 Found 45 hits with Last Name = 'hyun' and Initial = 'kh'
Found 45 hits with Last Name = 'hyun' and Initial = 'kh' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50008316

(CHEMBL3235164)Show SMILES COCCOCn1ccc(NC(=O)c2cc(Oc3ccc(cc3)S(C)(=O)=O)cc(c2)-c2ncccc2C)n1 Show InChI InChI=1S/C27H28N4O6S/c1-19-5-4-11-28-26(19)20-15-21(27(32)29-25-10-12-31(30-25)18-36-14-13-35-2)17-23(16-20)37-22-6-8-24(9-7-22)38(3,33)34/h4-12,15-17H,13-14,18H2,1-3H3,(H,29,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Yuhan Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes |
Bioorg Med Chem 22: 2280-93 (2014)
Article DOI: 10.1016/j.bmc.2014.02.009
BindingDB Entry DOI: 10.7270/Q2959K3X |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50089218
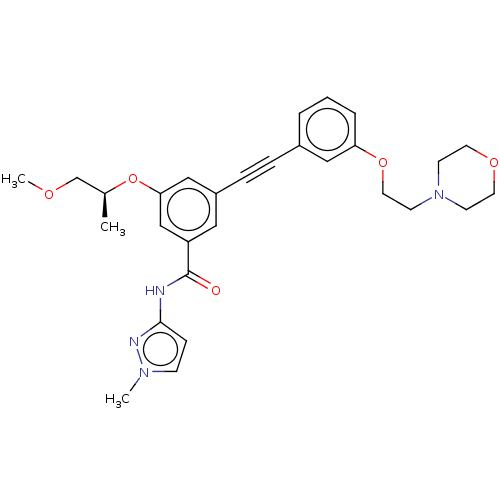
(CHEMBL3577737)Show SMILES COC[C@H](C)Oc1cc(cc(c1)C(=O)Nc1ccn(C)n1)C#Cc1cccc(OCCN2CCOCC2)c1 |r| Show InChI InChI=1S/C29H34N4O5/c1-22(21-35-3)38-27-19-24(17-25(20-27)29(34)30-28-9-10-32(2)31-28)8-7-23-5-4-6-26(18-23)37-16-13-33-11-14-36-15-12-33/h4-6,9-10,17-20,22H,11-16,21H2,1-3H3,(H,30,31,34)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a |
Yuhan Research Institute
Curated by ChEMBL
| Assay Description
Activation of recombinant human pancreatic glucokinase using 10 mM glucose as substrate by G6PDH coupled assay |
ACS Med Chem Lett 6: 296-301 (2015)
Article DOI: 10.1021/ml5004712
BindingDB Entry DOI: 10.7270/Q2ZP47VT |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50008282

(CHEMBL3235147)Show SMILES Cc1cccnc1-c1cc(Oc2ccc(cc2)S(C)(=O)=O)cc(c1)C(=O)Nc1nc(CC(=O)NC2CC2)cs1 Show InChI InChI=1S/C28H26N4O5S2/c1-17-4-3-11-29-26(17)18-12-19(14-23(13-18)37-22-7-9-24(10-8-22)39(2,35)36)27(34)32-28-31-21(16-38-28)15-25(33)30-20-5-6-20/h3-4,7-14,16,20H,5-6,15H2,1-2H3,(H,30,33)(H,31,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 103 | n/a | n/a | n/a | n/a |
Yuhan Research Institute
Curated by ChEMBL
| Assay Description
Activation of recombinant human pancreatic glucokinase using 10 mM glucose by spectrophotometry |
Bioorg Med Chem 22: 2280-93 (2014)
Article DOI: 10.1016/j.bmc.2014.02.009
BindingDB Entry DOI: 10.7270/Q2959K3X |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50008283
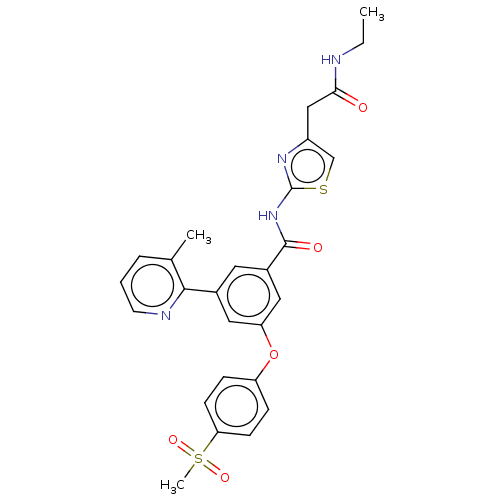
(CHEMBL3235148)Show SMILES CCNC(=O)Cc1csc(NC(=O)c2cc(Oc3ccc(cc3)S(C)(=O)=O)cc(c2)-c2ncccc2C)n1 Show InChI InChI=1S/C27H26N4O5S2/c1-4-28-24(32)15-20-16-37-27(30-20)31-26(33)19-12-18(25-17(2)6-5-11-29-25)13-22(14-19)36-21-7-9-23(10-8-21)38(3,34)35/h5-14,16H,4,15H2,1-3H3,(H,28,32)(H,30,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
Yuhan Research Institute
Curated by ChEMBL
| Assay Description
Activation of recombinant human pancreatic glucokinase using 10 mM glucose by spectrophotometry |
Bioorg Med Chem 22: 2280-93 (2014)
Article DOI: 10.1016/j.bmc.2014.02.009
BindingDB Entry DOI: 10.7270/Q2959K3X |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50008284
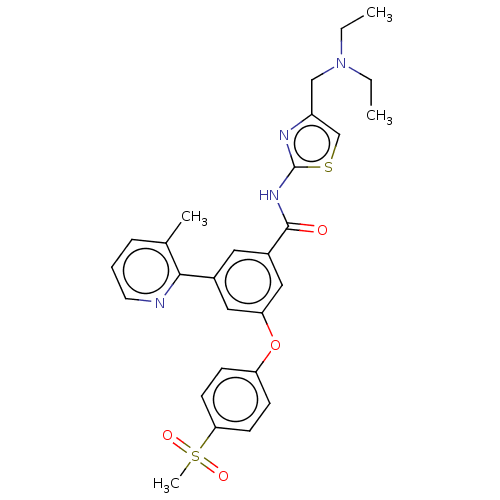
(CHEMBL3235149)Show SMILES CCN(CC)Cc1csc(NC(=O)c2cc(Oc3ccc(cc3)S(C)(=O)=O)cc(c2)-c2ncccc2C)n1 Show InChI InChI=1S/C28H30N4O4S2/c1-5-32(6-2)17-22-18-37-28(30-22)31-27(33)21-14-20(26-19(3)8-7-13-29-26)15-24(16-21)36-23-9-11-25(12-10-23)38(4,34)35/h7-16,18H,5-6,17H2,1-4H3,(H,30,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Yuhan Research Institute
Curated by ChEMBL
| Assay Description
Activation of recombinant human pancreatic glucokinase using 10 mM glucose by spectrophotometry |
Bioorg Med Chem 22: 2280-93 (2014)
Article DOI: 10.1016/j.bmc.2014.02.009
BindingDB Entry DOI: 10.7270/Q2959K3X |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50008296

