

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N(4)-(beta-N-acetylglucosaminyl)-L-asparaginase (Homo sapiens (Human)) | BDBM85476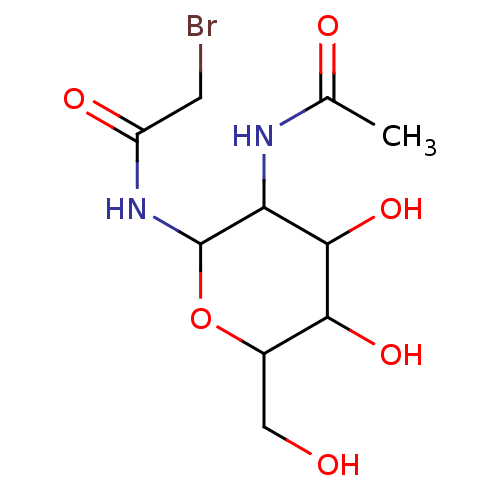 (GlcNAc-Asn analogue, 12) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.60E+5 | -19.3 | n/a | n/a | n/a | n/a | n/a | 5.8 | 37 |
University of North Carolina | Assay Description Glycosylasparaginase activity was measured in citrate-phosphate buffer at pH 5.8 at 37 C. N-Acetyl-D-glucosamine released during the reaction was me... | J Enzym Inhib 16: 269-74 (2001) Article DOI: 10.1080/14756360109162375 BindingDB Entry DOI: 10.7270/Q2P55M2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N(4)-(beta-N-acetylglucosaminyl)-L-asparaginase (Homo sapiens (Human)) | BDBM18125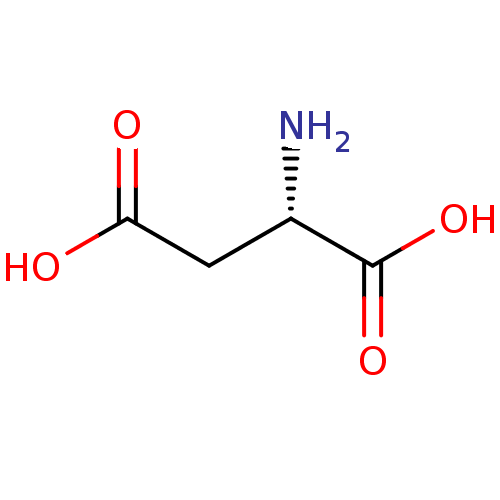 ((2S)-2-aminobutanedioic acid | Aspartate | CHEMBL2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 6.00E+5 | -19.1 | n/a | n/a | n/a | n/a | n/a | 5.8 | 37 |
University of North Carolina | Assay Description Glycosylasparaginase activity was measured in citrate-phosphate buffer at pH 5.8 at 37 C. N-Acetyl-D-glucosamine released during the reaction was me... | J Enzym Inhib 16: 269-74 (2001) Article DOI: 10.1080/14756360109162375 BindingDB Entry DOI: 10.7270/Q2P55M2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N(4)-(beta-N-acetylglucosaminyl)-L-asparaginase (Homo sapiens (Human)) | BDBM85475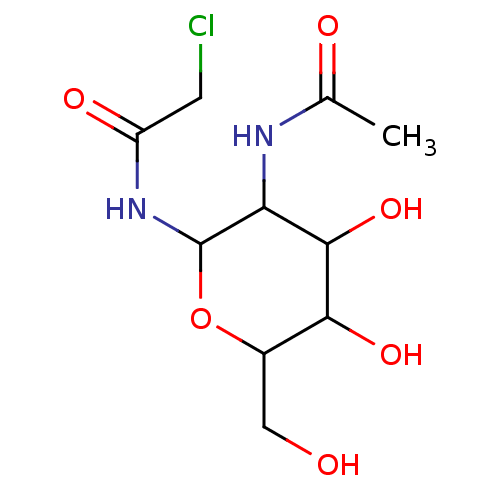 (GlcNAc-Asn analogue, 11) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.40E+5 | -19.0 | n/a | n/a | n/a | n/a | n/a | 5.8 | 37 |
University of North Carolina | Assay Description Glycosylasparaginase activity was measured in citrate-phosphate buffer at pH 5.8 at 37 C. N-Acetyl-D-glucosamine released during the reaction was me... | J Enzym Inhib 16: 269-74 (2001) Article DOI: 10.1080/14756360109162375 BindingDB