 Found 72 hits with Last Name = 'ikejiri' and Initial = 'm'
Found 72 hits with Last Name = 'ikejiri' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50335495
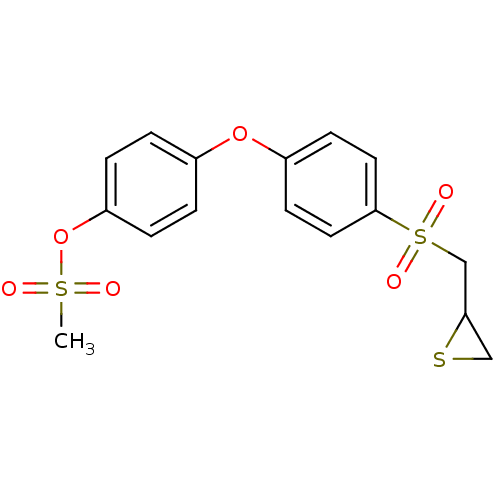
(4-(4-(thiiran-2-ylmethylsulfonyl)phenoxy)phenyl me...)Show SMILES CS(=O)(=O)Oc1ccc(Oc2ccc(cc2)S(=O)(=O)CC2CS2)cc1 Show InChI InChI=1S/C16H16O6S3/c1-24(17,18)22-14-4-2-12(3-5-14)21-13-6-8-16(9-7-13)25(19,20)11-15-10-23-15/h2-9,15H,10-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP9 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50388914
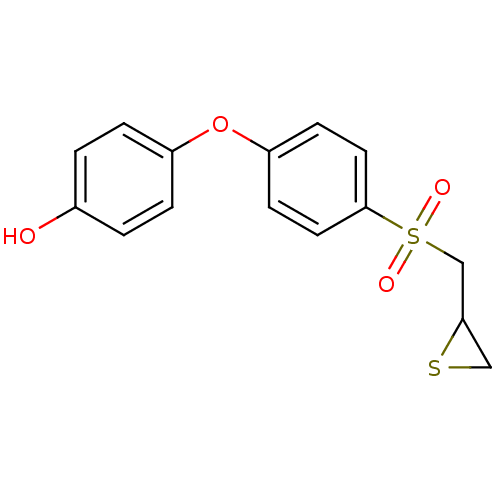
(CHEMBL2063274 | US10357546, p-OH SB-3CT)Show InChI InChI=1S/C15H14O4S2/c16-11-1-3-12(4-2-11)19-13-5-7-15(8-6-13)21(17,18)10-14-9-20-14/h1-8,14,16H,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP2 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50264809

(2-((4-phenoxyphenylsulfonyl)methyl)thiirane | CHEM...)Show InChI InChI=1S/C15H14O3S2/c16-20(17,11-14-10-19-14)15-8-6-13(7-9-15)18-12-4-2-1-3-5-12/h1-9,14H,10-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP2 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50335495

(4-(4-(thiiran-2-ylmethylsulfonyl)phenoxy)phenyl me...)Show SMILES CS(=O)(=O)Oc1ccc(Oc2ccc(cc2)S(=O)(=O)CC2CS2)cc1 Show InChI InChI=1S/C16H16O6S3/c1-24(17,18)22-14-4-2-12(3-5-14)21-13-6-8-16(9-7-13)25(19,20)11-15-10-23-15/h2-9,15H,10-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP2 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50354624
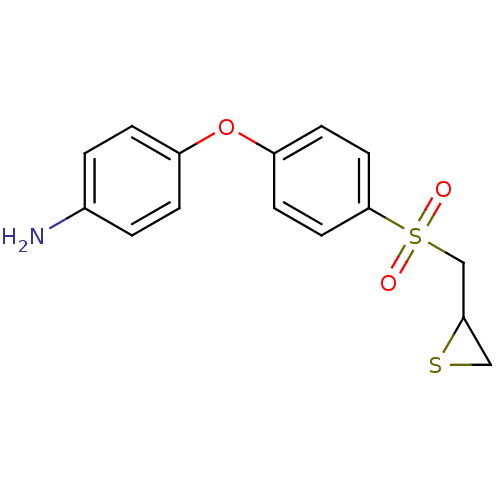
(CHEMBL1834422 | US10357546, ND-322 | US9951035, ND...)Show InChI InChI=1S/C15H15NO3S2/c16-11-1-3-12(4-2-11)19-13-5-7-15(8-6-13)21(17,18)10-14-9-20-14/h1-8,14H,9-10,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP2 assessed as hydrolysis of MOCAcPLGLA2pr(Dnp)AR-NH2 by fluorescence spectrofluorometry |
J Med Chem 54: 6676-90 (2011)
Article DOI: 10.1021/jm200566e
BindingDB Entry DOI: 10.7270/Q2D79BSM |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50264809

