 Found 74207 hits with Last Name = 'ho' and Initial = 'd'
Found 74207 hits with Last Name = 'ho' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50133817
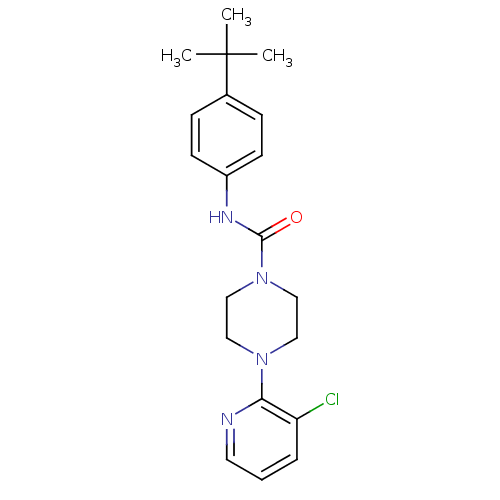
(4-(3-Chloro-pyridin-2-yl)-piperazine-1-carboxylic ...)Show SMILES CC(C)(C)c1ccc(NC(=O)N2CCN(CC2)c2ncccc2Cl)cc1 Show InChI InChI=1S/C20H25ClN4O/c1-20(2,3)15-6-8-16(9-7-15)23-19(26)25-13-11-24(12-14-25)18-17(21)5-4-10-22-18/h4-10H,11-14H2,1-3H3,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.000950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pudue Pharma Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 306: 377-86 (2003)
Article DOI: 10.1124/jpet.102.045674
BindingDB Entry DOI: 10.7270/Q2TX3CX5 |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM50160957
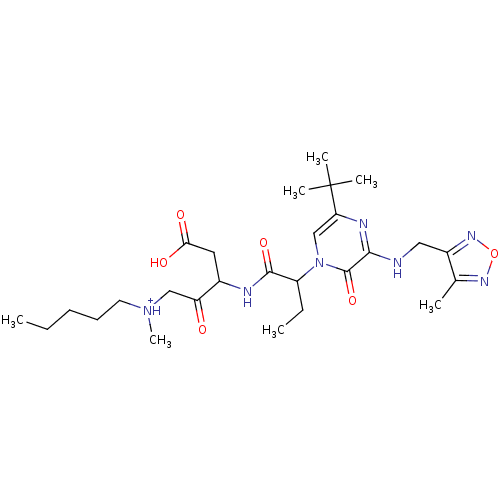
(CHEMBL179503 | [3-(2-{5-tert-Butyl-3-[(4-methyl-fu...)Show SMILES CCCCC[NH+](C)CC(=O)C(CC(O)=O)NC(=O)C(CC)n1cc(nc(NCc2nonc2C)c1=O)C(C)(C)C Show InChI InChI=1S/C27H43N7O6/c1-8-10-11-12-33(7)15-21(35)18(13-23(36)37)29-25(38)20(9-2)34-16-22(27(4,5)6)30-24(26(34)39)28-14-19-17(3)31-40-32-19/h16,18,20H,8-15H2,1-7H3,(H,28,30)(H,29,38)(H,36,37)/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human caspase-3 in neuronal precursor (NT2) cells |
Bioorg Med Chem Lett 15: 1173-80 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.006
BindingDB Entry DOI: 10.7270/Q2D50MGS |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50293089
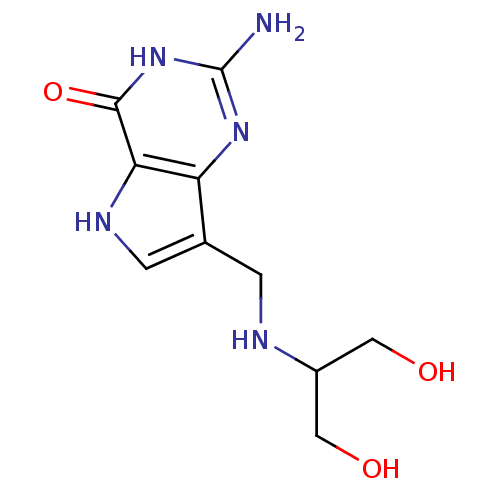
(2-Amino-7-{[(1,3-dihydroxypropan-2-yl)amino]methyl...)Show InChI InChI=1S/C10H15N5O3/c11-10-14-7-5(1-12-6(3-16)4-17)2-13-8(7)9(18)15-10/h2,6,12-13,16-17H,1,3-4H2,(H3,11,14,15,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human PNP by xanthine-oxidase coupled assay |
J Med Chem 52: 1126-43 (2009)
Article DOI: 10.1021/jm801421q
BindingDB Entry DOI: 10.7270/Q2QR4Z18 |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM22925

