ChEMBL
| Assay Description
Inhibition of human EphB4 by scintillation proximity method |
Bioorg Med Chem Lett 17: 250-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.050
BindingDB Entry DOI: 10.7270/Q2SB45D5 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50021590
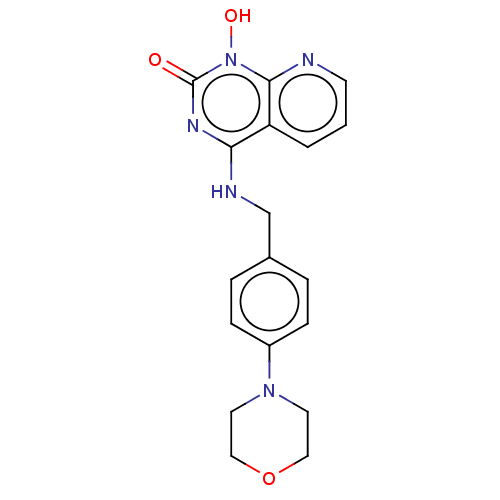
(CHEMBL3290945)Show InChI InChI=1S/C18H19N5O3/c24-18-21-16(15-2-1-7-19-17(15)23(18)25)20-12-13-3-5-14(6-4-13)22-8-10-26-11-9-22/h1-7,25H,8-12H2,(H,20,21,24) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of recombinant HIV1 integrase bound with donor DNA-SPA beads using [3H] target DNA as substrate assessed as strand transfer activity prein... |
Eur J Med Chem 83: 609-16 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.061
BindingDB Entry DOI: 10.7270/Q2MG7R34 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50241256
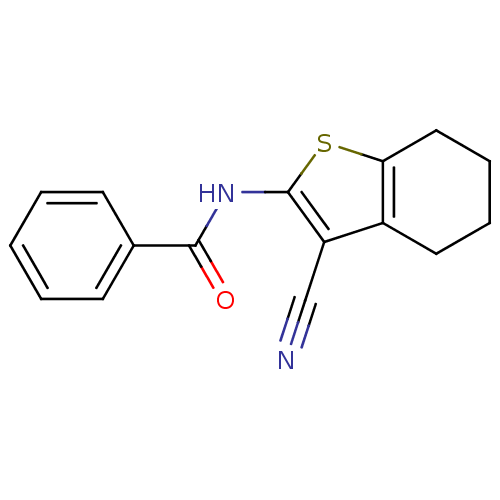
(CHEMBL233000 | N-(3-cyano-4,5,6,7-tetrahydrobenzo[...)Show InChI InChI=1S/C16H14N2OS/c17-10-13-12-8-4-5-9-14(12)20-16(13)18-15(19)11-6-2-1-3-7-11/h1-3,6-7H,4-5,8-9H2,(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of human truncated JNK3 |
Bioorg Med Chem Lett 17: 1296-301 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.003
BindingDB Entry DOI: 10.7270/Q2TB1840 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50411405
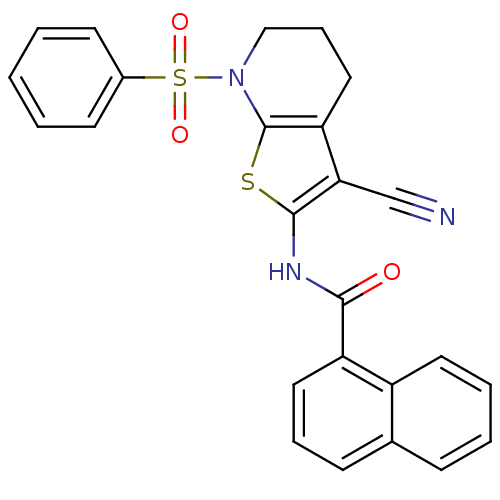
(CHEMBL233794)Show SMILES O=C(Nc1sc2N(CCCc2c1C#N)S(=O)(=O)c1ccccc1)c1cccc2ccccc12 Show InChI InChI=1S/C25H19N3O3S2/c26-16-22-21-14-7-15-28(33(30,31)18-10-2-1-3-11-18)25(21)32-24(22)27-23(29)20-13-6-9-17-8-4-5-12-19(17)20/h1-6,8-13H,7,14-15H2,(H,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of human truncated JNK3 |
Bioorg Med Chem Lett 17: 1296-301 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.003
BindingDB Entry DOI: 10.7270/Q2TB1840 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50195879

(3-(4-amino-3-(3-chloro-4-fluorophenyl)thieno[3,2-c...)Show SMILES Nc1ncc(-c2cccc(c2)S(N)(=O)=O)c2scc(-c3ccc(F)c(Cl)c3)c12 Show InChI InChI=1S/C19H13ClFN3O2S2/c20-15-7-11(4-5-16(15)21)14-9-27-18-13(8-24-19(22)17(14)18)10-2-1-3-12(6-10)28(23,25)26/h1-9H,(H2,22,24)(H2,23,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline K.K.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-6XHis-VEGFR2 by HTRF method |
Bioorg Med Chem Lett 17: 250-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.050
