 Found 194 hits with Last Name = 'hopper' and Initial = 'dw'
Found 194 hits with Last Name = 'hopper' and Initial = 'dw' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50142842
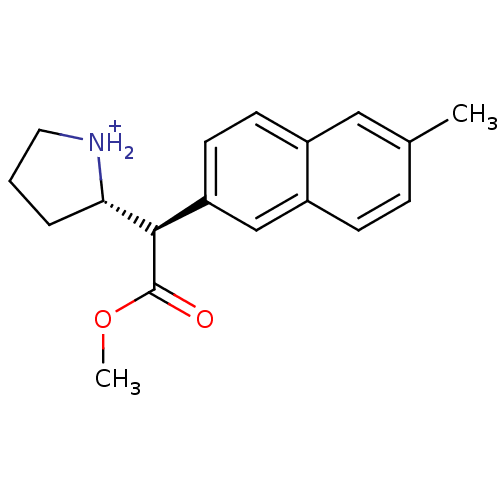
((S)-2-[(R)-Methoxycarbonyl-(6-methyl-naphthalen-2-...)Show SMILES COC(=O)[C@@H]([C@@H]1CCC[NH2+]1)c1ccc2cc(C)ccc2c1 Show InChI InChI=1S/C18H21NO2/c1-12-5-6-14-11-15(8-7-13(14)10-12)17(18(20)21-2)16-4-3-9-19-16/h5-8,10-11,16-17,19H,3-4,9H2,1-2H3/p+1/t16-,17+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York
Curated by ChEMBL
| Assay Description
Ability to displace 0.4 nM [3H]-paroxetine binding to serotonin transporter in rat frontal cortex |
Bioorg Med Chem Lett 14: 1799-802 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.097
BindingDB Entry DOI: 10.7270/Q2T43SJ8 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50142842

((S)-2-[(R)-Methoxycarbonyl-(6-methyl-naphthalen-2-...)Show SMILES COC(=O)[C@@H]([C@@H]1CCC[NH2+]1)c1ccc2cc(C)ccc2c1 Show InChI InChI=1S/C18H21NO2/c1-12-5-6-14-11-15(8-7-13(14)10-12)17(18(20)21-2)16-4-3-9-19-16/h5-8,10-11,16-17,19H,3-4,9H2,1-2H3/p+1/t16-,17+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York
Curated by ChEMBL
| Assay Description
Ability to displace 0.4 nM [3H]-paroxetine binding to serotonin transporter in rat frontal cortex |
Bioorg Med Chem Lett 14: 1799-802 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.097
BindingDB Entry DOI: 10.7270/Q2T43SJ8 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50142858
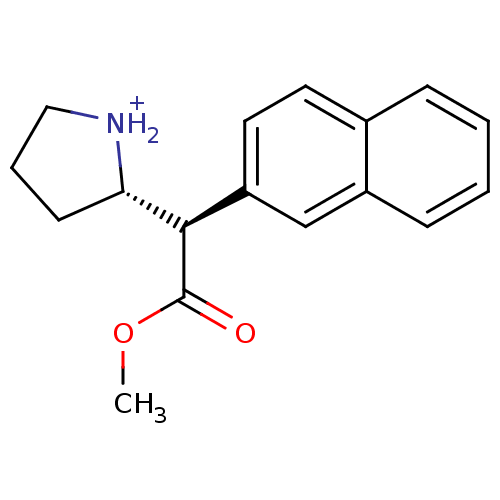
((S)-2-((R)-Methoxycarbonyl-naphthalen-2-yl-methyl)...)Show SMILES COC(=O)[C@@H]([C@@H]1CCC[NH2+]1)c1ccc2ccccc2c1 Show InChI InChI=1S/C17H19NO2/c1-20-17(19)16(15-7-4-10-18-15)14-9-8-12-5-2-3-6-13(12)11-14/h2-3,5-6,8-9,11,15-16,18H,4,7,10H2,1H3/p+1/t15-,16+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York
Curated by ChEMBL
| Assay Description
Ability to displace 0.4 nM [3H]-paroxetine binding to serotonin transporter in rat frontal cortex |
Bioorg Med Chem Lett 14: 1799-802 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.097
BindingDB Entry DOI: 10.7270/Q2T43SJ8 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50142858

((S)-2-((R)-Methoxycarbonyl-naphthalen-2-yl-methyl)...)Show SMILES COC(=O)[C@@H]([C@@H]1CCC[NH2+]1)c1ccc2ccccc2c1 Show InChI InChI=1S/C17H19NO2/c1-20-17(19)16(15-7-4-10-18-15)14-9-8-12-5-2-3-6-13(12)11-14/h2-3,5-6,8-9,11,15-16,18H,4,7,10H2,1H3/p+1/t15-,16+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York
Curated by ChEMBL
| Assay Description
Ability to displace 0.4 nM [3H]paroxetine binding to serotonin transporter in rat frontal cortex |
Bioorg Med Chem Lett 14: 1799-802 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.097
BindingDB Entry DOI: 10.7270/Q2T43SJ8 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50142846
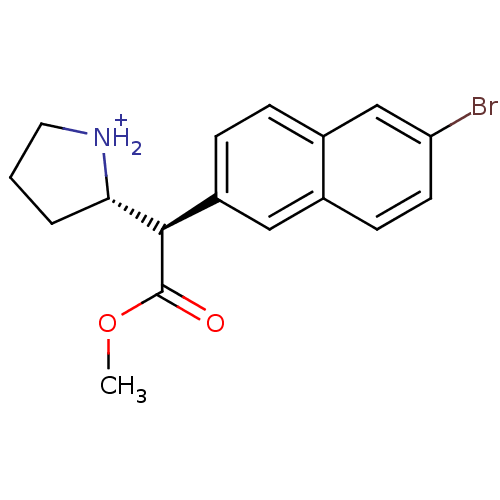
((S)-2-[(R)-(6-Bromo-naphthalen-2-yl)-methoxycarbon...)Show SMILES COC(=O)[C@@H]([C@@H]1CCC[NH2+]1)c1ccc2cc(Br)ccc2c1 Show InChI InChI=1S/C17H18BrNO2/c1-21-17(20)16(15-3-2-8-19-15)13-5-4-12-10-14(18)7-6-11(12)9-13/h4-7,9-10,15-16,19H,2-3,8H2,1H3/p+1/t15-,16+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York
Curated by ChEMBL
| Assay Description
Ability to displace 0.4 nM [3H]-paroxetine binding to serotonin transporter in rat frontal cortex |
Bioorg Med Chem Lett 14: 1799-802 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.097
BindingDB Entry DOI: 10.7270/Q2T43SJ8 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50142846

((S)-2-[(R)-(6-Bromo-naphthalen-2-yl)-methoxycarbon...)Show SMILES COC(=O)[C@@H]([C@@H]1CCC[NH2+]1)c1ccc2cc(Br)ccc2c1 Show InChI InChI=1S/C17H18BrNO2/c1-21-17(20)16(15-3-2-8-19-15)13-5-4-12-10-14(18)7-6-11(12)9-13/h4-7,9-10,15-16,19H,2-3,8H2,1H3/p+1/t15-,16+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York
Curated by ChEMBL
| Assay Description
Ability to displace 0.4 nM [3H]-paroxetine binding to serotonin transporter in rat frontal cortex |
Bioorg Med Chem Lett 14: 1799-802 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.097
BindingDB Entry DOI: 10.7270/Q2T43SJ8 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50142841
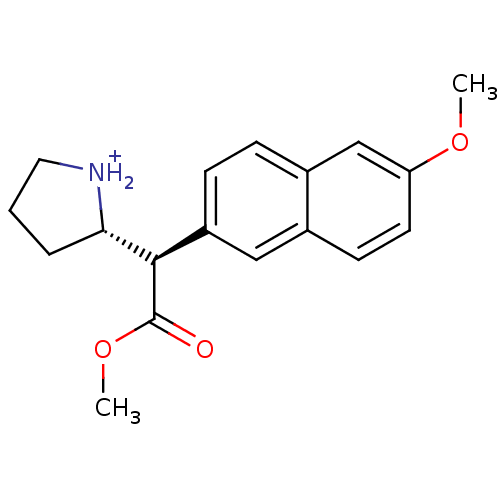
((S)-2-[(R)-Methoxycarbonyl-(6-methoxy-naphthalen-2...)Show SMILES COC(=O)[C@@H]([C@@H]1CCC[NH2+]1)c1ccc2cc(OC)ccc2c1 Show InChI InChI=1S/C18H21NO3/c1-21-15-8-7-12-10-14(6-5-13(12)11-15)17(18(20)22-2)16-4-3-9-19-16/h5-8,10-11,16-17,19H,3-4,9H2,1-2H3/p+1/t16-,17+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 245 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York
Curated by ChEMBL
| Assay Description
Ability to displace 0.4 nM [3H]-paroxetine binding to serotonin transporter in rat frontal cortex |
Bioorg Med Chem Lett 14: 1799-802 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.097
BindingDB Entry DOI: 10.7270/Q2T43SJ8 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50142841

