 Found 187 hits with Last Name = 'huppertz' and Initial = 'c'
Found 187 hits with Last Name = 'huppertz' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50348537

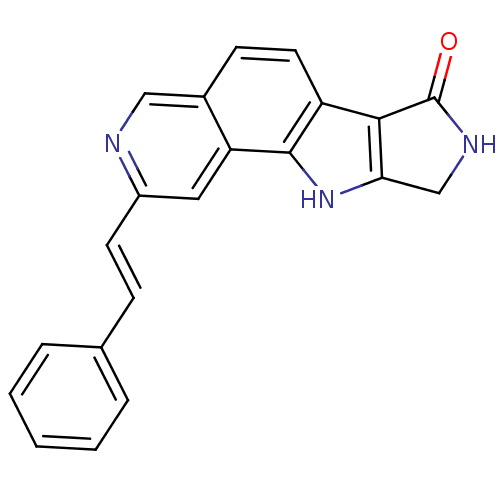
(CHEMBL1801376)Show SMILES Fc1cccc(Nc2cc3c4[nH]c5CNC(=O)c5c4ccc3cn2)c1 Show InChI InChI=1S/C19H13FN4O/c20-11-2-1-3-12(6-11)23-16-7-14-10(8-21-16)4-5-13-17-15(24-18(13)14)9-22-19(17)25/h1-8,24H,9H2,(H,21,23)(H,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of JNK2 |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348545

(CHEMBL1801384)Show SMILES O=C1NCCc2[nH]c3c(ccc4cnc(\C=C\c5ccc(CN6CCOCC6)cc5)cc34)c12 Show InChI InChI=1S/C27H26N4O2/c32-27-25-22-8-6-20-16-29-21(15-23(20)26(22)30-24(25)9-10-28-27)7-5-18-1-3-19(4-2-18)17-31-11-13-33-14-12-31/h1-8,15-16,30H,9-14,17H2,(H,28,32)/b7-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET assay |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50348534
(CHEMBL1801373)Show SMILES O=C1NCc2[nH]c3c(ccc4cnc(\C=C\c5ccccc5)cc34)c12 Show InChI InChI=1S/C21H15N3O/c25-21-19-16-9-7-14-11-22-15(8-6-13-4-2-1-3-5-13)10-17(14)20(16)24-18(19)12-23-21/h1-11,24H,12H2,(H,23,25)/b8-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of JNK2 |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348515
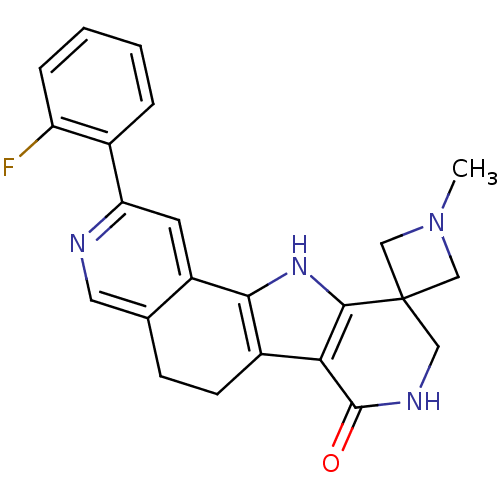
(CHEMBL1233942)Show SMILES CN1CC2(C1)CNC(=O)c1c3CCc4cnc(cc4-c3[nH]c21)-c1ccccc1F Show InChI InChI=1S/C23H21FN4O/c1-28-11-23(12-28)10-26-22(29)19-15-7-6-13-9-25-18(14-4-2-3-5-17(14)24)8-16(13)20(15)27-21(19)23/h2-5,8-9,27H,6-7,10-12H2,1H3,(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of MK2 mediated anisomycin-stimulated hsp27 phosphorylation in human THP-1 cells by fluorometric analysis |
Bioorg Med Chem Lett 20: 4719-23 (2010)
Checked by Author
Article DOI: 10.1016/j.bmcl.2010.04.023
BindingDB Entry DOI: 10.7270/Q2BZ66C2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50465455
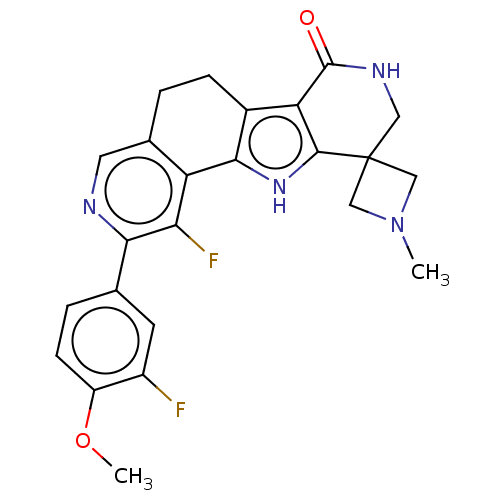
(CHEMBL4282514)Show SMILES COc1ccc(cc1F)-c1ncc2CCc3c([nH]c4c3C(=O)NCC43CN(C)C3)-c2c1F Show InChI InChI=1S/C24H22F2N4O2/c1-30-10-24(11-30)9-28-23(31)18-14-5-3-13-8-27-20(12-4-6-16(32-2)15(25)7-12)19(26)17(13)21(14)29-22(18)24/h4,6-8,29H,3,5,9-11H2,1-2H3,(H,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by TR-FRET assay |
ACS Med Chem Lett 9: 392-396 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00098
BindingDB Entry DOI: 10.7270/Q2VX0K5Z |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50465453

(CHEMBL4277929)Show SMILES CN1CC2(C1)CNC(=O)c1c3CCc4cnc(c(F)c4-c3[nH]c21)-c1cccc(F)c1 Show InChI InChI=1S/C23H20F2N4O/c1-29-10-23(11-29)9-27-22(30)17-15-6-5-13-8-26-19(12-3-2-4-14(24)7-12)18(25)16(13)20(15)28-21(17)23/h2-4,7-8,28H,5-6,9-11H2,1H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by TR-FRET assay |
ACS Med Chem Lett 9: 392-396 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00098
BindingDB Entry DOI: 10.7270/Q2VX0K5Z |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50465454