(2-((4-phenoxyphenylsulfonyl)methyl)thiirane | CHEM...)Show InChI InChI=1S/C15H14O3S2/c16-20(17,11-14-10-19-14)15-8-6-13(7-9-15)18-12-4-2-1-3-5-12/h1-9,14H,10-11H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP14 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50388911
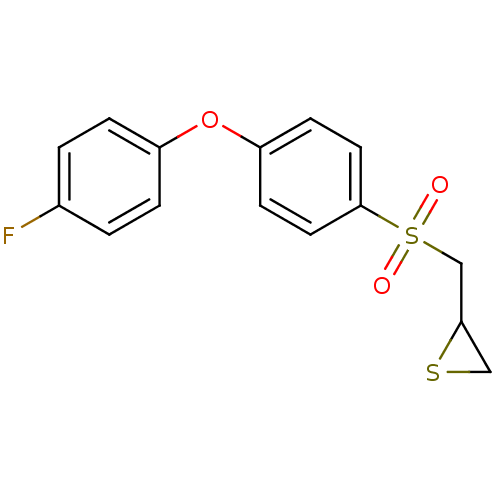
(CHEMBL2063270)Show InChI InChI=1S/C15H13FO3S2/c16-11-1-3-12(4-2-11)19-13-5-7-15(8-6-13)21(17,18)10-14-9-20-14/h1-8,14H,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP9 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50388911

(CHEMBL2063270)Show InChI InChI=1S/C15H13FO3S2/c16-11-1-3-12(4-2-11)19-13-5-7-15(8-6-13)21(17,18)10-14-9-20-14/h1-8,14H,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP2 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50354622

(CHEMBL1834418)Show SMILES NCCCC[C@H](N)C(=O)Nc1ccc(Oc2ccc(cc2)S(=O)(=O)CC2CS2)cc1 |r| Show InChI InChI=1S/C21H27N3O4S2/c22-12-2-1-3-20(23)21(25)24-15-4-6-16(7-5-15)28-17-8-10-19(11-9-17)30(26,27)14-18-13-29-18/h4-11,18,20H,1-3,12-14,22-23H2,(H,24,25)/t18?,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP2 assessed as hydrolysis of MOCAcPLGLA2pr(Dnp)AR-NH2 by fluorescence spectrofluorometry |
J Med Chem 54: 6676-90 (2011)
Article DOI: 10.1021/jm200566e
BindingDB Entry DOI: 10.7270/Q2D79BSM |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50354628