(CHEMBL3235150)Show SMILES Cc1cccnc1-c1cc(Oc2ccc(cc2)S(C)(=O)=O)cc(c1)C(=O)Nc1nc(CN2CCOCC2)cs1 Show InChI InChI=1S/C28H28N4O5S2/c1-19-4-3-9-29-26(19)20-14-21(16-24(15-20)37-23-5-7-25(8-6-23)39(2,34)35)27(33)31-28-30-22(18-38-28)17-32-10-12-36-13-11-32/h3-9,14-16,18H,10-13,17H2,1-2H3,(H,30,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 212 | n/a | n/a | n/a | n/a |
Yuhan Research Institute
Curated by ChEMBL
| Assay Description
Activation of recombinant human pancreatic glucokinase using 10 mM glucose by spectrophotometry |
Bioorg Med Chem 22: 2280-93 (2014)
Article DOI: 10.1016/j.bmc.2014.02.009
BindingDB Entry DOI: 10.7270/Q2959K3X |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50008298

(CHEMBL3235151)Show SMILES CN1CCN(Cc2csc(NC(=O)c3cc(Oc4ccc(cc4)S(C)(=O)=O)cc(c3)-c3ncccc3C)n2)CC1 Show InChI InChI=1S/C29H31N5O4S2/c1-20-5-4-10-30-27(20)21-15-22(17-25(16-21)38-24-6-8-26(9-7-24)40(3,36)37)28(35)32-29-31-23(19-39-29)18-34-13-11-33(2)12-14-34/h4-10,15-17,19H,11-14,18H2,1-3H3,(H,31,32,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 688 | n/a | n/a | n/a | n/a |
Yuhan Research Institute
Curated by ChEMBL
| Assay Description
Activation of recombinant human pancreatic glucokinase using 10 mM glucose by spectrophotometry |
Bioorg Med Chem 22: 2280-93 (2014)
Article DOI: 10.1016/j.bmc.2014.02.009
BindingDB Entry DOI: 10.7270/Q2959K3X |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50008300

(CHEMBL3235152)Show SMILES COCCN(CCOC)Cc1csc(NC(=O)c2cc(Oc3ccc(cc3)S(C)(=O)=O)cc(c2)-c2ncccc2C)n1 Show InChI InChI=1S/C30H34N4O6S2/c1-21-6-5-11-31-28(21)22-16-23(18-26(17-22)40-25-7-9-27(10-8-25)42(4,36)37)29(35)33-30-32-24(20-41-30)19-34(12-14-38-2)13-15-39-3/h5-11,16-18,20H,12-15,19H2,1-4H3,(H,32,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.43E+3 | n/a | n/a | n/a | n/a |
Yuhan Research Institute
Curated by ChEMBL
| Assay Description
Activation of recombinant human pancreatic glucokinase using 10 mM glucose by spectrophotometry |
Bioorg Med Chem 22: 2280-93 (2014)
Article DOI: 10.1016/j.bmc.2014.02.009
BindingDB Entry DOI: 10.7270/Q2959K3X |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50008301
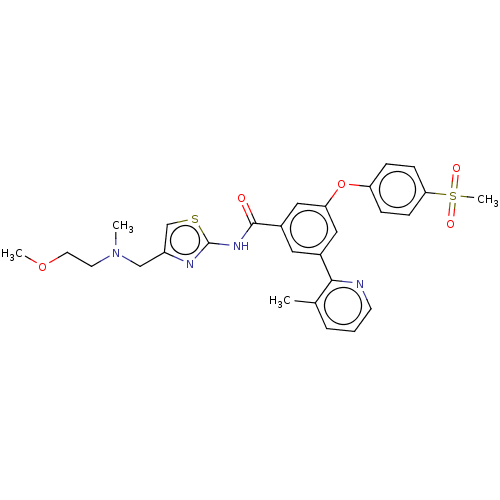
(CHEMBL3235153)Show SMILES COCCN(C)Cc1csc(NC(=O)c2cc(Oc3ccc(cc3)S(C)(=O)=O)cc(c2)-c2ncccc2C)n1 Show InChI InChI=1S/C28H30N4O5S2/c1-19-6-5-11-29-26(19)20-14-21(16-24(15-20)37-23-7-9-25(10-8-23)39(4,34)35)27(33)31-28-30-22(18-38-28)17-32(2)12-13-36-3/h5-11,14-16,18H,12-13,17H2,1-4H3,(H,30,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.46E+3 | n/a | n/a | n/a | n/a |
Yuhan Research Institute
Curated by ChEMBL
| Assay Description
Activation of recombinant human pancreatic glucokinase using 10 mM glucose by spectrophotometry |
Bioorg Med Chem 22: 2280-93 (2014)
Article DOI: 10.1016/j.bmc.2014.02.009
BindingDB Entry DOI: 10.7270/Q2959K3X |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50008302

(CHEMBL3235154)Show SMILES Cc1cccnc1-c1cc(Oc2ccc(cc2)S(C)(=O)=O)cc(c1)C(=O)Nc1nc(CN2CCNCC2)cs1 Show InChI InChI=1S/C28H29N5O4S2/c1-19-4-3-9-30-26(19)20-14-21(16-24(15-20)37-23-5-7-25(8-6-23)39(2,35)36)27(34)32-28-31-22(18-38-28)17-33-12-10-29-11-13-33/h3-9,14-16,18,29H,10-13,17H2,1-2H3,(H,31,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Yuhan Research Institute
Curated by ChEMBL
| Assay Description
Activation of recombinant human pancreatic glucokinase using 10 mM glucose by spectrophotometry |
Bioorg Med Chem 22: 2280-93 (2014)
Article DOI: 10.1016/j.bmc.2014.02.009
BindingDB Entry DOI: 10.7270/Q2959K3X |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50008306

(CHEMBL3235155)Show SMILES COCC(C)NCc1csc(NC(=O)c2cc(Oc3ccc(cc3)S(C)(=O)=O)cc(c2)-c2ncccc2C)n1 Show InChI InChI=1S/C28H30N4O5S2/c1-18-6-5-11-29-26(18)20-12-21(14-24(13-20)37-23-7-9-25(10-8-23)39(4,34)35)27(33)32-28-31-22(17-38-28)15-30-19(2)16-36-3/h5-14,17,19,30H,15-16H2,1-4H3,(H,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
Yuhan Research Institute
Curated by ChEMBL
| Assay Description
Activation of recombinant human pancreatic glucokinase using 10 mM glucose by spectrophotometry |
Bioorg Med Chem 22: 2280-93 (2014)
Article DOI: 10.1016/j.bmc.2014.02.009
BindingDB Entry DOI: 10.7270/Q2959K3X |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50008307

(CHEMBL3235156)Show SMILES COC(CNCc1csc(NC(=O)c2cc(Oc3ccc(cc3)S(C)(=O)=O)cc(c2)-c2ncccc2C)n1)OC Show InChI InChI=1S/C28H30N4O6S2/c1-18-6-5-11-30-26(18)19-12-20(14-23(13-19)38-22-7-9-24(10-8-22)40(4,34)35)27(33)32-28-31-21(17-39-28)15-29-16-25(36-2)37-3/h5-14,17,25,29H,15-16H2,1-4H3,(H,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 33 | n/a | n/a | n/a | n/a |
Yuhan Research Institute
Curated by ChEMBL
| Assay Description
Activation of recombinant human pancreatic glucokinase using 10 mM glucose by spectrophotometry |
Bioorg Med Chem 22: 2280-93 (2014)
Article DOI: 10.1016/j.bmc.2014.02.009
BindingDB Entry DOI: 10.7270/Q2959K3X |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50008308

(CHEMBL3235157)Show SMILES CC(C)NCc1csc(NC(=O)c2cc(Oc3ccc(cc3)S(C)(=O)=O)cc(c2)-c2ncccc2C)n1 Show InChI InChI=1S/C27H28N4O4S2/c1-17(2)29-15-21-16-36-27(30-21)31-26(32)20-12-19(25-18(3)6-5-11-28-25)13-23(14-20)35-22-7-9-24(10-8-22)37(4,33)34/h5-14,16-17,29H,15H2,1-4H3,(H,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 463 | n/a | n/a | n/a | n/a |
Yuhan Research Institute
Curated by ChEMBL
| Assay Description
Activation of recombinant human pancreatic glucokinase using 10 mM glucose by spectrophotometry |
Bioorg Med Chem 22: 2280-93 (2014)
Article DOI: 10.1016/j.bmc.2014.02.009
BindingDB Entry DOI: 10.7270/Q2959K3X |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50008309