((8R)-3-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxol...)Show SMILES OC[C@H]1O[C@H](C[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |c:17| Show InChI InChI=1S/C11H16N4O4/c16-3-8-6(17)1-9(19-8)15-5-14-10-7(18)2-12-4-13-11(10)15/h4-9,16-18H,1-3H2,(H,12,13)/t6-,7+,8+,9+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 0.00250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was tested in vitro for inhibition of human erythrocytic adenosine deaminase. |
J Med Chem 26: 1478-82 (1983)
Checked by Author
BindingDB Entry DOI: 10.7270/Q29Z95GT |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50293090
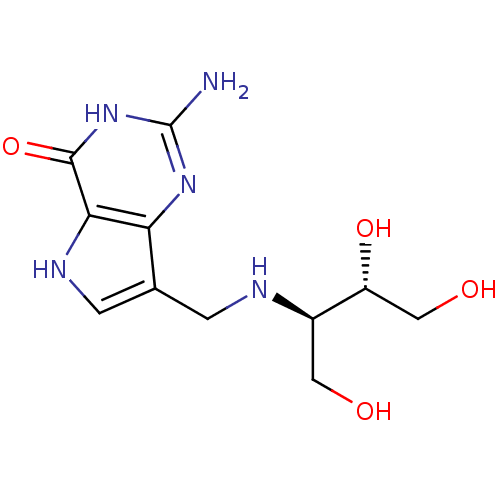
(2-Amino-7-({[(2R,3S)-1,3,4-trihydroxybutan-2-yl]am...)Show SMILES Nc1nc2c(CN[C@H](CO)[C@H](O)CO)c[nH]c2c(=O)[nH]1 |r| Show InChI InChI=1S/C11H17N5O4/c12-11-15-8-5(2-14-9(8)10(20)16-11)1-13-6(3-17)7(19)4-18/h2,6-7,13-14,17-19H,1,3-4H2,(H3,12,15,16,20)/t6-,7-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human PNP by xanthine-oxidase coupled assay |
J Med Chem 52: 1126-43 (2009)
Article DOI: 10.1021/jm801421q
BindingDB Entry DOI: 10.7270/Q2QR4Z18 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50246593
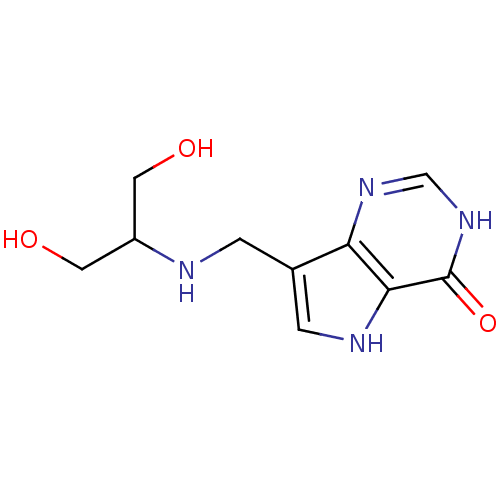
(7-((1,3-dihydroxypropan-2-ylamino)methyl)-3H-pyrro...)Show InChI InChI=1S/C10H14N4O3/c15-3-7(4-16)11-1-6-2-12-9-8(6)13-5-14-10(9)17/h2,5,7,11-12,15-16H,1,3-4H2,(H,13,14,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human PNP by xanthine-oxidase coupled assay |
J Med Chem 52: 1126-43 (2009)
Article DOI: 10.1021/jm801421q
BindingDB Entry DOI: 10.7270/Q2QR4Z18 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Caspase-3
(Homo sapiens (Human)) | BDBM50160974
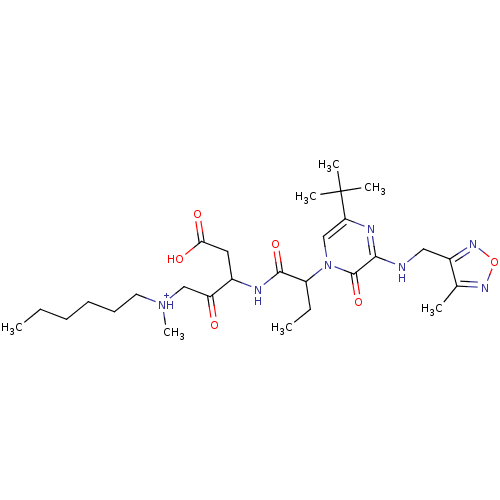
(CHEMBL366927 | [3-(2-{5-tert-Butyl-3-[(4-methyl-fu...)Show SMILES CCCCCC[NH+](C)CC(=O)C(CC(O)=O)NC(=O)C(CC)n1cc(nc(NCc2nonc2C)c1=O)C(C)(C)C Show InChI InChI=1S/C28H45N7O6/c1-8-10-11-12-13-34(7)16-22(36)19(14-24(37)38)30-26(39)21(9-2)35-17-23(28(4,5)6)31-25(27(35)40)29-15-20-18(3)32-41-33-20/h17,19,21H,8-16H2,1-7H3,(H,29,31)(H,30,39)(H,37,38)/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human caspase-3 in neuronal precursor (NT2) cells |
Bioorg Med Chem Lett 15: 1173-80 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.006
BindingDB Entry DOI: 10.7270/Q2D50MGS |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-2
(Rattus norvegicus (Rat)) | BDBM50295955
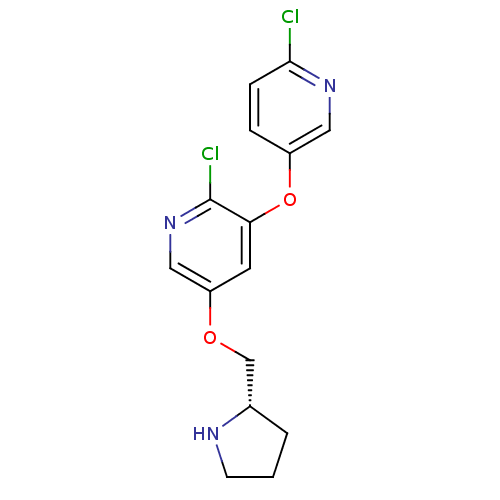
(5-(((S)-Pyrrolidin-2-yl)methoxy)-3-((6-chloropyrid...)Show SMILES Clc1ccc(Oc2cc(OC[C@@H]3CCCN3)cnc2Cl)cn1 |r| Show InChI InChI=1S/C15H15Cl2N3O2/c16-14-4-3-11(7-19-14)22-13-6-12(8-20-15(13)17)21-9-10-2-1-5-18-10/h3-4,6-8,10,18H,1-2,5,9H2/t10-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from rat alpha2beta2 nicotinic receptor expressed in human HEK293 cells by liquid scintillation counting |
Bioorg Med Chem 17: 4367-77 (2009)
Article DOI: 10.1016/j.bmc.2009.05.021
BindingDB Entry DOI: 10.7270/Q2DN453T |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50500526
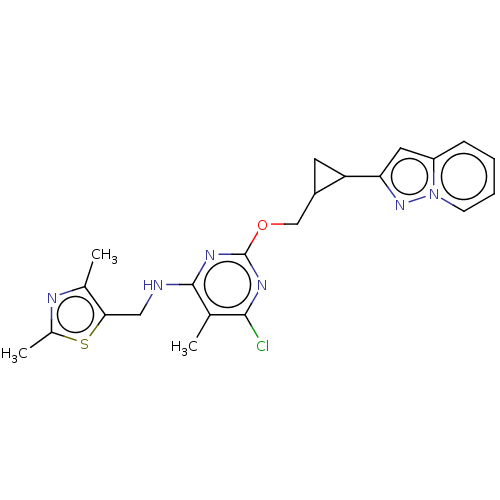
(CHEMBL3747517)Show SMILES Cc1nc(C)c(CNc2nc(OCC3CC3c3cc4ccccn4n3)nc(Cl)c2C)s1 Show InChI InChI=1S/C22H23ClN6OS/c1-12-20(23)26-22(27-21(12)24-10-19-13(2)25-14(3)31-19)30-11-15-8-17(15)18-9-16-6-4-5-7-29(16)28-18/h4-7,9,15,17H,8,10-11H2,1-3H3,(H,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50458766
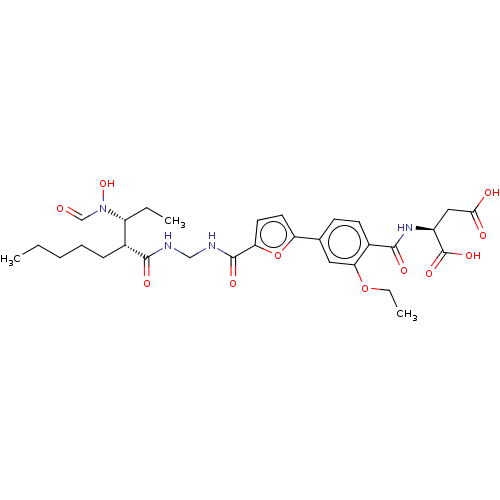
(CHEMBL4212386)Show SMILES CCCCC[C@H]([C@@H](CC)N(O)C=O)C(=O)NCNC(=O)c1ccc(o1)-c1ccc(C(=O)N[C@@H](CC(O)=O)C(O)=O)c(OCC)c1 |r| Show InChI InChI=1S/C30H40N4O11/c1-4-7-8-9-19(22(5-2)34(43)17-35)27(38)31-16-32-29(40)24-13-12-23(45-24)18-10-11-20(25(14-18)44-6-3)28(39)33-21(30(41)42)15-26(36)37/h10-14,17,19,21-22,43H,4-9,15-16H2,1-3H3,(H,31,38)(H,32,40)(H,33,39)(H,36,37)(H,41,42)/t19-,21+,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.00680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to BMP1 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate preincubated for 3 hrs followed by subs... |
ACS Med Chem Lett 9: 736-740 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00173
BindingDB Entry DOI: 10.7270/Q2X35127 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50293087
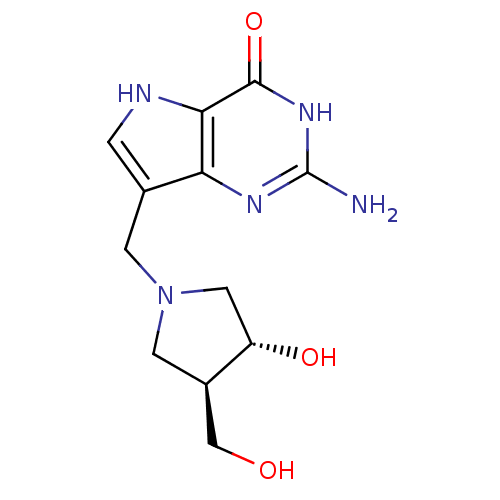
(2-amino-7-(((3R,4R)-3-hydroxy-4-(hydroxymethyl)pyr...)Show SMILES Nc1nc2c(CN3C[C@H](O)[C@@H](CO)C3)c[nH]c2c(=O)[nH]1 |r| Show InChI InChI=1S/C12H17N5O3/c13-12-15-9-6(1-14-10(9)11(20)16-12)2-17-3-7(5-18)8(19)4-17/h1,7-8,14,18-19H,2-5H2,(H3,13,15,16,20)/t7-,8+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human PNP by xanthine-oxidase coupled assay |
J Med Chem 52: 1126-43 (2009)
Article DOI: 10.1021/jm801421q
BindingDB Entry DOI: 10.7270/Q2QR4Z18 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50293091

