 Found 430 hits with Last Name = 'isabel' and Initial = 'e'
Found 430 hits with Last Name = 'isabel' and Initial = 'e' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cruzipain
(Trypanosoma cruzi) | BDBM50331776
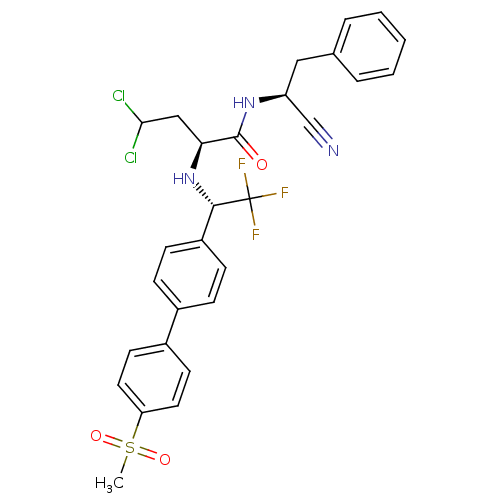
((S)-4,4-dichloro-N-((S)-1-cyano-2-phenylethyl)-2-(...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1ccc(cc1)[C@H](N[C@@H](CC(Cl)Cl)C(=O)N[C@@H](Cc1ccccc1)C#N)C(F)(F)F |r| Show InChI InChI=1S/C28H26Cl2F3N3O3S/c1-40(38,39)23-13-11-20(12-14-23)19-7-9-21(10-8-19)26(28(31,32)33)36-24(16-25(29)30)27(37)35-22(17-34)15-18-5-3-2-4-6-18/h2-14,22,24-26,36H,15-16H2,1H3,(H,35,37)/t22-,24-,26-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of trypanosoma cruzi Cruzipain |
Bioorg Med Chem Lett 20: 7444-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.015
BindingDB Entry DOI: 10.7270/Q29S1R8D |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50255753
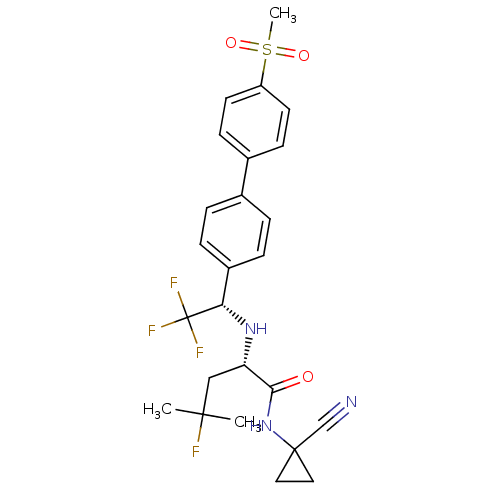
(CHEMBL481611 | MK-0822 | Odanacatib)Show SMILES CC(C)(F)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C25H27F4N3O3S/c1-23(2,26)14-20(22(33)32-24(15-30)12-13-24)31-21(25(27,28)29)18-6-4-16(5-7-18)17-8-10-19(11-9-17)36(3,34)35/h4-11,20-21,31H,12-14H2,1-3H3,(H,32,33)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 20: 887-92 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.083
BindingDB Entry DOI: 10.7270/Q27D2V75 |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50214542
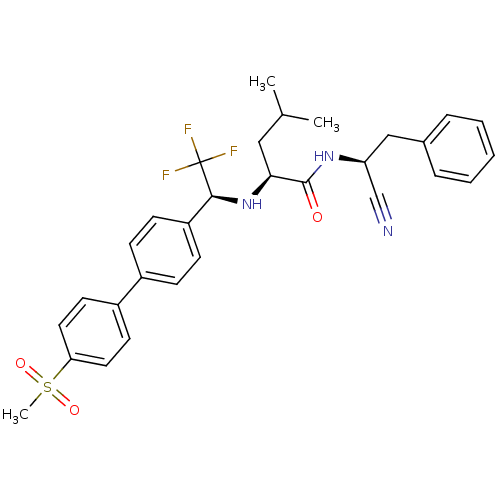
((S)-4-methyl-2-[(S)-2,2,2-trifluoro-1-(4'-methanes...)Show SMILES CC(C)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C(=O)N[C@@H](Cc1ccccc1)C#N Show InChI InChI=1S/C30H32F3N3O3S/c1-20(2)17-27(29(37)35-25(19-34)18-21-7-5-4-6-8-21)36-28(30(31,32)33)24-11-9-22(10-12-24)23-13-15-26(16-14-23)40(3,38)39/h4-16,20,25,27-28,36H,17-18H2,1-3H3,(H,35,37)/t25-,27-,28-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of trypanosoma cruzi Cruzipain |
Bioorg Med Chem Lett 20: 7444-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.015
BindingDB Entry DOI: 10.7270/Q29S1R8D |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50331769
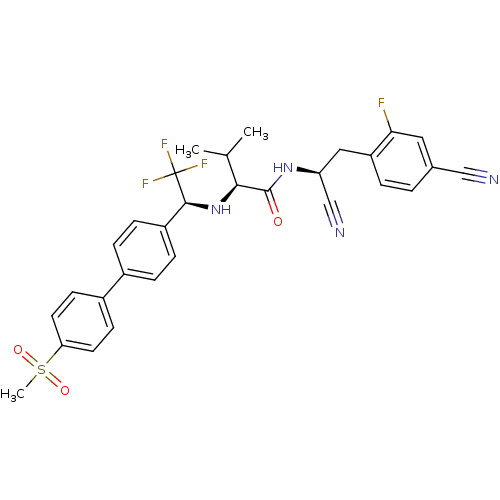