BindingDB Entry DOI: 10.7270/Q2SB45D5 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50021587
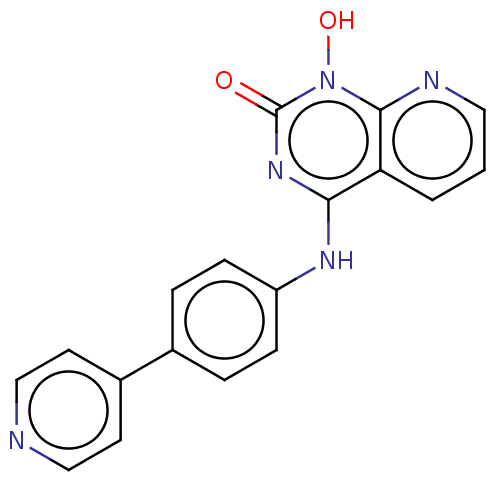
(CHEMBL3290942)Show InChI InChI=1S/C18H13N5O2/c24-18-22-16(15-2-1-9-20-17(15)23(18)25)21-14-5-3-12(4-6-14)13-7-10-19-11-8-13/h1-11,25H,(H,21,22,24) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of recombinant HIV1 integrase bound with donor DNA-SPA beads using [3H] target DNA as substrate assessed as strand transfer activity prein... |
Eur J Med Chem 83: 609-16 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.061
BindingDB Entry DOI: 10.7270/Q2MG7R34 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50021592
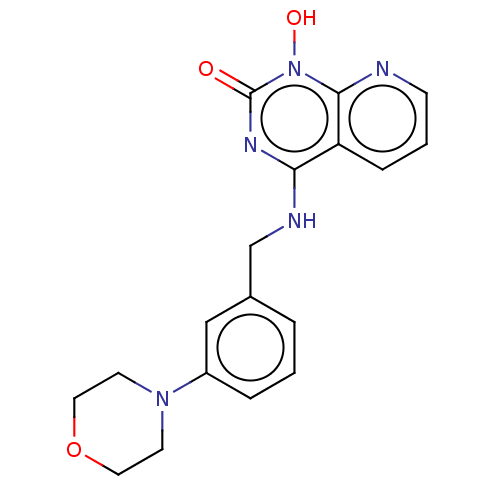
(CHEMBL3290947)Show InChI InChI=1S/C18H19N5O3/c24-18-21-16(15-5-2-6-19-17(15)23(18)25)20-12-13-3-1-4-14(11-13)22-7-9-26-10-8-22/h1-6,11,25H,7-10,12H2,(H,20,21,24) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of recombinant HIV1 integrase bound with donor DNA-SPA beads using [3H] target DNA as substrate assessed as strand transfer activity prein... |
Eur J Med Chem 83: 609-16 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.061
BindingDB Entry DOI: 10.7270/Q2MG7R34 |
More data for this
Ligand-Target Pair | |
Angiopoietin-1 receptor
(Homo sapiens (Human)) | BDBM50195885

(1-(4-(4-amino-7-(3-chlorophenyl)furo[3,2-c]pyridin...)Show SMILES Nc1ncc(-c2cccc(Cl)c2)c2occ(-c3ccc(NC(=O)Nc4cc(ccc4F)C(F)(F)F)cc3)c12 Show InChI InChI=1S/C27H17ClF4N4O2/c28-17-3-1-2-15(10-17)19-12-34-25(33)23-20(13-38-24(19)23)14-4-7-18(8-5-14)35-26(37)36-22-11-16(27(30,31)32)6-9-21(22)29/h1-13H,(H2,33,34)(H2,35,36,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline K.K.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-Tie2 by HTRF method |
Bioorg Med Chem Lett 17: 250-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.050
BindingDB Entry DOI: 10.7270/Q2SB45D5 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50195891
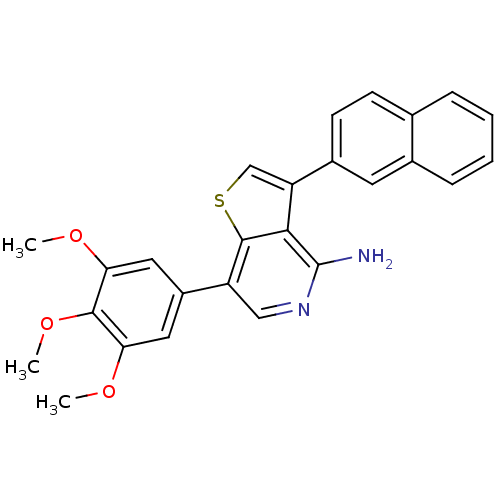
(3-(naphthalen-2-yl)-7-(3,4,5-trimethoxyphenyl)thie...)Show SMILES COc1cc(cc(OC)c1OC)-c1cnc(N)c2c(csc12)-c1ccc2ccccc2c1 Show InChI InChI=1S/C26H22N2O3S/c1-29-21-11-18(12-22(30-2)24(21)31-3)19-13-28-26(27)23-20(14-32-25(19)23)17-9-8-15-6-4-5-7-16(15)10-17/h4-14H,1-3H3,(H2,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline K.K.