(CHEMBL3235158)Show SMILES Cc1cccnc1-c1cc(Oc2ccc(cc2)S(C)(=O)=O)cc(c1)C(=O)Nc1nc(CNCC#C)cs1 Show InChI InChI=1S/C27H24N4O4S2/c1-4-11-28-16-21-17-36-27(30-21)31-26(32)20-13-19(25-18(2)6-5-12-29-25)14-23(15-20)35-22-7-9-24(10-8-22)37(3,33)34/h1,5-10,12-15,17,28H,11,16H2,2-3H3,(H,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 106 | n/a | n/a | n/a | n/a |
Yuhan Research Institute
Curated by ChEMBL
| Assay Description
Activation of recombinant human pancreatic glucokinase using 10 mM glucose by spectrophotometry |
Bioorg Med Chem 22: 2280-93 (2014)
Article DOI: 10.1016/j.bmc.2014.02.009
BindingDB Entry DOI: 10.7270/Q2959K3X |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50008311

(CHEMBL3235159)Show SMILES CCCNCc1csc(NC(=O)c2cc(Oc3ccc(cc3)S(C)(=O)=O)cc(c2)-c2ncccc2C)n1 Show InChI InChI=1S/C27H28N4O4S2/c1-4-11-28-16-21-17-36-27(30-21)31-26(32)20-13-19(25-18(2)6-5-12-29-25)14-23(15-20)35-22-7-9-24(10-8-22)37(3,33)34/h5-10,12-15,17,28H,4,11,16H2,1-3H3,(H,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Yuhan Research Institute
Curated by ChEMBL
| Assay Description
Activation of recombinant human pancreatic glucokinase using 10 mM glucose by spectrophotometry |
Bioorg Med Chem 22: 2280-93 (2014)
Article DOI: 10.1016/j.bmc.2014.02.009
BindingDB Entry DOI: 10.7270/Q2959K3X |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50008312

(CHEMBL3235160)Show SMILES Cc1cccnc1-c1cc(Oc2ccc(cc2)S(C)(=O)=O)cc(c1)C(=O)Nc1ccn(C)n1 Show InChI InChI=1S/C24H22N4O4S/c1-16-5-4-11-25-23(16)17-13-18(24(29)26-22-10-12-28(2)27-22)15-20(14-17)32-19-6-8-21(9-7-19)33(3,30)31/h4-15H,1-3H3,(H,26,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 807 | n/a | n/a | n/a | n/a |
Yuhan Research Institute
Curated by ChEMBL
| Assay Description
Activation of recombinant human pancreatic glucokinase using 10 mM glucose by spectrophotometry |
Bioorg Med Chem 22: 2280-93 (2014)
Article DOI: 10.1016/j.bmc.2014.02.009
BindingDB Entry DOI: 10.7270/Q2959K3X |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50008313

(CHEMBL3235161)Show SMILES CCOCn1ccc(NC(=O)c2cc(Oc3ccc(cc3)S(C)(=O)=O)cc(c2)-c2ncccc2C)n1 Show InChI InChI=1S/C26H26N4O5S/c1-4-34-17-30-13-11-24(29-30)28-26(31)20-14-19(25-18(2)6-5-12-27-25)15-22(16-20)35-21-7-9-23(10-8-21)36(3,32)33/h5-16H,4,17H2,1-3H3,(H,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Yuhan Research Institute
Curated by ChEMBL
| Assay Description
Activation of recombinant human pancreatic glucokinase using 10 mM glucose by spectrophotometry |
Bioorg Med Chem 22: 2280-93 (2014)
Article DOI: 10.1016/j.bmc.2014.02.009
BindingDB Entry DOI: 10.7270/Q2959K3X |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50008314

(CHEMBL3235162)Show SMILES CCN(CC)CCn1ccc(NC(=O)c2cc(Oc3ccc(cc3)S(C)(=O)=O)cc(c2)-c2ncccc2C)n1 Show InChI InChI=1S/C29H33N5O4S/c1-5-33(6-2)16-17-34-15-13-27(32-34)31-29(35)23-18-22(28-21(3)8-7-14-30-28)19-25(20-23)38-24-9-11-26(12-10-24)39(4,36)37/h7-15,18-20H,5-6,16-17H2,1-4H3,(H,31,32,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Yuhan Research Institute
Curated by ChEMBL
| Assay Description
Activation of recombinant human pancreatic glucokinase using 10 mM glucose by spectrophotometry |
Bioorg Med Chem 22: 2280-93 (2014)
Article DOI: 10.1016/j.bmc.2014.02.009
BindingDB Entry DOI: 10.7270/Q2959K3X |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50008315

(CHEMBL3235163)Show SMILES Cc1cccnc1-c1cc(Oc2ccc(cc2)S(C)(=O)=O)cc(c1)C(=O)Nc1ccn(CCN2CCOCC2)n1 Show InChI InChI=1S/C29H31N5O5S/c1-21-4-3-10-30-28(21)22-18-23(20-25(19-22)39-24-5-7-26(8-6-24)40(2,36)37)29(35)31-27-9-11-34(32-27)13-12-33-14-16-38-17-15-33/h3-11,18-20H,12-17H2,1-2H3,(H,31,32,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 441 | n/a | n/a | n/a | n/a |
Yuhan Research Institute
Curated by ChEMBL
| Assay Description
Activation of recombinant human pancreatic glucokinase using 10 mM glucose by spectrophotometry |
Bioorg Med Chem 22: 2280-93 (2014)
Article DOI: 10.1016/j.bmc.2014.02.009
BindingDB Entry DOI: 10.7270/Q2959K3X |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50008316

