 Found 654 hits with Last Name = 'inghardt' and Initial = 't'
Found 654 hits with Last Name = 'inghardt' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50214034
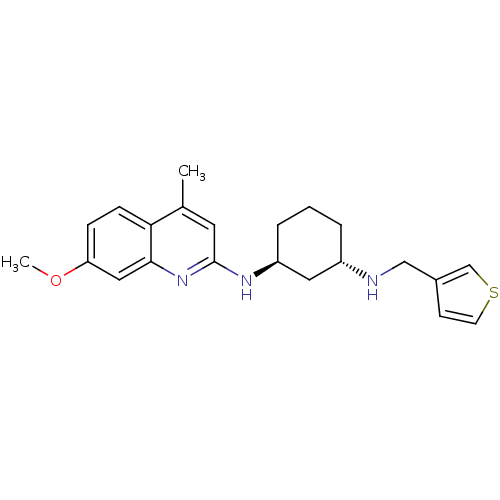
((1S,3S)-N1-(7-methoxy-4-methylquinolin-2-yl)-N3-(t...)Show SMILES COc1ccc2c(C)cc(N[C@H]3CCC[C@@H](C3)NCc3ccsc3)nc2c1 Show InChI InChI=1S/C22H27N3OS/c1-15-10-22(25-21-12-19(26-2)6-7-20(15)21)24-18-5-3-4-17(11-18)23-13-16-8-9-27-14-16/h6-10,12,14,17-18,23H,3-5,11,13H2,1-2H3,(H,24,25)/t17-,18-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Mölndal
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from human MCHR1 expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 4232-41 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.034
BindingDB Entry DOI: 10.7270/Q27W6BVC |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50214034

((1S,3S)-N1-(7-methoxy-4-methylquinolin-2-yl)-N3-(t...)Show SMILES COc1ccc2c(C)cc(N[C@H]3CCC[C@@H](C3)NCc3ccsc3)nc2c1 Show InChI InChI=1S/C22H27N3OS/c1-15-10-22(25-21-12-19(26-2)6-7-20(15)21)24-18-5-3-4-17(11-18)23-13-16-8-9-27-14-16/h6-10,12,14,17-18,23H,3-5,11,13H2,1-2H3,(H,24,25)/t17-,18-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Mölndal
Curated by ChEMBL
| Assay Description
Antagonist activity at human MCHR1 expressed in HEK293 cells assessed as [35S]GTPgammaS accumulation |
Bioorg Med Chem Lett 17: 4232-41 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.034
BindingDB Entry DOI: 10.7270/Q27W6BVC |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50296045
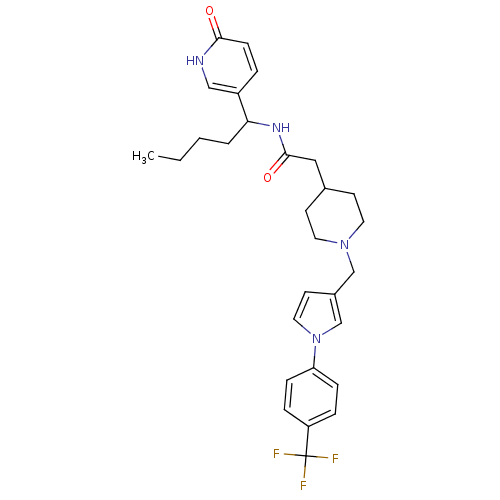
(CHEMBL562847 | N-(1-(6-oxo-1,6-dihydropyridin-3-yl...)Show SMILES CCCCC(NC(=O)CC1CCN(Cc2ccn(c2)-c2ccc(cc2)C(F)(F)F)CC1)c1ccc(=O)[nH]c1 Show InChI InChI=1S/C29H35F3N4O2/c1-2-3-4-26(23-5-10-27(37)33-18-23)34-28(38)17-21-11-14-35(15-12-21)19-22-13-16-36(20-22)25-8-6-24(7-9-25)29(30,31)32/h5-10,13,16,18,20-21,26H,2-4,11-12,14-15,17,19H2,1H3,(H,33,37)(H,34,38) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D M£lndal
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from human MCHR1 expressed in HEK293 cells |
Bioorg Med Chem Lett 19: 4268-73 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.067
BindingDB Entry DOI: 10.7270/Q2RX9C4H |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM312172

(Alternative Preparation | US10016430, Example 3 | ...)Show SMILES C[C@@H](N)c1cc(Cl)ccc1Cn1c2cc[nH]c2c(=O)[nH]c1=S |r,$;;;;;;;;;;;;;;;HN;;;;;;$| Show InChI InChI=1S/C15H15ClN4OS/c1-8(17)11-6-10(16)3-2-9(11)7-20-12-4-5-18-13(12)14(21)19-15(20)22/h2-6,8,18H,7,17H2,1H3,(H,19,21,22)/t8-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02141
BindingDB Entry DOI: 10.7270/Q2WD44KM |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM312172

(Alternative Preparation | US10016430, Example 3 | ...)Show SMILES C[C@@H](N)c1cc(Cl)ccc1Cn1c2cc[nH]c2c(=O)[nH]c1=S |r,$;;;;;;;;;;;;;;;HN;;;;;;$| Show InChI InChI=1S/C15H15ClN4OS/c1-8(17)11-6-10(16)3-2-9(11)7-20-12-4-5-18-13(12)14(21)19-15(20)22/h2-6,8,18H,7,17H2,1H3,(H,19,21,22)/t8-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02141
BindingDB Entry DOI: 10.7270/Q2WD44KM |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50296041

(CHEMBL553196 | N-((4-fluorophenyl)(6-methoxypyridi...)Show SMILES COc1ccc(cn1)C(NC(=O)CC1CCN(Cc2ccn(c2)-c2ccc(cc2)C(F)(F)F)CC1)c1ccc(F)cc1 Show InChI InChI=1S/C32H32F4N4O2/c1-42-30-11-4-25(19-37-30)31(24-2-7-27(33)8-3-24)38-29(41)18-22-12-15-39(16-13-22)20-23-14-17-40(21-23)28-9-5-26(6-10-28)32(34,35)36/h2-11,14,17,19,21-22,31H,12-13,15-16,18,20H2,1H3,(H,38,41) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D M£lndal
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from human MCHR1 expressed in HEK293 cells |
Bioorg Med Chem Lett 19: 4268-73 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.067
BindingDB Entry DOI: 10.7270/Q2RX9C4H |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50048722

