

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50095523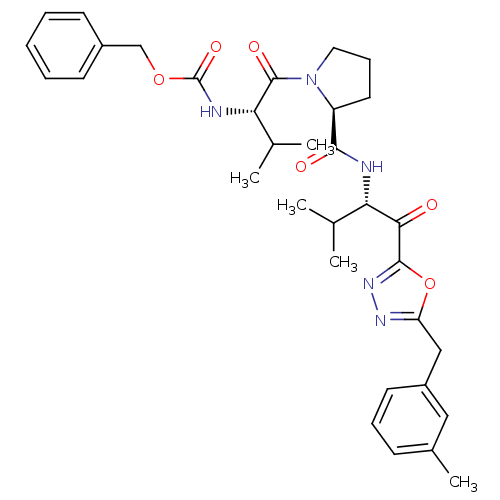 (CHEMBL285231 | [(S)-2-methyl-1-((S)-2-{(S)-2-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase HNE, hydrolysis of MeO-Suc-Ala-Ala-Pro-Val-pNa | J Med Chem 43: 4927-9 (2001) BindingDB Entry DOI: 10.7270/Q25D8R3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50095523 (CHEMBL285231 | [(S)-2-methyl-1-((S)-2-{(S)-2-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cortech, Inc. Curated by ChEMBL | Assay Description Binding constant derived from inhibition of elastase catalyzed hydrolysis of synthetic substrate | J Med Chem 44: 1268-85 (2001) BindingDB Entry DOI: 10.7270/Q2B858VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM22416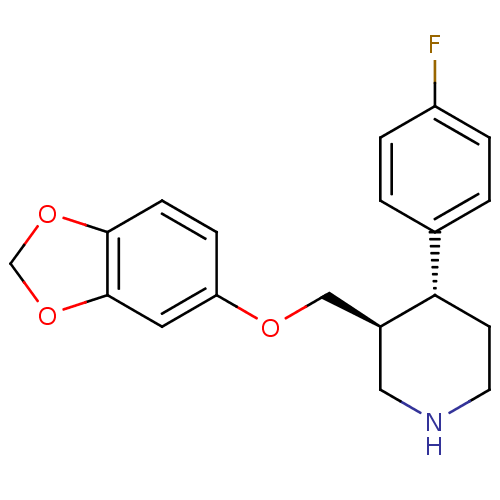 ((3S,4R)-3-[(1,3-benzodioxol-5-yloxy)methyl]-4-(4-f...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from human SERT expressed in HEK293 cell membranes after 1 hr by liquid scintillation counting method | Bioorg Med Chem 25: 293-304 (2017) Article DOI: 10.1016/j.bmc.2016.10.034 BindingDB Entry DOI: 10.7270/Q2571F0B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50206761 (CHEMBL3958278) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor expressed in CHOK1 cells after 30 mins by liquid scintillation counting analysis | Bioorg Med Chem 25: 293-304 (2017) Article DOI: 10.1016/j.bmc.2016.10.034 BindingDB Entry DOI: 10.7270/Q2571F0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50095526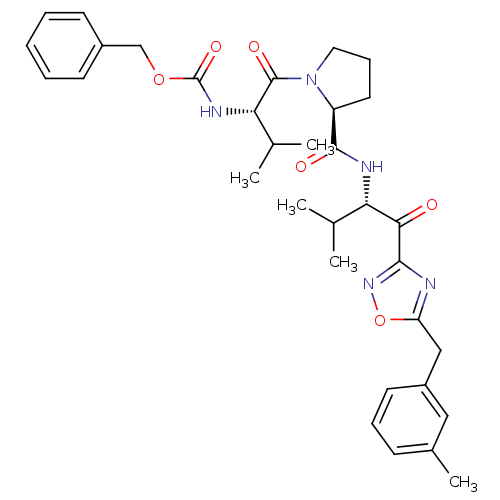 (CHEMBL24058 | [(S)-2-methyl-1-((S)-2-{(S)-2-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cortech, Inc. Curated by ChEMBL | Assay Description Binding constant derived from inhibition of elastase catalyzed hydrolysis of synthetic substrate | J Med Chem 44: 1268-85 (2001) BindingDB Entry DOI: 10.7270/Q2B858VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50095526 (CHEMBL24058 | [(S)-2-methyl-1-((S)-2-{(S)-2-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase HNE, hydrolysis of MeO-Suc-Ala-Ala-Pro-Val-pNa | J Med Chem 43: 4927-9 (2001) BindingDB Entry DOI: 10.7270/Q25D8R3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50095530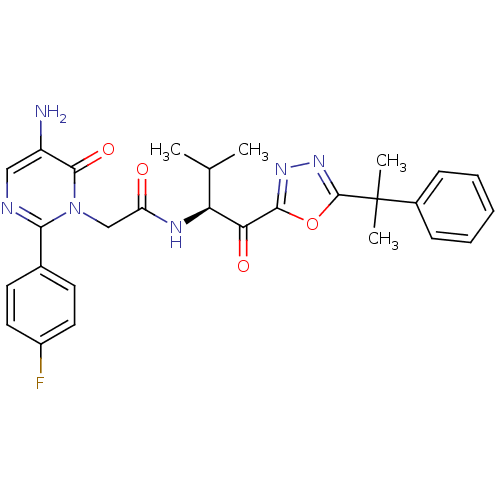 ((S)-2-(5-amino-2-(4-fluorophenyl)-6-oxopyrimidin-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cortech, Inc. Curated