(CHEMBL4293121)Show SMILES COc1ccc(cn1)-c1ncc2CCc3c([nH]c4c3C(=O)NCC43CN(C)C3)-c2c1F Show InChI InChI=1S/C23H22FN5O2/c1-29-10-23(11-29)9-27-22(30)17-14-5-3-12-7-26-19(13-4-6-15(31-2)25-8-13)18(24)16(12)20(14)28-21(17)23/h4,6-8,28H,3,5,9-11H2,1-2H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by TR-FRET assay |
ACS Med Chem Lett 9: 392-396 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00098
BindingDB Entry DOI: 10.7270/Q2VX0K5Z |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348492
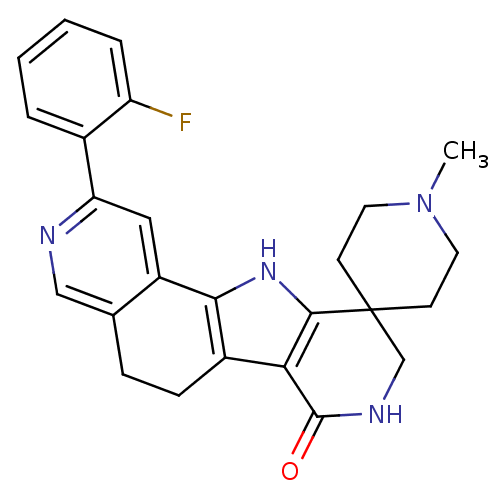
(CHEMBL1801296)Show SMILES CN1CCC2(CC1)CNC(=O)c1c3CCc4cnc(cc4-c3[nH]c21)-c1ccccc1F Show InChI InChI=1S/C25H25FN4O/c1-30-10-8-25(9-11-30)14-28-24(31)21-17-7-6-15-13-27-20(16-4-2-3-5-19(16)26)12-18(15)22(17)29-23(21)25/h2-5,12-13,29H,6-11,14H2,1H3,(H,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET analysis |
Bioorg Med Chem Lett 20: 4719-23 (2010)
Checked by Author
Article DOI: 10.1016/j.bmcl.2010.04.023
BindingDB Entry DOI: 10.7270/Q2BZ66C2 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348546
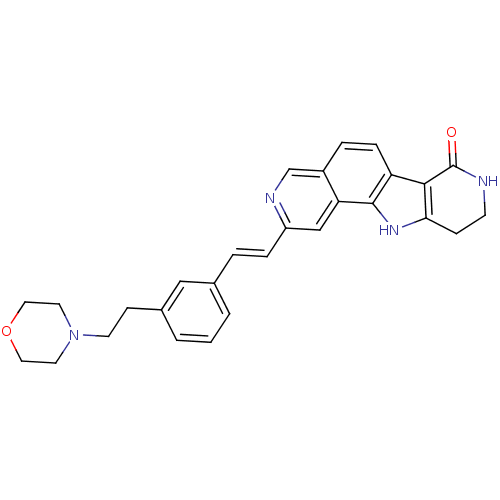
(CHEMBL1801385)Show SMILES O=C1NCCc2[nH]c3c(ccc4cnc(\C=C\c5cccc(CCN6CCOCC6)c5)cc34)c12 Show InChI InChI=1S/C28H28N4O2/c33-28-26-23-7-5-21-18-30-22(17-24(21)27(23)31-25(26)8-10-29-28)6-4-19-2-1-3-20(16-19)9-11-32-12-14-34-15-13-32/h1-7,16-18,31H,8-15H2,(H,29,33)/b6-4+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET assay |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348543

(CHEMBL1801382)Show SMILES COCCOc1cncc(c1)-c1cc2c3[nH]c4CNC(=O)c4c3ccc2cn1 Show InChI InChI=1S/C21H18N4O3/c1-27-4-5-28-14-6-13(8-22-10-14)17-7-16-12(9-23-17)2-3-15-19-18(25-20(15)16)11-24-21(19)26/h2-3,6-10,25H,4-5,11H2,1H3,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET assay |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348542
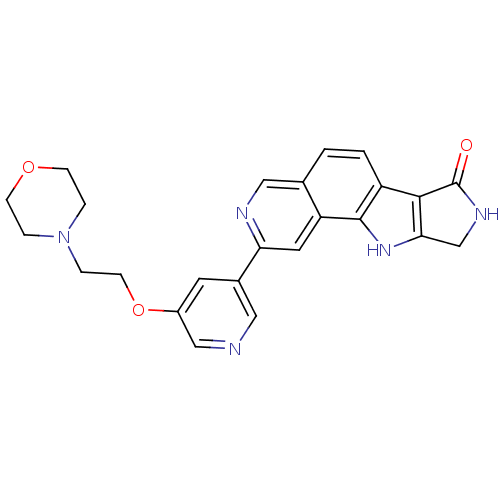
(CHEMBL1801381)Show SMILES O=C1NCc2[nH]c3c(ccc4cnc(cc34)-c3cncc(OCCN4CCOCC4)c3)c12 Show InChI InChI=1S/C24H23N5O3/c30-24-22-18-2-1-15-12-26-20(10-19(15)23(18)28-21(22)14-27-24)16-9-17(13-25-11-16)32-8-5-29-3-6-31-7-4-29/h1-2,9-13,28H,3-8,14H2,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET assay |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348491