(CHEMBL1834419)Show SMILES N[C@@H](CCC(O)=O)C(=O)Nc1ccc(Oc2ccc(cc2)S(=O)(=O)CC2CS2)cc1 |r| Show InChI InChI=1S/C20H22N2O6S2/c21-18(9-10-19(23)24)20(25)22-13-1-3-14(4-2-13)28-15-5-7-17(8-6-15)30(26,27)12-16-11-29-16/h1-8,16,18H,9-12,21H2,(H,22,25)(H,23,24)/t16?,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP2 assessed as hydrolysis of MOCAcPLGLA2pr(Dnp)AR-NH2 by fluorescence spectrofluorometry |
J Med Chem 54: 6676-90 (2011)
Article DOI: 10.1021/jm200566e
BindingDB Entry DOI: 10.7270/Q2D79BSM |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50388912
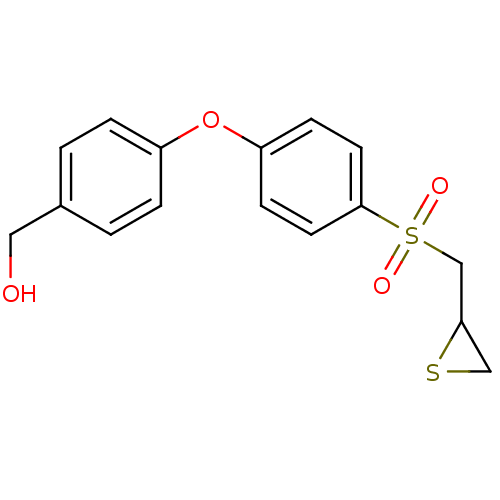
(CHEMBL2063273)Show InChI InChI=1S/C16H16O4S2/c17-9-12-1-3-13(4-2-12)20-14-5-7-16(8-6-14)22(18,19)11-15-10-21-15/h1-8,15,17H,9-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP2 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50388914

(CHEMBL2063274 | US10357546, p-OH SB-3CT)Show InChI InChI=1S/C15H14O4S2/c16-11-1-3-12(4-2-11)19-13-5-7-15(8-6-13)21(17,18)10-14-9-20-14/h1-8,14,16H,9-10H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP14 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50388917
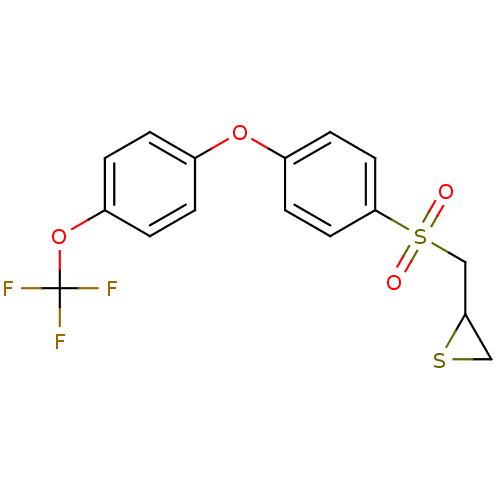
(CHEMBL2063277)Show SMILES FC(F)(F)Oc1ccc(Oc2ccc(cc2)S(=O)(=O)CC2CS2)cc1 Show InChI InChI=1S/C16H13F3O4S2/c17-16(18,19)23-13-3-1-11(2-4-13)22-12-5-7-15(8-6-12)25(20,21)10-14-9-24-14/h1-8,14H,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP2 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50264811

(CHEMBL485492 | N-(4-(4-(thiiran-2-ylmethylsulfonyl...)Show SMILES CC(=O)Nc1ccc(Oc2ccc(cc2)S(=O)(=O)CC2CS2)cc1 Show InChI InChI=1S/C17H17NO4S2/c1-12(19)18-13-2-4-14(5-3-13)22-15-6-8-17(9-7-15)24(20,21)11-16-10-23-16/h2-9,16H,10-11H2,1H3,(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP9 catalytic domain assessed as hydrolysis of MOCAcPLGLA2pr(Dnp)AR-NH2 by fluorescence spectrofluorometry |
J Med Chem 54: 6676-90 (2011)
Article DOI: 10.1021/jm200566e
BindingDB Entry DOI: 10.7270/Q2D79BSM |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50264811