(CHEMBL3319646)Show SMILES CC(Nc1cccc(F)c1)c1cc(cn2c1nc(cc2=O)N1CCOCC1)C(=O)NCCN(C)C Show InChI InChI=1S/C25H31FN6O3/c1-17(28-20-6-4-5-19(26)14-20)21-13-18(25(34)27-7-8-30(2)3)16-32-23(33)15-22(29-24(21)32)31-9-11-35-12-10-31/h4-6,13-17,28H,7-12H2,1-3H3,(H,27,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in human MAD-MB-468 cells assessed as inhibition of Ser473 Akt phosphorylation by cellular potency assay |
Bioorg Med Chem Lett 24: 3928-35 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.040
BindingDB Entry DOI: 10.7270/Q2BZ67PH |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50296044
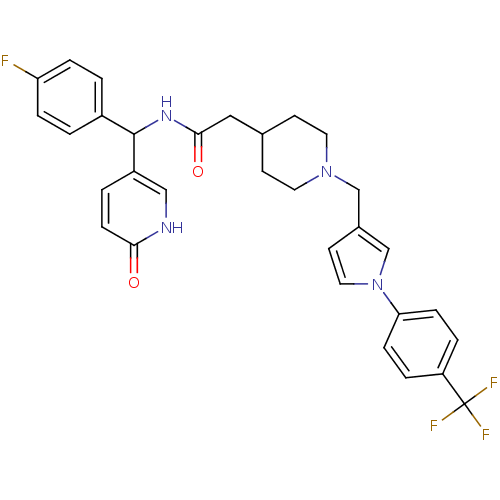
(CHEMBL554332 | N-((4-fluorophenyl)(6-oxo-1,6-dihyd...)Show SMILES Fc1ccc(cc1)C(NC(=O)CC1CCN(Cc2ccn(c2)-c2ccc(cc2)C(F)(F)F)CC1)c1ccc(=O)[nH]c1 Show InChI InChI=1S/C31H30F4N4O2/c32-26-6-1-23(2-7-26)30(24-3-10-28(40)36-18-24)37-29(41)17-21-11-14-38(15-12-21)19-22-13-16-39(20-22)27-8-4-25(5-9-27)31(33,34)35/h1-10,13,16,18,20-21,30H,11-12,14-15,17,19H2,(H,36,40)(H,37,41) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D M£lndal
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from human MCHR1 expressed in HEK293 cells |
Bioorg Med Chem Lett 19: 4268-73 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.067
BindingDB Entry DOI: 10.7270/Q2RX9C4H |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50048713
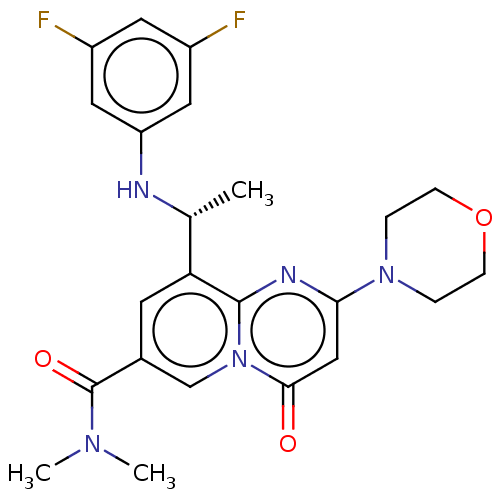
(CHEMBL3319490)Show SMILES C[C@@H](Nc1cc(F)cc(F)c1)c1cc(cn2c1nc(cc2=O)N1CCOCC1)C(=O)N(C)C |r| Show InChI InChI=1S/C23H25F2N5O3/c1-14(26-18-10-16(24)9-17(25)11-18)19-8-15(23(32)28(2)3)13-30-21(31)12-20(27-22(19)30)29-4-6-33-7-5-29/h8-14,26H,4-7H2,1-3H3/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in human MAD-MB-468 cells assessed as inhibition of Ser473 Akt phosphorylation by cellular potency assay |
Bioorg Med Chem Lett 24: 3928-35 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.040
BindingDB Entry DOI: 10.7270/Q2BZ67PH |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50214024

((1S,3S)-N1-(6-methoxy-4-methylquinolin-2-yl)-N3-(t...)Show SMILES COc1ccc2nc(N[C@H]3CCC[C@@H](C3)NCc3ccsc3)cc(C)c2c1 Show InChI InChI=1S/C22H27N3OS/c1-15-10-22(25-21-7-6-19(26-2)12-20(15)21)24-18-5-3-4-17(11-18)23-13-16-8-9-27-14-16/h6-10,12,14,17-18,23H,3-5,11,13H2,1-2H3,(H,24,25)/t17-,18-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Mölndal
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from human MCHR1 expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 4232-41 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.034
BindingDB Entry DOI: 10.7270/Q27W6BVC |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM50595667
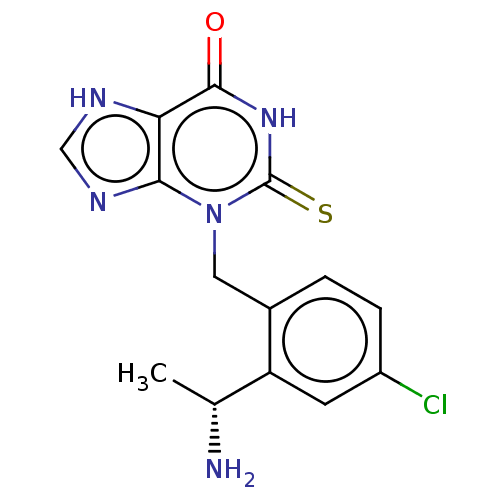
(CHEMBL5181350)Show SMILES C[C@@H](N)c1cc(Cl)ccc1Cn1c2nc[nH]c2c(=O)[nH]c1=S |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02141
BindingDB Entry DOI: 10.7270/Q2WD44KM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50048710
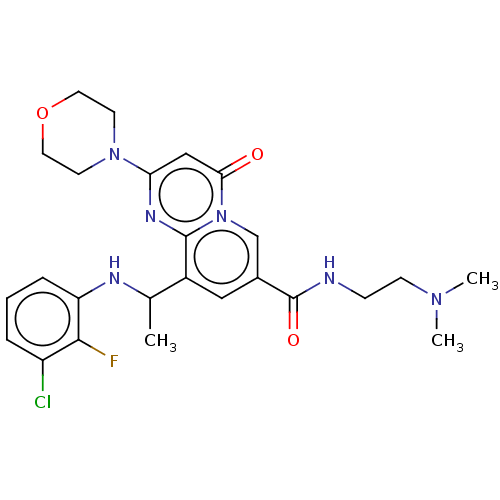
(CHEMBL3319485)Show SMILES CC(Nc1cccc(Cl)c1F)c1cc(cn2c1nc(cc2=O)N1CCOCC1)C(=O)NCCN(C)C Show InChI InChI=1S/C25H30ClFN6O3/c1-16(29-20-6-4-5-19(26)23(20)27)18-13-17(25(35)28-7-8-31(2)3)15-33-22(34)14-21(30-24(18)33)32-9-11-36-12-10-32/h4-6,13-16,29H,7-12H2,1-3H3,(H,28,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in human MAD-MB-468 cells assessed as inhibition of Ser473 Akt phosphorylation by cellular potency assay |
Bioorg Med Chem Lett 24: 3928-35 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.040
BindingDB Entry DOI: 10.7270/Q2BZ67PH |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50296019