Curated by ChEMBL
| Assay Description
Inhibition of human EphB4 by scintillation proximity method |
Bioorg Med Chem Lett 17: 250-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.050
BindingDB Entry DOI: 10.7270/Q2SB45D5 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50411421

(CHEMBL232600)Show SMILES CN(C)CC(=O)N1CCc2c(C1)sc(NC(=O)c1cccc3ccccc13)c2C#N Show InChI InChI=1S/C23H22N4O2S/c1-26(2)14-21(28)27-11-10-17-19(12-24)23(30-20(17)13-27)25-22(29)18-9-5-7-15-6-3-4-8-16(15)18/h3-9H,10-11,13-14H2,1-2H3,(H,25,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of human truncated JNK3 |
Bioorg Med Chem Lett 17: 1296-301 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.003
BindingDB Entry DOI: 10.7270/Q2TB1840 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50021582
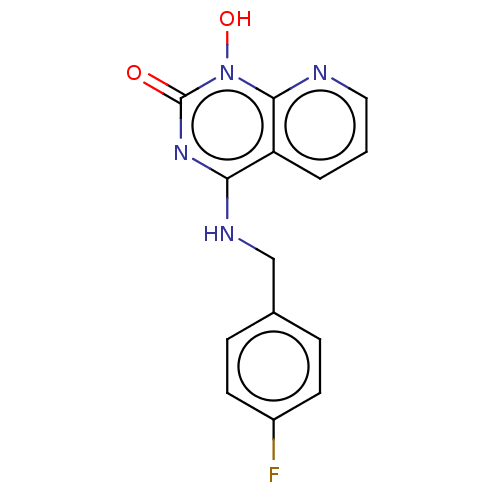
(CHEMBL3286442)Show InChI InChI=1S/C14H11FN4O2/c15-10-5-3-9(4-6-10)8-17-12-11-2-1-7-16-13(11)19(21)14(20)18-12/h1-7,21H,8H2,(H,17,18,20) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of recombinant HIV1 integrase bound with donor DNA-SPA beads using [3H] target DNA as substrate assessed as strand transfer activity prein... |
Eur J Med Chem 83: 609-16 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.061
BindingDB Entry DOI: 10.7270/Q2MG7R34 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50411416
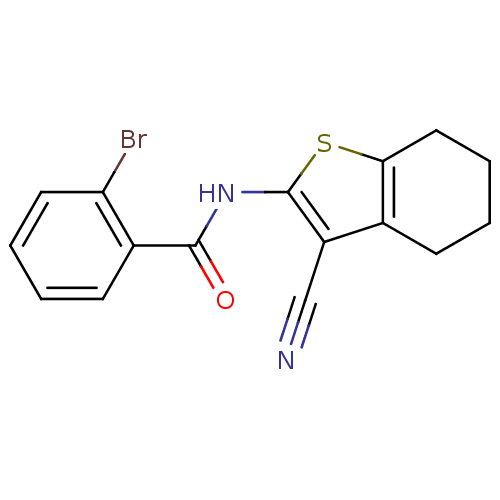
(CHEMBL234839)Show InChI InChI=1S/C16H13BrN2OS/c17-13-7-3-1-6-11(13)15(20)19-16-12(9-18)10-5-2-4-8-14(10)21-16/h1,3,6-7H,2,4-5,8H2,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of human truncated JNK3 |
Bioorg Med Chem Lett 17: 1296-301 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.003
BindingDB Entry DOI: 10.7270/Q2TB1840 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50411415
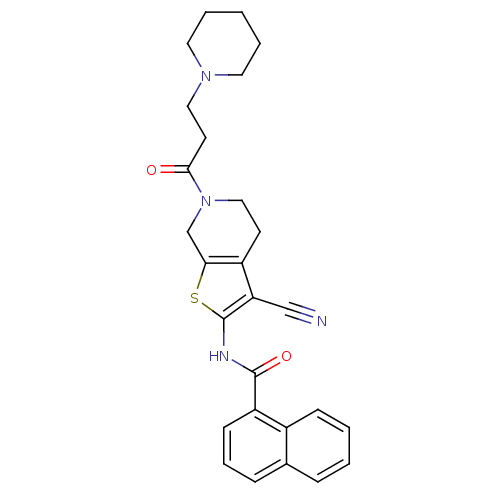