(CHEMBL485492 | N-(4-(4-(thiiran-2-ylmethylsulfonyl...)Show SMILES CC(=O)Nc1ccc(Oc2ccc(cc2)S(=O)(=O)CC2CS2)cc1 Show InChI InChI=1S/C17H17NO4S2/c1-12(19)18-13-2-4-14(5-3-13)22-15-6-8-17(9-7-15)24(20,21)11-16-10-23-16/h2-9,16H,10-11H2,1H3,(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP9 assessed as hydrolysis of MOCAcPLGLA2pr(Dnp)AR-NH2 by fluorescence spectrofluorometry |
J Med Chem 54: 6676-90 (2011)
Article DOI: 10.1021/jm200566e
BindingDB Entry DOI: 10.7270/Q2D79BSM |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50388916
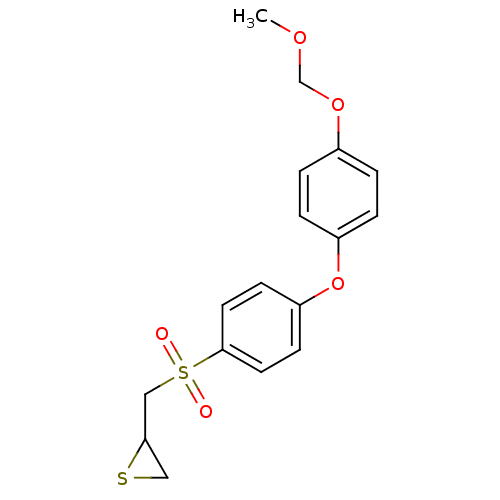
(CHEMBL2063276)Show InChI InChI=1S/C17H18O5S2/c1-20-12-21-13-2-4-14(5-3-13)22-15-6-8-17(9-7-15)24(18,19)11-16-10-23-16/h2-9,16H,10-12H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP14 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50335495

(4-(4-(thiiran-2-ylmethylsulfonyl)phenoxy)phenyl me...)Show SMILES CS(=O)(=O)Oc1ccc(Oc2ccc(cc2)S(=O)(=O)CC2CS2)cc1 Show InChI InChI=1S/C16H16O6S3/c1-24(17,18)22-14-4-2-12(3-5-14)21-13-6-8-16(9-7-13)25(19,20)11-15-10-23-15/h2-9,15H,10-11H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 145 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP14 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50388916

(CHEMBL2063276)Show InChI InChI=1S/C17H18O5S2/c1-20-12-21-13-2-4-14(5-3-13)22-15-6-8-17(9-7-15)24(18,19)11-16-10-23-16/h2-9,16H,10-12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP9 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50388914

(CHEMBL2063274 | US10357546, p-OH SB-3CT)Show InChI InChI=1S/C15H14O4S2/c16-11-1-3-12(4-2-11)19-13-5-7-15(8-6-13)21(17,18)10-14-9-20-14/h1-8,14,16H,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP9 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50388916

(CHEMBL2063276)Show InChI InChI=1S/C17H18O5S2/c1-20-12-21-13-2-4-14(5-3-13)22-15-6-8-17(9-7-15)24(18,19)11-16-10-23-16/h2-9,16H,10-12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP2 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50388910
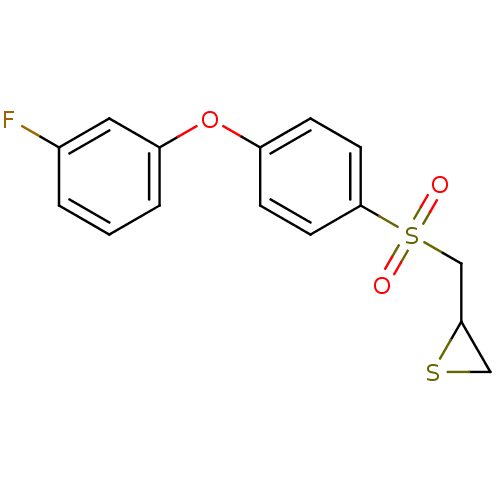
(CHEMBL2063271)Show InChI InChI=1S/C15H13FO3S2/c16-11-2-1-3-13(8-11)19-12-4-6-15(7-5-12)21(17,18)10-14-9-20-14/h1-8,14H,9-10H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP14 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50354624

(CHEMBL1834422 | US10357546, ND-322 | US9951035, ND...)Show InChI InChI=1S/C15H15NO3S2/c16-11-1-3-12(4-2-11)19-13-5-7-15(8-6-13)21(17,18)10-14-9-20-14/h1-8,14H,9-10,16H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP14 catalytic domain assessed as hydrolysis of MOCAcPLGLA2pr(Dnp)AR-NH2 by fluorescence spectrofluorometry |
J Med Chem 54: 6676-90 (2011)
Article DOI: 10.1021/jm200566e
BindingDB Entry DOI: 10.7270/Q2D79BSM |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50388912