by ChEMBL | Assay Description Binding constant derived from inhibition of elastase catalyzed hydrolysis of synthetic substrate | J Med Chem 44: 1268-85 (2001) BindingDB Entry DOI: 10.7270/Q2B858VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50095530 ((S)-2-(5-amino-2-(4-fluorophenyl)-6-oxopyrimidin-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase HNE, hydrolysis of MeO-Suc-Ala-Ala-Pro-Val-pNa | J Med Chem 43: 4927-9 (2001) BindingDB Entry DOI: 10.7270/Q25D8R3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50095521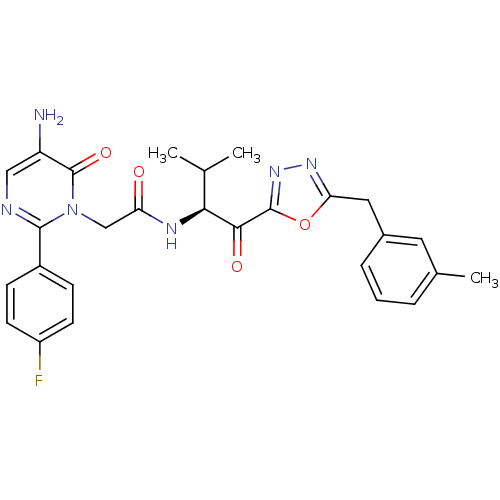 ((S)-2-[5-Amino-2-(4-fluoro-phenyl)-6-oxo-6H-pyrimi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase HNE, hydrolysis of MeO-Suc-Ala-Ala-Pro-Val-pNa | J Med Chem 43: 4927-9 (2001) BindingDB Entry DOI: 10.7270/Q25D8R3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50095521 ((S)-2-[5-Amino-2-(4-fluoro-phenyl)-6-oxo-6H-pyrimi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cortech, Inc. Curated by ChEMBL | Assay Description Binding constant derived from inhibition of elastase catalyzed hydrolysis of synthetic substrate | J Med Chem 44: 1268-85 (2001) BindingDB Entry DOI: 10.7270/Q2B858VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50206755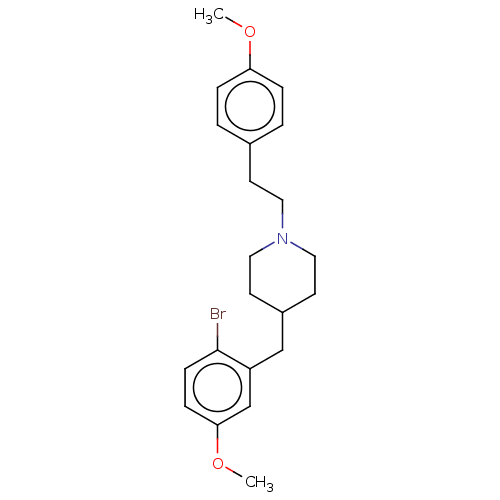 (CHEMBL3940484) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor expressed in CHOK1 cells after 30 mins by liquid scintillation counting analysis | Bioorg Med Chem 25: 293-304 (2017) Article DOI: 10.1016/j.bmc.2016.10.034 BindingDB Entry DOI: 10.7270/Q2571F0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50206797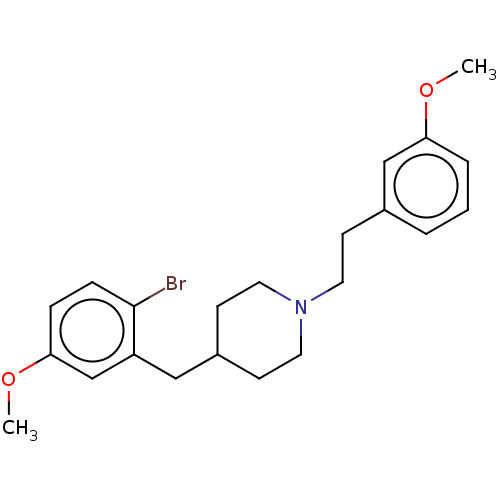 (CHEMBL3974952) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor expressed in CHOK1 cells after 30 mins by liquid scintillation counting analysis | Bioorg Med Chem 25: 293-304 (2017) Article DOI: 10.1016/j.bmc.2016.10.034 BindingDB Entry DOI: 10.7270/Q2571F0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50206788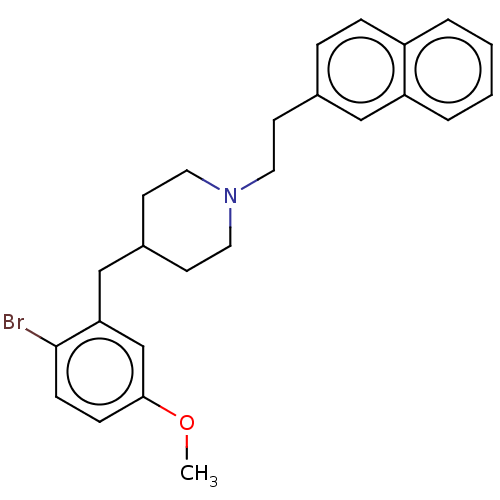 (CHEMBL3949638) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor expressed in CHOK1 cells after 30 mins by liquid scintillation counting analysis | Bioorg Med Chem 25: 293-304 (2017) Article DOI: 10.1016/j.bmc.2016.10.034 BindingDB Entry DOI: 