Entry DOI: 10.7270/Q2P55M2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N(4)-(beta-N-acetylglucosaminyl)-L-asparaginase (Homo sapiens (Human)) | BDBM85472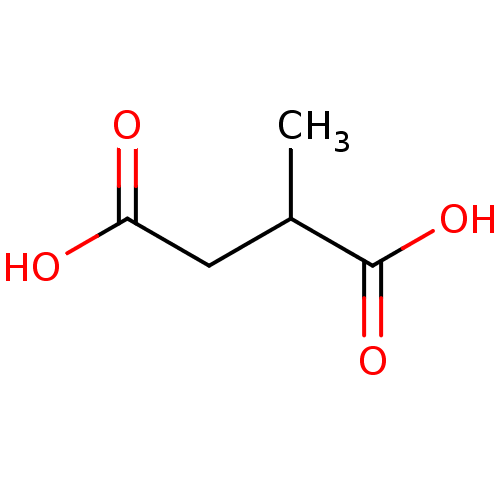 (D,L-2-Methylsuccinic acid, 5) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 7.00E+5 | -18.7 | n/a | n/a | n/a | n/a | n/a | 5.8 | 37 |
University of North Carolina | Assay Description Glycosylasparaginase activity was measured in citrate-phosphate buffer at pH 5.8 at 37 C. N-Acetyl-D-glucosamine released during the reaction was me... | J Enzym Inhib 16: 269-74 (2001) Article DOI: 10.1080/14756360109162375 BindingDB Entry DOI: 10.7270/Q2P55M2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N(4)-(beta-N-acetylglucosaminyl)-L-asparaginase (Homo sapiens (Human)) | BDBM85474 (GlcNAc-Asn analogue, 10) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.50E+5 | -18.6 | n/a | n/a | n/a | n/a | n/a | 5.8 | 37 |
University of North Carolina | Assay Description Glycosylasparaginase activity was measured in citrate-phosphate buffer at pH 5.8 at 37 C. N-Acetyl-D-glucosamine released during the reaction was me... | J Enzym Inhib 16: 269-74 (2001) Article DOI: 10.1080/14756360109162375 BindingDB Entry DOI: 10.7270/Q2P55M2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N(4)-(beta-N-acetylglucosaminyl)-L-asparaginase (Homo sapiens (Human)) | BDBM85077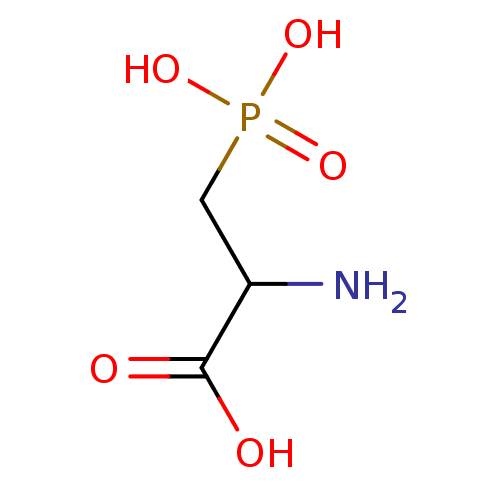 (3-Phosphono-D,L-2-aminopropionic acid, 7 | CAS_230...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents | Article PubMed | 9.00E+5 | -18.1 | n/a | n/a | n/a | n/a | n/a | 5.8 | 37 |