((S)-N-((S)-1-cyano-2-(4-cyano-2-fluorophenyl)ethyl...)Show SMILES CC(C)[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C(=O)N[C@@H](Cc1ccc(cc1F)C#N)C#N |r| Show InChI InChI=1S/C30H28F4N4O3S/c1-18(2)27(29(39)37-24(17-36)15-23-5-4-19(16-35)14-26(23)31)38-28(30(32,33)34)22-8-6-20(7-9-22)21-10-12-25(13-11-21)42(3,40)41/h4-14,18,24,27-28,38H,15H2,1-3H3,(H,37,39)/t24-,27-,28-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of trypanosoma cruzi Cruzipain |
Bioorg Med Chem Lett 20: 7444-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.015
BindingDB Entry DOI: 10.7270/Q29S1R8D |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50306304

((S)-N-(1-cyanocyclopropyl)-4-fluoro-4-methyl-2-((S...)Show SMILES CC(C)(F)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)C(C)(C)O)C(F)(F)F)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C27H31F4N3O2/c1-24(2,28)15-21(23(35)34-26(16-32)13-14-26)33-22(27(29,30)31)19-7-5-17(6-8-19)18-9-11-20(12-10-18)25(3,4)36/h5-12,21-22,33,36H,13-15H2,1-4H3,(H,34,35)/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 20: 887-92 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.083
BindingDB Entry DOI: 10.7270/Q27D2V75 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50255753

(CHEMBL481611 | MK-0822 | Odanacatib)Show SMILES CC(C)(F)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C25H27F4N3O3S/c1-23(2,26)14-20(22(33)32-24(15-30)12-13-24)31-21(25(27,28)29)18-6-4-16(5-7-18)17-8-10-19(11-9-17)36(3,34)35/h4-11,20-21,31H,12-14H2,1-3H3,(H,32,33)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 21: 920-3 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.070
BindingDB Entry DOI: 10.7270/Q2Z89CQF |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50306306
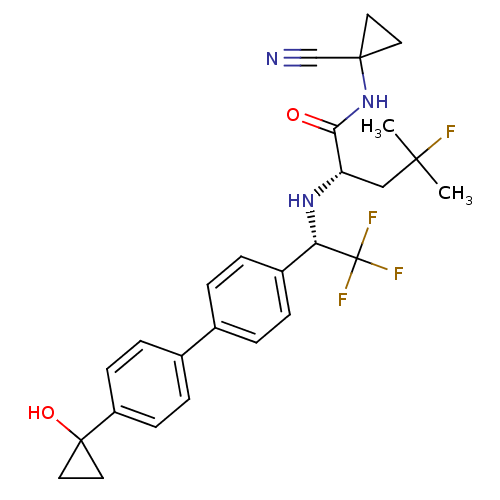
((S)-N-(1-cyanocyclopropyl)-4-fluoro-4-methyl-2-((S...)Show SMILES CC(C)(F)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)C1(O)CC1)C(F)(F)F)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C27H29F4N3O2/c1-24(2,28)15-21(23(35)34-25(16-32)11-12-25)33-22(27(29,30)31)19-5-3-17(4-6-19)18-7-9-20(10-8-18)26(36)13-14-26/h3-10,21-22,33,36H,11-15H2,1-2H3,(H,34,35)/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 20: 887-92 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.083