(7-({[(1R,2S)-2,3-DIHYDROXY-1-(HYDROXYMETHYL)PROPYL...)Show SMILES OC[C@@H](O)[C@@H](CO)NCc1c[nH]c2c1nc[nH]c2=O |r| Show InChI InChI=1S/C11H16N4O4/c16-3-7(8(18)4-17)12-1-6-2-13-10-9(6)14-5-15-11(10)19/h2,5,7-8,12-13,16-18H,1,3-4H2,(H,14,15,19)/t7-,8-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human PNP by xanthine-oxidase coupled assay |
J Med Chem 52: 1126-43 (2009)
Article DOI: 10.1021/jm801421q
BindingDB Entry DOI: 10.7270/Q2QR4Z18 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM22109
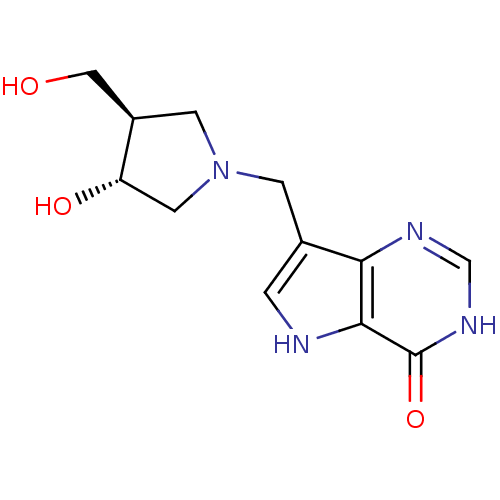
(7-{[(3R,4R)-3-hydroxy-4-(hydroxymethyl)pyrrolidin-...)Show SMILES OC[C@H]1CN(Cc2c[nH]c3c2nc[nH]c3=O)C[C@@H]1O |r| Show InChI InChI=1S/C12H16N4O3/c17-5-8-3-16(4-9(8)18)2-7-1-13-11-10(7)14-6-15-12(11)19/h1,6,8-9,13,17-18H,2-5H2,(H,14,15,19)/t8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human PNP by xanthine-oxidase coupled assay |
J Med Chem 52: 1126-43 (2009)
Article DOI: 10.1021/jm801421q
BindingDB Entry DOI: 10.7270/Q2QR4Z18 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50573166
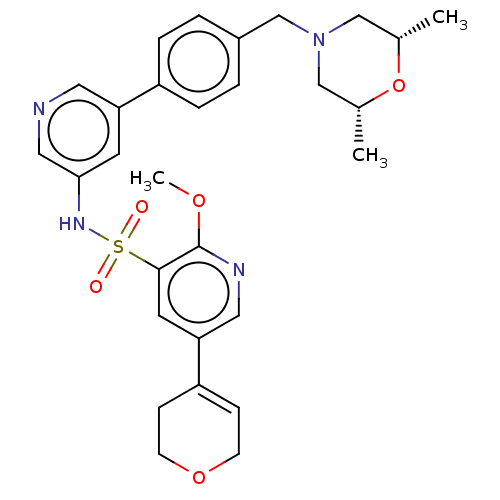
(CHEMBL4869783)Show SMILES COc1ncc(cc1S(=O)(=O)Nc1cncc(c1)-c1ccc(CN2C[C@H](C)O[C@H](C)C2)cc1)C1=CCOCC1 |r,t:37| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01102
BindingDB Entry DOI: 10.7270/Q2N87FKX |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(MOUSE) | BDBM82462
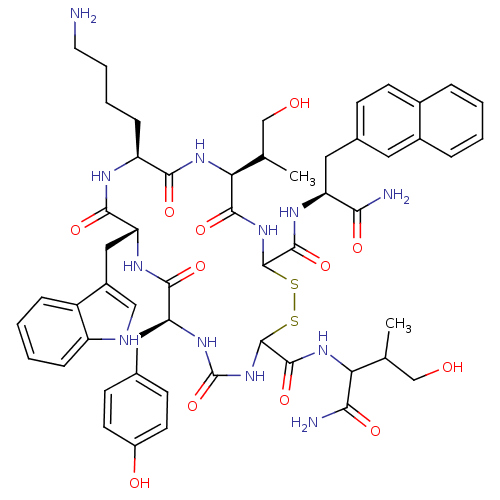
(BIM 23059 | D-Nal-c[Cys-Tyr-D-Trp-Lys-Thr-Cys]-Thr...)Show SMILES CC(CO)C(NC(=O)C1NC(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)CO)C(=O)NC(SS1)C(=O)N[C@@H](Cc1ccc2ccccc2c1)C(N)=O)C(N)=O |r| Show InChI InChI=1S/C54H68N12O12S2/c1-28(26-67)42(45(57)71)63-51(77)53-66-54(78)62-40(22-30-15-18-35(69)19-16-30)47(73)61-41(24-34-25-58-37-12-6-5-11-36(34)37)48(74)59-38(13-7-8-20-55)46(72)64-43(29(2)27-68)49(75)65-52(79-80-53)50(76)60-39(44(56)70)23-31-14-17-32-9-3-4-10-33(32)21-31/h3-6,9-12,14-19,21,25,28-29,38-43,52-53,58,67-69H,7-8,13,20,22-24,26-27,55H2,1-2H3,(H2,56,70)(H2,57,71)(H,59,74)(H,60,76)(H,61,73)(H,63,77)(H,64,72)(H,65,75)(H2,62,66,78)/t28?,29?,38-,39-,40-,41+,42?,43-,52?,53?/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Ltd.
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 350: 441-53 (1994)
Article DOI: 10.1007/BF00173012
BindingDB Entry DOI: 10.7270/Q2XD105R |
More data for this
Ligand-Target Pair | |
Galanin receptor type 1
(RAT) | BDBM50273370
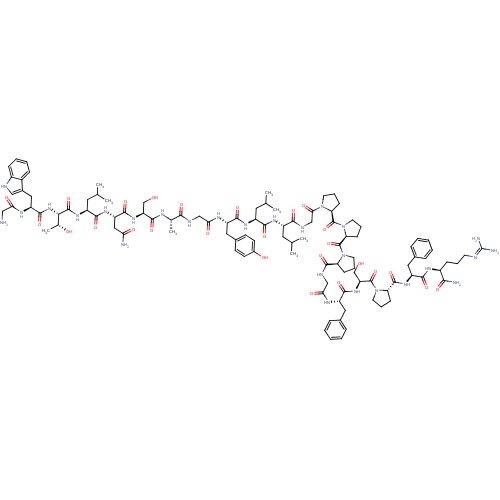
(CHEMBL503473 | GWTLNSAGYLLGPPPGFSPFR-CONH2 | Galan...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CN)[C@@H](C)O)C(=O)NCC(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCN=C(N)N)C(N)=O |r,wU:106.112,4.4,8.12,32.33,37.40,43.48,51.56,63.76,wD:149.157,138.145,134.142,125.131,114.119,99.104,92.96,16.25,59.60,79.83,(16.53,-9.13,;15.19,-9.9,;15.2,-11.44,;13.86,-9.13,;12.52,-9.9,;11.19,-9.13,;11.19,-7.59,;12.53,-6.82,;9.86,-6.82,;9.86,-5.28,;11.2,-4.51,;12.52,-5.28,;11.2,-2.97,;8.13,-7.82,;6.39,-6.82,;6.39,-5.28,;4.66,-7.83,;4.66,-9.36,;5.99,-10.13,;5.99,-11.67,;7.32,-12.44,;8.66,-11.67,;9.99,-12.44,;8.66,-10.13,;7.33,-9.36,;2.92,-6.82,;2.92,-5.28,;4.26,-4.52,;1.19,-4.28,;1.19,-2.75,;2.52,-1.97,;3.86,-2.74,;2.52,-.43,;3.86,.34,;1.2,.34,;1.19,1.88,;2.53,2.65,;-.14,2.65,;-.14,4.19,;1.19,4.96,;-1.48,1.88,;-2.81,2.65,;-2.81,4.19,;-4.14,1.88,;-5.48,2.64,;-5.47,4.19,;-6.81,4.96,;-4.14,4.96,;-4.15,.34,;-5.48,-.43,;-6.81,.34,;-5.48,-1.97,;-6.81,-2.74,;-8.14,-1.97,;-8.14,-.43,;-9.47,-2.74,;-4.14,-2.74,;-4.14,-4.28,;-5.48,-5.05,;-2.81,-5.05,;-2.81,-6.59,;-1.48,-7.36,;-.15,-6.59,;-1.48,-8.9,;-2.85,-9.59,;-4.14,-8.76,;-4.22,-7.22,;-5.71,-6.82,;-6.55,-8.12,;-8.07,-8.35,;-8.62,-9.79,;-7.65,-10.99,;-6.13,-10.75,;-5.58,-9.31,;-.14,-9.67,;1.19,-8.9,;1.19,-7.36,;2.53,-9.67,;2.53,-11.21,;-1.47,-4.28,;-.15,-5.05,;-1.47,-2.74,;12.53,-11.44,;13.86,-12.21,;11.19,-12.21,;11.19,-13.75,;12.53,-14.52,;13.86,-13.75,;12.53,-16.06,;11.28,-16.96,;11.76,-18.43,;13.29,-18.43,;13.77,-16.97,;15.24,-16.49,;15.64,-15,;16.65,-17.91,;16.41,-19.42,;17.78,-20.13,;18.87,-19.04,;18.18,-17.67,;19.08,-15.88,;20.62,-15.8,;18.31,-14.55,;16.78,-14.38,;16.46,-12.88,;17.8,-12.12,;18.94,-13.14,;20.31,-12.44,;21.65,-13.21,;20.31,-10.9,;21.64,-10.13,;22.97,-10.89,;24.3,-10.12,;22.98,-12.43,;24.31,-13.2,;24.32,-14.74,;25.65,-15.5,;25.66,-17.04,;26.99,-17.81,;28.33,-17.04,;28.32,-15.5,;26.99,-14.73,;25.64,-12.43,;25.64,-10.89,;26.98,-13.19,;28.31,-12.42,;29.65,-13.18,;30.98,-12.41,;28.31,-10.88,;26.97,-10.11,;29.64,-10.1,;31.05,-10.72,;32.07,-9.57,;31.3,-8.24,;29.8,-8.57,;28.51,-7.72,;27.17,-8.47,;28.53,-6.17,;29.88,-5.42,;31.2,-6.21,;32.54,-5.45,;32.56,-3.91,;33.91,-3.16,;35.23,-3.95,;35.21,-5.49,;33.87,-6.24,;29.9,-3.88,;31.24,-3.12,;28.57,-3.1,;28.59,-1.55,;29.93,-.8,;29.95,.74,;28.63,1.53,;28.65,3.07,;29.99,3.82,;30.01,5.36,;31.32,3.03,;27.27,-.77,;27.29,.77,;25.92,-1.52,)| Show InChI InChI=1S/C107H153N27O26/c1-57(2)42-70(123-93(147)71(43-58(3)4)124-95(149)74(47-64-33-35-66(138)36-34-64)121-86(141)52-115-91(145)60(7)118-100(154)78(55-135)128-98(152)77(49-84(109)139)125-94(148)72(44-59(5)6)127-103(157)89(61(8)137)130-99(153)76(119-85(140)50-108)48-65-51-114-68-27-16-15-26-67(65)68)92(146)117-54-88(143)131-38-20-31-82(131)105(159)134-41-21-32-83(134)106(160)133-40-18-29-80(133)101(155)116-53-87(142)120-73(45-62-22-11-9-12-23-62)96(150)129-79(56-136)104(158)132-39-19-30-81(132)102(156)126-75(46-63-24-13-10-14-25-63)97(151)122-69(90(110)144)28-17-37-113-107(111)112/h9-16,22-27,33-36,51,57-61,69-83,89,114,135-138H,17-21,28-32,37-50,52-56,108H2,1-8H3,(H2,109,139)(H2,110,144)(H,115,145)(H,116,155)(H,117,146)(H,118,154)(H,119,140)(H,120,142)(H,121,141)(H,122,151)(H,123,147)(H,124,149)(H,125,148)(H,126,156)(H,127,157)(H,128,152)(H,129,150)(H,130,153)(H4,111,112,113)/t60-,61+,69-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,82-,83-,89-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
FEBS Lett 405: 285-90 (1997)
Article DOI: 10.1016/s0014-5793(97)00196-8
BindingDB Entry DOI: 10.7270/Q2VH5MBC |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(RAT) | BDBM82463
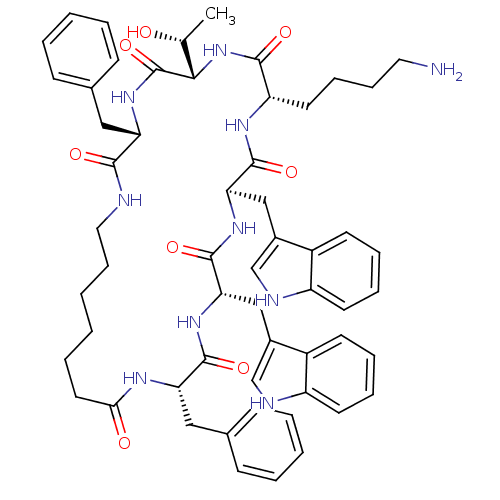
(Cyclo[L-Trp-D-Trp-L-Lys-L-Thr-L-Phe-(7-amino*hepta...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)CCCCCCNC(=O)[C@H](Cc2ccccc2)NC1=O Show InChI InChI=1S/C57H70N10O8/c1-36(68)51-57(75)66-46(30-37-18-6-4-7-19-37)52(70)59-29-17-3-2-10-27-50(69)62-47(31-38-20-8-5-9-21-38)54(72)64-49(33-40-35-61-44-25-14-12-23-42(40)44)56(74)65-48(32-39-34-60-43-24-13-11-22-41(39)43)55(73)63-45(53(71)67-51)26-15-16-28-58/h4-9,11-14,18-25,34-36,45-49,51,60-61,68H,2-3,10,15-17,26-33,58H2,1H3,(H,59,70)(H,62,69)(H,63,73)(H,64,72)(H,65,74)(H,66,75)(H,67,71)/t36-,45+,46+,47+,48-,49+,51+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Ltd.
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 350: 441-53 (1994)
Article DOI: 10.1007/BF00173012
BindingDB Entry DOI: 10.7270/Q2XD105R |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50109062
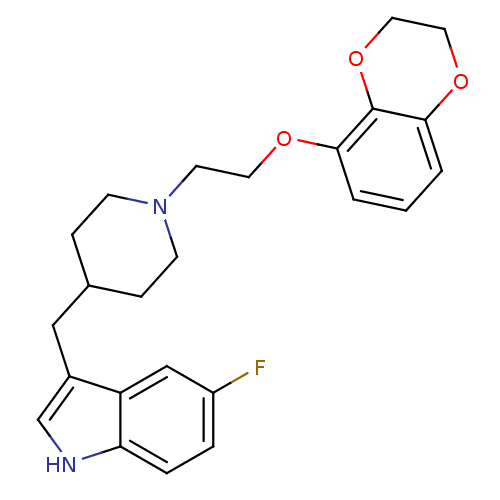
(3-{1-[2-(2,3-Dihydro-benzo[1,4]dioxin-5-yloxy)-eth...)Show SMILES Fc1ccc2[nH]cc(CC3CCN(CCOc4cccc5OCCOc45)CC3)c2c1 Show InChI InChI=1S/C24H27FN2O3/c25-19-4-5-21-20(15-19)18(16-26-21)14-17-6-8-27(9-7-17)10-11-28-22-2-1-3-23-24(22)30-13-12-29-23/h1-5,15-17,26H,6-14H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity of the compound for RB Serotonin transporter was determined in vitro by incubating compound |
Bioorg Med Chem Lett 12: 307-10 (2002)
BindingDB Entry DOI: 10.7270/Q2FJ2G2M |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(MOUSE) | BDBM82469
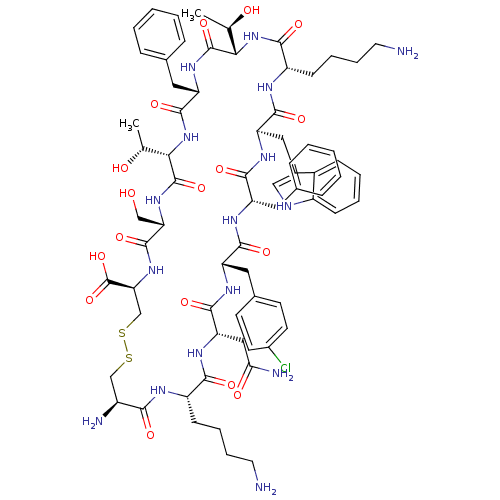
(BIM 23003 | BIM-23003 | EC5-21 | L-Cys(1)-L-Lys-L-...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc(Cl)cc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](Cc2ccccc2)NC1=O)[C@@H](C)O)C(O)=O Show InChI InChI=1S/C71H95ClN16O17S2/c1-38(90)58-69(102)84-52(30-41-17-7-4-8-18-41)67(100)88-59(39(2)91)70(103)85-55(35-89)68(101)86-56(71(104)105)37-107-106-36-46(75)60(93)78-48(21-11-13-27-73)61(94)83-54(33-57(76)92)66(99)81-51(31-42-23-25-44(72)26-24-42)63(96)80-50(29-40-15-5-3-6-16-40)64(97)82-53(32-43-34-77-47-20-10-9-19-45(43)47)65(98)79-49(62(95)87-58)22-12-14-28-74/h3-10,15-20,23-26,34,38-39,46,48-56,58-59,77,89-91H,11-14,21-22,27-33,35-37,73-75H2,1-2H3,(H2,76,92)(H,78,93)(H,79,98)(H,80,96)(H,81,99)(H,82,97)(H,83,94)(H,84,102)(H,85,103)(H,86,101)(H,87,95)(H,88,100)(H,104,105)/t38-,39-,46+,48+,49+,50+,51+,52+,53-,54+,55+,56+,58+,59+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Ltd.
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 350: 441-53 (1994)
Article DOI: 10.1007/BF00173012
BindingDB Entry DOI: 10.7270/Q2XD105R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50573157
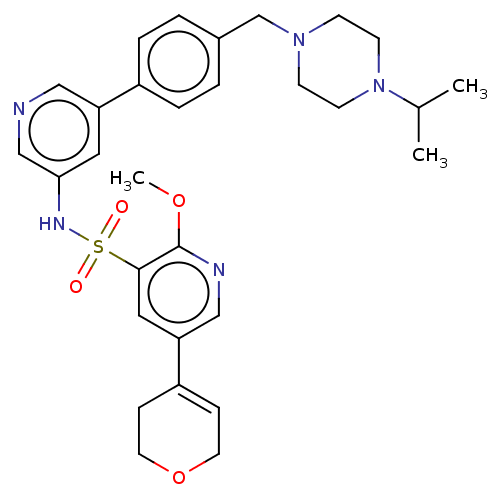
(CHEMBL4850297)Show SMILES COc1ncc(cc1S(=O)(=O)Nc1cncc(c1)-c1ccc(CN2CCN(CC2)C(C)C)cc1)C1=CCOCC1 |t:38| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01102
BindingDB Entry DOI: 10.7270/Q2N87FKX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50573157