(CHEMBL1801305)Show SMILES CN1CC2(C1)CNC(=O)c1c3CCc4cnc(nc4-c3[nH]c21)-c1cccc(F)c1 Show InChI InChI=1S/C22H20FN5O/c1-28-10-22(11-28)9-25-21(29)16-15-6-5-13-8-24-20(12-3-2-4-14(23)7-12)27-17(13)18(15)26-19(16)22/h2-4,7-8,26H,5-6,9-11H2,1H3,(H,25,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET analysis |
Bioorg Med Chem Lett 20: 4719-23 (2010)
Checked by Author
Article DOI: 10.1016/j.bmcl.2010.04.023
BindingDB Entry DOI: 10.7270/Q2BZ66C2 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348493
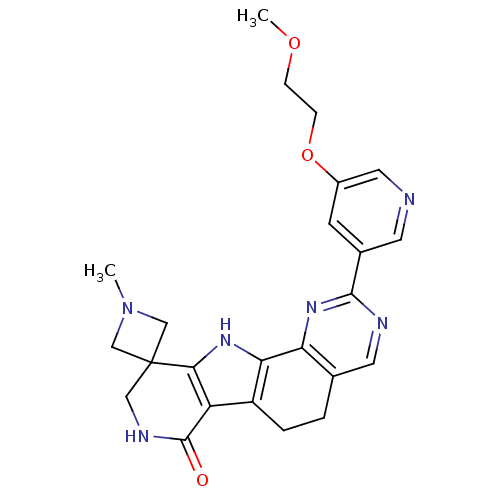
(CHEMBL1801304)Show SMILES COCCOc1cncc(c1)-c1ncc2CCc3c([nH]c4c3C(=O)NCC43CN(C)C3)-c2n1 Show InChI InChI=1S/C24H26N6O3/c1-30-12-24(13-30)11-27-23(31)18-17-4-3-14-9-26-22(29-19(14)20(17)28-21(18)24)15-7-16(10-25-8-15)33-6-5-32-2/h7-10,28H,3-6,11-13H2,1-2H3,(H,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET analysis |
Bioorg Med Chem Lett 20: 4719-23 (2010)
Checked by Author
Article DOI: 10.1016/j.bmcl.2010.04.023
BindingDB Entry DOI: 10.7270/Q2BZ66C2 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348481
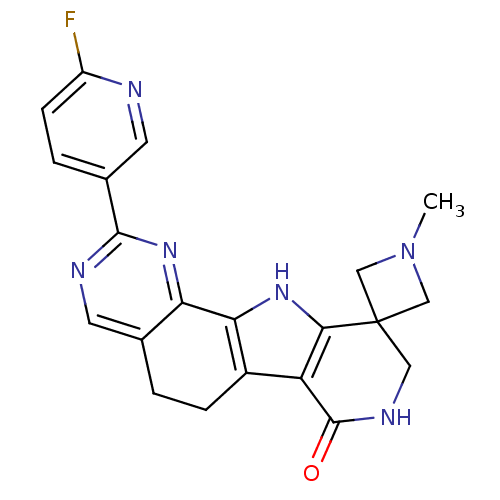
(CHEMBL1801307)Show SMILES CN1CC2(C1)CNC(=O)c1c3CCc4cnc(nc4-c3[nH]c21)-c1ccc(F)nc1 Show InChI InChI=1S/C21H19FN6O/c1-28-9-21(10-28)8-25-20(29)15-13-4-2-11-6-24-19(12-3-5-14(22)23-7-12)27-16(11)17(13)26-18(15)21/h3,5-7,26H,2,4,8-10H2,1H3,(H,25,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET analysis |
Bioorg Med Chem Lett 20: 4719-23 (2010)
Checked by Author
Article DOI: 10.1016/j.bmcl.2010.04.023
BindingDB Entry DOI: 10.7270/Q2BZ66C2 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348494
(CHEMBL1801274)Show SMILES Fc1cccc(c1)-c1cc2-c3[nH]c4CCNC(=O)c4c3CCc2cn1 Show InChI InChI=1S/C20H16FN3O/c21-13-3-1-2-11(8-13)17-9-15-12(10-23-17)4-5-14-18-16(24-19(14)15)6-7-22-20(18)25/h1-3,8-10,24H,4-7H2,(H,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET analysis |
Bioorg Med Chem Lett 20: 4719-23 (2010)
Checked by Author
Article DOI: 10.1016/j.bmcl.2010.04.023
BindingDB Entry DOI: 10.7270/Q2BZ66C2 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348535

(CHEMBL1801374)Show SMILES O=C1NCc2[nH]c3c(ccc4cnc(\C=C\c5ccc(CN6CCOCC6)cc5)cc34)c12 Show InChI InChI=1S/C26H24N4O2/c31-26-24-21-8-6-19-14-27-20(13-22(19)25(21)29-23(24)15-28-26)7-5-17-1-3-18(4-2-17)16-30-9-11-32-12-10-30/h1-8,13-14,29H,9-12,15-16H2,(H,28,31)/b7-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET assay |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348524
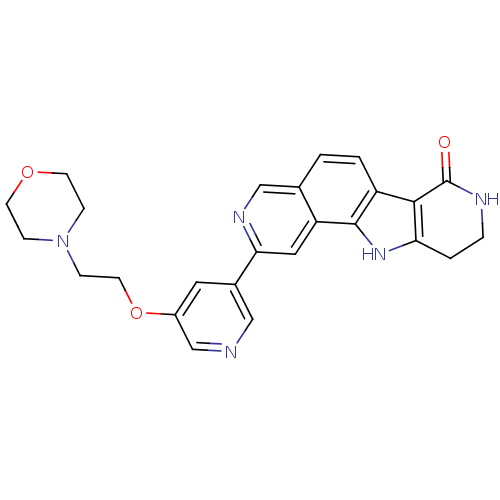
(CHEMBL1801388)Show SMILES O=C1NCCc2[nH]c3c(ccc4cnc(cc34)-c3cncc(OCCN4CCOCC4)c3)c12 Show InChI InChI=1S/C25H25N5O3/c31-25-23-19-2-1-16-14-28-22(12-20(16)24(19)29-21(23)3-4-27-25)17-11-18(15-26-13-17)33-10-7-30-5-8-32-9-6-30/h1-2,11-15,29H,3-10H2,(H,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET assay |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348536
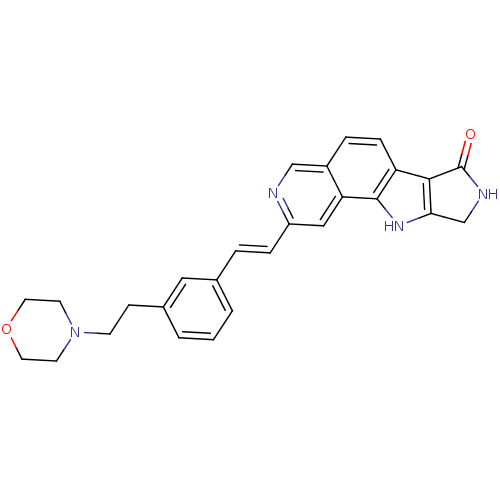
(CHEMBL1801375)Show SMILES O=C1NCc2[nH]c3c(ccc4cnc(\C=C\c5cccc(CCN6CCOCC6)c5)cc34)c12 Show InChI InChI=1S/C27H26N4O2/c32-27-25-22-7-5-20-16-28-21(15-23(20)26(22)30-24(25)17-29-27)6-4-18-2-1-3-19(14-18)8-9-31-10-12-33-13-11-31/h1-7,14-16,30H,8-13,17H2,(H,29,32)/b6-4+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET assay |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348534

(CHEMBL1801373)Show SMILES O=C1NCc2[nH]c3c(ccc4cnc(\C=C\c5ccccc5)cc34)c12 Show InChI InChI=1S/C21H15N3O/c25-21-19-16-9-7-14-11-22-15(8-6-13-4-2-1-3-5-13)10-17(14)20(16)24-18(19)12-23-21/h1-11,24H,12H2,(H,23,25)/b8-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET assay |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348495