BindingDB Entry DOI: 10.7270/Q27D2V75 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50306307

((S)-2-((S)-1-(4'-((S)-1-amino-1-oxopropan-2-yl)bip...)Show SMILES C[C@H](C(N)=O)c1ccc(cc1)-c1ccc(cc1)[C@H](N[C@@H](CC(C)(C)F)C(=O)NC1(CC1)C#N)C(F)(F)F |r| Show InChI InChI=1S/C27H30F4N4O2/c1-16(23(33)36)17-4-6-18(7-5-17)19-8-10-20(11-9-19)22(27(29,30)31)34-21(14-25(2,3)28)24(37)35-26(15-32)12-13-26/h4-11,16,21-22,34H,12-14H2,1-3H3,(H2,33,36)(H,35,37)/t16-,21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 20: 887-92 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.083
BindingDB Entry DOI: 10.7270/Q27D2V75 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50306309

(1-(4'-((S)-1-((S)-1-(1-cyanocyclopropylamino)-4-fl...)Show SMILES CC(C)(F)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)C1(CC1)C(N)=O)C(F)(F)F)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C28H30F4N4O2/c1-25(2,29)15-21(23(37)36-26(16-33)11-12-26)35-22(28(30,31)32)19-5-3-17(4-6-19)18-7-9-20(10-8-18)27(13-14-27)24(34)38/h3-10,21-22,35H,11-15H2,1-2H3,(H2,34,38)(H,36,37)/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 20: 887-92 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.083
BindingDB Entry DOI: 10.7270/Q27D2V75 |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50331767
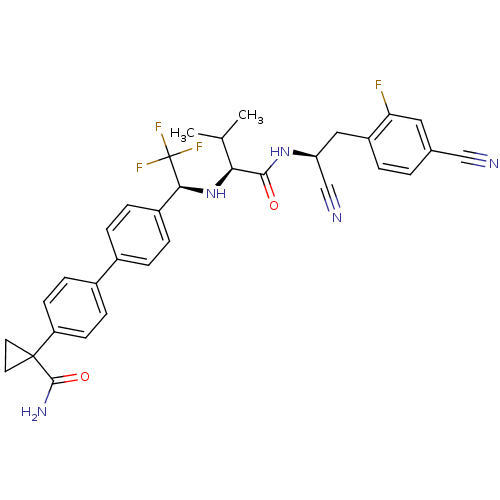
(1-(4'-((S)-1-((S)-1-((S)-1-cyano-2-(4-cyano-2-fluo...)Show SMILES CC(C)[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)C1(CC1)C(N)=O)C(F)(F)F)C(=O)N[C@@H](Cc1ccc(cc1F)C#N)C#N |r| Show InChI InChI=1S/C33H31F4N5O2/c1-19(2)28(30(43)41-26(18-39)16-24-4-3-20(17-38)15-27(24)34)42-29(33(35,36)37)23-7-5-21(6-8-23)22-9-11-25(12-10-22)32(13-14-32)31(40)44/h3-12,15,19,26,28-29,42H,13-14,16H2,1-2H3,(H2,40,44)(H,41,43)/t26-,28-,29-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of trypanosoma cruzi Cruzipain |
Bioorg Med Chem Lett 20: 7444-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.015
BindingDB Entry DOI: 10.7270/Q29S1R8D |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19489
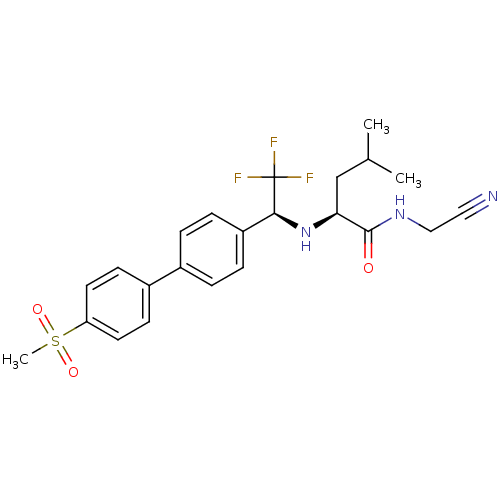