(CHEMBL549635 | N-(4-fluorobenzyl)-N-(3-hydroxyprop...)Show SMILES OCCCN(Cc1ccc(F)cc1)C(=O)CC1CCN(Cc2ccn(c2)-c2ccc(cc2)C(F)(F)F)CC1 Show InChI InChI=1S/C29H33F4N3O2/c30-26-6-2-23(3-7-26)20-36(13-1-17-37)28(38)18-22-10-14-34(15-11-22)19-24-12-16-35(21-24)27-8-4-25(5-9-27)29(31,32)33/h2-9,12,16,21-22,37H,1,10-11,13-15,17-20H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D M£lndal
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from human MCHR1 expressed in HEK293 cells |
Bioorg Med Chem Lett 19: 4268-73 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.067
BindingDB Entry DOI: 10.7270/Q2RX9C4H |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM50595661
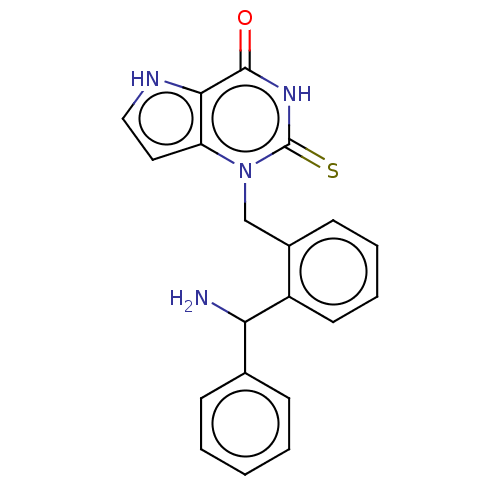
(CHEMBL5197968)Show SMILES NC(c1ccccc1)c1ccccc1Cn1c2cc[nH]c2c(=O)[nH]c1=S | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02141
BindingDB Entry DOI: 10.7270/Q2WD44KM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM50595661

(CHEMBL5197968)Show SMILES NC(c1ccccc1)c1ccccc1Cn1c2cc[nH]c2c(=O)[nH]c1=S | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02141
BindingDB Entry DOI: 10.7270/Q2WD44KM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50048713

(CHEMBL3319490)Show SMILES C[C@@H](Nc1cc(F)cc(F)c1)c1cc(cn2c1nc(cc2=O)N1CCOCC1)C(=O)N(C)C |r| Show InChI InChI=1S/C23H25F2N5O3/c1-14(26-18-10-16(24)9-17(25)11-18)19-8-15(23(32)28(2)3)13-30-21(31)12-20(27-22(19)30)29-4-6-33-7-5-29/h8-14,26H,4-7H2,1-3H3/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3Kbeta assessed as depletion of ATP substrate by Ultra Glo luciferase assay |
Bioorg Med Chem Lett 24: 3928-35 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.040
BindingDB Entry DOI: 10.7270/Q2BZ67PH |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50214036
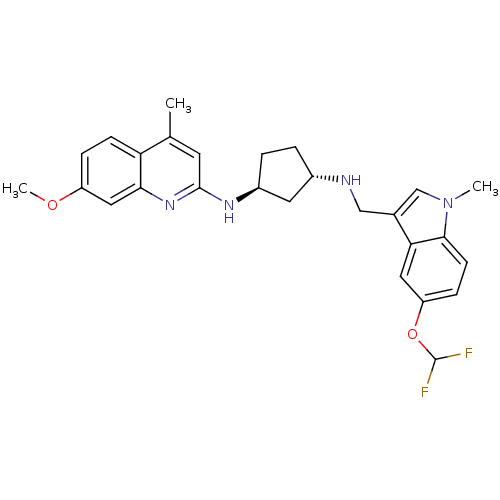
((1S,3S)-N1-((5-(difluoromethoxy)-1-methyl-1H-indol...)Show SMILES COc1ccc2c(C)cc(N[C@H]3CC[C@@H](C3)NCc3cn(C)c4ccc(OC(F)F)cc34)nc2c1 Show InChI InChI=1S/C27H30F2N4O2/c1-16-10-26(32-24-13-20(34-3)6-8-22(16)24)31-19-5-4-18(11-19)30-14-17-15-33(2)25-9-7-21(12-23(17)25)35-27(28)29/h6-10,12-13,15,18-19,27,30H,4-5,11,14H2,1-3H3,(H,31,32)/t18-,19-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Mölndal
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from human MCHR1 expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 4232-41 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.034
BindingDB Entry DOI: 10.7270/Q27W6BVC |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50296042

(CHEMBL549984 | N-(1-(6-methoxypyridin-3-yl)pentyl)...)Show SMILES CCCCC(NC(=O)CC1CCN(Cc2ccn(c2)-c2ccc(cc2)C(F)(F)F)CC1)c1ccc(OC)nc1 Show InChI InChI=1S/C30H37F3N4O2/c1-3-4-5-27(24-6-11-29(39-2)34-19-24)35-28(38)18-22-12-15-36(16-13-22)20-23-14-17-37(21-23)26-9-7-25(8-10-26)30(31,32)33/h6-11,14,17,19,21-22,27H,3-5,12-13,15-16,18,20H2,1-2H3,(H,35,38) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D M£lndal
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from human MCHR1 expressed in HEK293 cells |
Bioorg Med Chem Lett 19: 4268-73 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.067
BindingDB Entry DOI: 10.7270/Q2RX9C4H |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50296043