University of North Carolina | Assay Description Glycosylasparaginase activity was measured in citrate-phosphate buffer at pH 5.8 at 37 C. N-Acetyl-D-glucosamine released during the reaction was me... | J Enzym Inhib 16: 269-74 (2001) Article DOI: 10.1080/14756360109162375 BindingDB Entry DOI: 10.7270/Q2P55M2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N(4)-(beta-N-acetylglucosaminyl)-L-asparaginase (Homo sapiens (Human)) | BDBM85473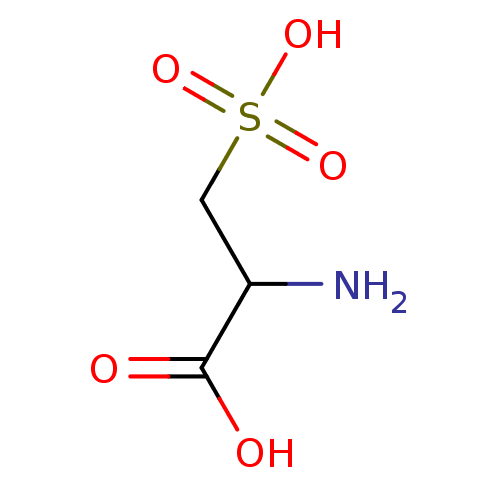 (L-Cysteic acid, 8 | L-cysteic acid) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.40E+6 | -16.9 | n/a | n/a | n/a | n/a | n/a | 5.8 | 37 |
University of North Carolina | Assay Description Glycosylasparaginase activity was measured in citrate-phosphate buffer at pH 5.8 at 37 C. N-Acetyl-D-glucosamine released during the reaction was me... | J Enzym Inhib 16: 269-74 (2001) Article DOI: 10.1080/14756360109162375 BindingDB Entry DOI: 10.7270/Q2P55M2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N(4)-(beta-N-acetylglucosaminyl)-L-asparaginase (Homo sapiens (Human)) | BDBM85471 (L-2-bromosuccinic acid, 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article PubMed | 2.70E+6 | -15.3 | n/a | n/a | n/a | n/a | n/a | 5.8 | 37 |
University of North Carolina | Assay Description Glycosylasparaginase activity was measured in citrate-phosphate buffer at pH 5.8 at 37 C. N-Acetyl-D-glucosamine released during the reaction was me... | J Enzym Inhib 16: 269-74 (2001) Article DOI: 10.1080/14756360109162375 BindingDB Entry DOI: 10.7270/Q2P55M2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N(4)-(beta-N-acetylglucosaminyl)-L-asparaginase (Homo sapiens (Human)) | BDBM26121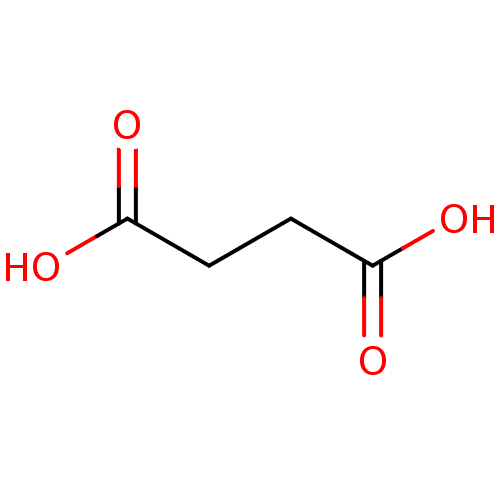 (SUCCINIC ACID | Substrate analogue, 11 | Succinate...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 5.00E+6 | -13.7 | n/a | n/a | n/a | n/a | n/a | 5.8 | 37 |