((2S)-N-(cyanomethyl)-4-methyl-2-[[(1S)-2,2,2-trifl...)Show SMILES CC(C)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C(=O)NCC#N |r| Show InChI InChI=1S/C23H26F3N3O3S/c1-15(2)14-20(22(30)28-13-12-27)29-21(23(24,25)26)18-6-4-16(5-7-18)17-8-10-19(11-9-17)33(3,31)32/h4-11,15,20-21,29H,13-14H2,1-3H3,(H,28,30)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 21: 920-3 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.070
BindingDB Entry DOI: 10.7270/Q2Z89CQF |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50336095
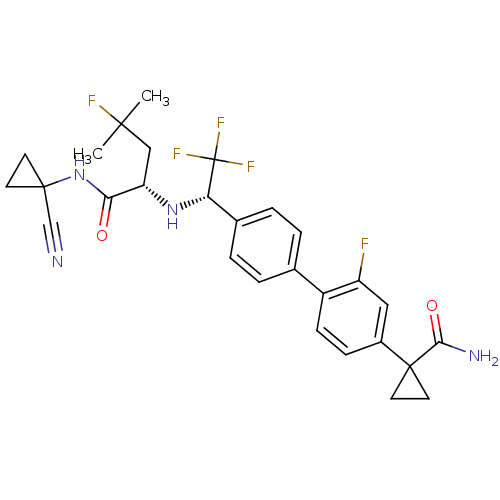
(1-(4'-((S)-1-((S)-1-(1-cyanocyclopropylamino)-4-fl...)Show SMILES CC(C)(F)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1F)C1(CC1)C(N)=O)C(F)(F)F)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C28H29F5N4O2/c1-25(2,30)14-21(23(38)37-26(15-34)9-10-26)36-22(28(31,32)33)17-5-3-16(4-6-17)19-8-7-18(13-20(19)29)27(11-12-27)24(35)39/h3-8,13,21-22,36H,9-12,14H2,1-2H3,(H2,35,39)(H,37,38)/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 21: 920-3 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.070
BindingDB Entry DOI: 10.7270/Q2Z89CQF |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50306310

((S)-N-(1-cyanocyclopropyl)-4-fluoro-4-methyl-2-((S...)Show SMILES Cc1csc(n1)-c1ccc(cc1)[C@H](N[C@@H](CC(C)(C)F)C(=O)NC1(CC1)C#N)C(F)(F)F |r| Show InChI InChI=1S/C22H24F4N4OS/c1-13-11-32-19(28-13)15-6-4-14(5-7-15)17(22(24,25)26)29-16(10-20(2,3)23)18(31)30-21(12-27)8-9-21/h4-7,11,16-17,29H,8-10H2,1-3H3,(H,30,31)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 20: 887-92 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.083
BindingDB Entry DOI: 10.7270/Q27D2V75 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50306305
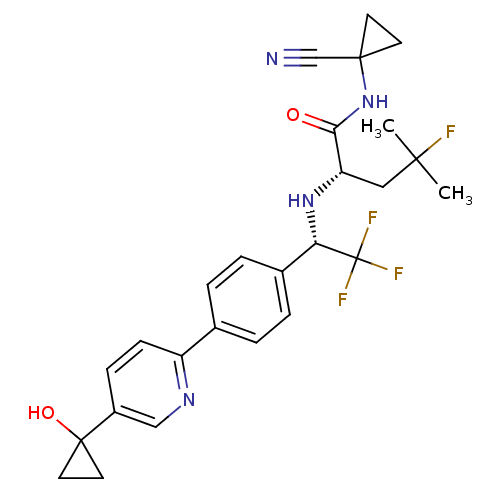
((S)-N-(1-cyanocyclopropyl)-4-fluoro-4-methyl-2-((S...)Show SMILES CC(C)(F)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cn1)C1(O)CC1)C(F)(F)F)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C26H28F4N4O2/c1-23(2,27)13-20(22(35)34-24(15-31)9-10-24)33-21(26(28,29)30)17-5-3-16(4-6-17)19-8-7-18(14-32-19)25(36)11-12-25/h3-8,14,20-21,33,36H,9-13H2,1-2H3,(H,34,35)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 20: 887-92 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.083
BindingDB Entry DOI: 10.7270/Q27D2V75 |
More data for this
Ligand-Target Pair | |
Cathepsin F
(Homo sapiens (Human)) | BDBM50214542