10.7270/Q2571F0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50206759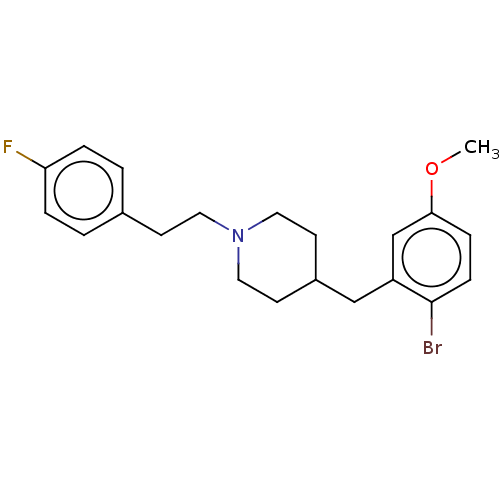 (CHEMBL3964504) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor expressed in CHOK1 cells after 30 mins by liquid scintillation counting analysis | Bioorg Med Chem 25: 293-304 (2017) Article DOI: 10.1016/j.bmc.2016.10.034 BindingDB Entry DOI: 10.7270/Q2571F0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50206756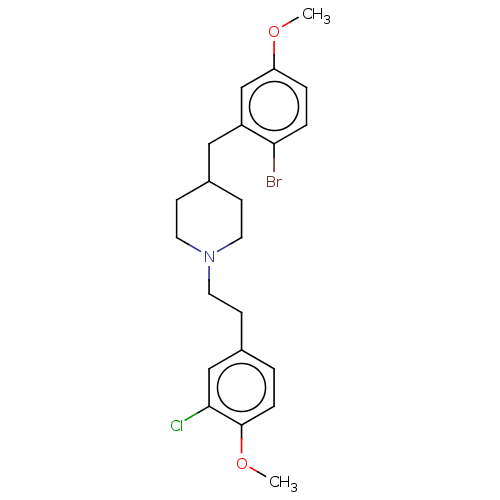 (CHEMBL3950161) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor expressed in CHOK1 cells after 30 mins by liquid scintillation counting analysis | Bioorg Med Chem 25: 293-304 (2017) Article DOI: 10.1016/j.bmc.2016.10.034 BindingDB Entry DOI: 10.7270/Q2571F0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50206753 (CHEMBL3940863) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor expressed in CHOK1 cells after 30 mins by liquid scintillation counting analysis | Bioorg Med Chem 25: 293-304 (2017) Article DOI: 10.1016/j.bmc.2016.10.034 BindingDB Entry DOI: 10.7270/Q2571F0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50095519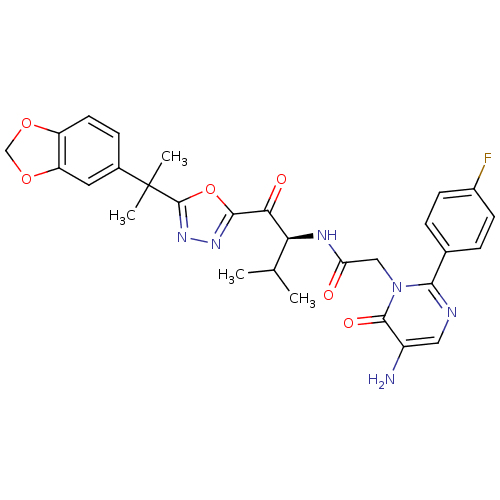 ((S)-2-[5-Amino-2-(4-fluoro-phenyl)-6-oxo-6H-pyrimi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase HNE, hydrolysis of MeO-Suc-Ala-Ala-Pro-Val-pNa | J Med Chem 43: 4927-9 (2001) BindingDB Entry DOI: 10.7270/Q25D8R3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50095519 ((S)-2-[5-Amino-2-(4-fluoro-phenyl)-6-oxo-6H-pyrimi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cortech, Inc. Curated by ChEMBL | Assay Description Binding constant derived from inhibition of elastase catalyzed hydrolysis of synthetic substrate | J Med Chem 44: 1268-85 (2001) BindingDB Entry DOI: 10.7270/Q2B858VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50206775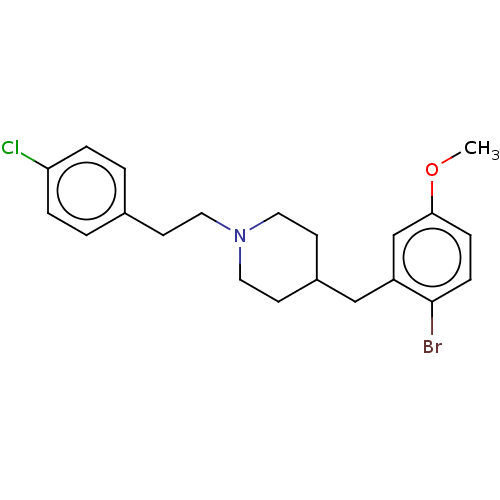 (CHEMBL3982923) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor expressed in CHOK1 cells after 30 mins by liquid scintillation counting analysis | Bioorg Med Chem 25: 293-304 (2017) Article DOI: 10.1016/j.bmc.2016.10.034 BindingDB Entry DOI: 10.7270/Q2571F0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50095525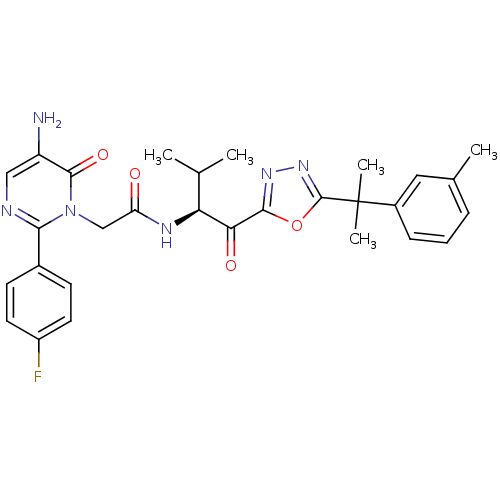 ((S)-2-(5-amino-2-(4-fluorophenyl)-6-oxopyrimidin-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cortech, Inc. Curated