University of North Carolina | Assay Description Glycosylasparaginase activity was measured in citrate-phosphate buffer at pH 5.8 at 37 C. N-Acetyl-D-glucosamine released during the reaction was me... | J Enzym Inhib 16: 269-74 (2001) Article DOI: 10.1080/14756360109162375 BindingDB Entry DOI: 10.7270/Q2P55M2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N(4)-(beta-N-acetylglucosaminyl)-L-asparaginase (Homo sapiens (Human)) | BDBM85470 (L-2-Chlorosuccinic acid, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article PubMed | 7.70E+6 | -12.5 | n/a | n/a | n/a | n/a | n/a | 5.8 | 37 |
University of North Carolina | Assay Description Glycosylasparaginase activity was measured in citrate-phosphate buffer at pH 5.8 at 37 C. N-Acetyl-D-glucosamine released during the reaction was me... | J Enzym Inhib 16: 269-74 (2001) Article DOI: 10.1080/14756360109162375 BindingDB Entry DOI: 10.7270/Q2P55M2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50108104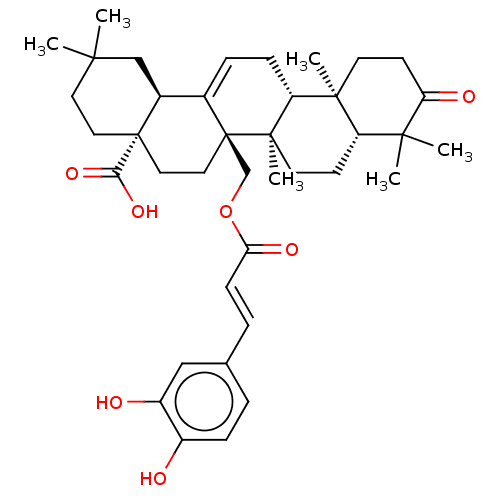 (CHEMBL3601500) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Kansas State University Curated by ChEMBL | Assay Description Antagonist activity at endothelin A receptor in rat A7r5 cells assessed as inhibition of ET-1-induced increase in cytosolic free Ca2+ level treated 1... | Bioorg Med Chem 23: 5985-98 (2015) Article DOI: 10.1016/j.bmc.2015.06.055 BindingDB Entry DOI: 10.7270/Q2862J7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Kansas State University Curated by ChEMBL | Assay Description Inhibition of GSK3beta (unknown origin) assessed as luminescence intensity by luminometry | Bioorg Med Chem Lett 24: 3392-7 (2014) Article DOI: 10.1016/j.bmcl.2014.05.085 BindingDB Entry DOI: 10.7270/Q2T72K25 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50046523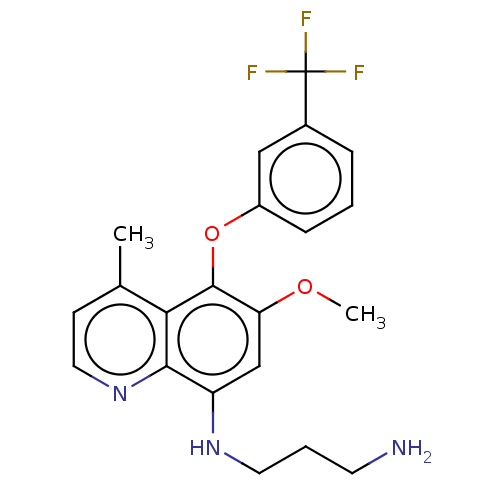 (CHEMBL495039) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Kansas State University Curated by ChEMBL | Assay Description Inhibition of GSK3beta (unknown origin) assessed as luminescence intensity by luminometry | Bioorg Med Chem Lett 24: 3392-7 (2014) Article DOI: 10.1016/j.bmcl.2014.05.085 