((S)-2-[(R)-Methoxycarbonyl-(6-methoxy-naphthalen-2...)Show SMILES COC(=O)[C@@H]([C@@H]1CCC[NH2+]1)c1ccc2cc(OC)ccc2c1 Show InChI InChI=1S/C18H21NO3/c1-21-15-8-7-12-10-14(6-5-13(12)11-15)17(18(20)22-2)16-4-3-9-19-16/h5-8,10-11,16-17,19H,3-4,9H2,1-2H3/p+1/t16-,17+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 245 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York
Curated by ChEMBL
| Assay Description
Ability to displace 0.4 nM [3H]paroxetine binding to serotonin transporter in rat frontal cortex |
Bioorg Med Chem Lett 14: 1799-802 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.097
BindingDB Entry DOI: 10.7270/Q2T43SJ8 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50142856
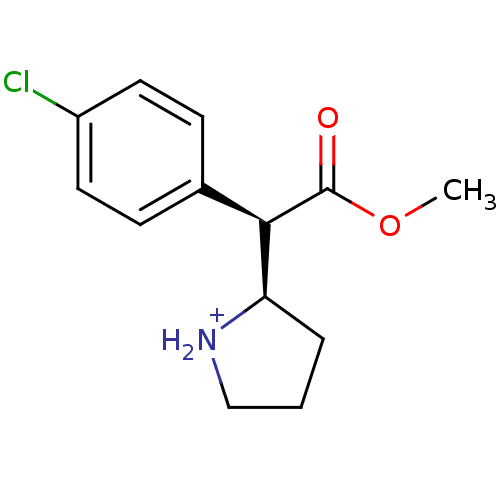
((R)-2-[(R)-(4-Chloro-phenyl)-methoxycarbonyl-methy...)Show SMILES COC(=O)[C@@H]([C@H]1CCC[NH2+]1)c1ccc(Cl)cc1 Show InChI InChI=1S/C13H16ClNO2/c1-17-13(16)12(11-3-2-8-15-11)9-4-6-10(14)7-5-9/h4-7,11-12,15H,2-3,8H2,1H3/p+1/t11-,12-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York
Curated by ChEMBL
| Assay Description
Ability to displace 0.4 nM [3H]-paroxetine binding to serotonin transporter in rat frontal cortex |
Bioorg Med Chem Lett 14: 1799-802 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.097
BindingDB Entry DOI: 10.7270/Q2T43SJ8 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50142855
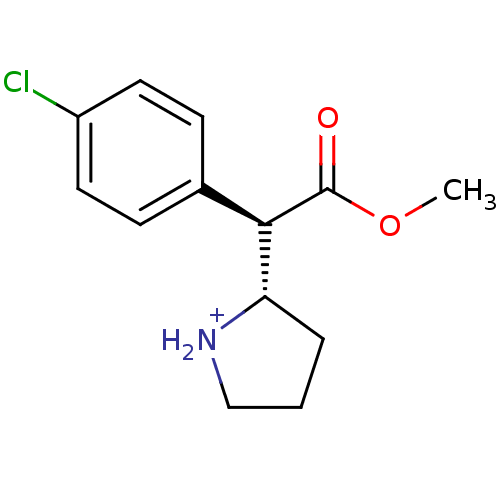
((S)-2-[(R)-(4-Chloro-phenyl)-methoxycarbonyl-methy...)Show SMILES COC(=O)[C@@H]([C@@H]1CCC[NH2+]1)c1ccc(Cl)cc1 Show InChI InChI=1S/C13H16ClNO2/c1-17-13(16)12(11-3-2-8-15-11)9-4-6-10(14)7-5-9/h4-7,11-12,15H,2-3,8H2,1H3/p+1/t11-,12+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York
Curated by ChEMBL
| Assay Description
Ability to displace 0.4 nM [3H]-paroxetine binding to serotonin transporter in rat frontal cortex |
Bioorg Med Chem Lett 14: 1799-802 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.097
BindingDB Entry DOI: 10.7270/Q2T43SJ8 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50142847
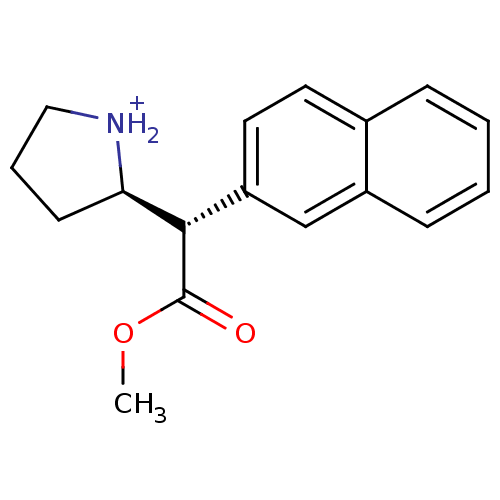
((R)-2-((S)-Methoxycarbonyl-naphthalen-2-yl-methyl)...)Show SMILES COC(=O)[C@H]([C@H]1CCC[NH2+]1)c1ccc2ccccc2c1 Show InChI InChI=1S/C17H19NO2/c1-20-17(19)16(15-7-4-10-18-15)14-9-8-12-5-2-3-6-13(12)11-14/h2-3,5-6,8-9,11,15-16,18H,4,7,10H2,1H3/p+1/t15-,16+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York
Curated by ChEMBL
| Assay Description
Ability to displace 0.4 nM [3H]-paroxetine binding to serotonin transporter in rat frontal cortex |
Bioorg Med Chem Lett 14: 1799-802 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.097
BindingDB Entry DOI: 10.7270/Q2T43SJ8 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50142852
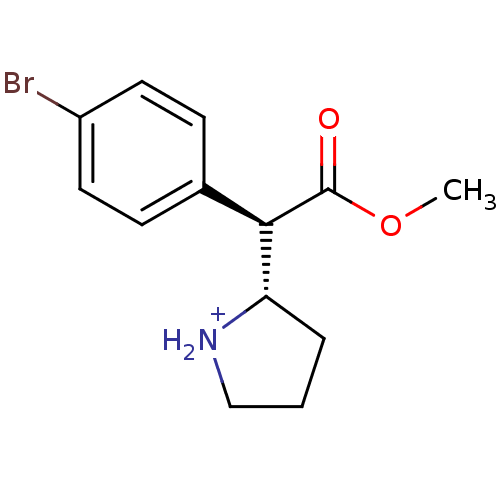
((S)-2-[(R)-(4-Bromo-phenyl)-methoxycarbonyl-methyl...)Show SMILES COC(=O)[C@@H]([C@@H]1CCC[NH2+]1)c1ccc(Br)cc1 Show InChI InChI=1S/C13H16BrNO2/c1-17-13(16)12(11-3-2-8-15-11)9-4-6-10(14)7-5-9/h4-7,11-12,15H,2-3,8H2,1H3/p+1/t11-,12+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York
Curated by ChEMBL
| Assay Description
Ability to displace 0.4 nM [3H]-paroxetine binding to serotonin transporter in rat frontal cortex |
Bioorg Med Chem Lett 14: 1799-802 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.097
BindingDB Entry DOI: 10.7270/Q2T43SJ8 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50142854
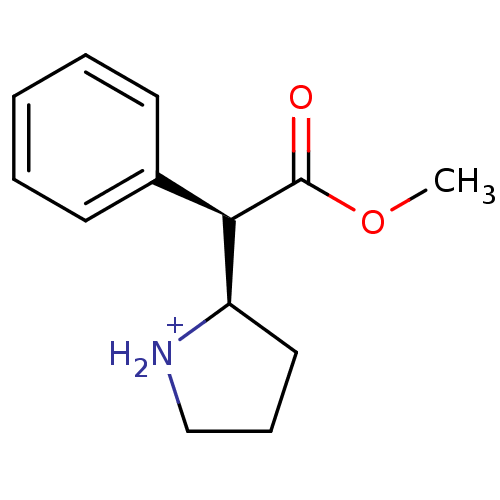
((R)-2-((R)-Methoxycarbonyl-phenyl-methyl)-pyrrolid...)Show InChI InChI=1S/C13H17NO2/c1-16-13(15)12(11-8-5-9-14-11)10-6-3-2-4-7-10/h2-4,6-7,11-12,14H,5,8-9H2,1H3/p+1/t11-,12-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York
Curated by ChEMBL
| Assay Description
Ability to displace 0.4 nM [3H]-paroxetine binding to serotonin transporter in rat frontal cortex |
Bioorg Med Chem Lett 14: 1799-802 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.097
BindingDB Entry DOI: 10.7270/Q2T43SJ8 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50142848
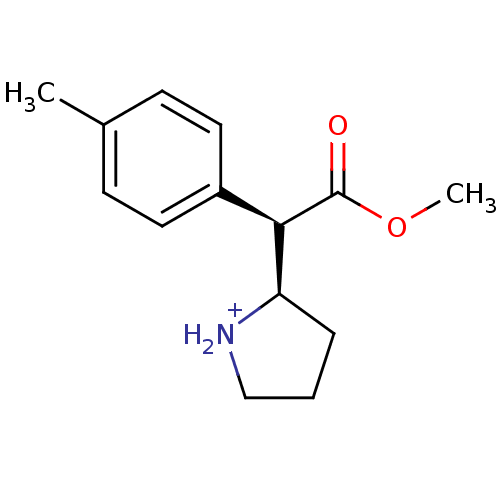
((R)-2-((R)-Methoxycarbonyl-p-tolyl-methyl)-pyrroli...)Show InChI InChI=1S/C14H19NO2/c1-10-5-7-11(8-6-10)13(14(16)17-2)12-4-3-9-15-12/h5-8,12-13,15H,3-4,9H2,1-2H3/p+1/t12-,13-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York
Curated by ChEMBL
| Assay Description
Ability to displace 0.4 nM [3H]-paroxetine binding to serotonin transporter in rat frontal cortex |
Bioorg Med Chem Lett 14: 1799-802 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.097
BindingDB Entry DOI: 10.7270/Q2T43SJ8 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50142850
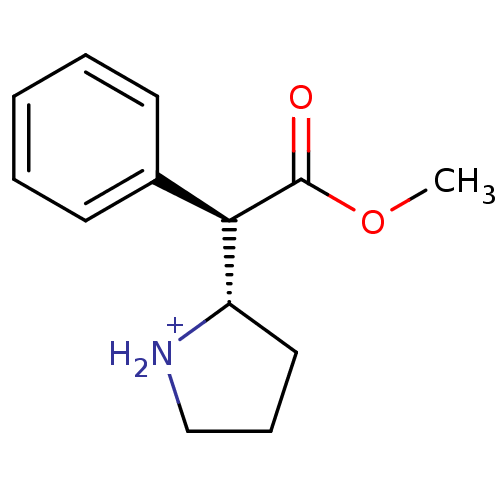
((S)-2-((R)-Methoxycarbonyl-phenyl-methyl)-pyrrolid...)Show InChI InChI=1S/C13H17NO2/c1-16-13(15)12(11-8-5-9-14-11)10-6-3-2-4-7-10/h2-4,6-7,11-12,14H,5,8-9H2,1H3/p+1/t11-,12+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York
Curated by ChEMBL
| Assay Description
Ability to displace 0.4 nM [3H]-paroxetine binding to serotonin transporter in rat frontal cortex |
Bioorg Med Chem Lett 14: 1799-802 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.097
BindingDB Entry DOI: 10.7270/Q2T43SJ8 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50142860
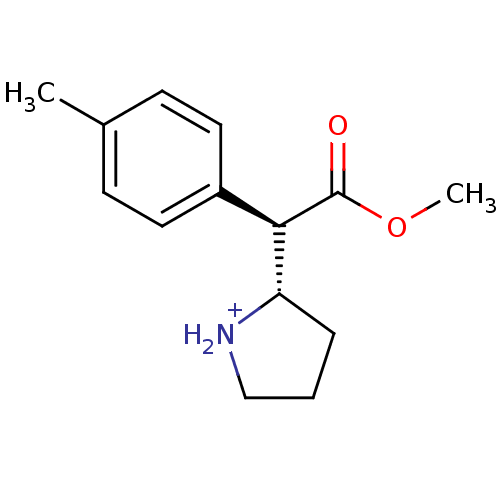
((S)-2-((R)-Methoxycarbonyl-p-tolyl-methyl)-pyrroli...)Show SMILES COC(=O)[C@@H]([C@@H]1CCC[NH2+]1)c1ccc(C)cc1 Show InChI InChI=1S/C14H19NO2/c1-10-5-7-11(8-6-10)13(14(16)17-2)12-4-3-9-15-12/h5-8,12-13,15H,3-4,9H2,1-2H3/p+1/t12-,13+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York
Curated by ChEMBL
| Assay Description
Ability to displace 0.4 nM [3H]-paroxetine binding to serotonin transporter in rat frontal cortex |
Bioorg Med Chem Lett 14: 1799-802 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.097
BindingDB Entry DOI: 10.7270/Q2T43SJ8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50303103
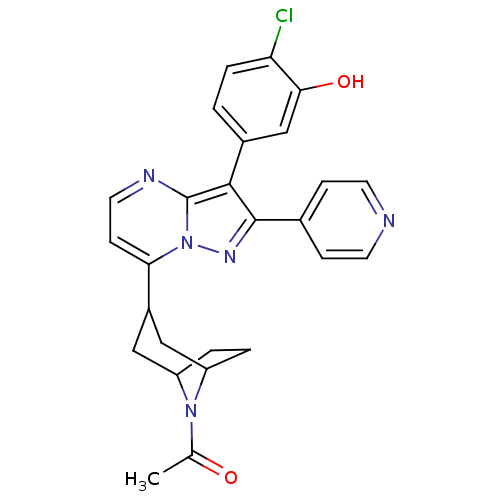
(1-(3-(3-(4-chloro-3-hydroxyphenyl)-2-(pyridin-4-yl...)Show SMILES CC(=O)N1C2CCC1CC(C2)c1ccnc2c(c(nn12)-c1ccncc1)-c1ccc(Cl)c(O)c1 |THB:11:9:3:5.6| Show InChI InChI=1S/C26H24ClN5O2/c1-15(33)31-19-3-4-20(31)13-18(12-19)22-8-11-29-26-24(17-2-5-21(27)23(34)14-17)25(30-32(22)26)16-6-9-28-10-7-16/h2,5-11,14,18-20,34H,3-4,12-13H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf |
Bioorg Med Chem Lett 19: 6957-61 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.058
BindingDB Entry DOI: 10.7270/Q2833S34 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50303123