by ChEMBL | Assay Description Binding constant derived from inhibition of elastase catalyzed hydrolysis of synthetic substrate | J Med Chem 44: 1268-85 (2001) BindingDB Entry DOI: 10.7270/Q2B858VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50095525 ((S)-2-(5-amino-2-(4-fluorophenyl)-6-oxopyrimidin-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase HNE, hydrolysis of MeO-Suc-Ala-Ala-Pro-Val-pNa | J Med Chem 43: 4927-9 (2001) BindingDB Entry DOI: 10.7270/Q25D8R3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50206772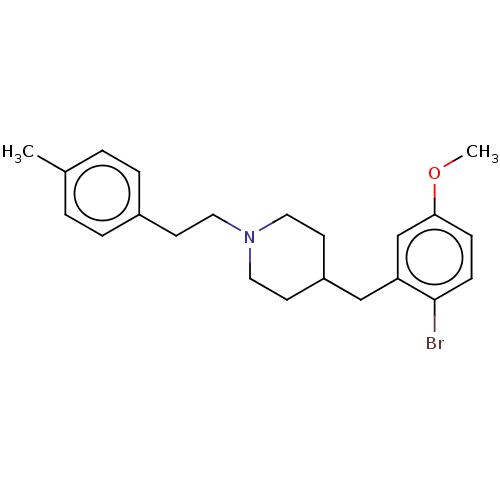 (CHEMBL3942037) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor expressed in CHOK1 cells after 30 mins by liquid scintillation counting analysis | Bioorg Med Chem 25: 293-304 (2017) Article DOI: 10.1016/j.bmc.2016.10.034 BindingDB Entry DOI: 10.7270/Q2571F0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50206785 (CHEMBL3963429) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor expressed in CHOK1 cells after 30 mins by liquid scintillation counting analysis | Bioorg Med Chem 25: 293-304 (2017) Article DOI: 10.1016/j.bmc.2016.10.034 BindingDB Entry DOI: 10.7270/Q2571F0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50098818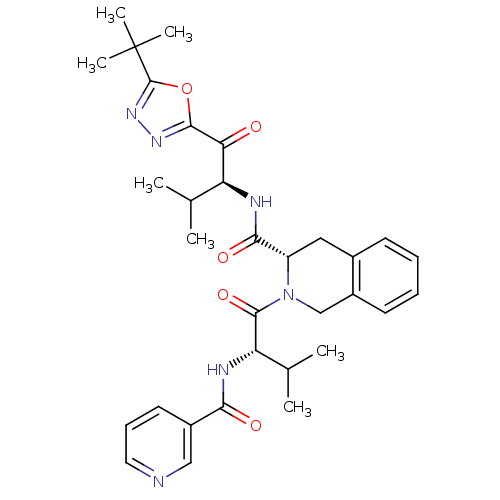 (2-{3-Methyl-2-[(pyridine-3-carbonyl)-amino]-butyry...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cortech, Inc. Curated by ChEMBL | Assay Description Binding constant derived from inhibition of elastase catalyzed hydrolysis of synthetic substrate | J Med Chem 44: 1268-85 (2001) BindingDB Entry DOI: 10.7270/Q2B858VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50010859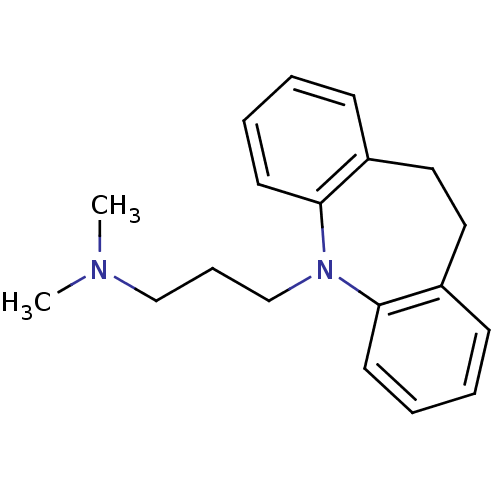 (CHEMBL11 | IMIPRAMINE HYDROCHLORIDE | IMIPRAMINE P...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from human SERT expressed in HEK293 cell membranes after 1 hr by liquid scintillation counting method | Bioorg Med Chem 25: 293-304 (2017) Article DOI: 10.1016/j.bmc.2016.10.034 BindingDB Entry DOI: 10.7270/Q2571F0B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50206763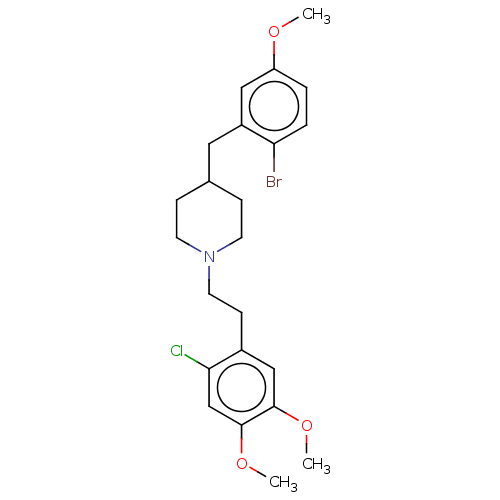 (CHEMBL3912020) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor expressed in CHOK1 cells after 30 mins by liquid scintillation counting analysis | Bioorg Med Chem 25: 