((S)-4-methyl-2-[(S)-2,2,2-trifluoro-1-(4'-methanes...)Show SMILES CC(C)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C(=O)N[C@@H](Cc1ccccc1)C#N Show InChI InChI=1S/C30H32F3N3O3S/c1-20(2)17-27(29(37)35-25(19-34)18-21-7-5-4-6-8-21)36-28(30(31,32)33)24-11-9-22(10-12-24)23-13-15-26(16-14-23)40(3,38)39/h4-16,20,25,27-28,36H,17-18H2,1-3H3,(H,35,37)/t25-,27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Cat F expressed in rabbit HIG82 cells |
Bioorg Med Chem Lett 20: 7444-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.015
BindingDB Entry DOI: 10.7270/Q29S1R8D |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50336094

(1-(4'-((S)-1-((S)-1-(1-cyanocyclopropylamino)-4-fl...)Show SMILES CC(C)(F)C[C@H](N[C@H](C(F)F)c1ccc(cc1)-c1ccc(cc1F)C1(CC1)C(N)=O)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C28H30F4N4O2/c1-26(2,32)14-21(24(37)36-27(15-33)9-10-27)35-22(23(30)31)17-5-3-16(4-6-17)19-8-7-18(13-20(19)29)28(11-12-28)25(34)38/h3-8,13,21-23,35H,9-12,14H2,1-2H3,(H2,34,38)(H,36,37)/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 21: 920-3 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.070
BindingDB Entry DOI: 10.7270/Q2Z89CQF |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50336091
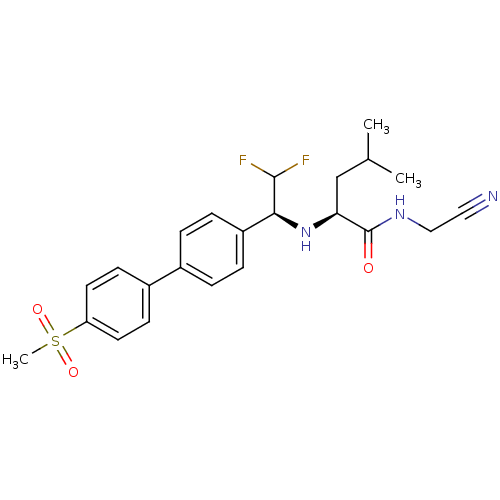
((S)-N-(cyanomethyl)-2-((S)-2,2-difluoro-1-(4'-(met...)Show SMILES CC(C)C[C@H](N[C@H](C(F)F)c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(=O)NCC#N |r| Show InChI InChI=1S/C23H27F2N3O3S/c1-15(2)14-20(23(29)27-13-12-26)28-21(22(24)25)18-6-4-16(5-7-18)17-8-10-19(11-9-17)32(3,30)31/h4-11,15,20-22,28H,13-14H2,1-3H3,(H,27,29)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 21: 920-3 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.070
BindingDB Entry DOI: 10.7270/Q2Z89CQF |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19491
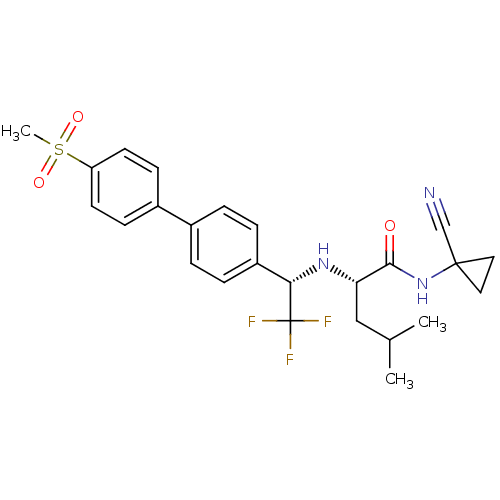
((2S)-N-(1-cyanocyclopropyl)-4-methyl-2-{[(1S)-2,2,...)Show SMILES CC(C)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C25H28F3N3O3S/c1-16(2)14-21(23(32)31-24(15-29)12-13-24)30-22(25(26,27)28)19-6-4-17(5-7-19)18-8-10-20(11-9-18)35(3,33)34/h4-11,16,21-22,30H,12-14H2,1-3H3,(H,31,32)/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 21: 920-3 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.070