(CHEMBL1614995)Show SMILES COCCOc1cncc(c1)-c1cc2c3[nH]c4CCNC(=O)c4c3ccc2cn1 Show InChI InChI=1S/C22H20N4O3/c1-28-6-7-29-15-8-14(10-23-12-15)19-9-17-13(11-25-19)2-3-16-20-18(26-21(16)17)4-5-24-22(20)27/h2-3,8-12,26H,4-7H2,1H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET analysis |
Bioorg Med Chem Lett 20: 4719-23 (2010)
Checked by Author
Article DOI: 10.1016/j.bmcl.2010.04.023
BindingDB Entry DOI: 10.7270/Q2BZ66C2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50348541
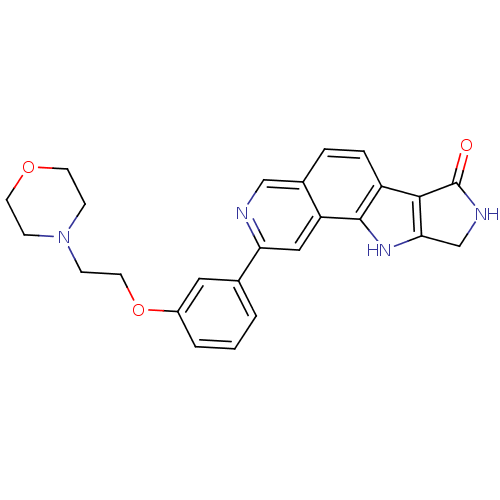
(CHEMBL1801380)Show SMILES O=C1NCc2[nH]c3c(ccc4cnc(cc34)-c3cccc(OCCN4CCOCC4)c3)c12 Show InChI InChI=1S/C25H24N4O3/c30-25-23-19-5-4-17-14-26-21(13-20(17)24(19)28-22(23)15-27-25)16-2-1-3-18(12-16)32-11-8-29-6-9-31-10-7-29/h1-5,12-14,28H,6-11,15H2,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of JNK2 |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348486
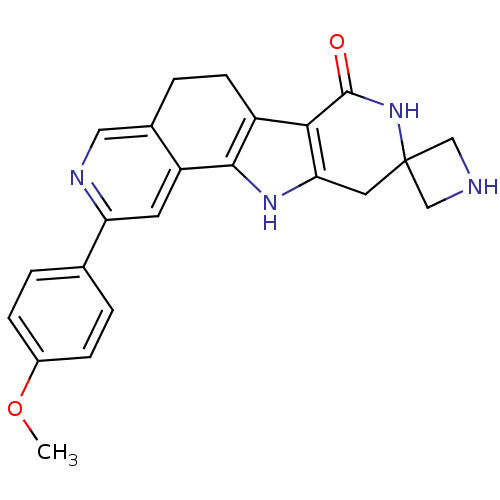
(CHEMBL1801294)Show SMILES COc1ccc(cc1)-c1cc2-c3[nH]c4CC5(CNC5)NC(=O)c4c3CCc2cn1 Show InChI InChI=1S/C23H22N4O2/c1-29-15-5-2-13(3-6-15)18-8-17-14(10-25-18)4-7-16-20-19(26-21(16)17)9-23(11-24-12-23)27-22(20)28/h2-3,5-6,8,10,24,26H,4,7,9,11-12H2,1H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET analysis |
Bioorg Med Chem Lett 20: 4719-23 (2010)
Checked by Author
Article DOI: 10.1016/j.bmcl.2010.04.023
BindingDB Entry DOI: 10.7270/Q2BZ66C2 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348495