BindingDB Entry DOI: 10.7270/Q2T72K25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50108104 (CHEMBL3601500) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Kansas State University Curated by ChEMBL | Assay Description Antagonist activity at endothelin A receptor in Wistar rat thoracic aorta strips assessed as inhibition of ET1-induced vasoconstriction preincubated ... | Bioorg Med Chem 23: 5985-98 (2015) Article DOI: 10.1016/j.bmc.2015.06.055 BindingDB Entry DOI: 10.7270/Q2862J7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50005944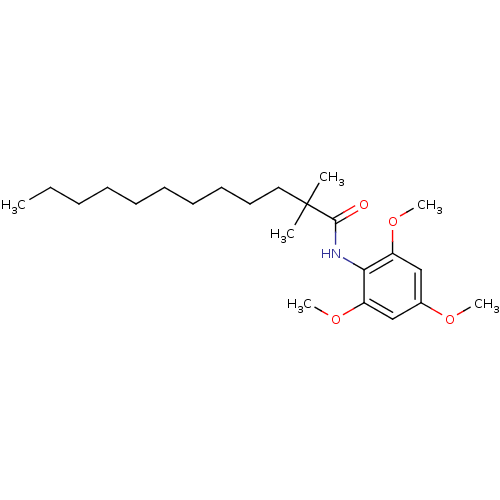 (2,2-Dimethyl-dodecanoic acid (2,4,6-trimethoxy-phe...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Kansas State University Curated by ChEMBL | Assay Description Inhibition of ACAT activity in human MC65 cells using NBD-cholesterol staining after 48 hrs by fluorescence assay | J Med Chem 55: 8969-73 (2012) Article DOI: 10.1021/jm3012189 BindingDB Entry DOI: 10.7270/Q2000370 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50273967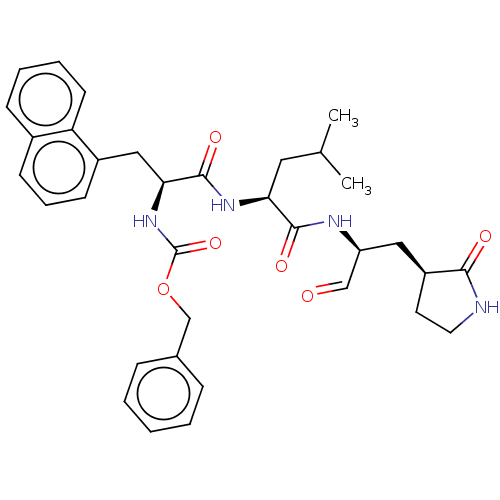 (CHEMBL2441741) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Kansas State University Curated by ChEMBL | Assay Description Inhibition of SARS coronavirus 3C-like protease by FRET assay | Bioorg Med Chem Lett 23: 6317-20 (2013) Article DOI: 10.1016/j.bmcl.2013.09.070 BindingDB Entry DOI: 10.7270/Q2VX0KG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50397451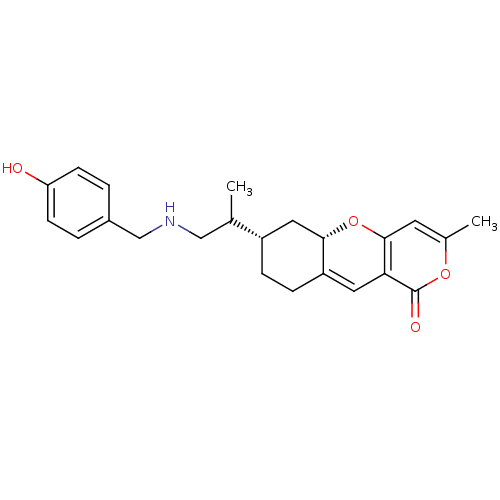 (CHEMBL2170736) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Kansas State University Curated by ChEMBL | Assay Description Inhibition of ACAT activity in human MC65 cells using NBD-cholesterol staining after 48 hrs by fluorescence assay | J Med Chem 55: 8969-73 (2012) Article DOI: 10.1021/jm3012189 BindingDB Entry DOI: 10.7270/Q2000370 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50494125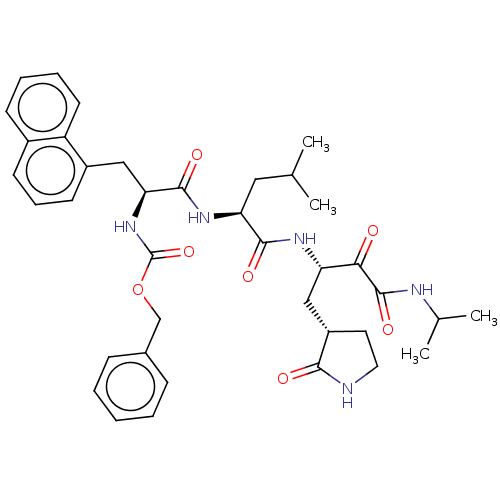 (CHEMBL2441745) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