293-304 (2017) Article DOI: 10.1016/j.bmc.2016.10.034 BindingDB Entry DOI: 10.7270/Q2571F0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50206757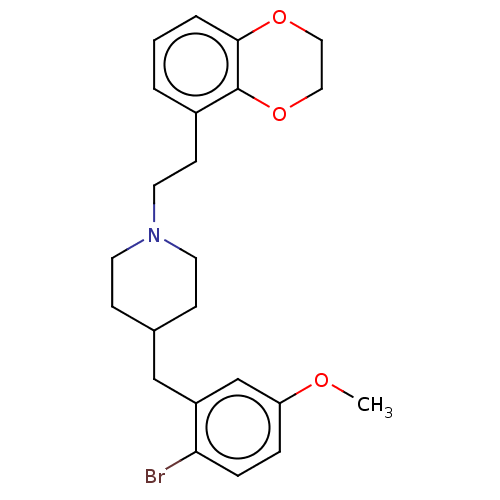 (CHEMBL3921860) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor expressed in CHOK1 cells after 30 mins by liquid scintillation counting analysis | Bioorg Med Chem 25: 293-304 (2017) Article DOI: 10.1016/j.bmc.2016.10.034 BindingDB Entry DOI: 10.7270/Q2571F0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50098833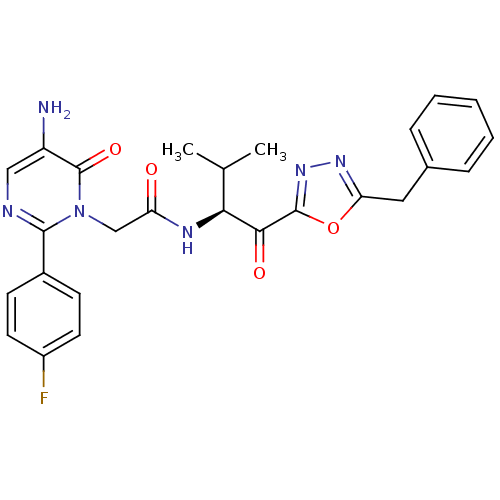 (2-[5-Amino-2-(4-fluoro-phenyl)-6-oxo-6H-pyrimidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cortech, Inc. Curated by ChEMBL | Assay Description Binding constant derived from inhibition of elastase catalyzed hydrolysis of synthetic substrate | J Med Chem 44: 1268-85 (2001) BindingDB Entry DOI: 10.7270/Q2B858VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50206762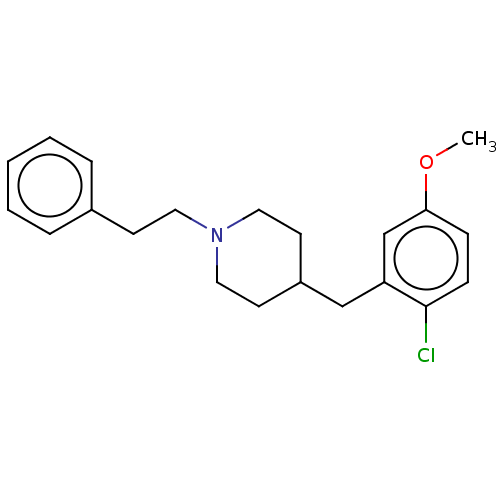 (CHEMBL3894524) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor expressed in CHOK1 cells after 30 mins by liquid scintillation counting analysis | Bioorg Med Chem 25: 293-304 (2017) Article DOI: 10.1016/j.bmc.2016.10.034 BindingDB Entry DOI: 10.7270/Q2571F0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50098827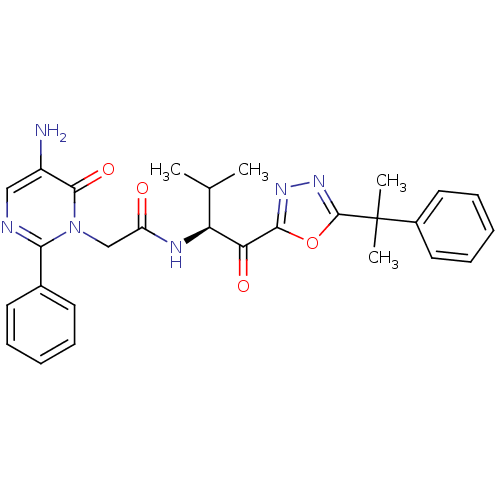 (2-(5-Amino-6-oxo-2-phenyl-6H-pyrimidin-1-yl)-N-{2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cortech, Inc. Curated by ChEMBL | Assay Description Binding constant derived from inhibition of elastase catalyzed hydrolysis of synthetic substrate | J Med Chem 44: 1268-85 (2001) BindingDB Entry DOI: 10.7270/Q2B858VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50206764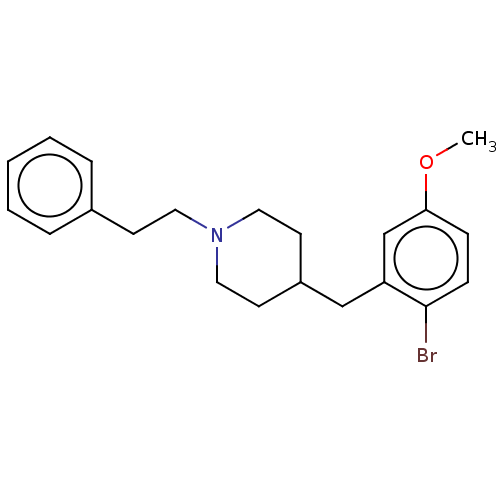 (CHEMBL3913541) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor expressed in CHOK1 cells after 30 mins by liquid scintillation counting analysis | Bioorg Med Chem 25: 293-304 (2017) Article DOI: 10.1016/j.bmc.2016.10.034 BindingDB Entry DOI: 10.7270/Q2571F0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50206776 (CHEMBL3896561) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor expressed in CHOK1 cells after 30 mins by liquid scintillation counting analysis | Bioorg Med Chem 25: 293-304 (2017) Article DOI: 10.1016/j.bmc.2016.10.034 BindingDB