(CHEMBL232599)Show SMILES O=C(CCN1CCCCC1)N1CCc2c(C1)sc(NC(=O)c1cccc3ccccc13)c2C#N Show InChI InChI=1S/C27H28N4O2S/c28-17-23-21-11-16-31(25(32)12-15-30-13-4-1-5-14-30)18-24(21)34-27(23)29-26(33)22-10-6-8-19-7-2-3-9-20(19)22/h2-3,6-10H,1,4-5,11-16,18H2,(H,29,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of human truncated JNK3 |
Bioorg Med Chem Lett 17: 1296-301 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.003
BindingDB Entry DOI: 10.7270/Q2TB1840 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50411414
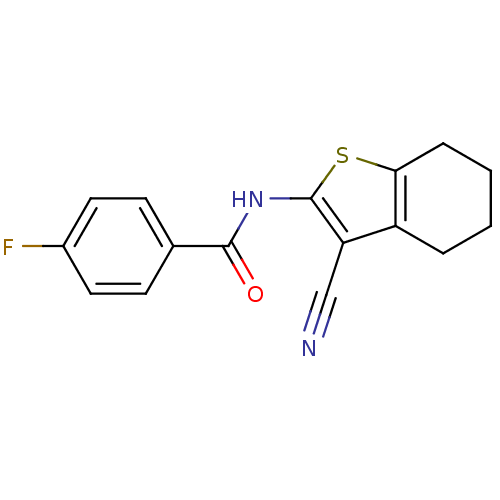
(CHEMBL397570 | SB-347804)Show InChI InChI=1S/C16H13FN2OS/c17-11-7-5-10(6-8-11)15(20)19-16-13(9-18)12-3-1-2-4-14(12)21-16/h5-8H,1-4H2,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of human truncated JNK3 |
Bioorg Med Chem Lett 17: 1296-301 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.003
BindingDB Entry DOI: 10.7270/Q2TB1840 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50411410
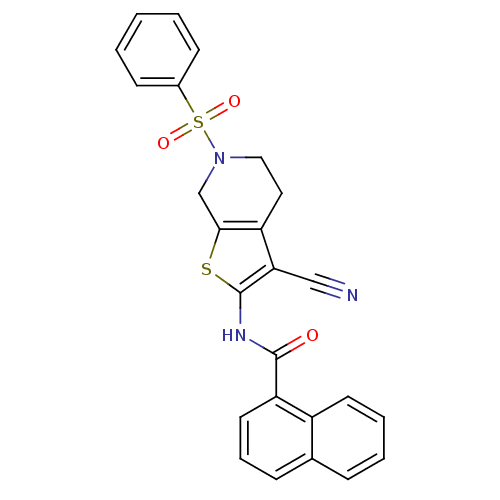
(CHEMBL398601)Show SMILES O=C(Nc1sc2CN(CCc2c1C#N)S(=O)(=O)c1ccccc1)c1cccc2ccccc12 Show InChI InChI=1S/C25H19N3O3S2/c26-15-22-20-13-14-28(33(30,31)18-9-2-1-3-10-18)16-23(20)32-25(22)27-24(29)21-12-6-8-17-7-4-5-11-19(17)21/h1-12H,13-14,16H2,(H,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of human truncated JNK3 |
Bioorg Med Chem Lett 17: 1296-301 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.003
BindingDB Entry DOI: 10.7270/Q2TB1840 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50195889
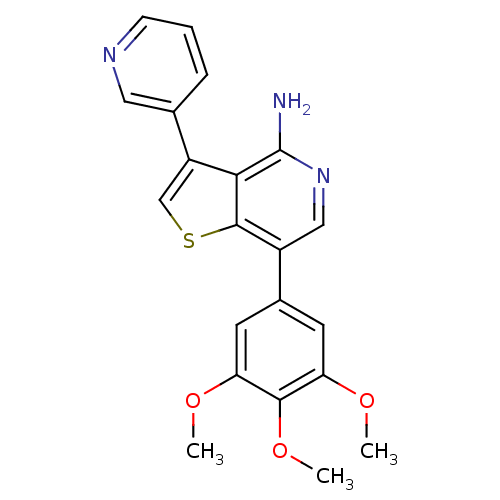
(3-(pyridin-3-yl)-7-(3,4,5-trimethoxyphenyl)thieno[...)Show SMILES COc1cc(cc(OC)c1OC)-c1cnc(N)c2c(csc12)-c1cccnc1 Show InChI InChI=1S/C21H19N3O3S/c1-25-16-7-13(8-17(26-2)19(16)27-3)14-10-24-21(22)18-15(11-28-20(14)18)12-5-4-6-23-9-12/h4-11H,1-3H3,(H2,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline K.K.