(CHEMBL570681 | ethyl 3-(3-(7-fluoro-1H-indazol-4-y...)Show SMILES CCOC(=O)N1C2CCC1CC(C2)c1ccnc2c(c(nn12)-c1ccncc1)-c1ccc(F)c2[nH]ncc12 |THB:13:11:5:7.8,(15.45,-16.9,;14.13,-17.7,;12.78,-16.95,;11.46,-17.75,;10.11,-17,;11.49,-19.29,;10.96,-20.75,;9.44,-21.43,;10.81,-21.4,;12,-20.54,;13.54,-21.38,;13,-22.85,;12.55,-21.67,;13,-24.39,;11.67,-25.16,;11.67,-26.71,;13,-27.48,;14.34,-26.71,;15.81,-27.18,;16.72,-25.93,;15.81,-24.68,;14.34,-25.16,;18.26,-25.93,;19.02,-27.26,;20.56,-27.26,;21.33,-25.93,;20.55,-24.59,;19.02,-24.6,;15.88,-28.72,;14.58,-29.54,;14.64,-31.08,;16,-31.8,;16.06,-33.34,;17.31,-30.98,;18.8,-31.39,;19.66,-30.1,;18.69,-28.88,;17.24,-29.43,)| Show InChI InChI=1S/C28H26FN7O2/c1-2-38-28(37)35-18-3-4-19(35)14-17(13-18)23-9-12-31-27-24(20-5-6-22(29)26-21(20)15-32-33-26)25(34-36(23)27)16-7-10-30-11-8-16/h5-12,15,17-19H,2-4,13-14H2,1H3,(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf |
Bioorg Med Chem Lett 19: 6957-61 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.058
BindingDB Entry DOI: 10.7270/Q2833S34 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50303098
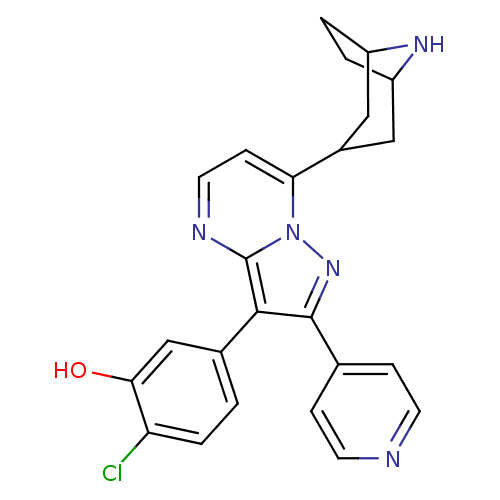
(5-(7-(8-azabicyclo[3.2.1]octan-3-yl)-2-(pyridin-4-...)Show SMILES Oc1cc(ccc1Cl)-c1c(nn2c(ccnc12)C1CC2CCC(C1)N2)-c1ccncc1 |TLB:12:17:24:20.21| Show InChI InChI=1S/C24H22ClN5O/c25-19-4-1-15(13-21(19)31)22-23(14-5-8-26-9-6-14)29-30-20(7-10-27-24(22)30)16-11-17-2-3-18(12-16)28-17/h1,4-10,13,16-18,28,31H,2-3,11-12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf |
Bioorg Med Chem Lett 19: 6957-61 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.058
BindingDB Entry DOI: 10.7270/Q2833S34 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50303121
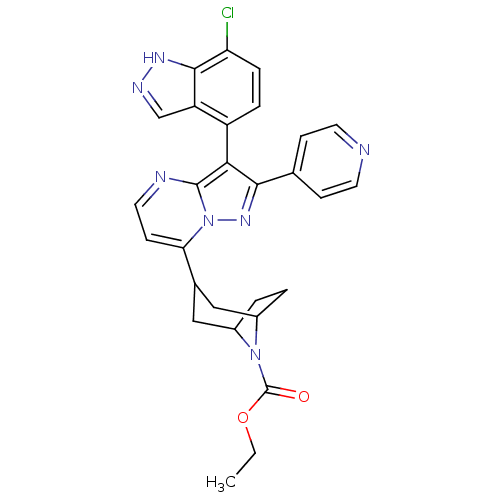
(CHEMBL585144 | ethyl 3-(3-(7-chloro-1H-indazol-4-y...)Show SMILES CCOC(=O)N1C2CCC1CC(C2)c1ccnc2c(c(nn12)-c1ccncc1)-c1ccc(Cl)c2[nH]ncc12 |THB:13:11:5:7.8,(29.95,2.2,;28.63,1.41,;27.29,2.16,;25.97,1.36,;24.62,2.11,;26,-.18,;25.46,-1.65,;23.94,-2.33,;25.31,-2.29,;26.51,-1.43,;28.04,-2.27,;27.5,-3.75,;27.06,-2.56,;27.51,-5.29,;26.18,-6.06,;26.18,-7.6,;27.51,-8.37,;28.84,-7.6,;30.32,-8.07,;31.23,-6.82,;30.31,-5.57,;28.84,-6.05,;32.76,-6.82,;33.53,-8.16,;35.07,-8.16,;35.84,-6.82,;35.06,-5.48,;33.52,-5.49,;30.38,-9.61,;29.08,-10.43,;29.14,-11.97,;30.5,-12.69,;30.56,-14.23,;31.81,-11.87,;33.31,-12.28,;34.16,-10.99,;33.2,-9.78,;31.74,-10.32,)| Show InChI InChI=1S/C28H26ClN7O2/c1-2-38-28(37)35-18-3-4-19(35)14-17(13-18)23-9-12-31-27-24(20-5-6-22(29)26-21(20)15-32-33-26)25(34-36(23)27)16-7-10-30-11-8-16/h5-12,15,17-19H,2-4,13-14H2,1H3,(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf |
Bioorg Med Chem Lett 19: 6957-61 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.058
BindingDB Entry DOI: 10.7270/Q2833S34 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50303100