(CHEMBL4850297)Show SMILES COc1ncc(cc1S(=O)(=O)Nc1cncc(c1)-c1ccc(CN2CCN(CC2)C(C)C)cc1)C1=CCOCC1 |t:38| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01102
BindingDB Entry DOI: 10.7270/Q2N87FKX |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50170654
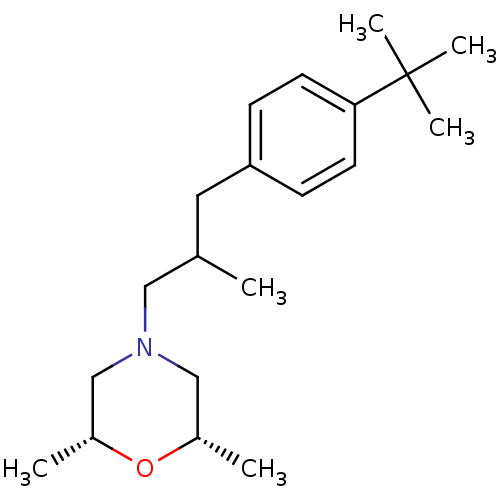
((+-)-cis-4-(3-(4-tert-butylphenyl)-2-methylpropyl)...)Show SMILES CC(CN1C[C@H](C)O[C@H](C)C1)Cc1ccc(cc1)C(C)(C)C |r| Show InChI InChI=1S/C20H33NO/c1-15(12-21-13-16(2)22-17(3)14-21)11-18-7-9-19(10-8-18)20(4,5)6/h7-10,15-17H,11-14H2,1-6H3/t15?,16-,17+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck
Curated by ChEMBL
| Assay Description
Affinity for sigma receptor type 1 of guinea pig using [3H]ifenprodil or (+)-[3H]pentazocine radioligand |
J Med Chem 48: 4754-64 (2005)
Article DOI: 10.1021/jm049073+
BindingDB Entry DOI: 10.7270/Q2639QHB |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM50367032
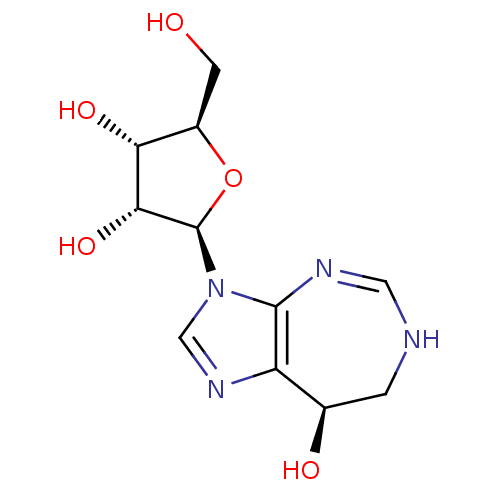
(COFORMYCIN)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |r,c:18| Show InChI InChI=1S/C11H16N4O5/c16-2-6-8(18)9(19)11(20-6)15-4-14-7-5(17)1-12-3-13-10(7)15/h3-6,8-9,11,16-19H,1-2H2,(H,12,13)/t5-,6-,8-,9-,11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was tested in vitro for inhibition of human erythrocytic adenosine deaminase |
J Med Chem 26: 1478-82 (1983)
Checked by Author
BindingDB Entry DOI: 10.7270/Q29Z95GT |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50500520