Kansas State University Curated by ChEMBL | Assay Description Inhibition of SARS coronavirus 3C-like protease by FRET assay | Bioorg Med Chem Lett 23: 6317-20 (2013) Article DOI: 10.1016/j.bmcl.2013.09.070 BindingDB Entry DOI: 10.7270/Q2VX0KG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50397448 (CHEMBL2170728) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Kansas State University Curated by ChEMBL | Assay Description Inhibition of ACAT activity in human MC65 cells using NBD-cholesterol staining after 48 hrs by fluorescence assay | J Med Chem 55: 8969-73 (2012) Article DOI: 10.1021/jm3012189 BindingDB Entry DOI: 10.7270/Q2000370 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50397452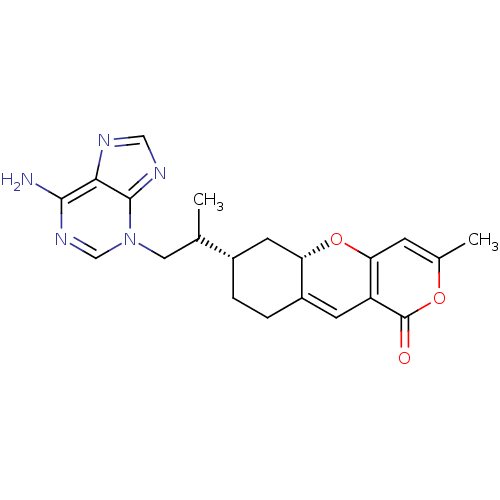 (CHEMBL520938) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kansas State University Curated by ChEMBL | Assay Description Inhibition of ACAT activity in human MC65 cells using NBD-cholesterol staining after 48 hrs by fluorescence assay | J Med Chem 55: 8969-73 (2012) Article DOI: 10.1021/jm3012189 BindingDB Entry DOI: 10.7270/Q2000370 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50397450 (CHEMBL2170821) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kansas State University Curated by ChEMBL | Assay Description Inhibition of ACAT activity in human MC65 cells using NBD-cholesterol staining after 48 hrs by fluorescence assay | J Med Chem 55: 8969-73 (2012) Article DOI: 10.1021/jm3012189 BindingDB Entry DOI: 10.7270/Q2000370 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50397449 (CHEMBL2170832) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kansas State University Curated by ChEMBL | Assay Description Inhibition of ACAT activity in human