(2-chloro-5-(7-(8-ethyl-8-azabicyclo[3.2.1]octan-3-...)Show SMILES CCN1C2CCC1CC(C2)c1ccnc2c(c(nn12)-c1ccncc1)-c1ccc(Cl)c(O)c1 |THB:10:8:2:4.5| Show InChI InChI=1S/C26H26ClN5O/c1-2-31-19-4-5-20(31)14-18(13-19)22-9-12-29-26-24(17-3-6-21(27)23(33)15-17)25(30-32(22)26)16-7-10-28-11-8-16/h3,6-12,15,18-20,33H,2,4-5,13-14H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf |
Bioorg Med Chem Lett 19: 6957-61 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.058
BindingDB Entry DOI: 10.7270/Q2833S34 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50303131
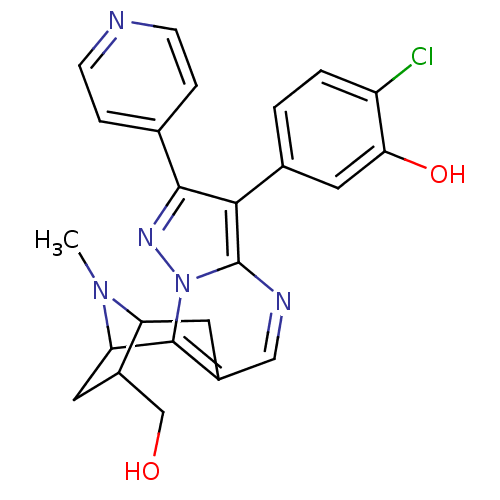
(2-chloro-5-[13-(hydroxymethyl)-15-methyl-5-(pyridi...)Show SMILES CN1C2Cc3cnc4c(c(nn4c3C1CC2CO)-c1ccncc1)-c1ccc(Cl)c(O)c1 |TLB:11:12:1:15.14,THB:5:4:1:15.14| Show InChI InChI=1S/C24H22ClN5O2/c1-29-18-8-15-11-27-24-21(14-2-3-17(25)20(32)10-14)22(13-4-6-26-7-5-13)28-30(24)23(15)19(29)9-16(18)12-31/h2-7,10-11,16,18-19,31-32H,8-9,12H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf |
Bioorg Med Chem Lett 19: 6957-61 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.058
BindingDB Entry DOI: 10.7270/Q2833S34 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50303127

(CHEMBL583133 | ethyl 3-(3-(7-methyl-1H-indazol-4-y...)Show SMILES CCOC(=O)N1C2CCC1CC(C2)c1ccnc2c(c(nn12)-c1ccncc1)-c1ccc(C)c2[nH]ncc12 |THB:13:11:5:7.8,(-1.88,2.01,;-3.2,1.22,;-4.55,1.96,;-5.86,1.17,;-7.21,1.91,;-5.83,-.37,;-6.37,-1.84,;-7.89,-2.52,;-6.52,-2.48,;-5.33,-1.62,;-3.79,-2.47,;-4.33,-3.94,;-4.77,-2.75,;-4.33,-5.48,;-5.65,-6.25,;-5.66,-7.79,;-4.32,-8.56,;-2.99,-7.79,;-1.52,-8.27,;-.6,-7.02,;-1.52,-5.76,;-2.99,-6.24,;.93,-7.01,;1.7,-8.35,;3.23,-8.35,;4.01,-7.02,;3.23,-5.68,;1.69,-5.68,;-1.45,-9.8,;-2.75,-10.63,;-2.69,-12.17,;-1.33,-12.89,;-1.27,-14.42,;-.02,-12.06,;1.48,-12.48,;2.33,-11.18,;1.36,-9.97,;-.09,-10.51,)| Show InChI InChI=1S/C29H29N7O2/c1-3-38-29(37)35-20-5-6-21(35)15-19(14-20)24-10-13-31-28-25(22-7-4-17(2)26-23(22)16-32-33-26)27(34-36(24)28)18-8-11-30-12-9-18/h4,7-13,16,19-21H,3,5-6,14-15H2,1-2H3,(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf |
Bioorg Med Chem Lett 19: 6957-61 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.058
BindingDB Entry DOI: 10.7270/Q2833S34 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50303132
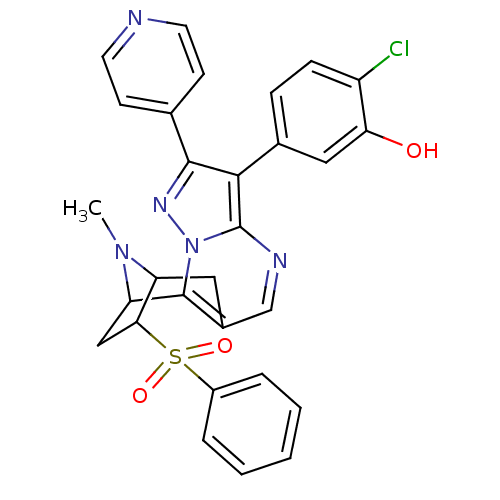
(2-chloro-5-[13-(hydroxymethyl)-15-methyl-5-(pyridi...)Show SMILES CN1C2Cc3cnc4c(c(nn4c3C1CC2S(=O)(=O)c1ccccc1)-c1ccncc1)-c1ccc(Cl)c(O)c1 |TLB:11:12:1:15.14,THB:5:4:1:15.14| Show InChI InChI=1S/C29H24ClN5O3S/c1-34-22-13-19-16-32-29-26(18-7-8-21(30)24(36)14-18)27(17-9-11-31-12-10-17)33-35(29)28(19)23(34)15-25(22)39(37,38)20-5-3-2-4-6-20/h2-12,14,16,22-23,25,36H,13,15H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf |
Bioorg Med Chem Lett 19: 6957-61 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.058
BindingDB Entry DOI: 10.7270/Q2833S34 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50303094
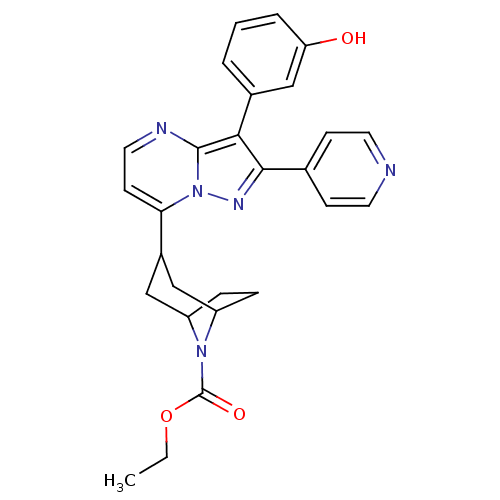
(CHEMBL571129 | ethyl 3-(3-(3-hydroxyphenyl)-2-(pyr...)Show SMILES CCOC(=O)N1C2CCC1CC(C2)c1ccnc2c(c(nn12)-c1ccncc1)-c1cccc(O)c1 |THB:13:11:5:7.8| Show InChI InChI=1S/C27H27N5O3/c1-2-35-27(34)31-20-6-7-21(31)15-19(14-20)23-10-13-29-26-24(18-4-3-5-22(33)16-18)25(30-32(23)26)17-8-11-28-12-9-17/h3-5,8-13,16,19-21,33H,2,6-7,14-15H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf |
Bioorg Med Chem Lett 19: 6957-61 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.058
BindingDB Entry DOI: 10.7270/Q2833S34 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50259007
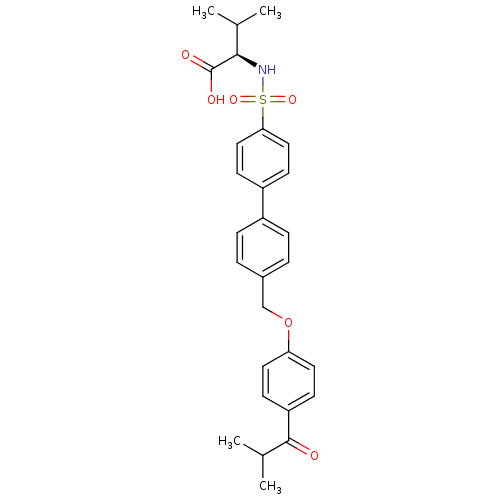
((R)-2-(4'-((4-isobutyrylphenoxy)methyl)biphenyl-4-...)Show SMILES CC(C)[C@@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(COc2ccc(cc2)C(=O)C(C)C)cc1)C(O)=O |r| Show InChI InChI=1S/C28H31NO6S/c1-18(2)26(28(31)32)29-36(33,34)25-15-11-22(12-16-25)21-7-5-20(6-8-21)17-35-24-13-9-23(10-14-24)27(30)19(3)4/h5-16,18-19,26,29H,17H2,1-4H3,(H,31,32)/t26-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 19: 2487-91 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.056
BindingDB Entry DOI: 10.7270/Q29886WB |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50259007