Curated by ChEMBL
| Assay Description
Inhibition of human EphB4 by scintillation proximity method |
Bioorg Med Chem Lett 17: 250-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.050
BindingDB Entry DOI: 10.7270/Q2SB45D5 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50021584
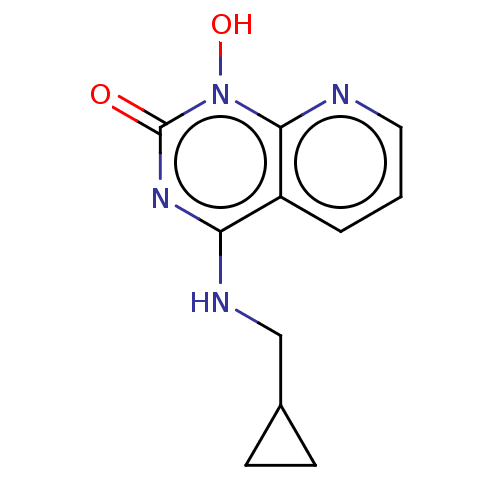
(CHEMBL3290939)Show InChI InChI=1S/C11H12N4O2/c16-11-14-9(13-6-7-3-4-7)8-2-1-5-12-10(8)15(11)17/h1-2,5,7,17H,3-4,6H2,(H,13,14,16) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of recombinant HIV1 integrase bound with donor DNA-SPA beads using [3H] target DNA as substrate assessed as strand transfer activity prein... |
Eur J Med Chem 83: 609-16 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.061
BindingDB Entry DOI: 10.7270/Q2MG7R34 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50411415

(CHEMBL232599)Show SMILES O=C(CCN1CCCCC1)N1CCc2c(C1)sc(NC(=O)c1cccc3ccccc13)c2C#N Show InChI InChI=1S/C27H28N4O2S/c28-17-23-21-11-16-31(25(32)12-15-30-13-4-1-5-14-30)18-24(21)34-27(23)29-26(33)22-10-6-8-19-7-2-3-9-20(19)22/h2-3,6-10H,1,4-5,11-16,18H2,(H,29,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 5.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of human JNK2alpha2 |
Bioorg Med Chem Lett 17: 1296-301 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.003
BindingDB Entry DOI: 10.7270/Q2TB1840 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50195881
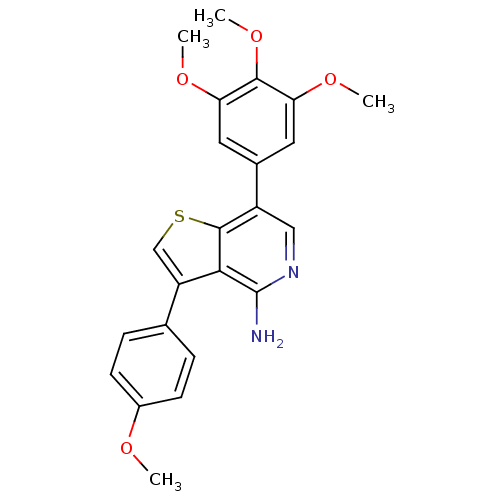
(3-(4-methoxyphenyl)-7-(3,4,5-trimethoxyphenyl)thie...)Show SMILES COc1ccc(cc1)-c1csc2c(cnc(N)c12)-c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C23H22N2O4S/c1-26-15-7-5-13(6-8-15)17-12-30-22-16(11-25-23(24)20(17)22)14-9-18(27-2)21(29-4)19(10-14)28-3/h5-12H,1-4H3,(H2,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline K.K.
Curated by ChEMBL
| Assay Description
Inhibition of human EphB4 by scintillation proximity method |
Bioorg Med Chem Lett 17: 250-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.050
BindingDB Entry DOI: 10.7270/Q2SB45D5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data