(CHEMBL3746993)Show SMILES Cc1nc(C)c(CNc2nc(OCC3CC3c3ccc4ccccc4n3)nc(Cl)c2C)s1 Show InChI InChI=1S/C24H24ClN5OS/c1-13-22(25)29-24(30-23(13)26-11-21-14(2)27-15(3)32-21)31-12-17-10-18(17)20-9-8-16-6-4-5-7-19(16)28-20/h4-9,17-18H,10-12H2,1-3H3,(H,26,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50500521

(CHEMBL3746277)Show SMILES Cc1nc(C)c(CNc2nc(OCC3CC3c3cc(C)ccn3)nc(Cl)c2C)s1 Show InChI InChI=1S/C21H24ClN5OS/c1-11-5-6-23-17(7-11)16-8-15(16)10-28-21-26-19(22)12(2)20(27-21)24-9-18-13(3)25-14(4)29-18/h5-7,15-16H,8-10H2,1-4H3,(H,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50500538

(CHEMBL3745790)Show SMILES COc1ccnc(c1)C1CC1COc1nc(Cl)c(C)c(NCc2sc(C)nc2C)n1 Show InChI InChI=1S/C21H24ClN5O2S/c1-11-19(22)26-21(27-20(11)24-9-18-12(2)25-13(3)30-18)29-10-14-7-16(14)17-8-15(28-4)5-6-23-17/h5-6,8,14,16H,7,9-10H2,1-4H3,(H,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(MOUSE) | BDBM82256
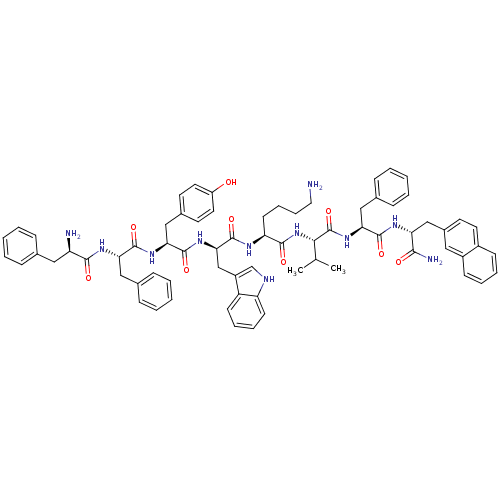
(BIM 23056 | CAS_150155-61-6 | D-Phe-Phe-Tyr-D-Trp-...)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](N)Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H](Cc1ccc2ccccc2c1)C(N)=O |r| Show InChI InChI=1S/C71H81N11O9/c1-44(2)63(71(91)81-61(39-47-22-10-5-11-23-47)67(87)77-58(64(74)84)41-49-29-32-50-24-12-13-25-51(50)36-49)82-66(86)57(28-16-17-35-72)76-70(90)62(42-52-43-75-56-27-15-14-26-54(52)56)80-69(89)60(40-48-30-33-53(83)34-31-48)79-68(88)59(38-46-20-8-4-9-21-46)78-65(85)55(73)37-45-18-6-3-7-19-45/h3-15,18-27,29-34,36,43-44,55,57-63,75,83H,16-17,28,35,37-42,72-73H2,1-2H3,(H2,74,84)(H,76,90)(H,77,87)(H,78,85)(H,79,88)(H,80,89)(H,81,91)(H,82,86)/t55-,57+,58-,59+,60+,61+,62-,63+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Ltd.
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 350: 441-53 (1994)
Article DOI: 10.1007/BF00173012
BindingDB Entry DOI: 10.7270/Q2XD105R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50573166

(CHEMBL4869783)Show SMILES COc1ncc(cc1S(=O)(=O)Nc1cncc(c1)-c1ccc(CN2C[C@H](C)O[C@H](C)C2)cc1)C1=CCOCC1 |r,t:37| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01102
BindingDB Entry DOI: 10.7270/Q2N87FKX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50573177

(CHEMBL4167702)Show SMILES COc1ncc(cc1S(=O)(=O)Nc1ccnc(c1)-c1ccc(CN2CCN(CC2)C(C)C)cc1F)N1CCOCC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01102
BindingDB Entry DOI: 10.7270/Q2N87FKX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50573177