(CHEMBL3235164)Show SMILES COCCOCn1ccc(NC(=O)c2cc(Oc3ccc(cc3)S(C)(=O)=O)cc(c2)-c2ncccc2C)n1 Show InChI InChI=1S/C27H28N4O6S/c1-19-5-4-11-28-26(19)20-15-21(27(32)29-25-10-12-31(30-25)18-36-14-13-35-2)17-23(16-20)37-22-6-8-24(9-7-22)38(3,33)34/h4-12,15-17H,13-14,18H2,1-3H3,(H,29,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 315 | n/a | n/a | n/a | n/a |
Yuhan Research Institute
Curated by ChEMBL
| Assay Description
Activation of recombinant human pancreatic glucokinase using 10 mM glucose by spectrophotometry |
Bioorg Med Chem 22: 2280-93 (2014)
Article DOI: 10.1016/j.bmc.2014.02.009
BindingDB Entry DOI: 10.7270/Q2959K3X |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50089103
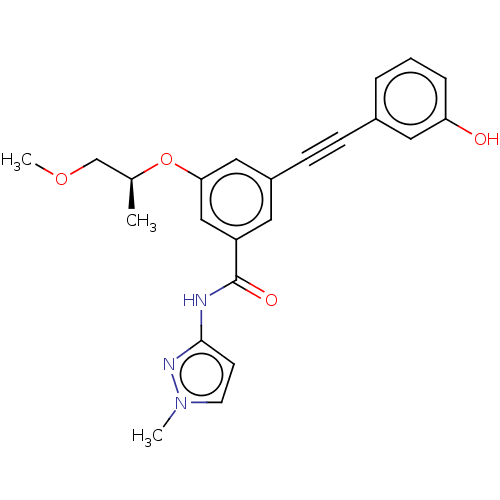
(CHEMBL3577724)Show SMILES COC[C@H](C)Oc1cc(cc(c1)C(=O)Nc1ccn(C)n1)C#Cc1cccc(O)c1 |r| Show InChI InChI=1S/C23H23N3O4/c1-16(15-29-3)30-21-13-18(8-7-17-5-4-6-20(27)12-17)11-19(14-21)23(28)24-22-9-10-26(2)25-22/h4-6,9-14,16,27H,15H2,1-3H3,(H,24,25,28)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 39 | n/a | n/a | n/a | n/a |
Yuhan Research Institute
Curated by ChEMBL
| Assay Description
Activation of recombinant human pancreatic glucokinase using 10 mM glucose as substrate by G6PDH coupled assay |
ACS Med Chem Lett 6: 296-301 (2015)
Article DOI: 10.1021/ml5004712
BindingDB Entry DOI: 10.7270/Q2ZP47VT |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50089104
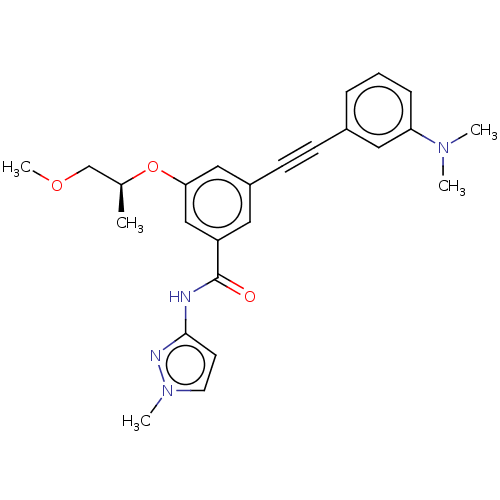
(CHEMBL3577725)Show SMILES COC[C@H](C)Oc1cc(cc(c1)C(=O)Nc1ccn(C)n1)C#Cc1cccc(c1)N(C)C |r| Show InChI InChI=1S/C25H28N4O3/c1-18(17-31-5)32-23-15-20(10-9-19-7-6-8-22(14-19)28(2)3)13-21(16-23)25(30)26-24-11-12-29(4)27-24/h6-8,11-16,18H,17H2,1-5H3,(H,26,27,30)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 37 | n/a | n/a | n/a | n/a |
Yuhan Research Institute
Curated by ChEMBL
| Assay Description
Activation of recombinant human pancreatic glucokinase using 10 mM glucose as substrate by G6PDH coupled assay |
ACS Med Chem Lett 6: 296-301 (2015)
Article DOI: 10.1021/ml5004712
BindingDB Entry DOI: 10.7270/Q2ZP47VT |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50089107
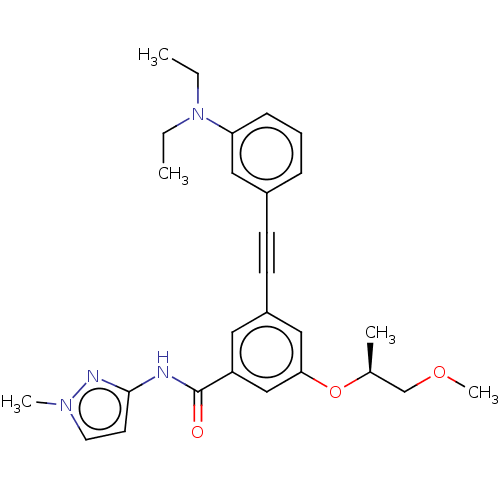
(CHEMBL3577726)Show SMILES CCN(CC)c1cccc(c1)C#Cc1cc(O[C@@H](C)COC)cc(c1)C(=O)Nc1ccn(C)n1 |r| Show InChI InChI=1S/C27H32N4O3/c1-6-31(7-2)24-10-8-9-21(16-24)11-12-22-15-23(18-25(17-22)34-20(3)19-33-5)27(32)28-26-13-14-30(4)29-26/h8-10,13-18,20H,6-7,19H2,1-5H3,(H,28,29,32)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 163 | n/a | n/a | n/a | n/a |
Yuhan Research Institute
Curated by ChEMBL
| Assay Description
Activation of recombinant human pancreatic glucokinase using 10 mM glucose as substrate by G6PDH coupled assay |
ACS Med Chem Lett 6: 296-301 (2015)
Article DOI: 10.1021/ml5004712
BindingDB Entry DOI: 10.7270/Q2ZP47VT |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50089108
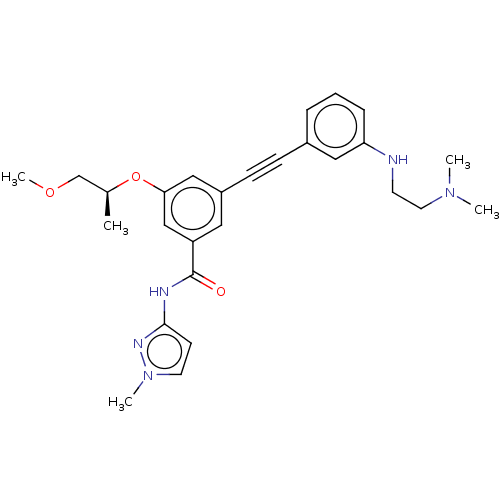
(CHEMBL3577727)Show SMILES COC[C@H](C)Oc1cc(cc(c1)C(=O)Nc1ccn(C)n1)C#Cc1cccc(NCCN(C)C)c1 |r| Show InChI InChI=1S/C27H33N5O3/c1-20(19-34-5)35-25-17-22(15-23(18-25)27(33)29-26-11-13-32(4)30-26)10-9-21-7-6-8-24(16-21)28-12-14-31(2)3/h6-8,11,13,15-18,20,28H,12,14,19H2,1-5H3,(H,29,30,33)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
Yuhan Research Institute
Curated by ChEMBL
| Assay Description
Activation of recombinant human pancreatic glucokinase using 10 mM glucose as substrate by G6PDH coupled assay |
ACS Med Chem Lett 6: 296-301 (2015)
Article DOI: 10.1021/ml5004712
BindingDB Entry DOI: 10.7270/Q2ZP47VT |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50089134
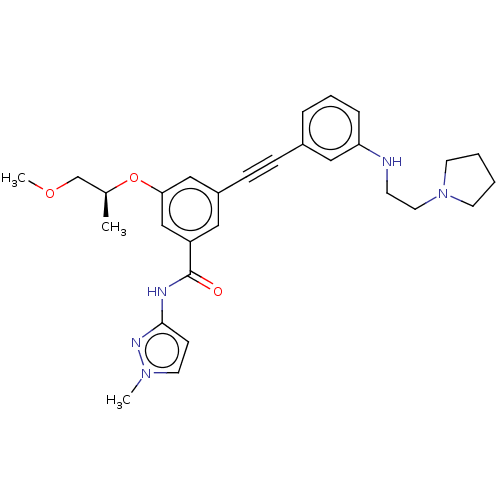
(CHEMBL3577728)Show SMILES COC[C@H](C)Oc1cc(cc(c1)C(=O)Nc1ccn(C)n1)C#Cc1cccc(NCCN2CCCC2)c1 |r| Show InChI InChI=1S/C29H35N5O3/c1-22(21-36-3)37-27-19-24(17-25(20-27)29(35)31-28-11-15-33(2)32-28)10-9-23-7-6-8-26(18-23)30-12-16-34-13-4-5-14-34/h6-8,11,15,17-20,22,30H,4-5,12-14,16,21H2,1-3H3,(H,31,32,35)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
Yuhan Research Institute
Curated by ChEMBL
| Assay Description
Activation of recombinant human pancreatic glucokinase using 10 mM glucose as substrate by G6PDH coupled assay |
ACS Med Chem Lett 6: 296-301 (2015)
Article DOI: 10.1021/ml5004712
BindingDB Entry DOI: 10.7270/Q2ZP47VT |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50089135
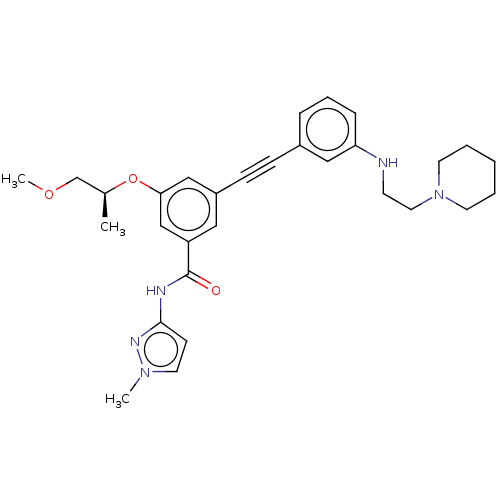
(CHEMBL3577729)Show SMILES COC[C@H](C)Oc1cc(cc(c1)C(=O)Nc1ccn(C)n1)C#Cc1cccc(NCCN2CCCCC2)c1 |r| Show InChI InChI=1S/C30H37N5O3/c1-23(22-37-3)38-28-20-25(18-26(21-28)30(36)32-29-12-16-34(2)33-29)11-10-24-8-7-9-27(19-24)31-13-17-35-14-5-4-6-15-35/h7-9,12,16,18-21,23,31H,4-6,13-15,17,22H2,1-3H3,(H,32,33,36)/t23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