(CHEMBL540930 | N-((5-chloro-6-oxo-1,6-dihydropyrid...)Show SMILES Fc1ccc(cc1)C(NC(=O)CC1CCN(Cc2ccn(c2)-c2ccc(cc2)C(F)(F)F)CC1)c1c[nH]c(=O)c(Cl)c1 Show InChI InChI=1S/C31H29ClF4N4O2/c32-27-16-23(17-37-30(27)42)29(22-1-5-25(33)6-2-22)38-28(41)15-20-9-12-39(13-10-20)18-21-11-14-40(19-21)26-7-3-24(4-8-26)31(34,35)36/h1-8,11,14,16-17,19-20,29H,9-10,12-13,15,18H2,(H,37,42)(H,38,41) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D M£lndal
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from human MCHR1 expressed in HEK293 cells |
Bioorg Med Chem Lett 19: 4268-73 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.067
BindingDB Entry DOI: 10.7270/Q2RX9C4H |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM312171
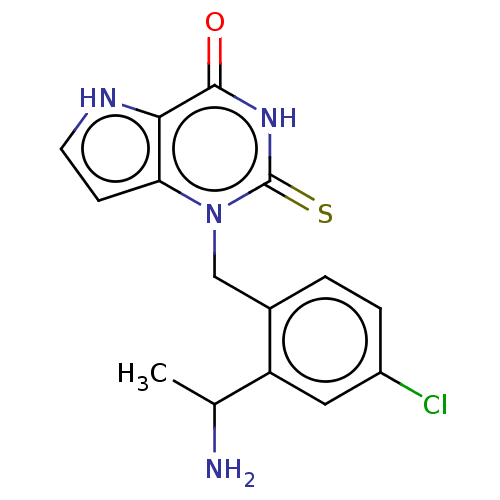
(1-[2-(1-Aminoethyl)-4-chlorobenzyl]-2-thioxo-1,2,3...)Show SMILES CC(N)c1cc(Cl)ccc1Cn1c2cc[nH]c2c(=O)[nH]c1=S |$;;;;;;;;;;;;;;;HN;;;;;;$| Show InChI InChI=1S/C15H15ClN4OS/c1-8(17)11-6-10(16)3-2-9(11)7-20-12-4-5-18-13(12)14(21)19-15(20)22/h2-6,8,18H,7,17H2,1H3,(H,19,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
MPO: The pharmacological activity of compounds disclosed herein was tested in the following screen (Test A) in which the compounds were tested in the... |
US Patent US10016430 (2018)
BindingDB Entry DOI: 10.7270/Q2WW7M2N |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM312172

(Alternative Preparation | US10016430, Example 3 | ...)Show SMILES C[C@@H](N)c1cc(Cl)ccc1Cn1c2cc[nH]c2c(=O)[nH]c1=S |r,$;;;;;;;;;;;;;;;HN;;;;;;$| Show InChI InChI=1S/C15H15ClN4OS/c1-8(17)11-6-10(16)3-2-9(11)7-20-12-4-5-18-13(12)14(21)19-15(20)22/h2-6,8,18H,7,17H2,1H3,(H,19,21,22)/t8-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
MPO: The pharmacological activity of compounds disclosed herein was tested in the following screen (Test A) in which the compounds were tested in the... |
US Patent US10016430 (2018)
BindingDB Entry DOI: 10.7270/Q2WW7M2N |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM312171

(1-[2-(1-Aminoethyl)-4-chlorobenzyl]-2-thioxo-1,2,3...)Show SMILES CC(N)c1cc(Cl)ccc1Cn1c2cc[nH]c2c(=O)[nH]c1=S |$;;;;;;;;;;;;;;;HN;;;;;;$| Show InChI InChI=1S/C15H15ClN4OS/c1-8(17)11-6-10(16)3-2-9(11)7-20-12-4-5-18-13(12)14(21)19-15(20)22/h2-6,8,18H,7,17H2,1H3,(H,19,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB
US Patent
| Assay Description
Methods for the determination of MPO inhibitory activity are disclosed in WO 02/090575. The pharmacological activity of compounds disclosed herein wa... |
US Patent US11000525 (2021)
BindingDB Entry DOI: 10.7270/Q2QJ7MD4 |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM312172

(Alternative Preparation | US10016430, Example 3 | ...)Show SMILES C[C@@H](N)c1cc(Cl)ccc1Cn1c2cc[nH]c2c(=O)[nH]c1=S |r,$;;;;;;;;;;;;;;;HN;;;;;;$| Show InChI InChI=1S/C15H15ClN4OS/c1-8(17)11-6-10(16)3-2-9(11)7-20-12-4-5-18-13(12)14(21)19-15(20)22/h2-6,8,18H,7,17H2,1H3,(H,19,21,22)/t8-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB
US Patent
| Assay Description
Methods for the determination of MPO inhibitory activity are disclosed in WO 02/090575. The pharmacological activity of compounds disclosed herein wa... |
US Patent US11000525 (2021)
BindingDB Entry DOI: 10.7270/Q2QJ7MD4 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50395803
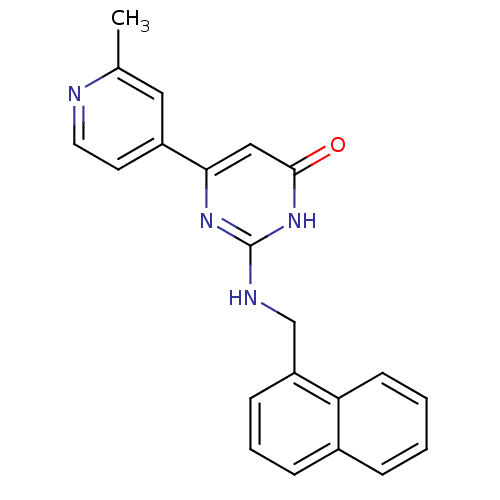
(CHEMBL2165175)Show SMILES Cc1cc(ccn1)-c1cc(=O)[nH]c(NCc2cccc3ccccc23)n1 Show InChI InChI=1S/C21H18N4O/c1-14-11-16(9-10-22-14)19-12-20(26)25-21(24-19)23-13-17-7-4-6-15-5-2-3-8-18(15)17/h2-12H,13H2,1H3,(H2,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His6-tagged PI3K p110beta expressed in baculovirus infected cells using DiC8-PI(4,5)P2 as substrate after 20 mins by ... |
Bioorg Med Chem Lett 22: 6671-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.102
BindingDB Entry DOI: 10.7270/Q2445NKM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50048720

(CHEMBL3319644)Show SMILES CC(Nc1ccccc1Cl)c1cc(cn2c1nc(cc2=O)N1CCOCC1)C(=O)NCCN(C)C Show InChI InChI=1S/C25H31ClN6O3/c1-17(28-21-7-5-4-6-20(21)26)19-14-18(25(34)27-8-9-30(2)3)16-32-23(33)15-22(29-24(19)32)31-10-12-35-13-11-31/h4-7,14-17,28H,8-13H2,1-3H3,(H,27,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in human MAD-MB-468 cells assessed as inhibition of Ser473 Akt phosphorylation by cellular potency assay |
Bioorg Med Chem Lett 24: 3928-35 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.040
BindingDB Entry DOI: 10.7270/Q2BZ67PH |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM312172