(CHEMBL1614995)Show SMILES COCCOc1cncc(c1)-c1cc2c3[nH]c4CCNC(=O)c4c3ccc2cn1 Show InChI InChI=1S/C22H20N4O3/c1-28-6-7-29-15-8-14(10-23-12-15)19-9-17-13(11-25-19)2-3-16-20-18(26-21(16)17)4-5-24-22(20)27/h2-3,8-12,26H,4-7H2,1H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET assay |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348480
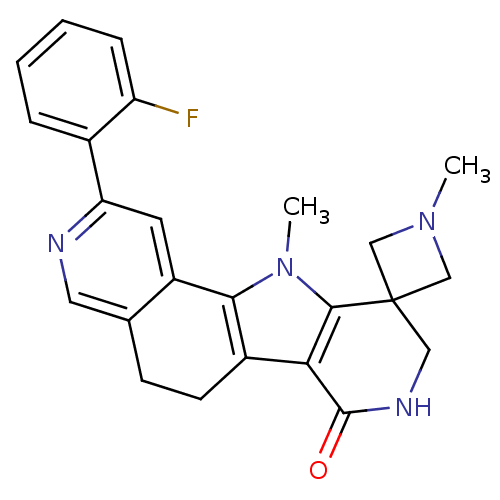
(CHEMBL1801312)Show SMILES CN1CC2(C1)CNC(=O)c1c3CCc4cnc(cc4-c3n(C)c21)-c1ccccc1F Show InChI InChI=1S/C24H23FN4O/c1-28-12-24(13-28)11-27-23(30)20-16-8-7-14-10-26-19(15-5-3-4-6-18(15)25)9-17(14)21(16)29(2)22(20)24/h3-6,9-10H,7-8,11-13H2,1-2H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET analysis |
Bioorg Med Chem Lett 20: 4719-23 (2010)
Checked by Author
Article DOI: 10.1016/j.bmcl.2010.04.023
BindingDB Entry DOI: 10.7270/Q2BZ66C2 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348530
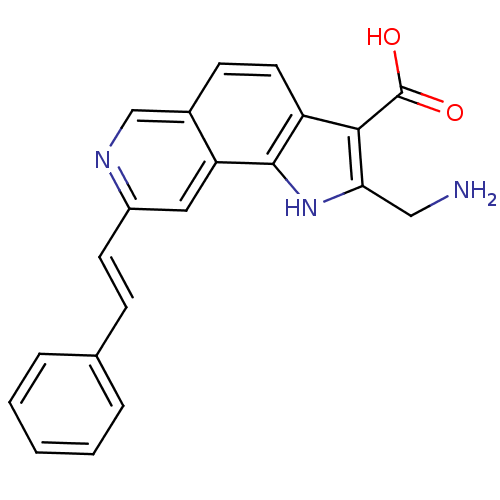
(CHEMBL1801313)Show SMILES NCc1[nH]c2c(ccc3cnc(\C=C\c4ccccc4)cc23)c1C(O)=O Show InChI InChI=1S/C21H17N3O2/c22-11-18-19(21(25)26)16-9-7-14-12-23-15(10-17(14)20(16)24-18)8-6-13-4-2-1-3-5-13/h1-10,12,24H,11,22H2,(H,25,26)/b8-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET assay |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348547
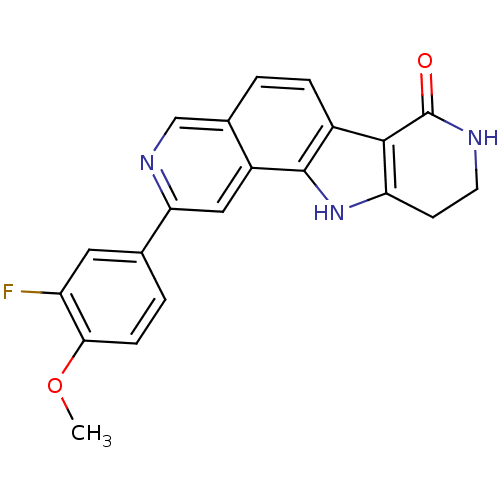
(CHEMBL1801386)Show SMILES COc1ccc(cc1F)-c1cc2c3[nH]c4CCNC(=O)c4c3ccc2cn1 Show InChI InChI=1S/C21H16FN3O2/c1-27-18-5-3-11(8-15(18)22)17-9-14-12(10-24-17)2-4-13-19-16(25-20(13)14)6-7-23-21(19)26/h2-5,8-10,25H,6-7H2,1H3,(H,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET assay |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348548
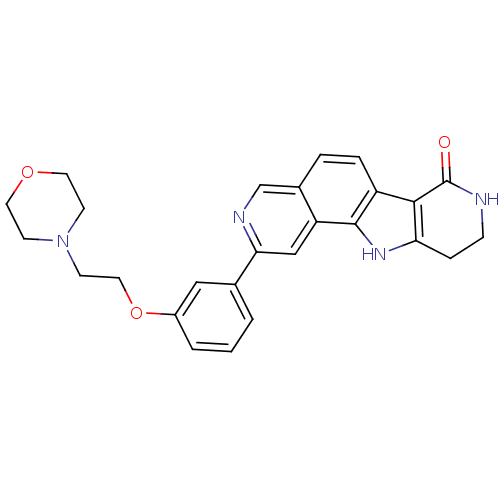
(CHEMBL1801387)Show SMILES O=C1NCCc2[nH]c3c(ccc4cnc(cc34)-c3cccc(OCCN4CCOCC4)c3)c12 Show InChI InChI=1S/C26H26N4O3/c31-26-24-20-5-4-18-16-28-23(15-21(18)25(20)29-22(24)6-7-27-26)17-2-1-3-19(14-17)33-13-10-30-8-11-32-12-9-30/h1-5,14-16,29H,6-13H2,(H,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET assay |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348496
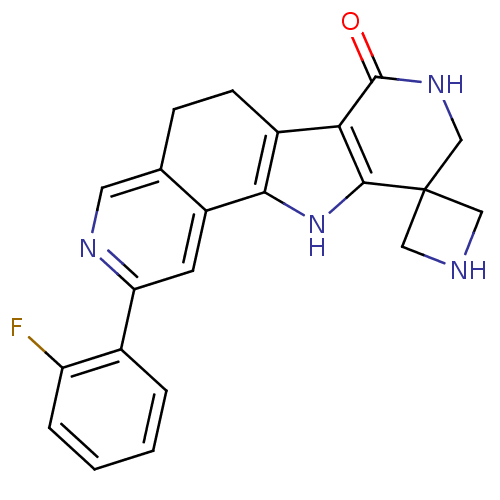
(CHEMBL1801284)Show SMILES Fc1ccccc1-c1cc2-c3[nH]c4c(c3CCc2cn1)C(=O)NCC41CNC1 Show InChI InChI=1S/C22H19FN4O/c23-16-4-2-1-3-13(16)17-7-15-12(8-25-17)5-6-14-18-20(27-19(14)15)22(9-24-10-22)11-26-21(18)28/h1-4,7-8,24,27H,5-6,9-11H2,(H,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET analysis |
Bioorg Med Chem Lett 20: 4719-23 (2010)
Checked by Author
Article DOI: 10.1016/j.bmcl.2010.04.023
BindingDB Entry DOI: 10.7270/Q2BZ66C2 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348497
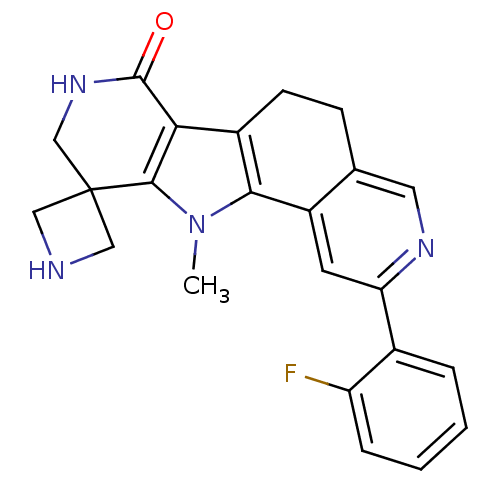
(CHEMBL1801311)Show SMILES Cn1c-2c(CCc3cnc(cc-23)-c2ccccc2F)c2c1C1(CNC1)CNC2=O Show InChI InChI=1S/C23H21FN4O/c1-28-20-15(19-21(28)23(10-25-11-23)12-27-22(19)29)7-6-13-9-26-18(8-16(13)20)14-4-2-3-5-17(14)24/h2-5,8-9,25H,6-7,10-12H2,1H3,(H,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET analysis |
Bioorg Med Chem Lett 20: 4719-23 (2010)
Checked by Author
Article DOI: 10.1016/j.bmcl.2010.04.023
BindingDB Entry DOI: 10.7270/Q2BZ66C2 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50348535

(CHEMBL1801374)Show SMILES O=C1NCc2[nH]c3c(ccc4cnc(\C=C\c5ccc(CN6CCOCC6)cc5)cc34)c12 Show InChI InChI=1S/C26H24N4O2/c31-26-24-21-8-6-19-14-27-20(13-22(19)25(21)29-23(24)15-28-26)7-5-17-1-3-18(4-2-17)16-30-9-11-32-12-10-30/h1-8,13-14,29H,9-12,15-16H2,(H,28,31)/b7-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of JNK2 |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348533
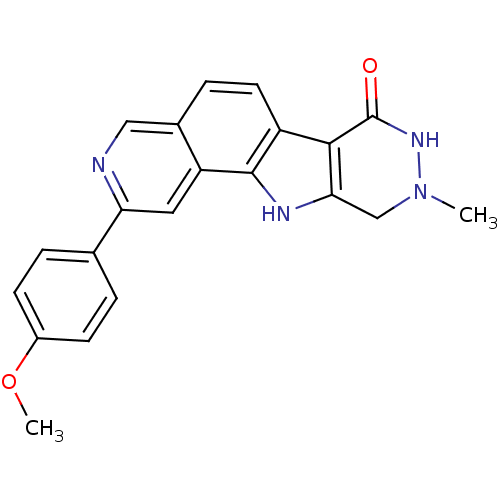
(CHEMBL1801316)Show SMILES COc1ccc(cc1)-c1cc2c3[nH]c4CN(C)NC(=O)c4c3ccc2cn1 Show InChI InChI=1S/C21H18N4O2/c1-25-11-18-19(21(26)24-25)15-8-5-13-10-22-17(9-16(13)20(15)23-18)12-3-6-14(27-2)7-4-12/h3-10,23H,11H2,1-2H3,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET assay |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348532