Yuhan Research Institute
Curated by ChEMBL
| Assay Description
Activation of recombinant human pancreatic glucokinase using 10 mM glucose as substrate by G6PDH coupled assay |
ACS Med Chem Lett 6: 296-301 (2015)
Article DOI: 10.1021/ml5004712
BindingDB Entry DOI: 10.7270/Q2ZP47VT |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50089136
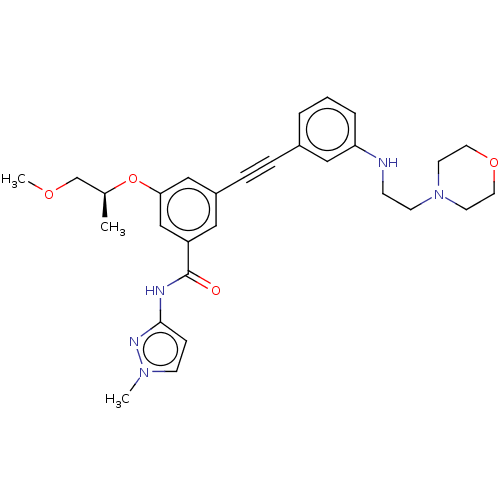
(CHEMBL3577730)Show SMILES COC[C@H](C)Oc1cc(cc(c1)C(=O)Nc1ccn(C)n1)C#Cc1cccc(NCCN2CCOCC2)c1 |r| Show InChI InChI=1S/C29H35N5O4/c1-22(21-36-3)38-27-19-24(17-25(20-27)29(35)31-28-9-11-33(2)32-28)8-7-23-5-4-6-26(18-23)30-10-12-34-13-15-37-16-14-34/h4-6,9,11,17-20,22,30H,10,12-16,21H2,1-3H3,(H,31,32,35)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Yuhan Research Institute
Curated by ChEMBL
| Assay Description
Activation of recombinant human pancreatic glucokinase using 10 mM glucose as substrate by G6PDH coupled assay |
ACS Med Chem Lett 6: 296-301 (2015)
Article DOI: 10.1021/ml5004712
BindingDB Entry DOI: 10.7270/Q2ZP47VT |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50089137
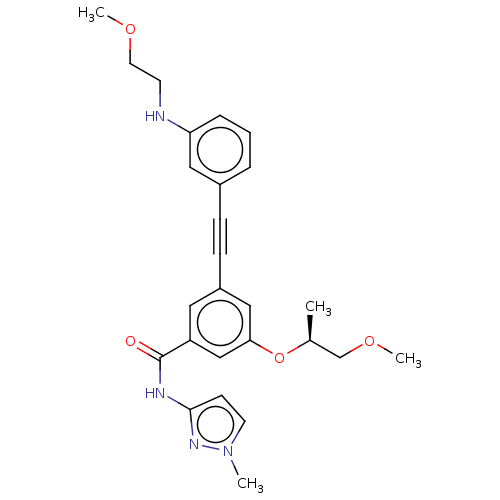
(CHEMBL3577731)Show SMILES COCCNc1cccc(c1)C#Cc1cc(O[C@@H](C)COC)cc(c1)C(=O)Nc1ccn(C)n1 |r| Show InChI InChI=1S/C26H30N4O4/c1-19(18-33-4)34-24-16-21(9-8-20-6-5-7-23(15-20)27-11-13-32-3)14-22(17-24)26(31)28-25-10-12-30(2)29-25/h5-7,10,12,14-17,19,27H,11,13,18H2,1-4H3,(H,28,29,31)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 39 | n/a | n/a | n/a | n/a |
Yuhan Research Institute
Curated by ChEMBL
| Assay Description
Activation of recombinant human pancreatic glucokinase using 10 mM glucose as substrate by G6PDH coupled assay |
ACS Med Chem Lett 6: 296-301 (2015)
Article DOI: 10.1021/ml5004712
BindingDB Entry DOI: 10.7270/Q2ZP47VT |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50089138
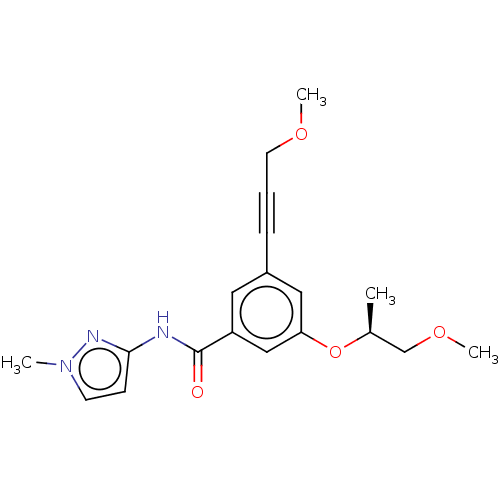
(CHEMBL3577714)Show SMILES COCC#Cc1cc(O[C@@H](C)COC)cc(c1)C(=O)Nc1ccn(C)n1 |r| Show InChI InChI=1S/C19H23N3O4/c1-14(13-25-4)26-17-11-15(6-5-9-24-3)10-16(12-17)19(23)20-18-7-8-22(2)21-18/h7-8,10-12,14H,9,13H2,1-4H3,(H,20,21,23)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.87E+3 | n/a | n/a | n/a | n/a |
Yuhan Research Institute
Curated by ChEMBL
| Assay Description
Activation of recombinant human pancreatic glucokinase using 10 mM glucose as substrate by G6PDH coupled assay |
ACS Med Chem Lett 6: 296-301 (2015)
Article DOI: 10.1021/ml5004712
BindingDB Entry DOI: 10.7270/Q2ZP47VT |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50089139
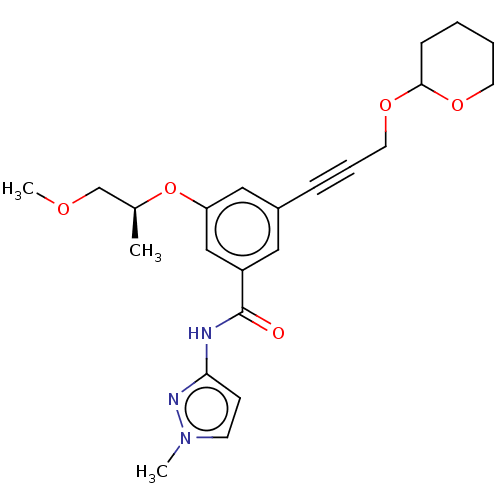
(CHEMBL3577715)Show SMILES COC[C@H](C)Oc1cc(cc(c1)C(=O)Nc1ccn(C)n1)C#CCOC1CCCCO1 |r| Show InChI InChI=1S/C23H29N3O5/c1-17(16-28-3)31-20-14-18(7-6-12-30-22-8-4-5-11-29-22)13-19(15-20)23(27)24-21-9-10-26(2)25-21/h9-10,13-15,17,22H,4-5,8,11-12,16H2,1-3H3,(H,24,25,27)/t17-,22?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
Yuhan Research Institute
Curated by ChEMBL
| Assay Description
Activation of recombinant human pancreatic glucokinase using 10 mM glucose as substrate by G6PDH coupled assay |
ACS Med Chem Lett 6: 296-301 (2015)
Article DOI: 10.1021/ml5004712
BindingDB Entry DOI: 10.7270/Q2ZP47VT |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50089140
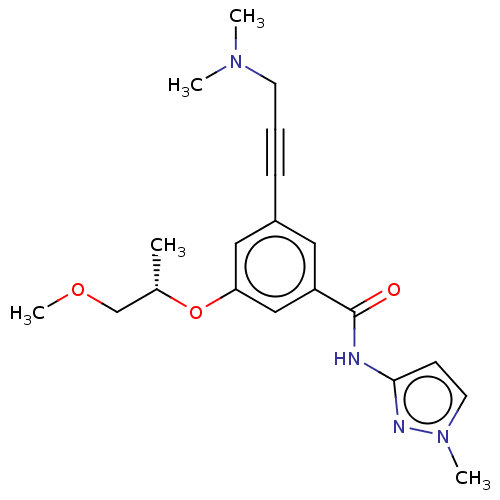
(CHEMBL3577716)Show SMILES COC[C@H](C)Oc1cc(cc(c1)C(=O)Nc1ccn(C)n1)C#CCN(C)C |r| Show InChI InChI=1S/C20H26N4O3/c1-15(14-26-5)27-18-12-16(7-6-9-23(2)3)11-17(13-18)20(25)21-19-8-10-24(4)22-19/h8,10-13,15H,9,14H2,1-5H3,(H,21,22,25)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a |
Yuhan Research Institute
Curated by ChEMBL
| Assay Description
Activation of recombinant human pancreatic glucokinase using 10 mM glucose as substrate by G6PDH coupled assay |
ACS Med Chem Lett 6: 296-301 (2015)
Article DOI: 10.1021/ml5004712
BindingDB Entry DOI: 10.7270/Q2ZP47VT |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50089141
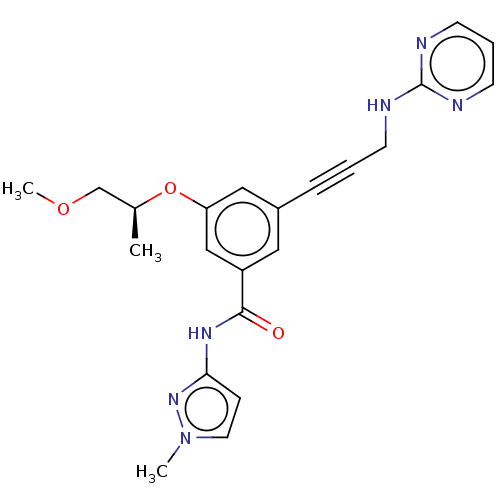
(CHEMBL3577717)Show SMILES COC[C@H](C)Oc1cc(cc(c1)C(=O)Nc1ccn(C)n1)C#CCNc1ncccn1 |r| Show InChI InChI=1S/C22H24N6O3/c1-16(15-30-3)31-19-13-17(6-4-8-23-22-24-9-5-10-25-22)12-18(14-19)21(29)26-20-7-11-28(2)27-20/h5,7,9-14,16H,8,15H2,1-3H3,(H,23,24,25)(H,26,27,29)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 301 | n/a | n/a | n/a | n/a |
Yuhan Research Institute
Curated by ChEMBL
| Assay Description
Activation of recombinant human pancreatic glucokinase using 10 mM glucose as substrate by G6PDH coupled assay |
ACS Med Chem Lett 6: 296-301 (2015)
Article DOI: 10.1021/ml5004712
BindingDB Entry DOI: 10.7270/Q2ZP47VT |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50089142