BindingDB Entry DOI: 10.7270/Q2Z89CQF |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM19847

((1R,2R)-N-(cyanomethyl)-5,5-difluoro-2-{2-[4-(meth...)Show SMILES CSc1ccc(cc1)-c1ccccc1[C@@H]1CCC(F)(F)C[C@H]1C(=O)NCC#N |r| Show InChI InChI=1S/C22H22F2N2OS/c1-28-16-8-6-15(7-9-16)17-4-2-3-5-18(17)19-10-11-22(23,24)14-20(19)21(27)26-13-12-25/h2-9,19-20H,10-11,13-14H2,1H3,(H,26,27)/t19-,20+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of rabbit cathepsin K |
J Med Chem 51: 6410-20 (2008)
Article DOI: 10.1021/jm800610j
BindingDB Entry DOI: 10.7270/Q261105F |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50306302

((S)-N-(1-cyanocyclopropyl)-2-((S)-1-(4'-((R)-2,2-d...)Show SMILES CC(C)(F)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)[C@@H](O)C(F)F)C(F)(F)F)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C26H27F6N3O2/c1-24(2,29)13-19(23(37)35-25(14-33)11-12-25)34-21(26(30,31)32)18-9-5-16(6-10-18)15-3-7-17(8-4-15)20(36)22(27)28/h3-10,19-22,34,36H,11-13H2,1-2H3,(H,35,37)/t19-,20+,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 20: 887-92 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.083
BindingDB Entry DOI: 10.7270/Q27D2V75 |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50331768
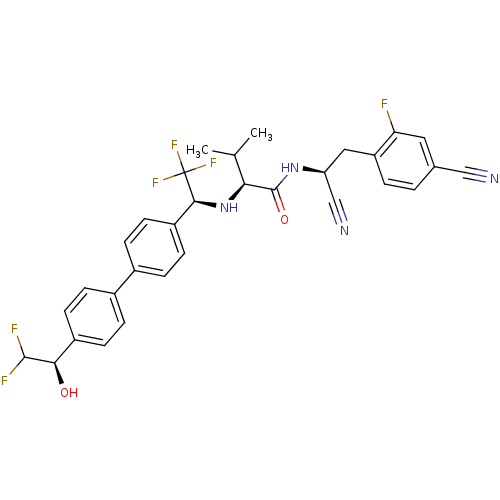
((S)-N-((S)-1-cyano-2-(4-cyano-2-fluorophenyl)ethyl...)Show SMILES CC(C)[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)[C@@H](O)C(F)F)C(F)(F)F)C(=O)N[C@@H](Cc1ccc(cc1F)C#N)C#N |r| Show InChI InChI=1S/C31H28F6N4O2/c1-17(2)26(30(43)40-24(16-39)14-23-4-3-18(15-38)13-25(23)32)41-28(31(35,36)37)22-11-7-20(8-12-22)19-5-9-21(10-6-19)27(42)29(33)34/h3-13,17,24,26-29,41-42H,14H2,1-2H3,(H,40,43)/t24-,26-,27+,28-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of trypanosoma cruzi Cruzipain |
Bioorg Med Chem Lett 20: 7444-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.015
BindingDB Entry DOI: 10.7270/Q29S1R8D |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50306311
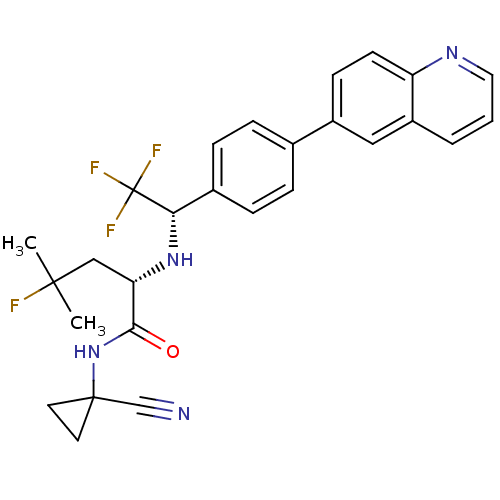