(CHEMBL1801315)Show SMILES OC1=NOCc2[nH]c3c(ccc4cnc(C=Cc5ccccc5)cc34)c12 |w:15.14,t:1| Show InChI InChI=1S/C21H15N3O2/c25-21-19-16-9-7-14-11-22-15(8-6-13-4-2-1-3-5-13)10-17(14)20(16)23-18(19)12-26-24-21/h1-11,23H,12H2,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET assay |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348498
(CHEMBL1801283)Show SMILES Fc1ccc(cn1)-c1cc2-c3[nH]c4c(c3CCc2cn1)C(=O)NCC41CC1 Show InChI InChI=1S/C21H17FN4O/c22-16-4-2-12(9-24-16)15-7-14-11(8-23-15)1-3-13-17-19(26-18(13)14)21(5-6-21)10-25-20(17)27/h2,4,7-9,26H,1,3,5-6,10H2,(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET analysis |
Bioorg Med Chem Lett 20: 4719-23 (2010)
Checked by Author
Article DOI: 10.1016/j.bmcl.2010.04.023
BindingDB Entry DOI: 10.7270/Q2BZ66C2 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348538
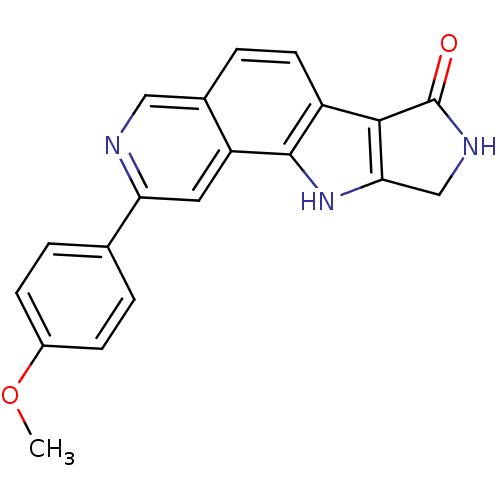
(CHEMBL1801377)Show SMILES COc1ccc(cc1)-c1cc2c3[nH]c4CNC(=O)c4c3ccc2cn1 Show InChI InChI=1S/C20H15N3O2/c1-25-13-5-2-11(3-6-13)16-8-15-12(9-21-16)4-7-14-18-17(23-19(14)15)10-22-20(18)24/h2-9,23H,10H2,1H3,(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET assay |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50348539
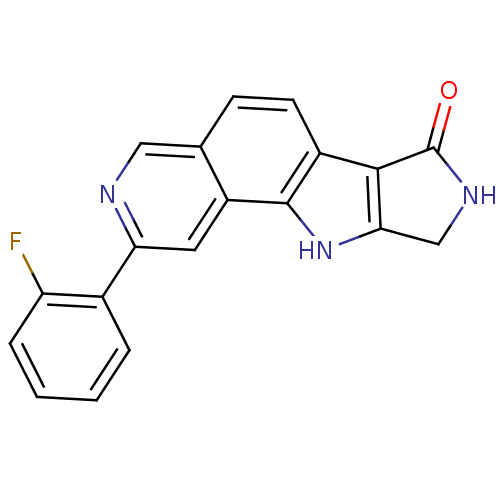
(CHEMBL1801378)Show InChI InChI=1S/C19H12FN3O/c20-14-4-2-1-3-11(14)15-7-13-10(8-21-15)5-6-12-17-16(23-18(12)13)9-22-19(17)24/h1-8,23H,9H2,(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of JNK2 |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348541

(CHEMBL1801380)Show SMILES O=C1NCc2[nH]c3c(ccc4cnc(cc34)-c3cccc(OCCN4CCOCC4)c3)c12 Show InChI InChI=1S/C25H24N4O3/c30-25-23-19-5-4-17-14-26-21(13-20(17)24(19)28-22(23)15-27-25)16-2-1-3-18(12-16)32-11-8-29-6-9-31-10-7-29/h1-5,12-14,28H,6-11,15H2,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET assay |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348499
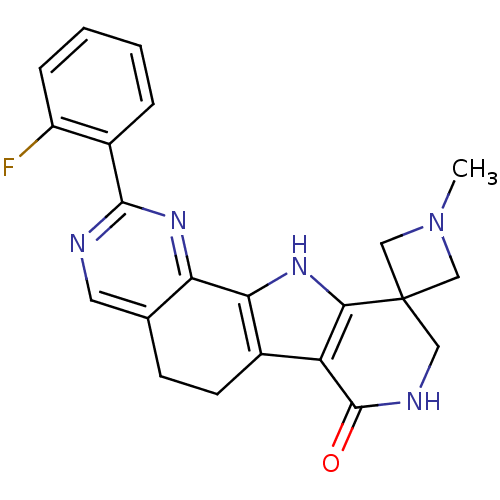
(CHEMBL1801303)Show SMILES CN1CC2(C1)CNC(=O)c1c3CCc4cnc(nc4-c3[nH]c21)-c1ccccc1F Show InChI InChI=1S/C22H20FN5O/c1-28-10-22(11-28)9-25-21(29)16-14-7-6-12-8-24-20(13-4-2-3-5-15(13)23)27-17(12)18(14)26-19(16)22/h2-5,8,26H,6-7,9-11H2,1H3,(H,25,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET analysis |
Bioorg Med Chem Lett 20: 4719-23 (2010)
Checked by Author
Article DOI: 10.1016/j.bmcl.2010.04.023
BindingDB Entry DOI: 10.7270/Q2BZ66C2 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50348536

(CHEMBL1801375)Show SMILES O=C1NCc2[nH]c3c(ccc4cnc(\C=C\c5cccc(CCN6CCOCC6)c5)cc34)c12 Show InChI InChI=1S/C27H26N4O2/c32-27-25-22-7-5-20-16-28-21(15-23(20)26(22)30-24(25)17-29-27)6-4-18-2-1-3-19(14-18)8-9-31-10-12-33-13-11-31/h1-7,14-16,30H,8-13,17H2,(H,29,32)/b6-4+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of JNK2 |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348483