Entry DOI: 10.7270/Q2571F0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50206774 (CHEMBL3924225) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor expressed in CHOK1 cells after 30 mins by liquid scintillation counting analysis | Bioorg Med Chem 25: 293-304 (2017) Article DOI: 10.1016/j.bmc.2016.10.034 BindingDB Entry DOI: 10.7270/Q2571F0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50098817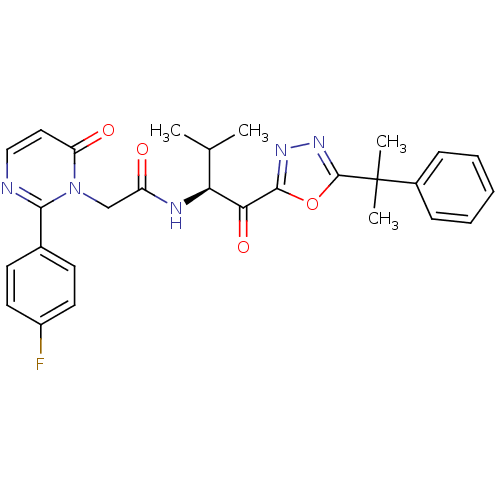 (2-[2-(4-Fluoro-phenyl)-6-oxo-6H-pyrimidin-1-yl]-N-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cortech, Inc. Curated by ChEMBL | Assay Description Binding constant derived from inhibition of elastase catalyzed hydrolysis of synthetic substrate | J Med Chem 44: 1268-85 (2001) BindingDB Entry DOI: 10.7270/Q2B858VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50095527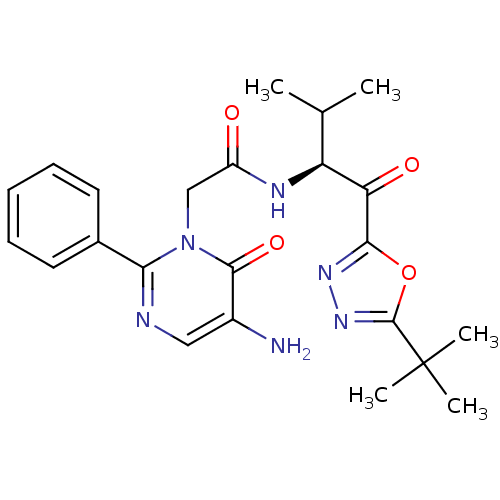 ((S)-2-(5-Amino-6-oxo-2-phenyl-6H-pyrimidin-1-yl)-N...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 3.59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cortech, Inc. Curated by ChEMBL | Assay Description Binding constant derived from inhibition of elastase catalyzed hydrolysis of synthetic substrate | J Med Chem 44: 1268-85 (2001) BindingDB Entry DOI: 10.7270/Q2B858VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50095527 ((S)-2-(5-Amino-6-oxo-2-phenyl-6H-pyrimidin-1-yl)-N...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 3.59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase HNE, hydrolysis of MeO-Suc-Ala-Ala-Pro-Val-pNa | J Med Chem 43: 4927-9 (2001) BindingDB Entry DOI: 10.7270/Q25D8R3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50190677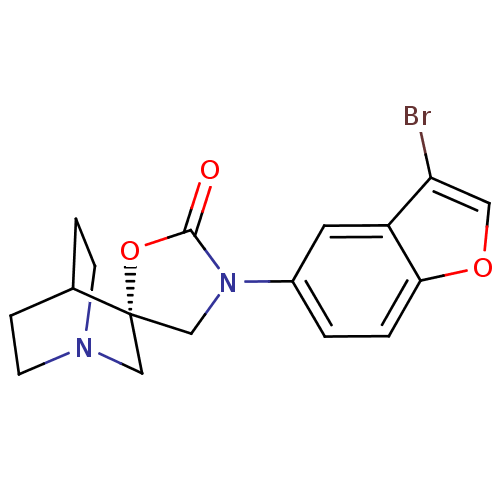 ((R)-3'-(3-bromobenzo[b]thiophen-5-yl)spiro[1-azabi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Displacement of [125I]alpha-BTX from alpha-7 nAChR in rat hippocampus membrane | J Med Chem 49: 4374-83 (2006) Article DOI: 10.1021/jm060249c BindingDB Entry DOI: 10.7270/Q2BR8T08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50190686 ((R)-3'-(2,3-dimethylbenzo[b]thiophen-5-yl)spiro[1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Displacement of [125I]alpha-BTX from alpha-7 nAChR in rat hippocampus membrane | J Med Chem 49: 4374-83 (2006) Article DOI: 10.1021/jm060249c BindingDB Entry DOI: 