((R)-2-(4'-((4-isobutyrylphenoxy)methyl)biphenyl-4-...)Show SMILES CC(C)[C@@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(COc2ccc(cc2)C(=O)C(C)C)cc1)C(O)=O |r| Show InChI InChI=1S/C28H31NO6S/c1-18(2)26(28(31)32)29-36(33,34)25-15-11-22(12-16-25)21-7-5-20(6-8-21)17-35-24-13-9-23(10-14-24)27(30)19(3)4/h5-16,18-19,26,29H,17H2,1-4H3,(H,31,32)/t26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 19: 2487-91 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.056
BindingDB Entry DOI: 10.7270/Q29886WB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50303130
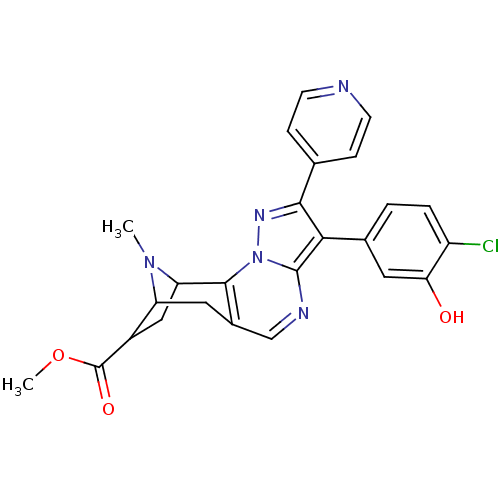
(CHEMBL585148 | Methyl 6-(4-chloro-3-hydroxyphenyl)...)Show SMILES COC(=O)C1CC2N(C)C1Cc1cnc3c(c(nn3c21)-c1ccncc1)-c1ccc(Cl)c(O)c1 |TLB:12:11:7:4.5,THB:18:19:7:4.5| Show InChI InChI=1S/C25H22ClN5O3/c1-30-18-9-15-12-28-24-21(14-3-4-17(26)20(32)10-14)22(13-5-7-27-8-6-13)29-31(24)23(15)19(30)11-16(18)25(33)34-2/h3-8,10,12,16,18-19,32H,9,11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf |
Bioorg Med Chem Lett 19: 6957-61 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.058
BindingDB Entry DOI: 10.7270/Q2833S34 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50303110
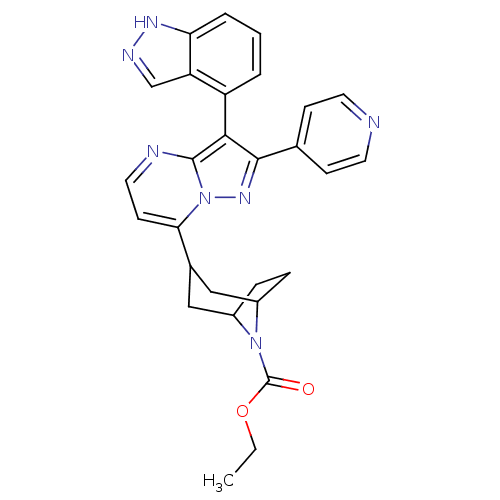
(CHEMBL585334 | ethyl 3-(3-(1H-indazol-4-yl)-2-(pyr...)Show SMILES CCOC(=O)N1C2CCC1CC(C2)c1ccnc2c(c(nn12)-c1ccncc1)-c1cccc2[nH]ncc12 |THB:13:11:5:7.8,(16.08,-21.46,;14.76,-22.25,;13.41,-21.51,;12.09,-22.3,;10.75,-21.56,;12.12,-23.84,;11.59,-25.31,;10.07,-25.99,;11.44,-25.95,;12.63,-25.09,;14.17,-25.94,;13.63,-27.41,;13.18,-26.22,;13.63,-28.95,;12.3,-29.72,;12.3,-31.26,;13.63,-32.03,;14.97,-31.26,;16.44,-31.73,;17.35,-30.49,;16.44,-29.23,;14.97,-29.71,;18.88,-30.48,;19.65,-31.82,;21.19,-31.82,;21.96,-30.48,;21.18,-29.15,;19.65,-29.15,;16.51,-33.27,;15.21,-34.09,;15.27,-35.64,;16.63,-36.35,;17.94,-35.53,;19.43,-35.94,;20.29,-34.65,;19.32,-33.44,;17.87,-33.98,)| Show InChI InChI=1S/C28H27N7O2/c1-2-37-28(36)34-19-6-7-20(34)15-18(14-19)24-10-13-30-27-25(21-4-3-5-23-22(21)16-31-32-23)26(33-35(24)27)17-8-11-29-12-9-17/h3-5,8-13,16,18-20H,2,6-7,14-15H2,1H3,(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf |
Bioorg Med Chem Lett 19: 6957-61 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.058
BindingDB Entry DOI: 10.7270/Q2833S34 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50303111
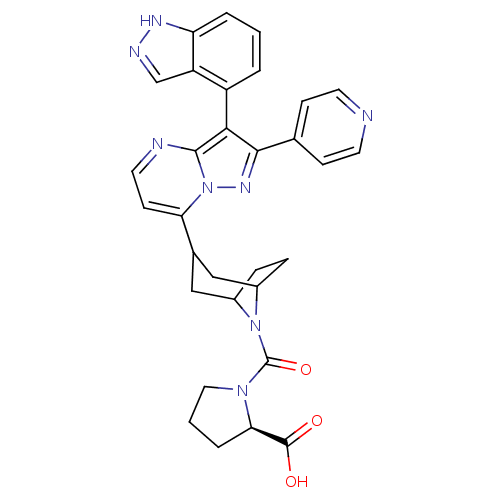
((2R)-1-(3-(3-(1H-indazol-4-yl)-2-(pyridin-4-yl)pyr...)Show SMILES OC(=O)[C@H]1CCCN1C(=O)N1C2CCC1CC(C2)c1ccnc2c(c(nn12)-c1ccncc1)-c1cccc2[nH]ncc12 |r,wU:3.2,THB:18:16:10:12.13,(28.67,-19.29,;28.61,-20.83,;27.25,-21.54,;29.91,-21.65,;31.37,-21.16,;32.29,-22.4,;31.39,-23.66,;29.92,-23.2,;28.69,-24.11,;27.34,-23.37,;28.71,-25.65,;28.18,-27.12,;26.66,-27.8,;28.03,-27.77,;29.22,-26.9,;30.76,-27.75,;30.22,-29.22,;29.78,-28.03,;30.22,-30.76,;28.89,-31.53,;28.89,-33.08,;30.23,-33.85,;31.56,-33.08,;33.03,-33.55,;33.94,-32.3,;33.03,-31.05,;31.56,-31.53,;35.48,-32.3,;36.25,-33.63,;37.79,-33.63,;38.56,-32.3,;37.78,-30.96,;36.24,-30.97,;33.1,-35.09,;31.8,-35.91,;31.86,-37.45,;33.22,-38.17,;34.53,-37.35,;36.03,-37.76,;36.88,-36.47,;35.91,-35.26,;34.46,-35.8,)| Show InChI InChI=1S/C31H30N8O3/c40-30(41)26-5-2-14-37(26)31(42)38-20-6-7-21(38)16-19(15-20)25-10-13-33-29-27(22-3-1-4-24-23(22)17-34-35-24)28(36-39(25)29)18-8-11-32-12-9-18/h1,3-4,8-13,17,19-21,26H,2,5-7,14-16H2,(H,34,35)(H,40,41)/t19?,20?,21?,26-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf |
Bioorg Med Chem Lett 19: 6957-61 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.058
BindingDB Entry DOI: 10.7270/Q2833S34 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50259009
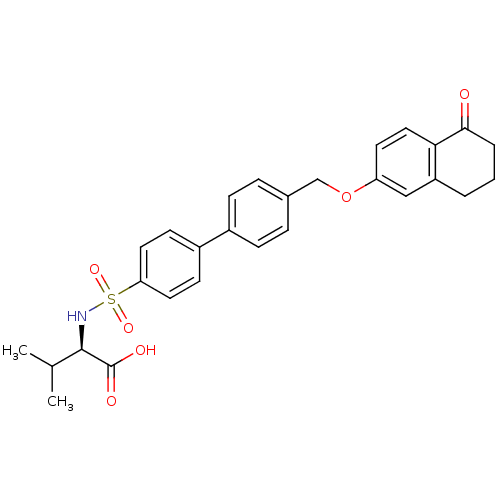
((R)-3-methyl-2-(4'-((5-oxo-5,6,7,8-tetrahydronapht...)Show SMILES CC(C)[C@@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(COc2ccc3C(=O)CCCc3c2)cc1)C(O)=O |r| Show InChI InChI=1S/C28H29NO6S/c1-18(2)27(28(31)32)29-36(33,34)24-13-10-21(11-14-24)20-8-6-19(7-9-20)17-35-23-12-15-25-22(16-23)4-3-5-26(25)30/h6-16,18,27,29H,3-5,17H2,1-2H3,(H,31,32)/t27-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 19: 2487-91 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.056
BindingDB Entry DOI: 10.7270/Q29886WB |
More data for this
Ligand-Target Pair | |
D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor
(Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM50327157