MC65 cells using NBD-cholesterol staining after 48 hrs by fluorescence assay | J Med Chem 55: 8969-73 (2012) Article DOI: 10.1021/jm3012189 BindingDB Entry DOI: 10.7270/Q2000370 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM50436488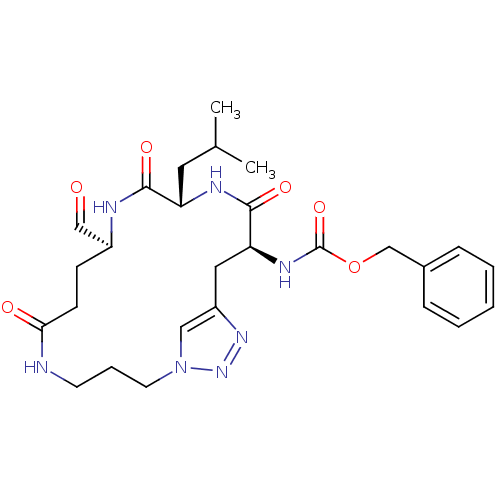 (CHEMBL2397338) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wichita State University Curated by ChEMBL | Assay Description Inhibition of Coxsackievirus B3 Nancy 3Cpro | Bioorg Med Chem Lett 23: 3709-12 (2013) Article DOI: 10.1016/j.bmcl.2013.05.021 BindingDB Entry DOI: 10.7270/Q22Z16XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50046524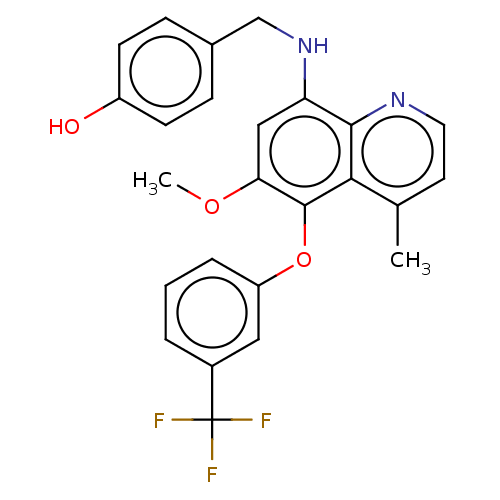 (CHEMBL3311235) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kansas State University Curated by ChEMBL | Assay Description Inhibition of GSK3beta (unknown origin) assessed as luminescence intensity by luminometry | Bioorg Med Chem Lett 24: 3392-7 (2014) Article DOI: 10.1016/j.bmcl.2014.05.085 BindingDB Entry DOI: 10.7270/Q2T72K25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50046525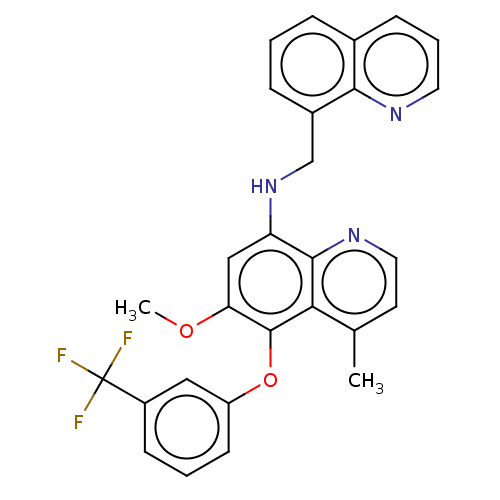 (CHEMBL3311239) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kansas State University Curated by ChEMBL | Assay Description Inhibition of GSK3beta (unknown origin) assessed as luminescence intensity by luminometry | Bioorg Med Chem Lett 24: 3392-7 (2014) Article DOI: 10.1016/j.bmcl.2014.05.085 BindingDB Entry DOI: 10.7270/Q2T72K25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50046526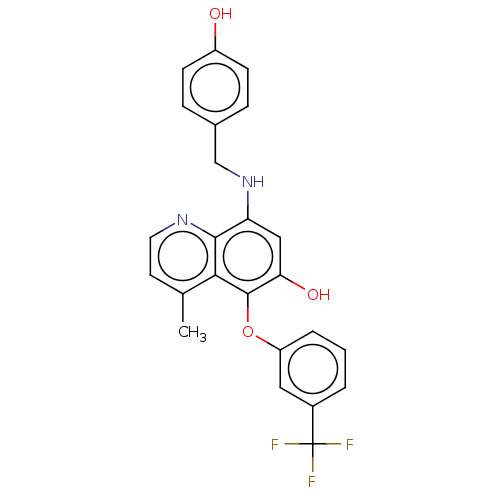 (CHEMBL3311242) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kansas State University Curated by ChEMBL | Assay Description Inhibition of GSK3beta (unknown origin) assessed as luminescence intensity by luminometry | Bioorg Med Chem Lett 24: 3392-7 (2014) Article DOI: 10.1016/j.bmcl.2014.05.085 BindingDB Entry DOI: 10.7270/Q2T72K25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50254160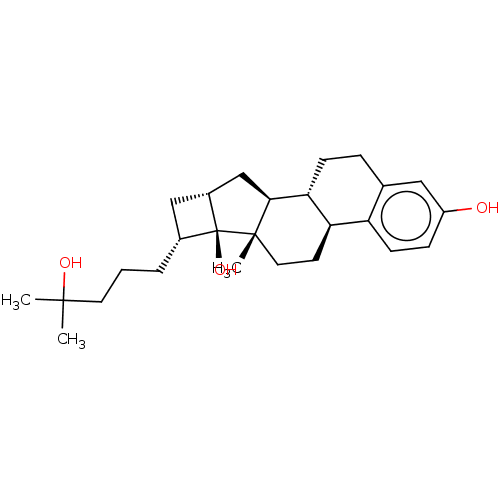 (CHEMBL4062601) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a |