(CHEMBL4167702)Show SMILES COc1ncc(cc1S(=O)(=O)Nc1ccnc(c1)-c1ccc(CN2CCN(CC2)C(C)C)cc1F)N1CCOCC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01102
BindingDB Entry DOI: 10.7270/Q2N87FKX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neurotensin receptor type 1
(MOUSE) | BDBM50281791
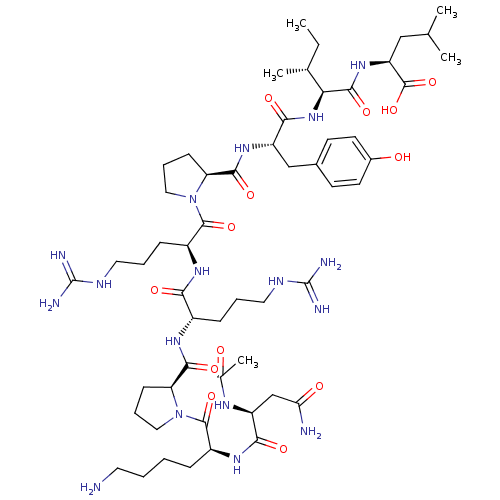
(Ac-Asn-Lys-Pro-Arg-Arg-Pro-Tyr-Ile-Leu-OH | CHEMBL...)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@H](CC(N)=O)NC(C)=O)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C55H91N17O13/c1-6-31(4)44(50(81)69-40(53(84)85)27-30(2)3)70-47(78)38(28-33-18-20-34(74)21-19-33)68-49(80)42-17-12-26-72(42)52(83)37(15-10-24-63-55(60)61)66-45(76)35(14-9-23-62-54(58)59)65-48(79)41-16-11-25-71(41)51(82)36(13-7-8-22-56)67-46(77)39(29-43(57)75)64-32(5)73/h18-21,30-31,35-42,44,74H,6-17,22-29,56H2,1-5H3,(H2,57,75)(H,64,73)(H,65,79)(H,66,76)(H,67,77)(H,68,80)(H,69,81)(H,70,78)(H,84,85)(H4,58,59,62)(H4,60,61,63)/t31-,35+,36+,37+,38+,39+,40+,41+,42+,44+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [125I]-Tyr3-NT(1-13) to NT receptors in neonatal mouse whole brain (minus cerebellum) |
Bioorg Med Chem Lett 3: 949-952 (1993)
Article DOI: 10.1016/S0960-894X(00)80698-8
BindingDB Entry DOI: 10.7270/Q2CJ8DDX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50573167
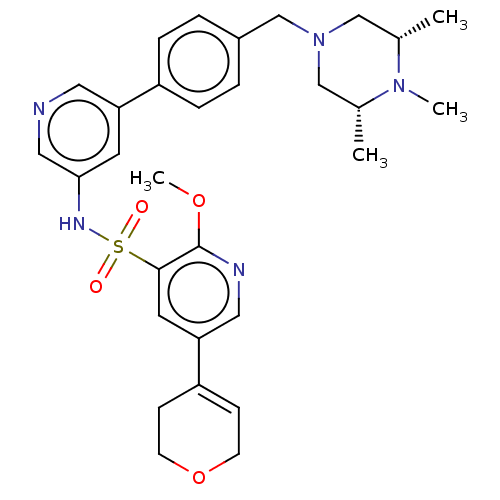
(CHEMBL4858875)Show SMILES COc1ncc(cc1S(=O)(=O)Nc1cncc(c1)-c1ccc(CN2C[C@H](C)N(C)[C@H](C)C2)cc1)C1=CCOCC1 |r,t:38| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01102
BindingDB Entry DOI: 10.7270/Q2N87FKX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50573167

(CHEMBL4858875)Show SMILES COc1ncc(cc1S(=O)(=O)Nc1cncc(c1)-c1ccc(CN2C[C@H](C)N(C)[C@H](C)C2)cc1)C1=CCOCC1 |r,t:38| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01102
BindingDB Entry DOI: 10.7270/Q2N87FKX |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440788
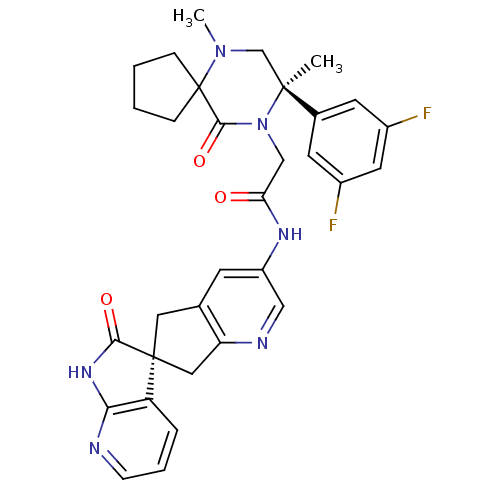
(CHEMBL2431249)Show SMILES CN1C[C@](C)(N(CC(=O)Nc2cnc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)C11CCCC1)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C32H32F2N6O3/c1-30(20-11-21(33)13-22(34)12-20)18-39(2)32(7-3-4-8-32)29(43)40(30)17-26(41)37-23-10-19-14-31(15-25(19)36-16-23)24-6-5-9-35-27(24)38-28(31)42/h5-6,9-13,16H,3-4,7-8,14-15,17-18H2,1-2H3,(H,37,41)(H,35,38,42)/t30-,31-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(MOUSE) | BDBM50240845
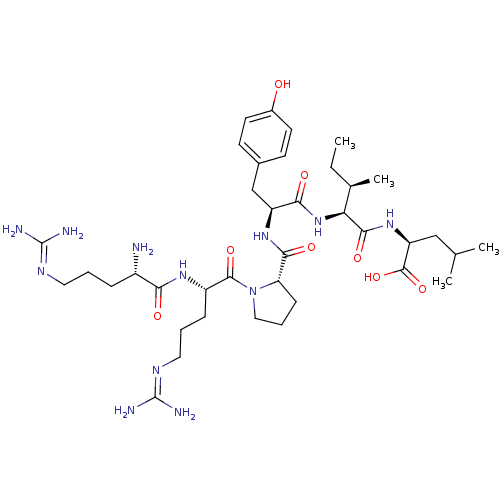
((S)-2-{(2S,3R)-2-[(S)-2-({(S)-1-[(S)-2-((S)-2-Amin...)Show SMILES [#6]-[#6]-[#6@@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](-[#8])=O |r| Show InChI InChI=1S/C38H64N12O8/c1-5-22(4)30(34(55)48-28(36(57)58)19-21(2)3)49-32(53)27(20-23-12-14-24(51)15-13-23)47-33(54)29-11-8-18-50(29)35(56)26(10-7-17-45-38(42)43)46-31(52)25(39)9-6-16-44-37(40)41/h12-15,21-22,25-30,51H,5-11,16-20,39H2,1-4H3,(H,46,52)(H,47,54)(H,48,55)(H,49,53)(H,57,58)(H4,40,41,44)(H4,42,43,45)/t22-,25+,26+,27+,28+,29+,30+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [125I]-Tyr3-NT(1-13) to NT receptors in neonatal mouse whole brain (minus cerebellum) |
Bioorg Med Chem Lett 3: 949-952 (1993)
Article DOI: 10.1016/S0960-894X(00)80698-8
BindingDB Entry DOI: 10.7270/Q2CJ8DDX |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440782
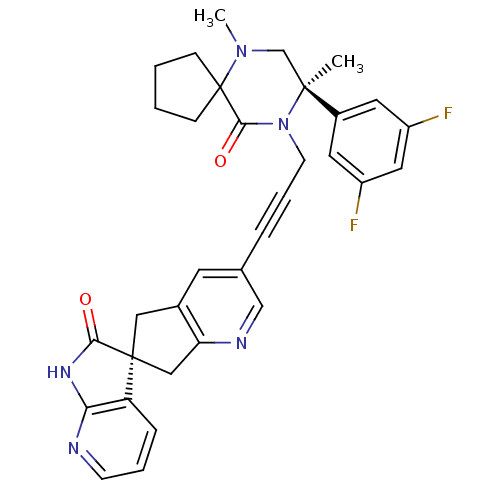
(CHEMBL2431246)Show SMILES CN1C[C@](C)(N(CC#Cc2cnc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)C11CCCC1)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C33H31F2N5O2/c1-31(23-14-24(34)16-25(35)15-23)20-39(2)33(9-3-4-10-33)30(42)40(31)12-6-7-21-13-22-17-32(18-27(22)37-19-21)26-8-5-11-36-28(26)38-29(32)41/h5,8,11,13-16,19H,3-4,9-10,12,17-18,20H2,1-2H3,(H,36,38,41)/t31-,32-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50068612
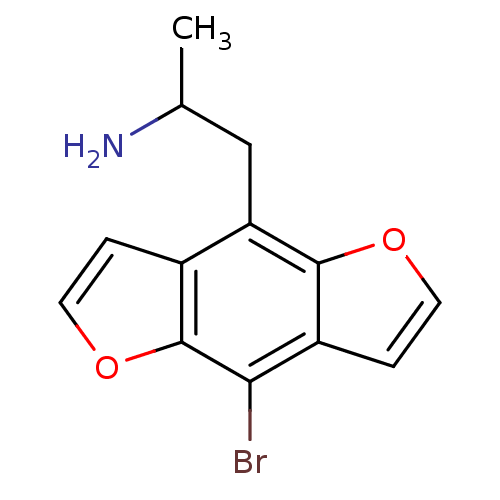
(2-(8-Bromo-benzo[1,2-b;4,5-b']difuran-4-yl)-1-meth...)Show InChI InChI=1S/C13H12BrNO2/c1-7(15)6-10-8-2-4-17-13(8)11(14)9-3-5-16-12(9)10/h2-5,7H,6,15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Binding activity against cloned human 5-hydroxytryptamine 2C receptor using [125I]-DOI as the radioligand. |
J Med Chem 41: 5148-9 (1999)
Article DOI: 10.1021/jm9803525
BindingDB Entry DOI: 10.7270/Q2862FKX |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50500519
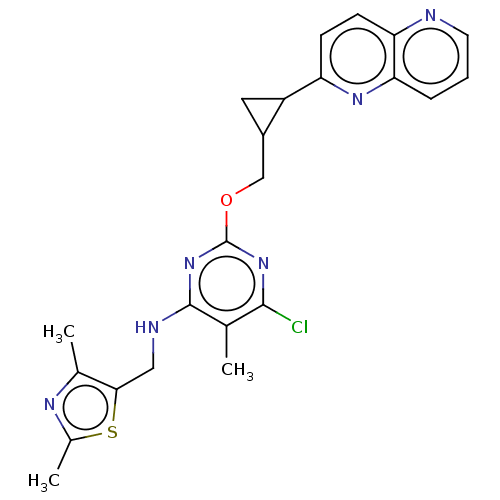
(CHEMBL3746917)Show SMILES Cc1nc(C)c(CNc2nc(OCC3CC3c3ccc4ncccc4n3)nc(Cl)c2C)s1 Show InChI InChI=1S/C23H23ClN6OS/c1-12-21(24)29-23(30-22(12)26-10-20-13(2)27-14(3)32-20)31-11-15-9-16(15)17-6-7-18-19(28-17)5-4-8-25-18/h4-8,15-16H,9-11H2,1-3H3,(H,26,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50500535