((R)-methyl 2-(3,4-dichlorophenyl)-2-((R)-piperidin...)Show SMILES COC(=O)[C@@H]([C@H]1CCCCN1)c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C14H17Cl2NO2/c1-19-14(18)13(12-4-2-3-7-17-12)9-5-6-10(15)11(16)8-9/h5-6,8,12-13,17H,2-4,7H2,1H3/t12-,13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York
Curated by ChEMBL
| Assay Description
Potency of inhibiting 10 pM [125I]-RTI-55 binding to dopamine receptor in rat striatal membranes |
Bioorg Med Chem Lett 14: 1799-802 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.097
BindingDB Entry DOI: 10.7270/Q2T43SJ8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50303102
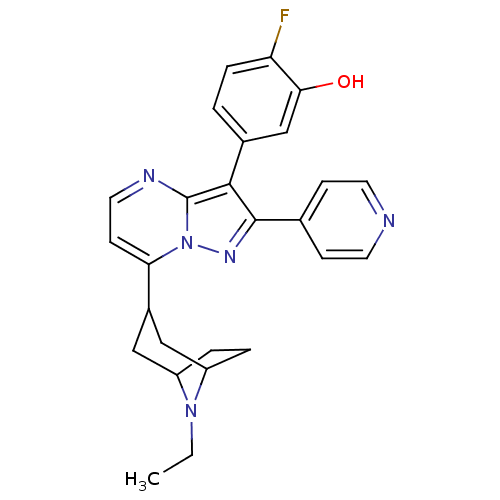
(5-(7-(8-ethyl-8-azabicyclo[3.2.1]octan-3-yl)-2-(py...)Show SMILES CCN1C2CCC1CC(C2)c1ccnc2c(c(nn12)-c1ccncc1)-c1ccc(F)c(O)c1 |THB:10:8:2:4.5| Show InChI InChI=1S/C26H26FN5O/c1-2-31-19-4-5-20(31)14-18(13-19)22-9-12-29-26-24(17-3-6-21(27)23(33)15-17)25(30-32(22)26)16-7-10-28-11-8-16/h3,6-12,15,18-20,33H,2,4-5,13-14H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf |
Bioorg Med Chem Lett 19: 6957-61 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.058
BindingDB Entry DOI: 10.7270/Q2833S34 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50258875

((R)-2-(4'-((4-(4-fluorobenzoyl)phenoxy)methyl)biph...)Show SMILES CC(C)[C@@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(COc2ccc(cc2)C(=O)c2ccc(F)cc2)cc1)C(O)=O |r| Show InChI InChI=1S/C31H28FNO6S/c1-20(2)29(31(35)36)33-40(37,38)28-17-11-23(12-18-28)22-5-3-21(4-6-22)19-39-27-15-9-25(10-16-27)30(34)24-7-13-26(32)14-8-24/h3-18,20,29,33H,19H2,1-2H3,(H,35,36)/t29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 19: 2487-91 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.056
BindingDB Entry DOI: 10.7270/Q29886WB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50303124
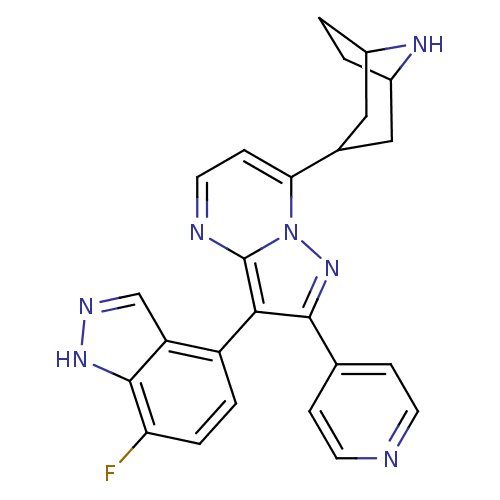
(7-(8-azabicyclo[3.2.1]octan-3-yl)-3-(7-fluoro-1H-i...)Show SMILES Fc1ccc(-c2c(nn3c(ccnc23)C2CC3CCC(C2)N3)-c2ccncc2)c2cn[nH]c12 |TLB:9:14:21:17.18,(31.91,-31.64,;31.85,-30.1,;30.48,-29.38,;30.43,-27.84,;31.72,-27.02,;31.66,-25.48,;32.57,-24.24,;31.66,-22.98,;30.18,-23.46,;28.85,-22.7,;27.52,-23.47,;27.52,-25.01,;28.85,-25.78,;30.18,-25.01,;28.84,-21.16,;28.4,-19.97,;26.81,-19.06,;25.29,-19.74,;26.66,-19.7,;27.85,-18.84,;29.39,-19.69,;27.34,-17.59,;34.1,-24.23,;34.87,-25.57,;36.41,-25.57,;37.18,-24.23,;36.4,-22.9,;34.86,-22.9,;33.09,-27.73,;34.54,-27.19,;35.5,-28.4,;34.65,-29.69,;33.16,-29.28,)| Show InChI InChI=1S/C25H22FN7/c26-20-4-3-18(19-13-29-31-24(19)20)22-23(14-5-8-27-9-6-14)32-33-21(7-10-28-25(22)33)15-11-16-1-2-17(12-15)30-16/h3-10,13,15-17,30H,1-2,11-12H2,(H,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf |
Bioorg Med Chem Lett 19: 6957-61 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.058
BindingDB Entry DOI: 10.7270/Q2833S34 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50303097
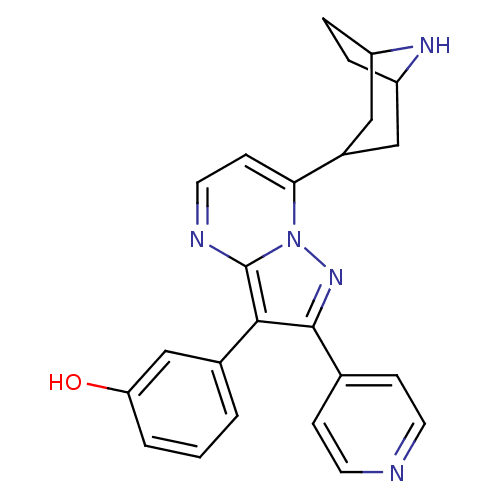
(3-(7-(8-azabicyclo[3.2.1]octan-3-yl)-2-(pyridin-4-...)Show SMILES Oc1cccc(c1)-c1c(nn2c(ccnc12)C1CC2CCC(C1)N2)-c1ccncc1 |TLB:11:16:23:19.20| Show InChI InChI=1S/C24H23N5O/c30-20-3-1-2-16(14-20)22-23(15-6-9-25-10-7-15)28-29-21(8-11-26-24(22)29)17-12-18-4-5-19(13-17)27-18/h1-3,6-11,14,17-19,27,30H,4-5,12-13H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf |
Bioorg Med Chem Lett 19: 6957-61 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.058
BindingDB Entry DOI: 10.7270/Q2833S34 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50303122

(3-(7-chloro-1H-indazol-4-yl)-7-(8-ethyl-8-azabicyc...)Show SMILES CCN1C2CCC1CC(C2)c1ccnc2c(c(nn12)-c1ccncc1)-c1ccc(Cl)c2[nH]ncc12 |THB:10:8:2:4.5,(-4.54,-15.7,;-5.86,-16.5,;-5.83,-18.03,;-6.37,-19.5,;-7.89,-20.18,;-6.52,-20.15,;-5.33,-19.28,;-3.79,-20.13,;-4.33,-21.6,;-4.77,-20.41,;-4.33,-23.14,;-5.65,-23.91,;-5.66,-25.45,;-4.32,-26.22,;-2.99,-25.45,;-1.52,-25.93,;-.6,-24.68,;-1.52,-23.42,;-2.99,-23.9,;.93,-24.68,;1.7,-26.01,;3.23,-26.01,;4.01,-24.68,;3.23,-23.34,;1.69,-23.35,;-1.45,-27.47,;-2.75,-28.29,;-2.69,-29.83,;-1.33,-30.55,;-1.27,-32.09,;-.02,-29.73,;1.48,-30.14,;2.33,-28.84,;1.36,-27.63,;-.09,-28.18,)| Show InChI InChI=1S/C27H26ClN7/c1-2-34-18-3-4-19(34)14-17(13-18)23-9-12-30-27-24(20-5-6-22(28)26-21(20)15-31-32-26)25(33-35(23)27)16-7-10-29-11-8-16/h5-12,15,17-19H,2-4,13-14H2,1H3,(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf |
Bioorg Med Chem Lett 19: 6957-61 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.058
BindingDB Entry DOI: 10.7270/Q2833S34 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50303125
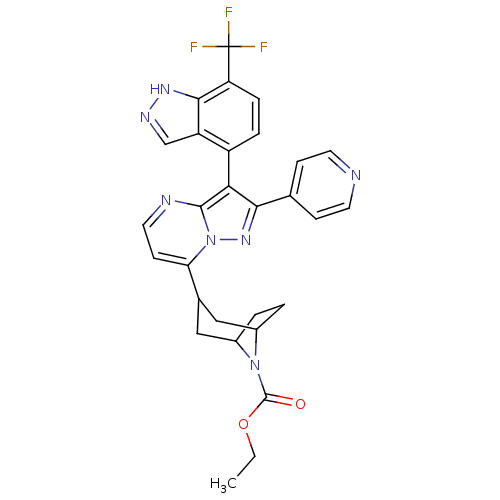
(CHEMBL571744 | ethyl 3-(2-(pyridin-4-yl)-3-(7-(tri...)Show SMILES CCOC(=O)N1C2CCC1CC(C2)c1ccnc2c(c(nn12)-c1ccncc1)-c1ccc(c2[nH]ncc12)C(F)(F)F |THB:13:11:5:7.8,(.29,-34.91,;-1.03,-35.7,;-2.38,-34.96,;-3.7,-35.75,;-5.05,-35.01,;-3.67,-37.29,;-4.2,-38.76,;-5.73,-39.44,;-4.35,-39.41,;-3.16,-38.54,;-1.62,-39.39,;-2.16,-40.86,;-2.61,-39.67,;-2.16,-42.4,;-3.49,-43.17,;-3.49,-44.72,;-2.15,-45.49,;-.82,-44.72,;.65,-45.19,;1.56,-43.94,;.65,-42.69,;-.82,-43.17,;3.1,-43.94,;3.87,-45.28,;5.41,-45.28,;6.18,-43.94,;5.4,-42.6,;3.86,-42.61,;.72,-46.73,;-.58,-47.55,;-.52,-49.1,;.84,-49.81,;2.15,-48.99,;3.65,-49.4,;4.5,-48.11,;3.53,-46.9,;2.08,-47.44,;.9,-51.35,;-.4,-52.17,;2.26,-52.07,;.89,-52.89,)| Show InChI InChI=1S/C29H26F3N7O2/c1-2-41-28(40)38-18-3-4-19(38)14-17(13-18)23-9-12-34-27-24(25(37-39(23)27)16-7-10-33-11-8-16)20-5-6-22(29(30,31)32)26-21(20)15-35-36-26/h5-12,15,17-19H,2-4,13-14H2,1H3,(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf |
Bioorg Med Chem Lett 19: 6957-61 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.058
BindingDB Entry DOI: 10.7270/Q2833S34 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50303112
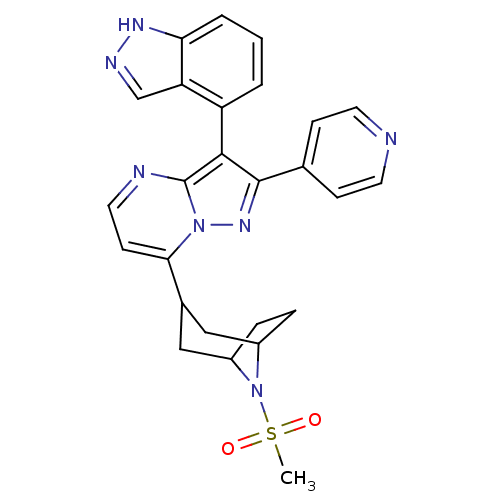
(3-(1H-indazol-4-yl)-7-(8-(methylsulfonyl)-8-azabic...)Show SMILES CS(=O)(=O)N1C2CCC1CC(C2)c1ccnc2c(c(nn12)-c1ccncc1)-c1cccc2[nH]ncc12 |THB:12:10:4:6.7,(-3.77,1.28,;-5.1,.49,;-6.44,-.27,;-6.43,1.27,;-5.06,-1.05,;-5.6,-2.52,;-7.12,-3.2,;-5.75,-3.16,;-4.56,-2.3,;-3.02,-3.14,;-3.56,-4.62,;-4,-3.43,;-3.56,-6.16,;-4.88,-6.93,;-4.89,-8.47,;-3.55,-9.24,;-2.22,-8.47,;-.75,-8.94,;.16,-7.69,;-.75,-6.44,;-2.22,-6.92,;1.7,-7.69,;2.47,-9.03,;4,-9.03,;4.77,-7.69,;4,-6.35,;2.46,-6.36,;-.68,-10.48,;-1.98,-11.3,;-1.92,-12.84,;-.56,-13.56,;.75,-12.74,;2.25,-13.15,;3.1,-11.86,;2.13,-10.65,;.68,-11.19,)| Show InChI InChI=1S/C26H25N7O2S/c1-36(34,35)33-18-5-6-19(33)14-17(13-18)23-9-12-28-26-24(20-3-2-4-22-21(20)15-29-30-22)25(31-32(23)26)16-7-10-27-11-8-16/h2-4,7-12,15,17-19H,5-6,13-14H2,1H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf |
Bioorg Med Chem Lett 19: 6957-61 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.058
BindingDB Entry DOI: 10.7270/Q2833S34 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50258917