Graduate School of Pharmaceutical Sciences, Kyoto University, Yoshida, Sakyo-ku, Kyoto 606-8501, Japan. Curated by ChEMBL | Assay Description Agonist activity at vitamin D receptor (unknown origin) expressed in HEK293 cells co-expressing pCMX-RXRalpha and pCMX-beta-galactosidase assessed as... | Bioorg Med Chem Lett 27: 3408-3411 (2017) Article DOI: 10.1016/j.bmcl.2017.05.089 BindingDB Entry DOI: 10.7270/Q2X350WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50254164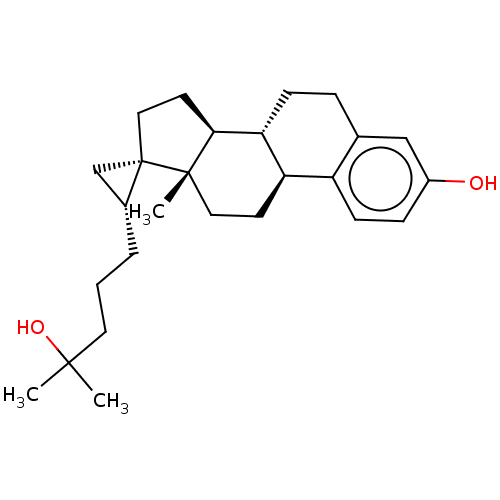 (CHEMBL4075325) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a |
Graduate School of Pharmaceutical Sciences, Kyoto University, Yoshida, Sakyo-ku, Kyoto 606-8501, Japan. Curated by ChEMBL | Assay Description Agonist activity at vitamin D receptor (unknown origin) expressed in HEK293 cells co-expressing pCMX-RXRalpha and pCMX-beta-galactosidase assessed as... | Bioorg Med Chem Lett 27: 3408-3411 (2017) Article DOI: 10.1016/j.bmcl.2017.05.089 BindingDB Entry DOI: 10.7270/Q2X350WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50254165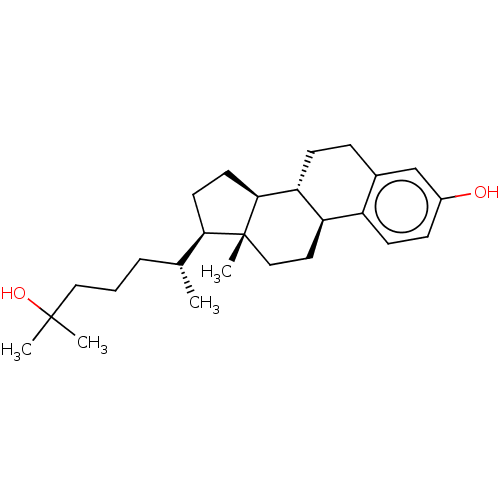 (CHEMBL4080769) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 850 | n/a | n/a | n/a | n/a |
Graduate School of Pharmaceutical Sciences, Kyoto University, Yoshida, Sakyo-ku, Kyoto 606-8501, Japan. Curated by ChEMBL | Assay Description Agonist activity at vitamin D receptor (unknown origin) expressed in HEK293 cells co-expressing pCMX-RXRalpha and pCMX-beta-galactosidase assessed as... | Bioorg Med Chem Lett 27: 3408-3411 (2017) Article DOI: 10.1016/j.bmcl.2017.05.089 BindingDB Entry DOI: 10.7270/Q2X350WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||