10.7270/Q2BR8T08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50190696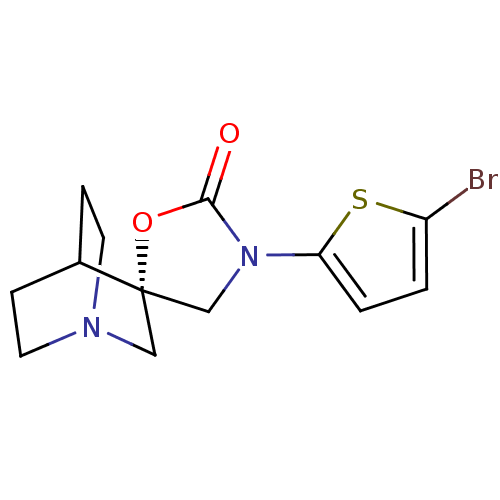 ((2S)-3'-(5-bromothien-2-yl)-2'H-spiro[4-azabicyclo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Displacement of [125I]alpha-BTX from alpha-7 nAChR in rat hippocampus membrane | J Med Chem 49: 4374-83 (2006) Article DOI: 10.1021/jm060249c BindingDB Entry DOI: 10.7270/Q2BR8T08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50206784 (CHEMBL3913057) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor expressed in CHOK1 cells after 30 mins by liquid scintillation counting analysis | Bioorg Med Chem 25: 293-304 (2017) Article DOI: 10.1016/j.bmc.2016.10.034 BindingDB Entry DOI: 10.7270/Q2571F0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50206770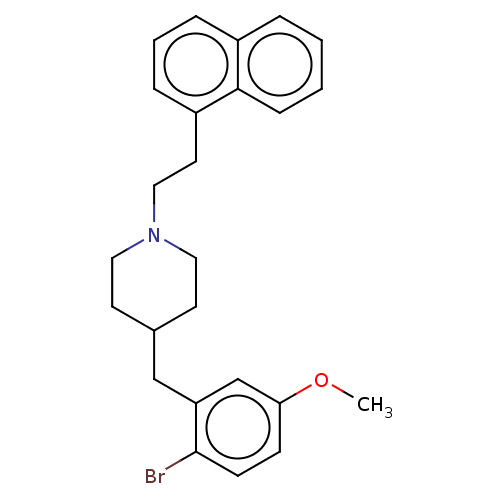 (CHEMBL3939995) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor expressed in CHOK1 cells after 30 mins by liquid scintillation counting analysis | Bioorg Med Chem 25: 293-304 (2017) Article DOI: 10.1016/j.bmc.2016.10.034 BindingDB Entry DOI: 10.7270/Q2571F0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50098830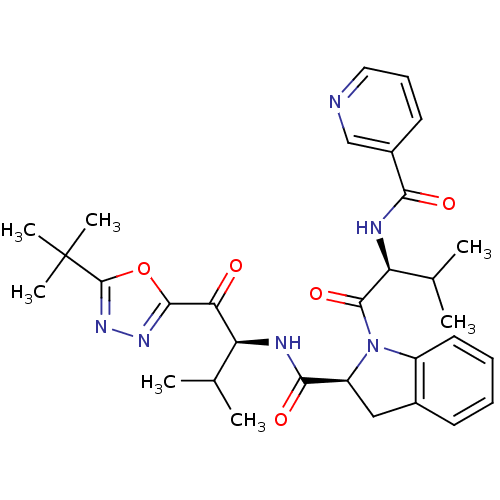 (1-{3-Methyl-2-[(pyridine-3-carbonyl)-amino]-butyry...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cortech, Inc. Curated by ChEMBL | Assay Description Binding constant derived from inhibition of elastase catalyzed hydrolysis of synthetic substrate | J Med Chem 44: 1268-85 (2001) BindingDB Entry DOI: 10.7270/Q2B858VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50206760 (CHEMBL3892582) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor expressed in CHOK1 cells after 30 mins by liquid scintillation counting analysis | Bioorg Med Chem 25: 293-304 (2017) Article DOI: 10.1016/j.bmc.2016.10.034 BindingDB Entry DOI: 10.7270/Q2571F0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50095520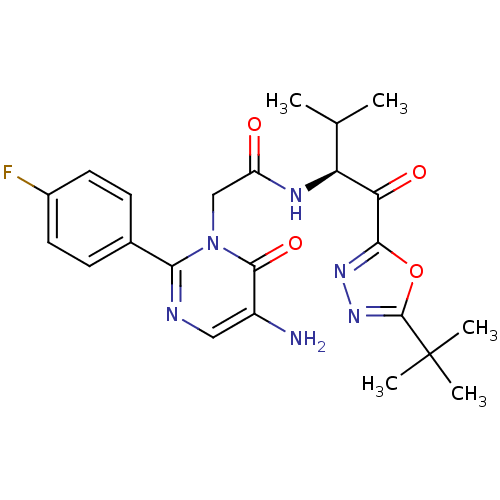 ((S)-2-(5-amino-2-(4-fluorophenyl)-6-oxopyrimidin-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 6.38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase HNE, hydrolysis of MeO-Suc-Ala-Ala-Pro-Val-pNa | J Med Chem 43: 4927-9 (2001) BindingDB Entry DOI: 10.7270/Q25D8R3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50095520 ((S)-2-(5-amino-2-(4-fluorophenyl)-6-oxopyrimidin-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 6.38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cortech, Inc. Curated by ChEMBL | Assay Description Binding constant derived from inhibition of elastase catalyzed hydrolysis of synthetic substrate | J Med Chem 44: 1268-85 (2001) BindingDB Entry DOI: 10.7270/Q2B858VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50206777 (CHEMBL3942269) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor expressed in CHOK1 cells after 30 mins by liquid scintillation counting analysis | Bioorg Med Chem 25: 293-304 (2017) Article DOI: 10.1016/j.bmc.2016.10.034 BindingDB Entry DOI: 10.7270/Q2571F0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50098823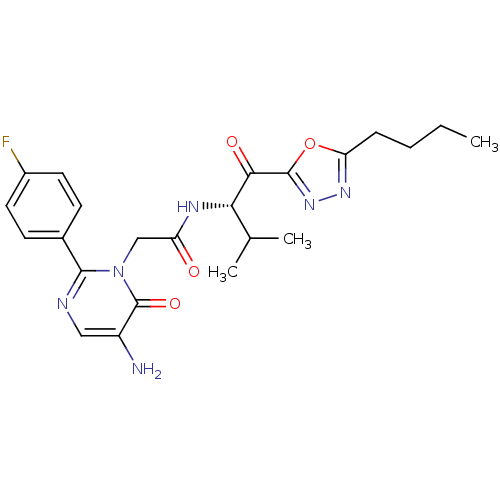 (2-[5-Amino-2-(4-fluoro-phenyl)-6-oxo-6H-pyrimidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 6.76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cortech, Inc. Curated by ChEMBL | Assay Description Binding constant derived from inhibition of elastase catalyzed hydrolysis of synthetic substrate | J Med Chem 44: 1268-85 (2001) BindingDB Entry DOI: 10.7270/Q2B858VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50098826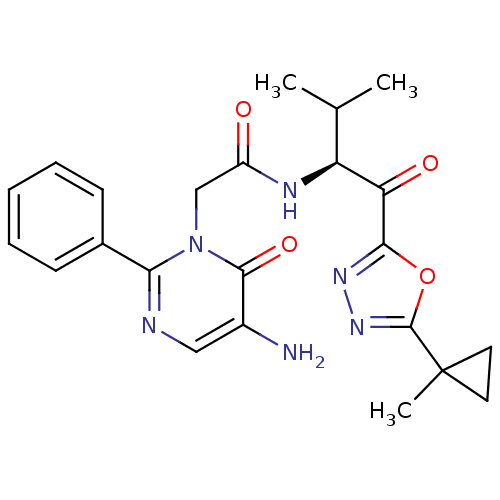 (2-(5-Amino-6-oxo-2-phenyl-6H-pyrimidin-1-yl)-N-{2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 8.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cortech, Inc. Curated by ChEMBL | Assay Description Binding constant derived from inhibition of elastase catalyzed hydrolysis of synthetic substrate | J Med Chem 44: 1268-85 (2001) BindingDB Entry DOI: 10.7270/Q2B858VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50095778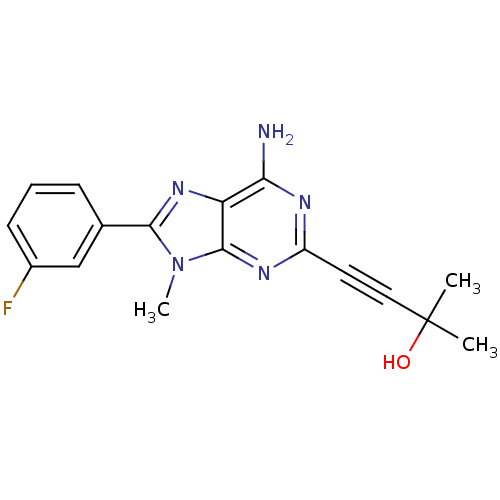 (4-[6-Amino-8-(3-fluoro-phenyl)-9-methyl-9H-purin-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding Affinity towards Adenosine A2A receptor expressed in HEK-293 cells versus [3H]-CGS-21,680 | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50206765 (CHEMBL3906576) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor expressed in CHOK1 cells after 30 mins by liquid scintillation counting analysis | Bioorg Med Chem 25: 293-304 (2017) Article DOI: 10.1016/j.bmc.2016.10.034 BindingDB Entry DOI: 10.7270/Q2571F0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3691 total ) | Next | Last >> |