(CHEMBL3747450)Show SMILES Cc1nc(C)c(CNc2nc(OCC3CC3c3cn(C)cn3)nc(Cl)c2C)s1 Show InChI InChI=1S/C19H23ClN6OS/c1-10-17(20)24-19(25-18(10)21-6-16-11(2)23-12(3)28-16)27-8-13-5-14(13)15-7-26(4)9-22-15/h7,9,13-14H,5-6,8H2,1-4H3,(H,21,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50051568

((3S,6S,9S,12R,15S,18S)-9-(4-Amino-butyl)-3-benzyl-...)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](C)N(C)C(=O)[C@H](Cc2ccccc2)NC1=O Show InChI InChI=1S/C44H56N8O7/c1-26(2)38-43(58)50-37(23-28-12-6-5-7-13-28)44(59)52(4)27(3)39(54)48-35(22-29-17-19-31(53)20-18-29)41(56)49-36(24-30-25-46-33-15-9-8-14-32(30)33)42(57)47-34(40(55)51-38)16-10-11-21-45/h5-9,12-15,17-20,25-27,34-38,46,53H,10-11,16,21-24,45H2,1-4H3,(H,47,57)(H,48,54)(H,49,56)(H,50,58)(H,51,55)/t27-,34-,35-,36+,37-,38-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma
Curated by PDSP Ki Database
| |
Eur J Pharmacol 348: 311-20 (1998)
Article DOI: 10.1016/s0014-2999(98)00159-9
BindingDB Entry DOI: 10.7270/Q2MK6BF9 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440784
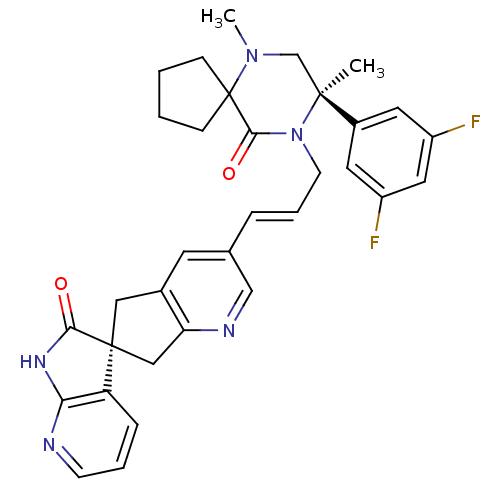
(CHEMBL2431253)Show SMILES CN1C[C@](C)(N(C\C=C\c2cnc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)C11CCCC1)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C33H33F2N5O2/c1-31(23-14-24(34)16-25(35)15-23)20-39(2)33(9-3-4-10-33)30(42)40(31)12-6-7-21-13-22-17-32(18-27(22)37-19-21)26-8-5-11-36-28(26)38-29(32)41/h5-8,11,13-16,19H,3-4,9-10,12,17-18,20H2,1-2H3,(H,36,38,41)/b7-6+/t31-,32-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50356282
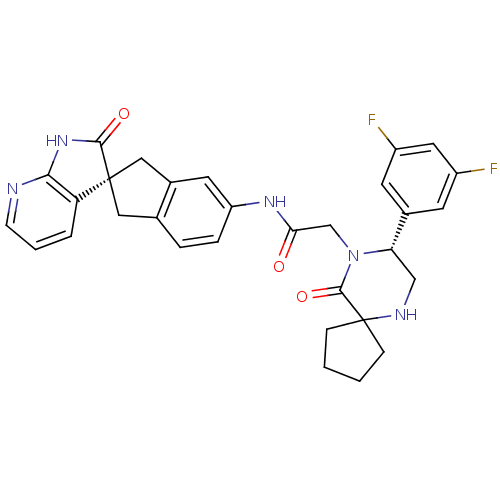
(CHEMBL1910936)Show SMILES Fc1cc(F)cc(c1)[C@@H]1CNC2(CCCC2)C(=O)N1CC(=O)Nc1ccc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31 |r| Show InChI InChI=1S/C31H29F2N5O3/c32-21-10-19(11-22(33)13-21)25-16-35-31(7-1-2-8-31)29(41)38(25)17-26(39)36-23-6-5-18-14-30(15-20(18)12-23)24-4-3-9-34-27(24)37-28(30)40/h3-6,9-13,25,35H,1-2,7-8,14-17H2,(H,36,39)(H,34,37,40)/t25-,30+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-2
(Rattus norvegicus (Rat)) | BDBM50295954
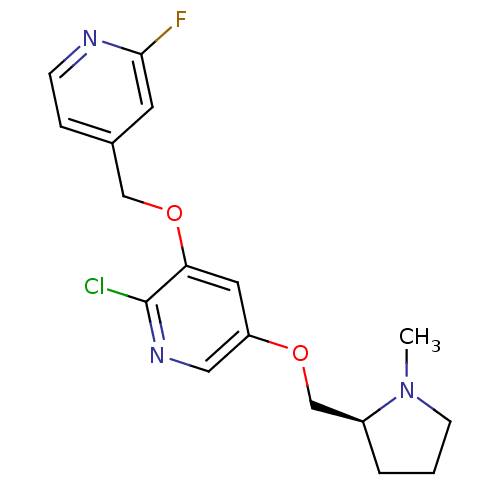
(2-Chloro-3-(2-fluoro-4-(pyridinyl)methoxy)-5-((1-m...)Show SMILES CN1CCC[C@H]1COc1cnc(Cl)c(OCc2ccnc(F)c2)c1 |r| Show InChI InChI=1S/C17H19ClFN3O2/c1-22-6-2-3-13(22)11-23-14-8-15(17(18)21-9-14)24-10-12-4-5-20-16(19)7-12/h4-5,7-9,13H,2-3,6,10-11H2,1H3/t13-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from rat alpha2beta2 nicotinic receptor expressed in human HEK293 cells by liquid scintillation counting |
Bioorg Med Chem 17: 4367-77 (2009)
Article DOI: 10.1016/j.bmc.2009.05.021
BindingDB Entry DOI: 10.7270/Q2DN453T |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440791
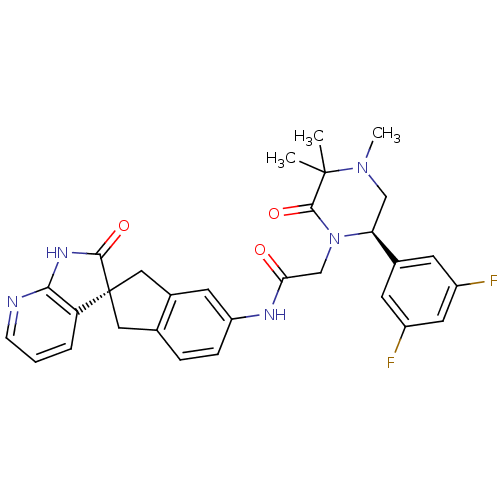
(CHEMBL2431256)Show SMILES CN1C[C@H](N(CC(=O)Nc2ccc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)C1(C)C)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C30H29F2N5O3/c1-29(2)28(40)37(24(15-36(29)3)18-9-20(31)12-21(32)10-18)16-25(38)34-22-7-6-17-13-30(14-19(17)11-22)23-5-4-8-33-26(23)35-27(30)39/h4-12,24H,13-16H2,1-3H3,(H,34,38)(H,33,35,39)/t24-,30+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(MOUSE) | BDBM50281781