(Alternative Preparation | US10016430, Example 3 | ...)Show SMILES C[C@@H](N)c1cc(Cl)ccc1Cn1c2cc[nH]c2c(=O)[nH]c1=S |r,$;;;;;;;;;;;;;;;HN;;;;;;$| Show InChI InChI=1S/C15H15ClN4OS/c1-8(17)11-6-10(16)3-2-9(11)7-20-12-4-5-18-13(12)14(21)19-15(20)22/h2-6,8,18H,7,17H2,1H3,(H,19,21,22)/t8-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The pharmacological activity of compounds disclosed herein was tested in the following screen (Test A) in which the compounds were tested in the pres... |
US Patent US9616063 (2017)
BindingDB Entry DOI: 10.7270/Q2Q24296 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50214037
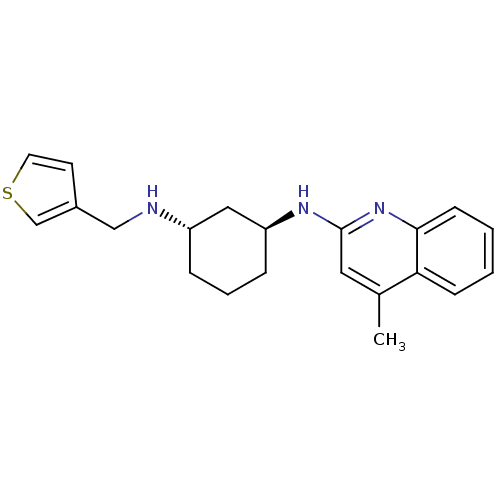
((1S,3S)-N1-(4-methylquinolin-2-yl)-N3-(thiophen-3-...)Show SMILES Cc1cc(N[C@H]2CCC[C@@H](C2)NCc2ccsc2)nc2ccccc12 Show InChI InChI=1S/C21H25N3S/c1-15-11-21(24-20-8-3-2-7-19(15)20)23-18-6-4-5-17(12-18)22-13-16-9-10-25-14-16/h2-3,7-11,14,17-18,22H,4-6,12-13H2,1H3,(H,23,24)/t17-,18-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Mölndal
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from human MCHR1 expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 4232-41 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.034
BindingDB Entry DOI: 10.7270/Q27W6BVC |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50048710

(CHEMBL3319485)Show SMILES CC(Nc1cccc(Cl)c1F)c1cc(cn2c1nc(cc2=O)N1CCOCC1)C(=O)NCCN(C)C Show InChI InChI=1S/C25H30ClFN6O3/c1-16(29-20-6-4-5-19(26)23(20)27)18-13-17(25(35)28-7-8-31(2)3)15-33-22(34)14-21(30-24(18)33)32-9-11-36-12-10-32/h4-6,13-16,29H,7-12H2,1-3H3,(H,28,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3Kbeta assessed as depletion of ATP substrate by Ultra Glo luciferase assay |
Bioorg Med Chem Lett 24: 3928-35 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.040
BindingDB Entry DOI: 10.7270/Q2BZ67PH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
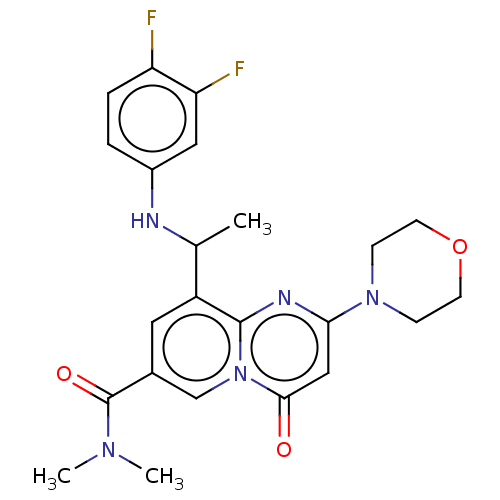
(Homo sapiens (Human)) | BDBM50048712
(CHEMBL3319489)Show SMILES CC(Nc1ccc(F)c(F)c1)c1cc(cn2c1nc(cc2=O)N1CCOCC1)C(=O)N(C)C Show InChI InChI=1S/C23H25F2N5O3/c1-14(26-16-4-5-18(24)19(25)11-16)17-10-15(23(32)28(2)3)13-30-21(31)12-20(27-22(17)30)29-6-8-33-9-7-29/h4-5,10-14,26H,6-9H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3Kbeta assessed as depletion of ATP substrate by Ultra Glo luciferase assay |
Bioorg Med Chem Lett 24: 3928-35 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.040
BindingDB Entry DOI: 10.7270/Q2BZ67PH |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM312171

(1-[2-(1-Aminoethyl)-4-chlorobenzyl]-2-thioxo-1,2,3...)Show SMILES CC(N)c1cc(Cl)ccc1Cn1c2cc[nH]c2c(=O)[nH]c1=S |$;;;;;;;;;;;;;;;HN;;;;;;$| Show InChI InChI=1S/C15H15ClN4OS/c1-8(17)11-6-10(16)3-2-9(11)7-20-12-4-5-18-13(12)14(21)19-15(20)22/h2-6,8,18H,7,17H2,1H3,(H,19,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The pharmacological activity of compounds disclosed herein was tested in the following screen (Test A) in which the compounds were tested in the pres... |
US Patent US9616063 (2017)
BindingDB Entry DOI: 10.7270/Q2Q24296 |
More data for this
Ligand-Target Pair | |
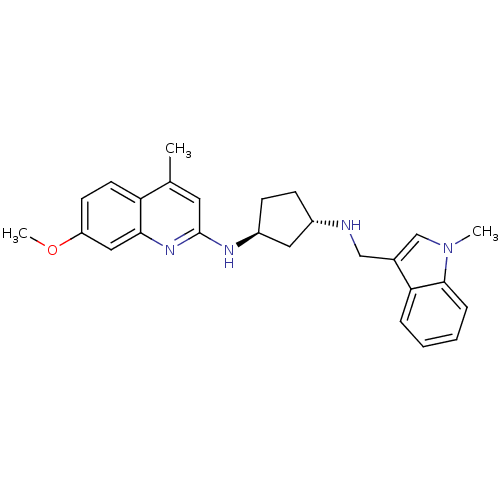
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50048709
(CHEMBL3318715)Show SMILES CC(Nc1ccccc1)c1cc(cn2c1nc(cc2=O)N1CCOCC1)C(=O)NCCN(C)C Show InChI InChI=1S/C25H32N6O3/c1-18(27-20-7-5-4-6-8-20)21-15-19(25(33)26-9-10-29(2)3)17-31-23(32)16-22(28-24(21)31)30-11-13-34-14-12-30/h4-8,15-18,27H,9-14H2,1-3H3,(H,26,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in human MAD-MB-468 cells assessed as inhibition of Ser473 Akt phosphorylation by cellular potency assay |
Bioorg Med Chem Lett 24: 3928-35 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.040
BindingDB Entry DOI: 10.7270/Q2BZ67PH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50048709