(CHEMBL3577718)Show SMILES COC[C@H](C)Oc1cc(cc(c1)C(=O)Nc1ccn(C)n1)C#CCSC1CCCC1 |r| Show InChI InChI=1S/C23H29N3O3S/c1-17(16-28-3)29-20-14-18(7-6-12-30-21-8-4-5-9-21)13-19(15-20)23(27)24-22-10-11-26(2)25-22/h10-11,13-15,17,21H,4-5,8-9,12,16H2,1-3H3,(H,24,25,27)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
Yuhan Research Institute
Curated by ChEMBL
| Assay Description
Activation of recombinant human pancreatic glucokinase using 10 mM glucose as substrate by G6PDH coupled assay |
ACS Med Chem Lett 6: 296-301 (2015)
Article DOI: 10.1021/ml5004712
BindingDB Entry DOI: 10.7270/Q2ZP47VT |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50089143
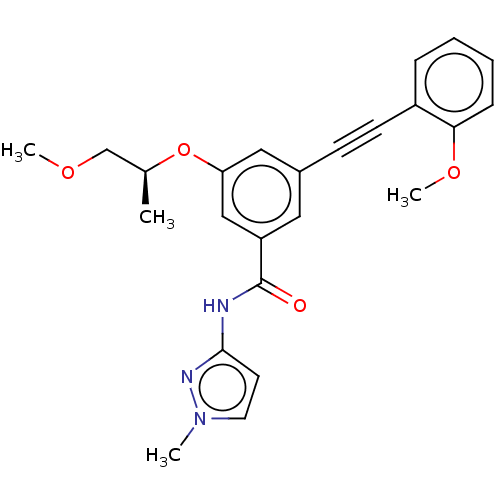
(CHEMBL3577719)Show SMILES COC[C@H](C)Oc1cc(cc(c1)C(=O)Nc1ccn(C)n1)C#Cc1ccccc1OC |r| Show InChI InChI=1S/C24H25N3O4/c1-17(16-29-3)31-21-14-18(9-10-19-7-5-6-8-22(19)30-4)13-20(15-21)24(28)25-23-11-12-27(2)26-23/h5-8,11-15,17H,16H2,1-4H3,(H,25,26,28)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 21 | n/a | n/a | n/a | n/a |
Yuhan Research Institute
Curated by ChEMBL
| Assay Description
Activation of recombinant human pancreatic glucokinase using 10 mM glucose as substrate by G6PDH coupled assay |
ACS Med Chem Lett 6: 296-301 (2015)
Article DOI: 10.1021/ml5004712
BindingDB Entry DOI: 10.7270/Q2ZP47VT |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50089173
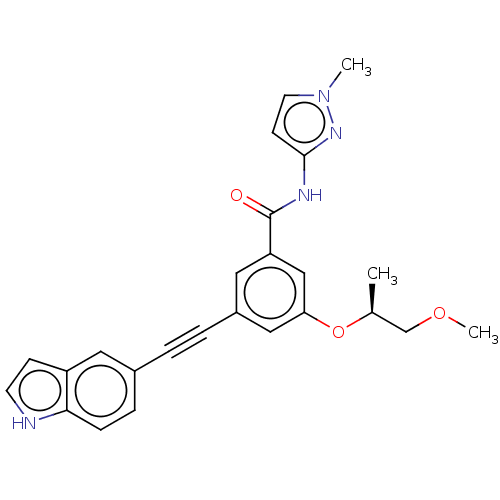
(CHEMBL3577720)Show SMILES COC[C@H](C)Oc1cc(cc(c1)C(=O)Nc1ccn(C)n1)C#Cc1ccc2[nH]ccc2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
Yuhan Research Institute
Curated by ChEMBL
| Assay Description
Activation of recombinant human pancreatic glucokinase using 10 mM glucose as substrate by G6PDH coupled assay |
ACS Med Chem Lett 6: 296-301 (2015)
Article DOI: 10.1021/ml5004712
BindingDB Entry DOI: 10.7270/Q2ZP47VT |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50089174
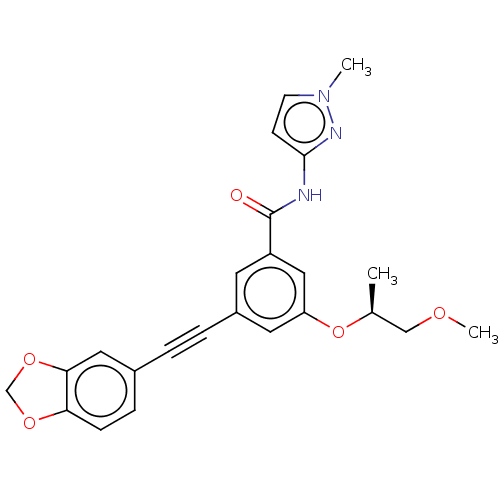
(CHEMBL3577721)Show SMILES COC[C@H](C)Oc1cc(cc(c1)C(=O)Nc1ccn(C)n1)C#Cc1ccc2OCOc2c1 |r| Show InChI InChI=1S/C24H23N3O5/c1-16(14-29-3)32-20-11-18(5-4-17-6-7-21-22(12-17)31-15-30-21)10-19(13-20)24(28)25-23-8-9-27(2)26-23/h6-13,16H,14-15H2,1-3H3,(H,25,26,28)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a |
Yuhan Research Institute
Curated by ChEMBL
| Assay Description
Activation of recombinant human pancreatic glucokinase using 10 mM glucose as substrate by G6PDH coupled assay |
ACS Med Chem Lett 6: 296-301 (2015)
Article DOI: 10.1021/ml5004712
BindingDB Entry DOI: 10.7270/Q2ZP47VT |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50089175
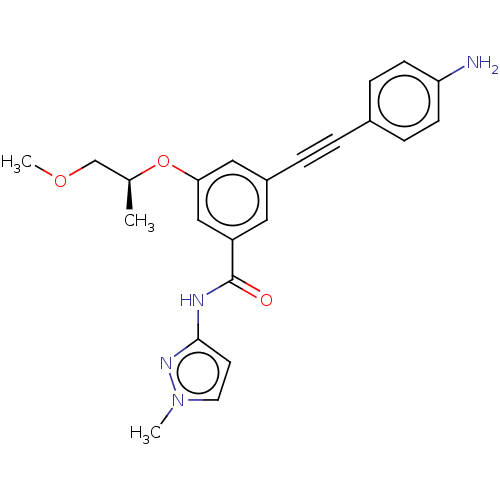
(CHEMBL3577722)Show SMILES COC[C@H](C)Oc1cc(cc(c1)C(=O)Nc1ccn(C)n1)C#Cc1ccc(N)cc1 |r| Show InChI InChI=1S/C23H24N4O3/c1-16(15-29-3)30-21-13-18(5-4-17-6-8-20(24)9-7-17)12-19(14-21)23(28)25-22-10-11-27(2)26-22/h6-14,16H,15,24H2,1-3H3,(H,25,26,28)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 34 | n/a | n/a | n/a | n/a |
Yuhan Research Institute
Curated by ChEMBL
| Assay Description
Activation of recombinant human pancreatic glucokinase using 10 mM glucose as substrate by G6PDH coupled assay |
ACS Med Chem Lett 6: 296-301 (2015)
Article DOI: 10.1021/ml5004712
BindingDB Entry DOI: 10.7270/Q2ZP47VT |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50089176
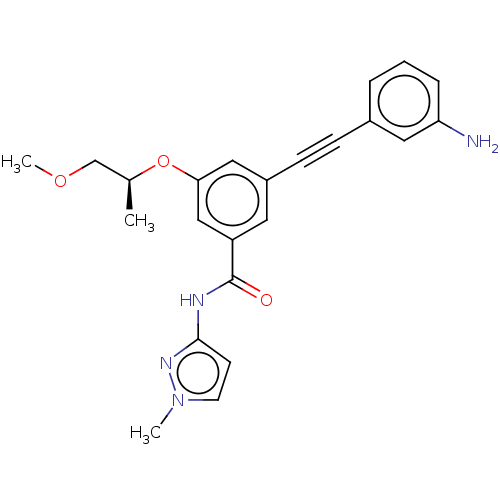