(CHEMBL2063273)Show InChI InChI=1S/C16H16O4S2/c17-9-12-1-3-13(4-2-12)20-14-5-7-16(8-6-14)22(18,19)11-15-10-21-15/h1-8,15,17H,9-11H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 215 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP14 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50354625
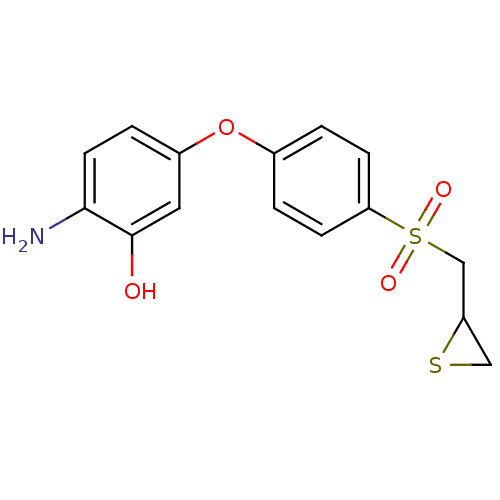
(CHEMBL1834423)Show InChI InChI=1S/C15H15NO4S2/c16-14-6-3-11(7-15(14)17)20-10-1-4-13(5-2-10)22(18,19)9-12-8-21-12/h1-7,12,17H,8-9,16H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP14 catalytic domain assessed as hydrolysis of MOCAcPLGLA2pr(Dnp)AR-NH2 by fluorescence spectrofluorometry |
J Med Chem 54: 6676-90 (2011)
Article DOI: 10.1021/jm200566e
BindingDB Entry DOI: 10.7270/Q2D79BSM |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50388915
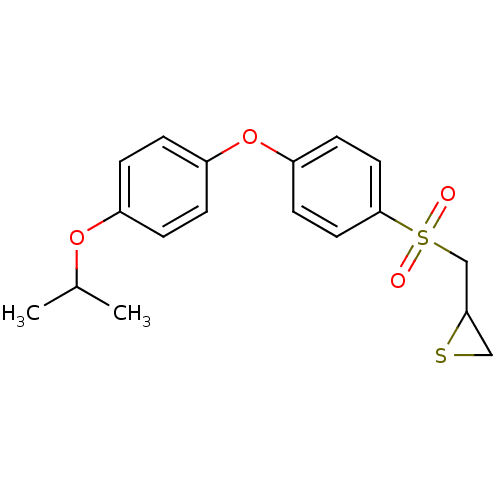
(CHEMBL2063275)Show InChI InChI=1S/C18H20O4S2/c1-13(2)21-14-3-5-15(6-4-14)22-16-7-9-18(10-8-16)24(19,20)12-17-11-23-17/h3-10,13,17H,11-12H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP14 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50388913
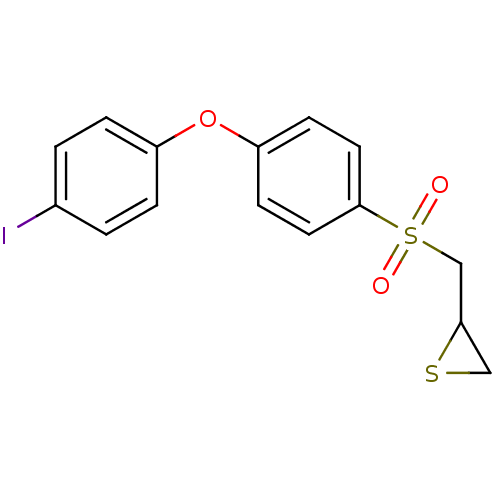
(CHEMBL2063272)Show InChI InChI=1S/C15H13IO3S2/c16-11-1-3-12(4-2-11)19-13-5-7-15(8-6-13)21(17,18)10-14-9-20-14/h1-8,14H,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP9 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50388915