(CHEMBL3318715)Show SMILES CC(Nc1ccccc1)c1cc(cn2c1nc(cc2=O)N1CCOCC1)C(=O)NCCN(C)C Show InChI InChI=1S/C25H32N6O3/c1-18(27-20-7-5-4-6-8-20)21-15-19(25(33)26-9-10-29(2)3)17-31-23(32)16-22(28-24(21)31)30-11-13-34-14-12-30/h4-8,15-18,27H,9-14H2,1-3H3,(H,26,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3Kbeta assessed as depletion of ATP substrate by Ultra Glo luciferase assay |
Bioorg Med Chem Lett 24: 3928-35 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.040
BindingDB Entry DOI: 10.7270/Q2BZ67PH |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50296047
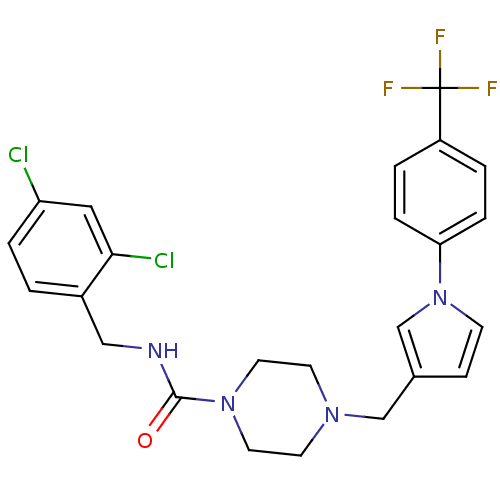
(CHEMBL554511 | N-(2,4-dichlorobenzyl)-4-((1-(4-(tr...)Show SMILES FC(F)(F)c1ccc(cc1)-n1ccc(CN2CCN(CC2)C(=O)NCc2ccc(Cl)cc2Cl)c1 Show InChI InChI=1S/C24H23Cl2F3N4O/c25-20-4-1-18(22(26)13-20)14-30-23(34)32-11-9-31(10-12-32)15-17-7-8-33(16-17)21-5-2-19(3-6-21)24(27,28)29/h1-8,13,16H,9-12,14-15H2,(H,30,34) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D M£lndal
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from human MCHR1 expressed in HEK293 cells |
Bioorg Med Chem Lett 19: 4268-73 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.067
BindingDB Entry DOI: 10.7270/Q2RX9C4H |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50048838

(CHEMBL3319494)Show SMILES CC(Nc1cc(F)cc(F)c1)c1cc(cn2c1nc(cc2=O)N1CCOCC1)C(=O)N(C)CCO Show InChI InChI=1S/C24H27F2N5O4/c1-15(27-19-11-17(25)10-18(26)12-19)20-9-16(24(34)29(2)3-6-32)14-31-22(33)13-21(28-23(20)31)30-4-7-35-8-5-30/h9-15,27,32H,3-8H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in human MAD-MB-468 cells assessed as inhibition of Ser473 Akt phosphorylation by cellular potency assay |
Bioorg Med Chem Lett 24: 3928-35 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.040
BindingDB Entry DOI: 10.7270/Q2BZ67PH |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50214052

((1S,3S)-N1-(4-methoxyquinolin-2-yl)-N3-(thiophen-3...)Show SMILES COc1cc(N[C@H]2CCC[C@@H](C2)NCc2ccsc2)nc2ccccc12 Show InChI InChI=1S/C21H25N3OS/c1-25-20-12-21(24-19-8-3-2-7-18(19)20)23-17-6-4-5-16(11-17)22-13-15-9-10-26-14-15/h2-3,7-10,12,14,16-17,22H,4-6,11,13H2,1H3,(H,23,24)/t16-,17-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Mölndal
Curated by ChEMBL
| Assay Description
Antagonist activity at human MCHR1 expressed in HEK293 cells assessed as [35S]GTPgammaS accumulation |
Bioorg Med Chem Lett 17: 4232-41 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.034
BindingDB Entry DOI: 10.7270/Q27W6BVC |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM312157
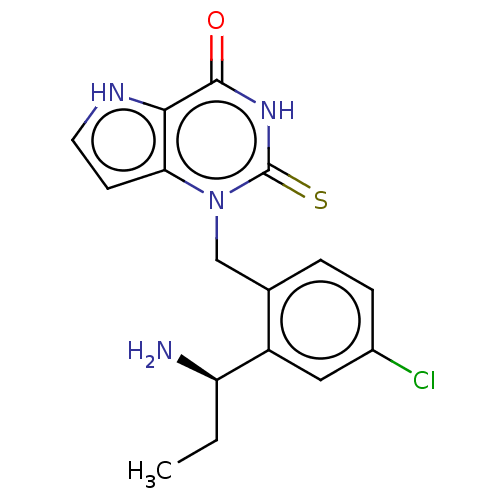
(1-{2-[(1R)-1-Aminopropyl]-4-chlorobenzyl}-2-thioxo...)Show SMILES CC[C@@H](N)c1cc(Cl)ccc1Cn1c2cc[nH]c2c(=O)[nH]c1=S |r,$;;;;;;;;;;;;;;;;HN;;;;;;$| Show InChI InChI=1S/C16H17ClN4OS/c1-2-12(18)11-7-10(17)4-3-9(11)8-21-13-5-6-19-14(13)15(22)20-16(21)23/h3-7,12,19H,2,8,18H2,1H3,(H,20,22,23)/t12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB
US Patent
| Assay Description
Methods for the determination of MPO inhibitory activity are disclosed in WO 02/090575. The pharmacological activity of compounds disclosed herein wa... |
US Patent US11000525 (2021)
BindingDB Entry DOI: 10.7270/Q2QJ7MD4 |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM312157

(1-{2-[(1R)-1-Aminopropyl]-4-chlorobenzyl}-2-thioxo...)Show SMILES CC[C@@H](N)c1cc(Cl)ccc1Cn1c2cc[nH]c2c(=O)[nH]c1=S |r,$;;;;;;;;;;;;;;;;HN;;;;;;$| Show InChI InChI=1S/C16H17ClN4OS/c1-2-12(18)11-7-10(17)4-3-9(11)8-21-13-5-6-19-14(13)15(22)20-16(21)23/h3-7,12,19H,2,8,18H2,1H3,(H,20,22,23)/t12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
MPO: The pharmacological activity of compounds disclosed herein was tested in the following screen (Test A) in which the compounds were tested in the... |
US Patent US10016430 (2018)
BindingDB Entry DOI: 10.7270/Q2WW7M2N |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM312157

(1-{2-[(1R)-1-Aminopropyl]-4-chlorobenzyl}-2-thioxo...)Show SMILES CC[C@@H](N)c1cc(Cl)ccc1Cn1c2cc[nH]c2c(=O)[nH]c1=S |r,$;;;;;;;;;;;;;;;;HN;;;;;;$| Show InChI InChI=1S/C16H17ClN4OS/c1-2-12(18)11-7-10(17)4-3-9(11)8-21-13-5-6-19-14(13)15(22)20-16(21)23/h3-7,12,19H,2,8,18H2,1H3,(H,20,22,23)/t12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The pharmacological activity of compounds disclosed herein was tested in the following screen (Test A) in which the compounds were tested in the pres... |
US Patent US9616063 (2017)
BindingDB Entry DOI: 10.7270/Q2Q24296 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50214052