(CHEMBL3577723)Show SMILES COC[C@H](C)Oc1cc(cc(c1)C(=O)Nc1ccn(C)n1)C#Cc1cccc(N)c1 |r| Show InChI InChI=1S/C23H24N4O3/c1-16(15-29-3)30-21-13-18(8-7-17-5-4-6-20(24)12-17)11-19(14-21)23(28)25-22-9-10-27(2)26-22/h4-6,9-14,16H,15,24H2,1-3H3,(H,25,26,28)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 27 | n/a | n/a | n/a | n/a |
Yuhan Research Institute
Curated by ChEMBL
| Assay Description
Activation of recombinant human pancreatic glucokinase using 10 mM glucose as substrate by G6PDH coupled assay |
ACS Med Chem Lett 6: 296-301 (2015)
Article DOI: 10.1021/ml5004712
BindingDB Entry DOI: 10.7270/Q2ZP47VT |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50089177
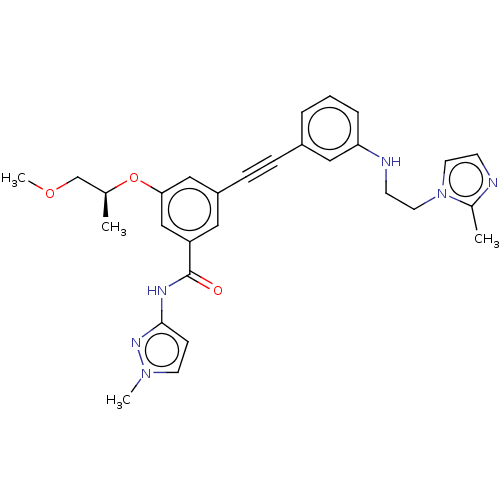
(CHEMBL3577732)Show SMILES COC[C@H](C)Oc1cc(cc(c1)C(=O)Nc1ccn(C)n1)C#Cc1cccc(NCCn2ccnc2C)c1 |r| Show InChI InChI=1S/C29H32N6O3/c1-21(20-37-4)38-27-18-24(16-25(19-27)29(36)32-28-10-13-34(3)33-28)9-8-23-6-5-7-26(17-23)31-12-15-35-14-11-30-22(35)2/h5-7,10-11,13-14,16-19,21,31H,12,15,20H2,1-4H3,(H,32,33,36)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 9 | n/a | n/a | n/a | n/a |
Yuhan Research Institute
Curated by ChEMBL
| Assay Description
Activation of recombinant human pancreatic glucokinase using 10 mM glucose as substrate by G6PDH coupled assay |
ACS Med Chem Lett 6: 296-301 (2015)
Article DOI: 10.1021/ml5004712
BindingDB Entry DOI: 10.7270/Q2ZP47VT |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50089178
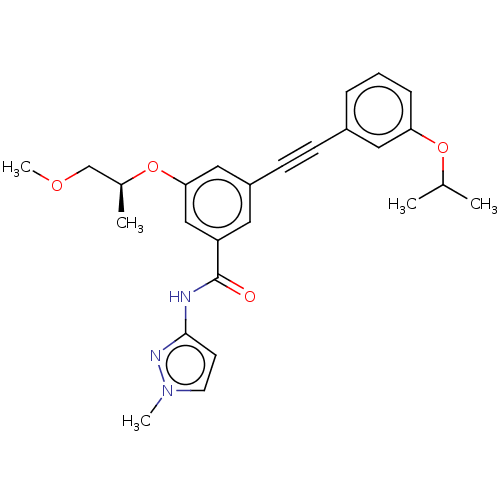
(CHEMBL3577733)Show SMILES COC[C@H](C)Oc1cc(cc(c1)C(=O)Nc1ccn(C)n1)C#Cc1cccc(OC(C)C)c1 |r| Show InChI InChI=1S/C26H29N3O4/c1-18(2)32-23-8-6-7-20(14-23)9-10-21-13-22(16-24(15-21)33-19(3)17-31-5)26(30)27-25-11-12-29(4)28-25/h6-8,11-16,18-19H,17H2,1-5H3,(H,27,28,30)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 53 | n/a | n/a | n/a | n/a |
Yuhan Research Institute
Curated by ChEMBL
| Assay Description
Activation of recombinant human pancreatic glucokinase using 10 mM glucose as substrate by G6PDH coupled assay |
ACS Med Chem Lett 6: 296-301 (2015)
Article DOI: 10.1021/ml5004712
BindingDB Entry DOI: 10.7270/Q2ZP47VT |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50089179
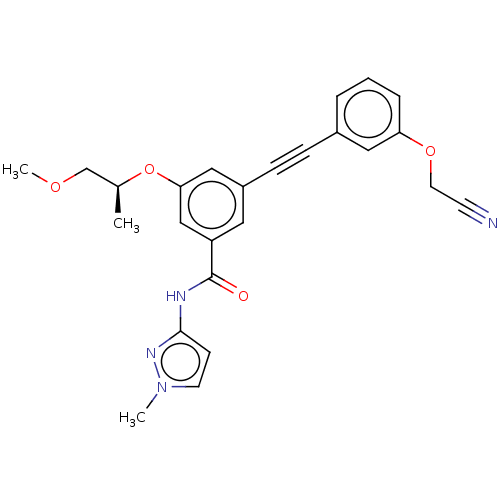
(CHEMBL3577734)Show SMILES COC[C@H](C)Oc1cc(cc(c1)C(=O)Nc1ccn(C)n1)C#Cc1cccc(OCC#N)c1 |r| Show InChI InChI=1S/C25H24N4O4/c1-18(17-31-3)33-23-15-20(8-7-19-5-4-6-22(14-19)32-12-10-26)13-21(16-23)25(30)27-24-9-11-29(2)28-24/h4-6,9,11,13-16,18H,12,17H2,1-3H3,(H,27,28,30)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 46 | n/a | n/a | n/a | n/a |
Yuhan Research Institute
Curated by ChEMBL
| Assay Description
Activation of recombinant human pancreatic glucokinase using 10 mM glucose as substrate by G6PDH coupled assay |
ACS Med Chem Lett 6: 296-301 (2015)
Article DOI: 10.1021/ml5004712
BindingDB Entry DOI: 10.7270/Q2ZP47VT |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50089180
(CHEMBL3577735)Show SMILES COC[C@H](C)Oc1cc(cc(c1)C(=O)Nc1ccn(C)n1)C#Cc1cccc(OCCN2CCCC2)c1 |r| Show InChI InChI=1S/C29H34N4O4/c1-22(21-35-3)37-27-19-24(17-25(20-27)29(34)30-28-11-14-32(2)31-28)10-9-23-7-6-8-26(18-23)36-16-15-33-12-4-5-13-33/h6-8,11,14,17-20,22H,4-5,12-13,15-16,21H2,1-3H3,(H,30,31,34)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
Yuhan Research Institute
Curated by ChEMBL
| Assay Description
Activation of recombinant human pancreatic glucokinase using 10 mM glucose as substrate by G6PDH coupled assay |
ACS Med Chem Lett 6: 296-301 (2015)
Article DOI: 10.1021/ml5004712
BindingDB Entry DOI: 10.7270/Q2ZP47VT |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50089181