(Ac-Lys-Pro-Arg-Arg-Pro-Tyr-Ile-Leu-OH | CHEMBL2645...)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(C)=O)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C51H85N15O11/c1-6-30(4)41(46(73)63-38(49(76)77)27-29(2)3)64-43(70)37(28-32-18-20-33(68)21-19-32)62-45(72)40-17-12-26-66(40)48(75)36(15-10-24-58-51(55)56)61-42(69)34(14-9-23-57-50(53)54)60-44(71)39-16-11-25-65(39)47(74)35(59-31(5)67)13-7-8-22-52/h18-21,29-30,34-41,68H,6-17,22-28,52H2,1-5H3,(H,59,67)(H,60,71)(H,61,69)(H,62,72)(H,63,73)(H,64,70)(H,76,77)(H4,53,54,57)(H4,55,56,58)/t30-,34+,35+,36+,37+,38+,39+,40+,41+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [125I]-Tyr3-NT(1-13) to NT receptors in neonatal mouse whole brain (minus cerebellum) |
Bioorg Med Chem Lett 3: 949-952 (1993)
Article DOI: 10.1016/S0960-894X(00)80698-8
BindingDB Entry DOI: 10.7270/Q2CJ8DDX |
More data for this
Ligand-Target Pair | |
Lutropin-choriogonadotropic hormone receptor
(Rattus norvegicus) | BDBM50230127
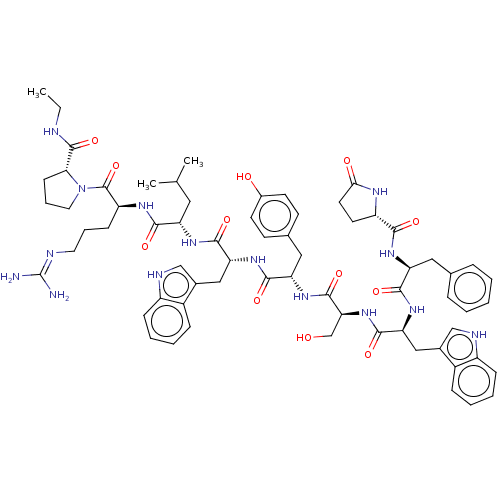
(CHEMBL407606)Show SMILES CCNC(=O)[C@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCC(=O)N1 |wU:45.57,31.44,63.79,23.28,5.4,88.94,wD:57.63,77.91,12.20,(27.09,-8.95,;27.47,-10.44,;28.96,-10.86,;30.04,-11.96,;31.53,-11.6,;29.61,-13.45,;30.56,-14.67,;29.69,-15.95,;28.21,-15.53,;28.16,-13.97,;26.88,-13.11,;26.98,-11.58,;25.49,-13.78,;25.38,-15.33,;26.66,-16.2,;26.55,-17.73,;27.83,-18.59,;27.72,-20.13,;29,-21,;26.33,-20.8,;24.22,-12.92,;22.82,-13.6,;22.72,-15.13,;21.54,-12.73,;21.65,-11.2,;23.05,-10.52,;24.32,-11.38,;23.15,-8.98,;20.15,-13.41,;18.89,-12.55,;18.99,-11.01,;17.49,-13.23,;17.39,-14.76,;18.14,-16.08,;19.64,-16.43,;19.78,-17.94,;18.38,-18.56,;17.91,-20.01,;16.43,-20.33,;15.39,-19.17,;15.88,-17.73,;17.36,-17.42,;16.22,-12.36,;14.83,-13.03,;14.71,-14.57,;13.55,-12.17,;13.66,-10.62,;15.04,-9.95,;15.16,-8.42,;16.54,-7.74,;17.82,-8.6,;19.2,-7.93,;17.71,-10.14,;16.32,-10.81,;12.16,-12.85,;10.88,-11.97,;10.99,-10.44,;9.49,-12.65,;9.39,-14.19,;10.67,-15.06,;8.21,-11.79,;6.83,-12.46,;6.71,-14.01,;5.54,-11.6,;5.65,-10.07,;7.05,-9.39,;8.58,-9.18,;8.85,-7.67,;7.49,-6.93,;7.12,-5.44,;5.63,-5,;4.53,-6.07,;4.89,-7.56,;6.37,-8,;4.17,-12.27,;2.88,-11.41,;3,-9.87,;1.49,-12.09,;1.38,-13.62,;2.15,-14.95,;3.66,-14.95,;4.41,-16.27,;3.65,-17.59,;2.12,-17.59,;1.37,-16.26,;.22,-11.23,;-1.18,-11.9,;-1.28,-13.44,;-2.34,-11.11,;-2.34,-9.69,;-3.87,-9.46,;-4.55,-10.83,;-6.06,-11.09,;-3.45,-11.92,)| Show InChI InChI=1S/C67H85N15O12/c1-4-70-65(93)56-21-13-29-82(56)66(94)49(20-12-28-71-67(68)69)75-59(87)50(30-38(2)3)76-62(90)53(33-41-35-72-46-18-10-8-16-44(41)46)79-61(89)52(32-40-22-24-43(84)25-23-40)78-64(92)55(37-83)81-63(91)54(34-42-36-73-47-19-11-9-17-45(42)47)80-60(88)51(31-39-14-6-5-7-15-39)77-58(86)48-26-27-57(85)74-48/h5-11,14-19,22-25,35-36,38,48-56,72-73,83-84H,4,12-13,20-21,26-34,37H2,1-3H3,(H,70,93)(H,74,85)(H,75,87)(H,76,90)(H,77,86)(H,78,92)(H,79,89)(H,80,88)(H,81,91)(H4,68,69,71)/t48-,49-,50-,51-,52-,53+,54-,55-,56+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0245 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Negative logarithm of equilibrium dissociation constant in the rat pituitary luteinizing releasing hormone receptor binding assay |
J Med Chem 35: 3890-4 (1992)
BindingDB Entry DOI: 10.7270/Q24Q7X6M |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-2
(Rattus norvegicus (Rat)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from rat alpha2beta2 nicotinic receptor expressed in human HEK293 cells by liquid scintillation counting |
Bioorg Med Chem 17: 4367-77 (2009)
Article DOI: 10.1016/j.bmc.2009.05.021
BindingDB Entry DOI: 10.7270/Q2DN453T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50125967

(CHEMBL3627846)Show SMILES Cc1ccc2c(c1)nc(CCN1C(=O)c3ccccc3C1=O)n(-c1ccc3n(C)ncc3c1)c2=O Show InChI InChI=1S/C27H21N5O3/c1-16-7-9-21-22(13-16)29-24(11-12-31-25(33)19-5-3-4-6-20(19)26(31)34)32(27(21)35)18-8-10-23-17(14-18)15-28-30(23)2/h3-10,13-15H,11-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50573182
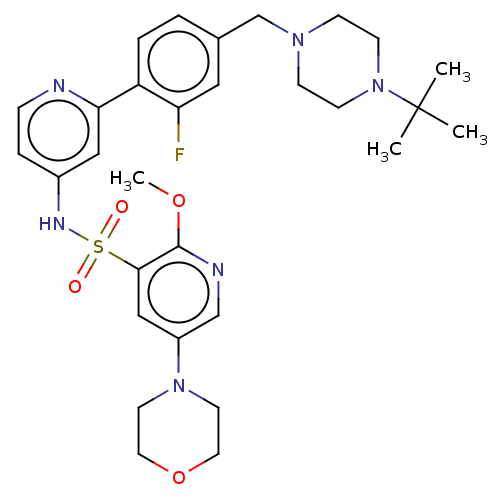
(CHEMBL4175571)Show SMILES COc1ncc(cc1S(=O)(=O)Nc1ccnc(c1)-c1ccc(CN2CCN(CC2)C(C)(C)C)cc1F)N1CCOCC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01102
BindingDB Entry DOI: 10.7270/Q2N87FKX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50573182

(CHEMBL4175571)Show SMILES COc1ncc(cc1S(=O)(=O)Nc1ccnc(c1)-c1ccc(CN2CCN(CC2)C(C)(C)C)cc1F)N1CCOCC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01102
BindingDB Entry DOI: 10.7270/Q2N87FKX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data