((1S,3S)-N1-(4-methoxyquinolin-2-yl)-N3-(thiophen-3...)Show SMILES COc1cc(N[C@H]2CCC[C@@H](C2)NCc2ccsc2)nc2ccccc12 Show InChI InChI=1S/C21H25N3OS/c1-25-20-12-21(24-19-8-3-2-7-18(19)20)23-17-6-4-5-16(11-17)22-13-15-9-10-26-14-15/h2-3,7-10,12,14,16-17,22H,4-6,11,13H2,1H3,(H,23,24)/t16-,17-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Mölndal
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from human MCHR1 expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 4232-41 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.034
BindingDB Entry DOI: 10.7270/Q27W6BVC |
More data for this
Ligand-Target Pair | |
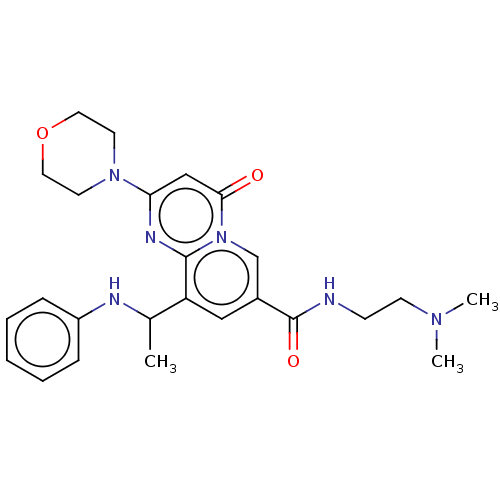
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50214035
((1S,3S)-N1-(7-methoxy-4-methylquinolin-2-yl)-N3-((...)Show SMILES COc1ccc2c(C)cc(N[C@H]3CC[C@@H](C3)NCc3cn(C)c4ccccc34)nc2c1 Show InChI InChI=1S/C26H30N4O/c1-17-12-26(29-24-14-21(31-3)10-11-22(17)24)28-20-9-8-19(13-20)27-15-18-16-30(2)25-7-5-4-6-23(18)25/h4-7,10-12,14,16,19-20,27H,8-9,13,15H2,1-3H3,(H,28,29)/t19-,20-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Mölndal
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from human MCHR1 expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 4232-41 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.034
BindingDB Entry DOI: 10.7270/Q27W6BVC |
More data for this
Ligand-Target Pair | |
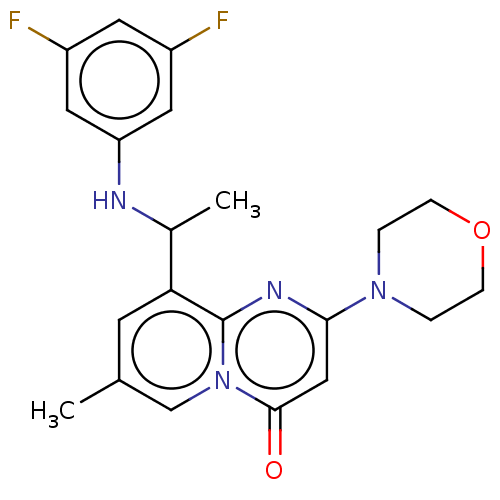
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50048717
(CHEMBL3319642)Show SMILES CC(Nc1cc(F)cc(F)c1)c1cc(C)cn2c1nc(cc2=O)N1CCOCC1 Show InChI InChI=1S/C21H22F2N4O2/c1-13-7-18(14(2)24-17-9-15(22)8-16(23)10-17)21-25-19(11-20(28)27(21)12-13)26-3-5-29-6-4-26/h7-12,14,24H,3-6H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in human MAD-MB-468 cells assessed as inhibition of Ser473 Akt phosphorylation by cellular potency assay |
Bioorg Med Chem Lett 24: 3928-35 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.040
BindingDB Entry DOI: 10.7270/Q2BZ67PH |
More data for this
Ligand-Target Pair | |
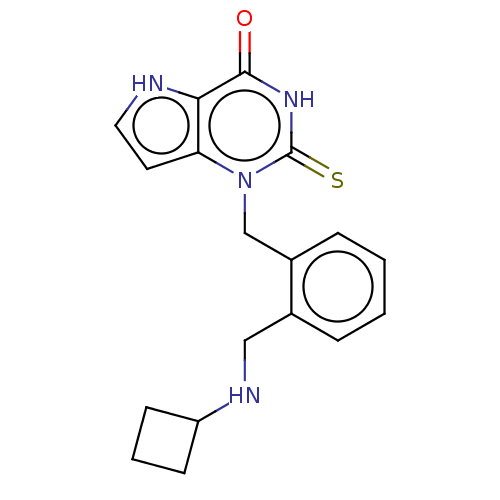
Myeloperoxidase
(Homo sapiens (Human)) | BDBM312256
(1-{2-[(Cyclobutylamino)methyl]benzyl}-2-thioxo-1,2...)Show SMILES O=c1[nH]c(=S)n(Cc2ccccc2CNC2CCC2)c2cc[nH]c12 |$;;;;;;;;;;;;;;HN;;;;;;;;HN;$| Show InChI InChI=1S/C18H20N4OS/c23-17-16-15(8-9-19-16)22(18(24)21-17)11-13-5-2-1-4-12(13)10-20-14-6-3-7-14/h1-2,4-5,8-9,14,19-20H,3,6-7,10-11H2,(H,21,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
MPO: The pharmacological activity of compounds disclosed herein was tested in the following screen (Test A) in which the compounds were tested in the... |
US Patent US10016430 (2018)
BindingDB Entry DOI: 10.7270/Q2WW7M2N |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM312256

(1-{2-[(Cyclobutylamino)methyl]benzyl}-2-thioxo-1,2...)Show SMILES O=c1[nH]c(=S)n(Cc2ccccc2CNC2CCC2)c2cc[nH]c12 |$;;;;;;;;;;;;;;HN;;;;;;;;HN;$| Show InChI InChI=1S/C18H20N4OS/c23-17-16-15(8-9-19-16)22(18(24)21-17)11-13-5-2-1-4-12(13)10-20-14-6-3-7-14/h1-2,4-5,8-9,14,19-20H,3,6-7,10-11H2,(H,21,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB
US Patent
| Assay Description
Methods for the determination of MPO inhibitory activity are disclosed in WO 02/090575. The pharmacological activity of compounds disclosed herein wa... |
US Patent US11000525 (2021)
BindingDB Entry DOI: 10.7270/Q2QJ7MD4 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50297624