(CHEMBL3577736)Show SMILES COC[C@H](C)Oc1cc(cc(c1)C(=O)Nc1ccn(C)n1)C#Cc1cccc(OCCN2CCCCC2)c1 |r| Show InChI InChI=1S/C30H36N4O4/c1-23(22-36-3)38-28-20-25(18-26(21-28)30(35)31-29-12-15-33(2)32-29)11-10-24-8-7-9-27(19-24)37-17-16-34-13-5-4-6-14-34/h7-9,12,15,18-21,23H,4-6,13-14,16-17,22H2,1-3H3,(H,31,32,35)/t23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 7 | n/a | n/a | n/a | n/a |
Yuhan Research Institute
Curated by ChEMBL
| Assay Description
Activation of recombinant human pancreatic glucokinase using 10 mM glucose as substrate by G6PDH coupled assay |
ACS Med Chem Lett 6: 296-301 (2015)
Article DOI: 10.1021/ml5004712
BindingDB Entry DOI: 10.7270/Q2ZP47VT |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50008280

(CHEMBL3235145)Show SMILES Cc1cccnc1-c1cc(Oc2ccc(cc2)S(C)(=O)=O)cc(c1)C(=O)Nc1nccs1 Show InChI InChI=1S/C23H19N3O4S2/c1-15-4-3-9-24-21(15)16-12-17(22(27)26-23-25-10-11-31-23)14-19(13-16)30-18-5-7-20(8-6-18)32(2,28)29/h3-14H,1-2H3,(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 95 | n/a | n/a | n/a | n/a |
Yuhan Research Institute
Curated by ChEMBL
| Assay Description
Activation of recombinant human pancreatic glucokinase using 10 mM glucose by spectrophotometry |
Bioorg Med Chem 22: 2280-93 (2014)
Article DOI: 10.1016/j.bmc.2014.02.009
BindingDB Entry DOI: 10.7270/Q2959K3X |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50008281

(CHEMBL3235146)Show SMILES Cc1cccnc1-c1cc(Oc2ccc(cc2)S(C)(=O)=O)cc(c1)C(=O)Nc1ncc(F)s1 Show InChI InChI=1S/C23H18FN3O4S2/c1-14-4-3-9-25-21(14)15-10-16(22(28)27-23-26-13-20(24)32-23)12-18(11-15)31-17-5-7-19(8-6-17)33(2,29)30/h3-13H,1-2H3,(H,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 238 | n/a | n/a | n/a | n/a |
Yuhan Research Institute
Curated by ChEMBL
| Assay Description
Activation of recombinant human pancreatic glucokinase using 10 mM glucose by spectrophotometry |
Bioorg Med Chem 22: 2280-93 (2014)
Article DOI: 10.1016/j.bmc.2014.02.009
BindingDB Entry DOI: 10.7270/Q2959K3X |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data