(CHEMBL1801194)Show SMILES Fc1ccccc1-c1cc2-c3[nH]c4CCNC(=O)c4c3CCc2cn1 Show InChI InChI=1S/C20H16FN3O/c21-15-4-2-1-3-12(15)17-9-14-11(10-23-17)5-6-13-18-16(24-19(13)14)7-8-22-20(18)25/h1-4,9-10,24H,5-8H2,(H,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET analysis |
Bioorg Med Chem Lett 20: 4719-23 (2010)
Checked by Author
Article DOI: 10.1016/j.bmcl.2010.04.023
BindingDB Entry DOI: 10.7270/Q2BZ66C2 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50348543

(CHEMBL1801382)Show SMILES COCCOc1cncc(c1)-c1cc2c3[nH]c4CNC(=O)c4c3ccc2cn1 Show InChI InChI=1S/C21H18N4O3/c1-27-4-5-28-14-6-13(8-22-10-14)17-7-16-12(9-23-17)2-3-15-19-18(25-20(15)16)11-24-21(19)26/h2-3,6-10,25H,4-5,11H2,1H3,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of JNK2 |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348479

(CHEMBL1801195)Show SMILES COCCOc1cncc(c1)-c1cc2-c3[nH]c4CCNC(=O)c4c3CCc2cn1 Show InChI InChI=1S/C22H22N4O3/c1-28-6-7-29-15-8-14(10-23-12-15)19-9-17-13(11-25-19)2-3-16-20-18(26-21(16)17)4-5-24-22(20)27/h8-12,26H,2-7H2,1H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET analysis |
Bioorg Med Chem Lett 20: 4719-23 (2010)
Checked by Author
Article DOI: 10.1016/j.bmcl.2010.04.023
BindingDB Entry DOI: 10.7270/Q2BZ66C2 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM30322
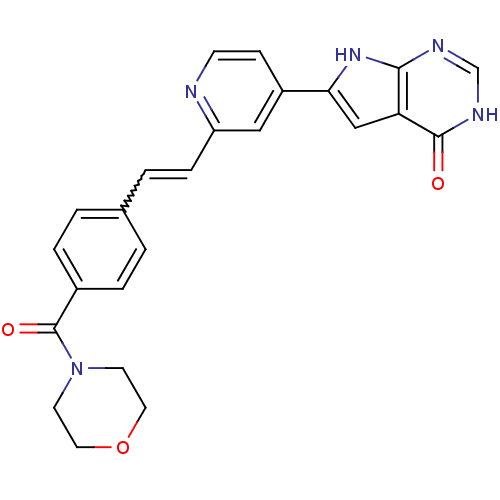
(Pyrrolo-pyrimidone, 15)Show SMILES O=C(N1CCOCC1)c1ccc(C=Cc2cc(ccn2)-c2cc3c(nc[nH]c3=O)[nH]2)cc1 |w:12.12| Show InChI InChI=1S/C24H21N5O3/c30-23-20-14-21(28-22(20)26-15-27-23)18-7-8-25-19(13-18)6-3-16-1-4-17(5-2-16)24(31)29-9-11-32-12-10-29/h1-8,13-15H,9-12H2,(H2,26,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis
| Assay Description
For MK2 inhibition, reactions contained compound or vehicle control, hsp27 peptide substrate and activated MK2 mix containing ATP. After incubation, ... |
Bioorg Med Chem Lett 18: 6142-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.039
BindingDB Entry DOI: 10.7270/Q2V40SKW |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348500
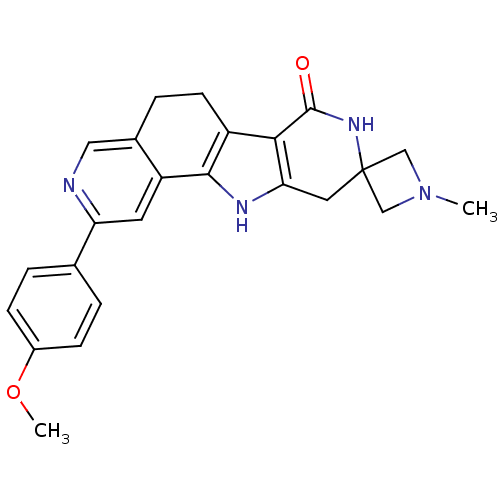
(CHEMBL1801295)Show SMILES COc1ccc(cc1)-c1cc2-c3[nH]c4CC5(CN(C)C5)NC(=O)c4c3CCc2cn1 Show InChI InChI=1S/C24H24N4O2/c1-28-12-24(13-28)10-20-21(23(29)27-24)17-8-5-15-11-25-19(9-18(15)22(17)26-20)14-3-6-16(30-2)7-4-14/h3-4,6-7,9,11,26H,5,8,10,12-13H2,1-2H3,(H,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET analysis |
Bioorg Med Chem Lett 20: 4719-23 (2010)
Checked by Author
Article DOI: 10.1016/j.bmcl.2010.04.023
BindingDB Entry DOI: 10.7270/Q2BZ66C2 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348501