(1-phenyl-N-(1-((1-(4-(trifluoromethyl)phenyl)-1H-p...)Show SMILES FC(F)(F)c1ccc(cc1)-n1ccc(CN2CCC(CC2)NC(=O)N2CCn3cccc3C2c2ccccc2)c1 Show InChI InChI=1S/C31H32F3N5O/c32-31(33,34)25-8-10-27(11-9-25)38-18-12-23(22-38)21-36-16-13-26(14-17-36)35-30(40)39-20-19-37-15-4-7-28(37)29(39)24-5-2-1-3-6-24/h1-12,15,18,22,26,29H,13-14,16-17,19-21H2,(H,35,40) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D M£lndal
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from human MCH1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 19: 4274-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.066
BindingDB Entry DOI: 10.7270/Q24Q7V1J |
More data for this
Ligand-Target Pair | |
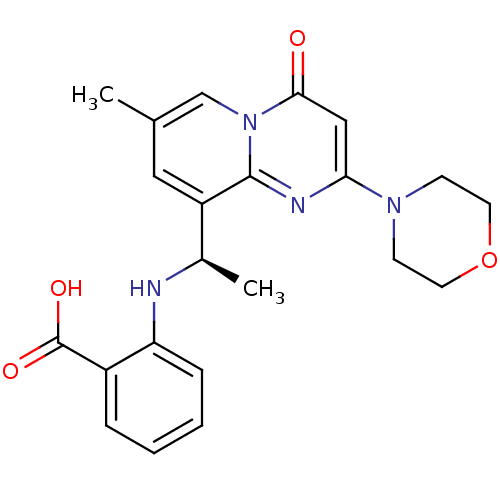
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50395821
(CHEMBL2165191)Show SMILES C[C@@H](Nc1ccccc1C(O)=O)c1cc(C)cn2c1nc(cc2=O)N1CCOCC1 |r| Show InChI InChI=1S/C22H24N4O4/c1-14-11-17(15(2)23-18-6-4-3-5-16(18)22(28)29)21-24-19(12-20(27)26(21)13-14)25-7-9-30-10-8-25/h3-6,11-13,15,23H,7-10H2,1-2H3,(H,28,29)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3Kbeta assessed as PIP3 production by AlphaScreen assay |
Bioorg Med Chem Lett 24: 3936-43 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.007
BindingDB Entry DOI: 10.7270/Q2BP04F3 |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM312256

(1-{2-[(Cyclobutylamino)methyl]benzyl}-2-thioxo-1,2...)Show SMILES O=c1[nH]c(=S)n(Cc2ccccc2CNC2CCC2)c2cc[nH]c12 |$;;;;;;;;;;;;;;HN;;;;;;;;HN;$| Show InChI InChI=1S/C18H20N4OS/c23-17-16-15(8-9-19-16)22(18(24)21-17)11-13-5-2-1-4-12(13)10-20-14-6-3-7-14/h1-2,4-5,8-9,14,19-20H,3,6-7,10-11H2,(H,21,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The pharmacological activity of compounds disclosed herein was tested in the following screen (Test A) in which the compounds were tested in the pres... |
US Patent US9616063 (2017)
BindingDB Entry DOI: 10.7270/Q2Q24296 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50214050

((1S,3S)-N1-(benzo[c][1,2,5]thiadiazol-4-ylmethyl)-...)Show SMILES COc1ccc2c(C)cc(N[C@H]3CCC[C@@H](C3)NCc3cccc4nsnc34)nc2c1 Show InChI InChI=1S/C24H27N5OS/c1-15-11-23(27-22-13-19(30-2)9-10-20(15)22)26-18-7-4-6-17(12-18)25-14-16-5-3-8-21-24(16)29-31-28-21/h3,5,8-11,13,17-18,25H,4,6-7,12,14H2,1-2H3,(H,26,27)/t17-,18-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Mölndal
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from human MCHR1 expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 4232-41 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.034
BindingDB Entry DOI: 10.7270/Q27W6BVC |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50048719

(CHEMBL3319643)Show SMILES CC(Nc1cccc(Cl)c1)c1cc(cn2c1nc(cc2=O)N1CCOCC1)C(=O)NCCN(C)C Show InChI InChI=1S/C25H31ClN6O3/c1-17(28-20-6-4-5-19(26)14-20)21-13-18(25(34)27-7-8-30(2)3)16-32-23(33)15-22(29-24(21)32)31-9-11-35-12-10-31/h4-6,13-17,28H,7-12H2,1-3H3,(H,27,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in human MAD-MB-468 cells assessed as inhibition of Ser473 Akt phosphorylation by cellular potency assay |
Bioorg Med Chem Lett 24: 3928-35 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.040
BindingDB Entry DOI: 10.7270/Q2BZ67PH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50395821

(CHEMBL2165191)Show SMILES C[C@@H](Nc1ccccc1C(O)=O)c1cc(C)cn2c1nc(cc2=O)N1CCOCC1 |r| Show InChI InChI=1S/C22H24N4O4/c1-14-11-17(15(2)23-18-6-4-3-5-16(18)22(28)29)21-24-19(12-20(27)26(21)13-14)25-7-9-30-10-8-25/h3-6,11-13,15,23H,7-10H2,1-2H3,(H,28,29)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His6-tagged PI3K p110beta expressed in baculovirus infected cells using DiC8-PI(4,5)P2 as substrate after 20 mins by ... |
Bioorg Med Chem Lett 22: 6671-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.102
BindingDB Entry DOI: 10.7270/Q2445NKM |
More data for this
Ligand-Target Pair | |
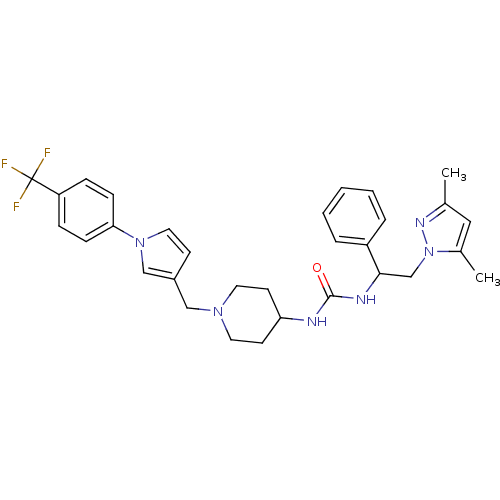
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50297625
(1-(2-(3,5-dimethyl-1H-pyrazol-1-yl)-1-phenylethyl)...)Show SMILES Cc1cc(C)n(CC(NC(=O)NC2CCN(Cc3ccn(c3)-c3ccc(cc3)C(F)(F)F)CC2)c2ccccc2)n1 Show InChI InChI=1S/C31H35F3N6O/c1-22-18-23(2)40(37-22)21-29(25-6-4-3-5-7-25)36-30(41)35-27-13-15-38(16-14-27)19-24-12-17-39(20-24)28-10-8-26(9-11-28)31(32,33)34/h3-12,17-18,20,27,29H,13-16,19,21H2,1-2H3,(H2,35,36,41) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D M£lndal
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from human MCH1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 19: 4274-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.066
BindingDB Entry DOI: 10.7270/Q24Q7V1J |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data