((R)-2-(4'-((4-(cyclohexanecarbonyl)phenoxy)methyl)...)Show SMILES CC(C)[C@@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(COc2ccc(cc2)C(=O)C2CCCCC2)cc1)C(O)=O |r| Show InChI InChI=1S/C31H35NO6S/c1-21(2)29(31(34)35)32-39(36,37)28-18-14-24(15-19-28)23-10-8-22(9-11-23)20-38-27-16-12-26(13-17-27)30(33)25-6-4-3-5-7-25/h8-19,21,25,29,32H,3-7,20H2,1-2H3,(H,34,35)/t29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 19: 2487-91 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.056
BindingDB Entry DOI: 10.7270/Q29886WB |
More data for this
Ligand-Target Pair | |
D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor
(Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM50327157

((R)-methyl 2-(3,4-dichlorophenyl)-2-((R)-piperidin...)Show SMILES COC(=O)[C@@H]([C@H]1CCCCN1)c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C14H17Cl2NO2/c1-19-14(18)13(12-4-2-3-7-17-12)9-5-6-10(15)11(16)8-9/h5-6,8,12-13,17H,2-4,7H2,1H3/t12-,13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York
Curated by ChEMBL
| Assay Description
Potency of inhibiting 10 pM [125I]-RTI-55 binding to dopamine transporter in rat striatal membranes |
Bioorg Med Chem Lett 14: 1799-802 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.097
BindingDB Entry DOI: 10.7270/Q2T43SJ8 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50258875

((R)-2-(4'-((4-(4-fluorobenzoyl)phenoxy)methyl)biph...)Show SMILES CC(C)[C@@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(COc2ccc(cc2)C(=O)c2ccc(F)cc2)cc1)C(O)=O |r| Show InChI InChI=1S/C31H28FNO6S/c1-20(2)29(31(35)36)33-40(37,38)28-17-11-23(12-18-28)22-5-3-21(4-6-22)19-39-27-15-9-25(10-16-27)30(34)24-7-13-26(32)14-8-24/h3-18,20,29,33H,19H2,1-2H3,(H,35,36)/t29-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 19: 2487-91 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.056
BindingDB Entry DOI: 10.7270/Q29886WB |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50259009

((R)-3-methyl-2-(4'-((5-oxo-5,6,7,8-tetrahydronapht...)Show SMILES CC(C)[C@@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(COc2ccc3C(=O)CCCc3c2)cc1)C(O)=O |r| Show InChI InChI=1S/C28H29NO6S/c1-18(2)27(28(31)32)29-36(33,34)24-13-10-21(11-14-24)20-8-6-19(7-9-20)17-35-23-12-15-25-22(16-23)4-3-5-26(25)30/h6-16,18,27,29H,3-5,17H2,1-2H3,(H,31,32)/t27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 19: 2487-91 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.056
BindingDB Entry DOI: 10.7270/Q29886WB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50303113
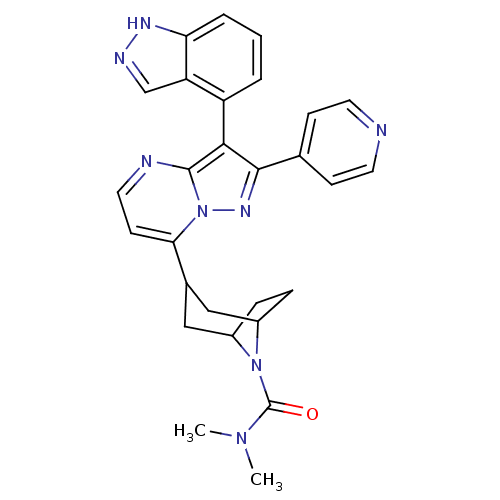
(3-(3-(1H-indazol-4-yl)-2-(pyridin-4-yl)-1,2-dihydr...)Show SMILES CN(C)C(=O)N1C2CCC1CC(C2)c1ccnc2c(c(nn12)-c1ccncc1)-c1cccc2[nH]ncc12 |THB:13:11:5:7.8,(13.39,-1.4,;12.04,-.65,;12.02,.89,;10.72,-1.45,;9.38,-.7,;10.75,-2.98,;10.22,-4.45,;8.7,-5.13,;10.07,-5.1,;11.26,-4.23,;12.8,-5.08,;12.26,-6.55,;11.81,-5.36,;12.26,-8.09,;10.93,-8.86,;10.93,-10.4,;12.26,-11.17,;13.6,-10.4,;15.07,-10.88,;15.98,-9.63,;15.07,-8.37,;13.6,-8.85,;17.51,-9.62,;18.28,-10.96,;19.82,-10.96,;20.59,-9.63,;19.81,-8.29,;18.28,-8.3,;15.14,-12.41,;13.84,-13.24,;13.9,-14.78,;15.26,-15.5,;16.57,-14.67,;18.06,-15.09,;18.92,-13.79,;17.95,-12.58,;16.5,-13.13,)| Show InChI InChI=1S/C28H28N8O/c1-34(2)28(37)35-19-6-7-20(35)15-18(14-19)24-10-13-30-27-25(21-4-3-5-23-22(21)16-31-32-23)26(33-36(24)27)17-8-11-29-12-9-17/h3-5,8-13,16,18-20H,6-7,14-15H2,1-2H3,(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf |
Bioorg Med Chem Lett 19: 6957-61 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.058
BindingDB Entry DOI: 10.7270/Q2833S34 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50303114