(CHEMBL1801279)Show SMILES Fc1ccc(cn1)-c1cc2-c3[nH]c4CC5(CC5)NC(=O)c4c3CCc2cn1 Show InChI InChI=1S/C21H17FN4O/c22-17-4-2-12(10-24-17)15-7-14-11(9-23-15)1-3-13-18-16(25-19(13)14)8-21(5-6-21)26-20(18)27/h2,4,7,9-10,25H,1,3,5-6,8H2,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET analysis |
Bioorg Med Chem Lett 20: 4719-23 (2010)
Checked by Author
Article DOI: 10.1016/j.bmcl.2010.04.023
BindingDB Entry DOI: 10.7270/Q2BZ66C2 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM30323
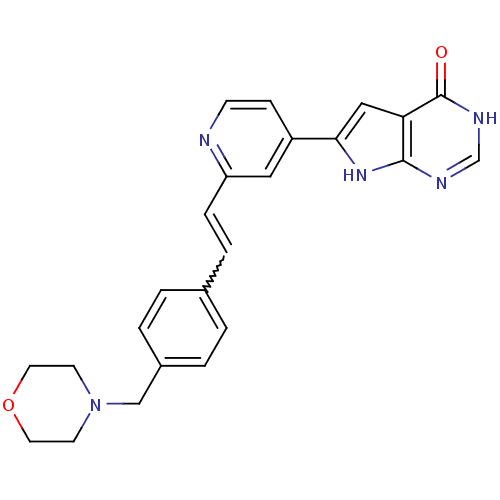
(Pyrrolo-pyrimidone, 16)Show SMILES O=c1[nH]cnc2[nH]c(cc12)-c1ccnc(C=Cc2ccc(CN3CCOCC3)cc2)c1 |w:16.18| Show InChI InChI=1S/C24H23N5O2/c30-24-21-14-22(28-23(21)26-16-27-24)19-7-8-25-20(13-19)6-5-17-1-3-18(4-2-17)15-29-9-11-31-12-10-29/h1-8,13-14,16H,9-12,15H2,(H2,26,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis
| Assay Description
For MK2 inhibition, reactions contained compound or vehicle control, hsp27 peptide substrate and activated MK2 mix containing ATP. After incubation, ... |
Bioorg Med Chem Lett 18: 6142-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.039
BindingDB Entry DOI: 10.7270/Q2V40SKW |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348531
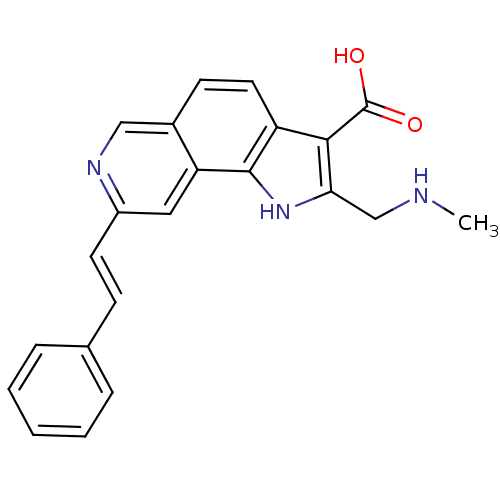
(CHEMBL1801314)Show SMILES CNCc1[nH]c2c(ccc3cnc(\C=C\c4ccccc4)cc23)c1C(O)=O Show InChI InChI=1S/C22H19N3O2/c1-23-13-19-20(22(26)27)17-10-8-15-12-24-16(11-18(15)21(17)25-19)9-7-14-5-3-2-4-6-14/h2-12,23,25H,13H2,1H3,(H,26,27)/b9-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET assay |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50310343

(6-(1-(3-(1H-indol-6-yl)phenyl)-3-amino-1H-pyrazol-...)Show SMILES Nc1nn(cc1-c1ccc2C(=O)NCCc2c1)-c1cccc(c1)-c1ccc2cc[nH]c2c1 Show InChI InChI=1S/C26H21N5O/c27-25-23(19-6-7-22-20(12-19)9-11-29-26(22)32)15-31(30-25)21-3-1-2-17(13-21)18-5-4-16-8-10-28-24(16)14-18/h1-8,10,12-15,28H,9,11H2,(H2,27,30)(H,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 by FRET assay |
Bioorg Med Chem Lett 20: 1293-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.138
BindingDB Entry DOI: 10.7270/Q2MP53C5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348550
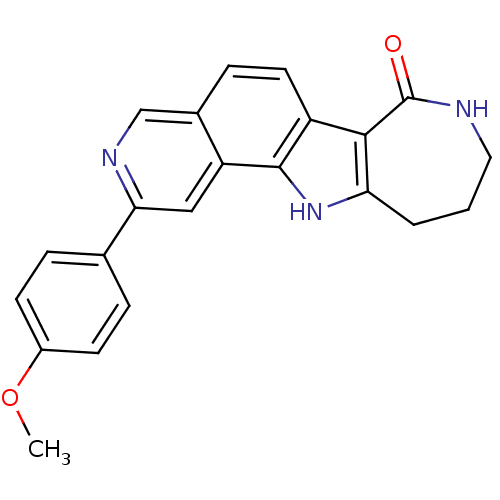
(CHEMBL1801091)Show SMILES COc1ccc(cc1)-c1cc2c3[nH]c4CCCNC(=O)c4c3ccc2cn1 Show InChI InChI=1S/C22H19N3O2/c1-27-15-7-4-13(5-8-15)19-11-17-14(12-24-19)6-9-16-20-18(25-21(16)17)3-2-10-23-22(20)26/h4-9,11-12,25H,2-3,10H2,1H3,(H,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET assay |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348537

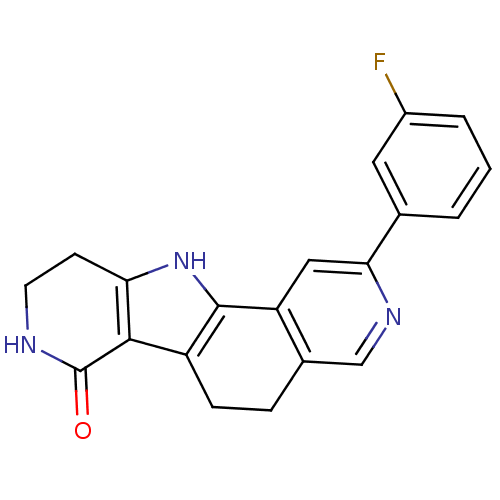
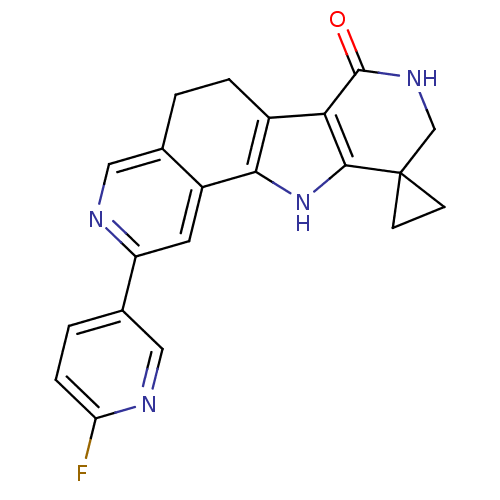
(CHEMBL1801376)Show SMILES Fc1cccc(Nc2cc3c4[nH]c5CNC(=O)c5c4ccc3cn2)c1 Show InChI InChI=1S/C19H13FN4O/c20-11-2-1-3-12(6-11)23-16-7-14-10(8-21-16)4-5-13-17-15(24-18(13)14)9-22-19(17)25/h1-8,24H,9H2,(H,21,23)(H,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET assay |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50348542

(CHEMBL1801381)Show SMILES O=C1NCc2[nH]c3c(ccc4cnc(cc34)-c3cncc(OCCN4CCOCC4)c3)c12 Show InChI InChI=1S/C24H23N5O3/c30-24-22-18-2-1-15-12-26-20(10-19(15)23(18)28-21(22)14-27-24)16-9-17(13-25-11-16)32-8-5-29-3-6-31-7-4-29/h1-2,9-13,28H,3-8,14H2,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of JNK2 |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data