(CHEMBL2063275)Show InChI InChI=1S/C18H20O4S2/c1-13(2)21-14-3-5-15(6-4-14)22-16-7-9-18(10-8-16)24(19,20)12-17-11-23-17/h3-10,13,17H,11-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP2 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50388912

(CHEMBL2063273)Show InChI InChI=1S/C16H16O4S2/c17-9-12-1-3-13(4-2-12)20-14-5-7-16(8-6-14)22(18,19)11-15-10-21-15/h1-8,15,17H,9-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP9 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50354626

(CHEMBL1834421)Show SMILES [#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-c1ccc(-[#8]-c2ccc(cc2)S(=O)(=O)[#6]-[#6]-2-[#6]-[#16]-2)cc1 |r| Show InChI InChI=1S/C27H39N9O5S2/c28-22(3-1-13-33-26(29)30)24(37)36-23(4-2-14-34-27(31)32)25(38)35-17-5-7-18(8-6-17)41-19-9-11-21(12-10-19)43(39,40)16-20-15-42-20/h5-12,20,22-23H,1-4,13-16,28H2,(H,35,38)(H,36,37)(H4,29,30,33)(H4,31,32,34)/t20?,22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP2 assessed as hydrolysis of MOCAcPLGLA2pr(Dnp)AR-NH2 by fluorescence spectrofluorometry |
J Med Chem 54: 6676-90 (2011)
Article DOI: 10.1021/jm200566e
BindingDB Entry DOI: 10.7270/Q2D79BSM |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50354623

(CHEMBL1834417)Show SMILES NCC(=O)Nc1ccc(Oc2ccc(cc2)S(=O)(=O)CC2CS2)cc1 Show InChI InChI=1S/C17H18N2O4S2/c18-9-17(20)19-12-1-3-13(4-2-12)23-14-5-7-16(8-6-14)25(21,22)11-15-10-24-15/h1-8,15H,9-11,18H2,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP2 assessed as hydrolysis of MOCAcPLGLA2pr(Dnp)AR-NH2 by fluorescence spectrofluorometry |
J Med Chem 54: 6676-90 (2011)
Article DOI: 10.1021/jm200566e
BindingDB Entry DOI: 10.7270/Q2D79BSM |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50388910

(CHEMBL2063271)Show InChI InChI=1S/C15H13FO3S2/c16-11-2-1-3-13(8-11)19-12-4-6-15(7-5-12)21(17,18)10-14-9-20-14/h1-8,14H,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP2 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50388915

(CHEMBL2063275)Show InChI InChI=1S/C18H20O4S2/c1-13(2)21-14-3-5-15(6-4-14)22-16-7-9-18(10-8-16)24(19,20)12-17-11-23-17/h3-10,13,17H,11-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP9 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50354627

(CHEMBL1834420)Show SMILES [#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-c1ccc(-[#8]-c2ccc(cc2)S(=O)(=O)[#6]-[#6]-2-[#6]-[#16]-2)cc1 |r| Show InChI InChI=1S/C21H27N5O4S2/c22-19(2-1-11-25-21(23)24)20(27)26-14-3-5-15(6-4-14)30-16-7-9-18(10-8-16)32(28,29)13-17-12-31-17/h3-10,17,19H,1-2,11-13,22H2,(H,26,27)(H4,23,24,25)/t17?,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP2 assessed as hydrolysis of MOCAcPLGLA2pr(Dnp)AR-NH2 by fluorescence spectrofluorometry |
J Med Chem 54: 6676-90 (2011)
Article DOI: 10.1021/jm200566e
BindingDB Entry DOI: 10.7270/Q2D79BSM |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50335495

(4-(4-(thiiran-2-ylmethylsulfonyl)phenoxy)phenyl me...)Show SMILES CS(=O)(=O)Oc1ccc(Oc2ccc(cc2)S(=O)(=O)CC2CS2)cc1 Show InChI InChI=1S/C16H16O6S3/c1-24(17,18)22-14-4-2-12(3-5-14)21-13-6-8-16(9-7-13)25(19,20)11-15-10-23-15/h2-9,15H,10-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 575 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP3 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50388911