(3-(3-(1H-indazol-4-yl)-2-(pyridin-4-yl)-1,2-dihydr...)Show SMILES CCNC(=O)N1C2CCC1CC(C2)c1ccnc2c(c(nn12)-c1ccncc1)-c1cccc2[nH]ncc12 |THB:13:11:5:7.8,(29.22,-.78,;27.9,-1.58,;26.56,-.83,;25.24,-1.63,;23.89,-.88,;25.27,-3.16,;24.73,-4.63,;23.21,-5.31,;24.58,-5.28,;25.77,-4.41,;27.31,-5.26,;26.77,-6.73,;26.33,-5.54,;26.78,-8.27,;25.45,-9.04,;25.45,-10.58,;26.78,-11.35,;28.11,-10.58,;29.59,-11.06,;30.5,-9.81,;29.58,-8.55,;28.11,-9.03,;32.03,-9.8,;32.8,-11.14,;34.34,-11.14,;35.11,-9.81,;34.33,-8.47,;32.79,-8.48,;29.65,-12.6,;28.35,-13.42,;28.41,-14.96,;29.77,-15.68,;31.08,-14.85,;32.58,-15.27,;33.43,-13.97,;32.47,-12.76,;31.01,-13.31,)| Show InChI InChI=1S/C28H28N8O/c1-2-30-28(37)35-19-6-7-20(35)15-18(14-19)24-10-13-31-27-25(21-4-3-5-23-22(21)16-32-33-23)26(34-36(24)27)17-8-11-29-12-9-17/h3-5,8-13,16,18-20H,2,6-7,14-15H2,1H3,(H,30,37)(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf |
Bioorg Med Chem Lett 19: 6957-61 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.058
BindingDB Entry DOI: 10.7270/Q2833S34 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50303129
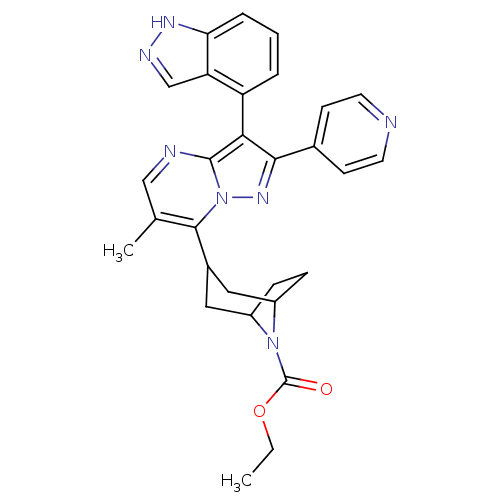
(CHEMBL585335 | ethyl 3-(3-(1H-indazol-4-yl)-6-meth...)Show SMILES CCOC(=O)N1C2CCC1CC(C2)c1c(C)cnc2c(c(nn12)-c1ccncc1)-c1cccc2[nH]ncc12 |THB:13:11:5:7.8,(5.99,.06,;4.67,-.73,;3.32,.01,;2,-.78,;.65,-.04,;2.03,-2.32,;1.5,-3.79,;-.02,-4.47,;1.35,-4.44,;2.54,-3.57,;4.08,-4.42,;3.54,-5.89,;3.09,-4.7,;3.54,-7.43,;2.21,-8.2,;.88,-7.43,;2.21,-9.74,;3.54,-10.51,;4.88,-9.74,;6.35,-10.22,;7.26,-8.97,;6.35,-7.71,;4.88,-8.19,;8.79,-8.97,;9.56,-10.3,;11.1,-10.3,;11.87,-8.97,;11.09,-7.63,;9.56,-7.64,;6.42,-11.76,;5.12,-12.58,;5.18,-14.12,;6.54,-14.84,;7.85,-14.02,;9.34,-14.43,;10.2,-13.13,;9.23,-11.92,;7.78,-12.47,)| Show InChI InChI=1S/C29H29N7O2/c1-3-38-29(37)35-20-7-8-21(35)14-19(13-20)27-17(2)15-31-28-25(22-5-4-6-24-23(22)16-32-33-24)26(34-36(27)28)18-9-11-30-12-10-18/h4-6,9-12,15-16,19-21H,3,7-8,13-14H2,1-2H3,(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf |
Bioorg Med Chem Lett 19: 6957-61 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.058
BindingDB Entry DOI: 10.7270/Q2833S34 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50303096
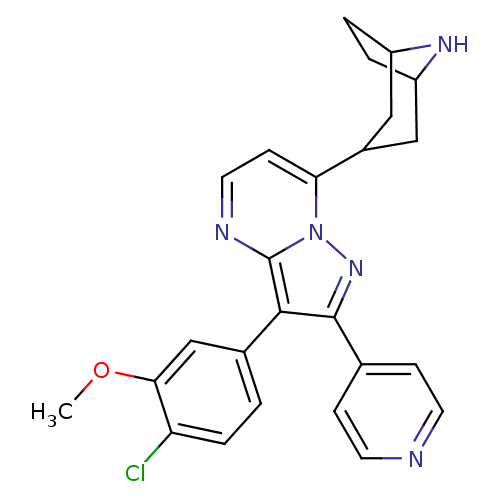
(7-(8-azabicyclo[3.2.1]octan-3-yl)-3-(4-chloro-3-me...)Show SMILES COc1cc(ccc1Cl)-c1c(nn2c(ccnc12)C1CC2CCC(C1)N2)-c1ccncc1 |TLB:13:18:25:21.22| Show InChI InChI=1S/C25H24ClN5O/c1-32-22-14-16(2-5-20(22)26)23-24(15-6-9-27-10-7-15)30-31-21(8-11-28-25(23)31)17-12-18-3-4-19(13-17)29-18/h2,5-11,14,17-19,29H,3-4,12-13H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf |
Bioorg Med Chem Lett 19: 6957-61 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.058
BindingDB Entry DOI: 10.7270/Q2833S34 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50303115
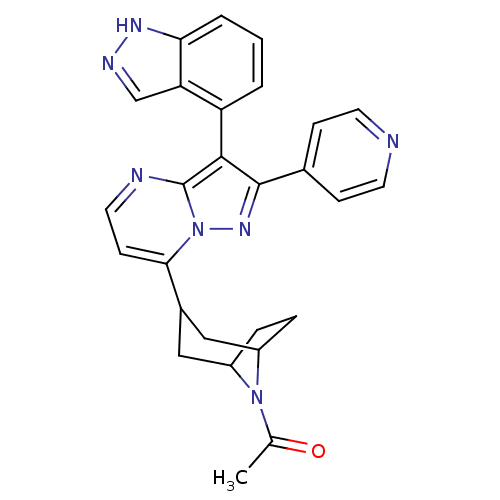
(1-(3-(3-(1H-indazol-4-yl)-2-(pyridin-4-yl)-1,2-dih...)Show SMILES CC(=O)N1C2CCC1CC(C2)c1ccnc2c(c(nn12)-c1ccncc1)-c1cccc2[nH]ncc12 |THB:11:9:3:5.6,(42.06,-.04,;40.74,-.83,;39.39,-.09,;40.77,-2.37,;40.23,-3.84,;38.71,-4.52,;40.08,-4.48,;41.27,-3.62,;42.81,-4.47,;42.27,-5.94,;41.83,-4.75,;42.28,-7.48,;40.95,-8.25,;40.95,-9.79,;42.28,-10.56,;43.61,-9.79,;45.09,-10.26,;46,-9.02,;45.08,-7.76,;43.61,-8.24,;47.53,-9.01,;48.3,-10.35,;49.84,-10.35,;50.61,-9.01,;49.83,-7.68,;48.29,-7.68,;45.15,-11.8,;43.85,-12.62,;43.91,-14.17,;45.27,-14.88,;46.58,-14.06,;48.08,-14.47,;48.93,-13.18,;47.97,-11.97,;46.51,-12.51,)| Show InChI InChI=1S/C27H25N7O/c1-16(35)33-19-5-6-20(33)14-18(13-19)24-9-12-29-27-25(21-3-2-4-23-22(21)15-30-31-23)26(32-34(24)27)17-7-10-28-11-8-17/h2-4,7-12,15,18-20H,5-6,13-14H2,1H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf |
Bioorg Med Chem Lett 19: 6957-61 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.058
BindingDB Entry DOI: 10.7270/Q2833S34 |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50319739
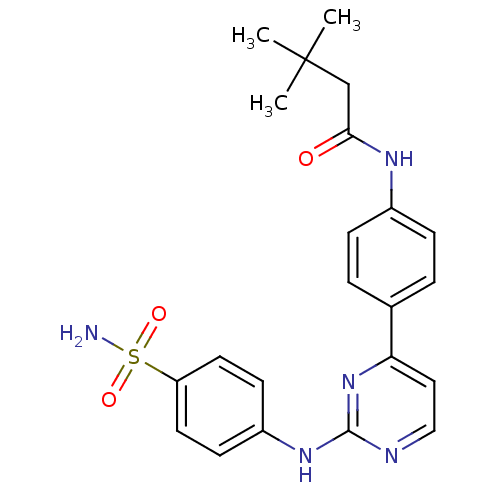
(3,3-dimethyl-N-(4-(2-(4-sulfamoylphenylamino)pyrim...)Show SMILES CC(C)(C)CC(=O)Nc1ccc(cc1)-c1ccnc(Nc2ccc(cc2)S(N)(=O)=O)n1 Show InChI InChI=1S/C22H25N5O3S/c1-22(2,3)14-20(28)25-16-6-4-15(5-7-16)19-12-13-24-21(27-19)26-17-8-10-18(11-9-17)31(23,29)30/h4-13H,14H2,1-3H3,(H,25,28)(H2,23,29,30)(H,24,26,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged IKKbeta after 30 mins |
Bioorg Med Chem Lett 20: 3821-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.022
BindingDB Entry DOI: 10.7270/Q2833S7X |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50258917

((R)-2-(4'-((4-(cyclohexanecarbonyl)phenoxy)methyl)...)Show SMILES CC(C)[C@@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(COc2ccc(cc2)C(=O)C2CCCCC2)cc1)C(O)=O |r| Show InChI InChI=1S/C31H35NO6S/c1-21(2)29(31(34)35)32-39(36,37)28-18-14-24(15-19-28)23-10-8-22(9-11-23)20-38-27-16-12-26(13-17-27)30(33)25-6-4-3-5-7-25/h8-19,21,25,29,32H,3-7,20H2,1-2H3,(H,34,35)/t29-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 19: 2487-91 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.056
BindingDB Entry DOI: 10.7270/Q29886WB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data