(CHEMBL2063270)Show InChI InChI=1S/C15H13FO3S2/c16-11-1-3-12(4-2-11)19-13-5-7-15(8-6-13)21(17,18)10-14-9-20-14/h1-8,14H,9-10H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP14 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50264809

(2-((4-phenoxyphenylsulfonyl)methyl)thiirane | CHEM...)Show InChI InChI=1S/C15H14O3S2/c16-20(17,11-14-10-19-14)15-8-6-13(7-9-15)18-12-4-2-1-3-5-12/h1-9,14H,10-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP9 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50388913

(CHEMBL2063272)Show InChI InChI=1S/C15H13IO3S2/c16-11-1-3-12(4-2-11)19-13-5-7-15(8-6-13)21(17,18)10-14-9-20-14/h1-8,14H,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP2 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50264811

(CHEMBL485492 | N-(4-(4-(thiiran-2-ylmethylsulfonyl...)Show SMILES CC(=O)Nc1ccc(Oc2ccc(cc2)S(=O)(=O)CC2CS2)cc1 Show InChI InChI=1S/C17H17NO4S2/c1-12(19)18-13-2-4-14(5-3-13)22-15-6-8-17(9-7-15)24(20,21)11-16-10-23-16/h2-9,16H,10-11H2,1H3,(H,18,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP14 assessed as hydrolysis of MOCAcPLGLA2pr(Dnp)AR-NH2 by fluorescence spectrofluorometry |
J Med Chem 54: 6676-90 (2011)
Article DOI: 10.1021/jm200566e
BindingDB Entry DOI: 10.7270/Q2D79BSM |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50354624

(CHEMBL1834422 | US10357546, ND-322 | US9951035, ND...)Show InChI InChI=1S/C15H15NO3S2/c16-11-1-3-12(4-2-11)19-13-5-7-15(8-6-13)21(17,18)10-14-9-20-14/h1-8,14H,9-10,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP9 catalytic domain assessed as hydrolysis of MOCAcPLGLA2pr(Dnp)AR-NH2 by fluorescence spectrofluorometry |
J Med Chem 54: 6676-90 (2011)
Article DOI: 10.1021/jm200566e
BindingDB Entry DOI: 10.7270/Q2D79BSM |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50388917

(CHEMBL2063277)Show SMILES FC(F)(F)Oc1ccc(Oc2ccc(cc2)S(=O)(=O)CC2CS2)cc1 Show InChI InChI=1S/C16H13F3O4S2/c17-16(18,19)23-13-3-1-11(2-4-13)22-12-5-7-15(8-6-12)25(20,21)10-14-9-24-14/h1-8,14H,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP9 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50354622

(CHEMBL1834418)Show SMILES NCCCC[C@H](N)C(=O)Nc1ccc(Oc2ccc(cc2)S(=O)(=O)CC2CS2)cc1 |r| Show InChI InChI=1S/C21H27N3O4S2/c22-12-2-1-3-20(23)21(25)24-15-4-6-16(7-5-15)28-17-8-10-19(11-9-17)30(26,27)14-18-13-29-18/h4-11,18,20H,1-3,12-14,22-23H2,(H,24,25)/t18?,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP9 catalytic domain assessed as hydrolysis of MOCAcPLGLA2pr(Dnp)AR-NH2 by fluorescence spectrofluorometry |
J Med Chem 54: 6676-90 (2011)
Article DOI: 10.1021/jm200566e
BindingDB Entry DOI: 10.7270/Q2D79BSM |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50388914

(CHEMBL2063274 | US10357546, p-OH SB-3CT)Show InChI InChI=1S/C15H14O4S2/c16-11-1-3-12(4-2-11)19-13-5-7-15(8-6-13)21(17,18)10-14-9-20-14/h1-8,14,16H,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP3 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50388910

(CHEMBL2063271)Show InChI InChI=1S/C15H13FO3S2/c16-11-2-1-3-13(8-11)19-12-4-6-15(7-5-12)21(17,18)10-14-9-20-14/h1-8,14H,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP9 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50354623

(CHEMBL1834417)Show SMILES NCC(=O)Nc1ccc(Oc2ccc(cc2)S(=O)(=O)CC2CS2)cc1 Show InChI InChI=1S/C17H18N2O4S2/c18-9-17(20)19-12-1-3-13(4-2-12)23-14-5-7-16(8-6-14)25(21,22)11-15-10-24-15/h1-8,15H,9-11,18H2,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP9 catalytic domain assessed as hydrolysis of MOCAcPLGLA2pr(Dnp)AR-NH2 by fluorescence spectrofluorometry |
J Med Chem 54: 6676-90 (2011)
Article DOI: 10.1021/jm200566e
BindingDB Entry DOI: 10.7270/Q2D79BSM |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50388912

(CHEMBL2063273)Show InChI InChI=1S/C16H16O4S2/c17-9-12-1-3-13(4-2-12)20-14-5-7-16(8-6-14)22(18,19)11-15-10-21-15/h1-8,15,17H,9-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP3 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50354628

(CHEMBL1834419)Show SMILES N[C@@H](CCC(O)=O)C(=O)Nc1ccc(Oc2ccc(cc2)S(=O)(=O)CC2CS2)cc1 |r| Show InChI InChI=1S/C20H22N2O6S2/c21-18(9-10-19(23)24)20(25)22-13-1-3-14(4-2-13)28-15-5-7-17(8-6-15)30(26,27)12-16-11-29-16/h1-8,16,18H,9-12,21H2,(H,22,25)(H,23,24)/t16?,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP9 catalytic domain assessed as hydrolysis of MOCAcPLGLA2pr(Dnp)AR-NH2 by fluorescence spectrofluorometry |
J Med Chem 54: 6676-90 (2011)
Article DOI: 10.1021/jm200566e
BindingDB Entry DOI: 10.7270/Q2D79BSM |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50388917

(CHEMBL2063277)Show SMILES FC(F)(F)Oc1ccc(Oc2ccc(cc2)S(=O)(=O)CC2CS2)cc1 Show InChI InChI=1S/C16H13F3O4S2/c17-16(18,19)23-13-3-1-11(2-4-13)22-12-5-7-15(8-6-12)25(20,21)10-14-9-24-14/h1-8,14H,9-10H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP14 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50264811

(CHEMBL485492 | N-(4-(4-(thiiran-2-ylmethylsulfonyl...)Show SMILES CC(=O)Nc1ccc(Oc2ccc(cc2)S(=O)(=O)CC2CS2)cc1 Show InChI InChI=1S/C17H17NO4S2/c1-12(19)18-13-2-4-14(5-3-13)22-15-6-8-17(9-7-15)24(20,21)11-16-10-23-16/h2-9,16H,10-11H2,1H3,(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP1 assessed as hydrolysis of MOCAcPLGLA2pr(Dnp)AR-NH2 by fluorescence spectrofluorometry |
J Med Chem 54: 6676-90 (2011)
Article DOI: 10.1021/jm200566e
BindingDB Entry DOI: 10.7270/Q2D79BSM |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50388916

(CHEMBL2063276)Show InChI InChI=1S/C17H18O5S2/c1-20-12-21-13-2-4-14(5-3-13)22-15-6-8-17(9-7-15)24(18,19)11-16-10-23-16/h2-9,16H,10-12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP3 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50264811

(CHEMBL485492 | N-(4-(4-(thiiran-2-ylmethylsulfonyl...)Show SMILES CC(=O)Nc1ccc(Oc2ccc(cc2)S(=O)(=O)CC2CS2)cc1 Show InChI InChI=1S/C17H17NO4S2/c1-12(19)18-13-2-4-14(5-3-13)22-15-6-8-17(9-7-15)24(20,21)11-16-10-23-16/h2-9,16H,10-11H2,1H3,(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP3 assessed as hydrolysis of MOCAcPLGLA2pr(Dnp)AR-NH2 by fluorescence spectrofluorometry |
J Med Chem 54: 6676-90 (2011)
Article DOI: 10.1021/jm200566e
BindingDB Entry DOI: 10.7270/Q2D79BSM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data