((S)-N-(1-cyanocyclopropyl)-4-fluoro-4-methyl-2-((S...)Show SMILES CC(C)(F)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc2ncccc2c1)C(F)(F)F)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C27H26F4N4O/c1-25(2,28)15-22(24(36)35-26(16-32)11-12-26)34-23(27(29,30)31)18-7-5-17(6-8-18)19-9-10-21-20(14-19)4-3-13-33-21/h3-10,13-14,22-23,34H,11-12,15H2,1-2H3,(H,35,36)/t22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 20: 887-92 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.083
BindingDB Entry DOI: 10.7270/Q27D2V75 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50306303

((S)-N-(1-cyanocyclopropyl)-2-((S)-1-(4'-((S)-2,2-d...)Show SMILES CC(C)(F)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)[C@H](O)C(F)F)C(F)(F)F)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C26H27F6N3O2/c1-24(2,29)13-19(23(37)35-25(14-33)11-12-25)34-21(26(30,31)32)18-9-5-16(6-10-18)15-3-7-17(8-4-15)20(36)22(27)28/h3-10,19-22,34,36H,11-13H2,1-2H3,(H,35,37)/t19-,20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 20: 887-92 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.083
BindingDB Entry DOI: 10.7270/Q27D2V75 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM19909

((1R,2R)-N-(cyanomethyl)-5,5-difluoro-2-{1-methyl-4...)Show SMILES CSc1ccc(cc1)-c1cn(C)nc1[C@@H]1CCC(F)(F)C[C@H]1C(=O)NCC#N |r| Show InChI InChI=1S/C20H22F2N4OS/c1-26-12-17(13-3-5-14(28-2)6-4-13)18(25-26)15-7-8-20(21,22)11-16(15)19(27)24-10-9-23/h3-6,12,15-16H,7-8,10-11H2,1-2H3,(H,24,27)/t15-,16-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of rabbit cathepsin K |
J Med Chem 51: 6410-20 (2008)
Article DOI: 10.1021/jm800610j
BindingDB Entry DOI: 10.7270/Q261105F |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50306308
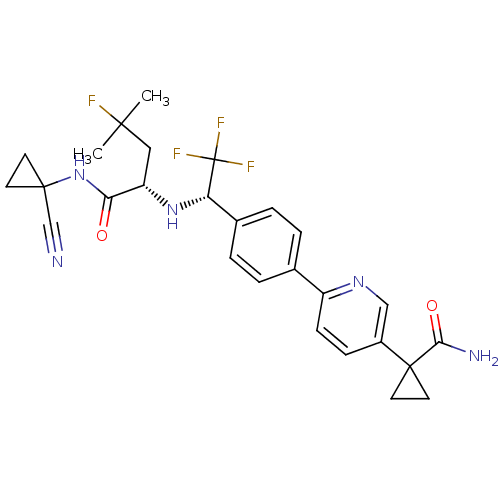
(1-(6-(4-((S)-1-((S)-1-(1-cyanocyclopropylamino)-4-...)Show SMILES CC(C)(F)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cn1)C1(CC1)C(N)=O)C(F)(F)F)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C27H29F4N5O2/c1-24(2,28)13-20(22(37)36-25(15-32)9-10-25)35-21(27(29,30)31)17-5-3-16(4-6-17)19-8-7-18(14-34-19)26(11-12-26)23(33)38/h3-8,14,20-21,35H,9-13H2,1-2H3,(H2,33,38)(H,36,37)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 20: 887-92 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.083
BindingDB Entry DOI: 10.7270/Q27D2V75 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50336089
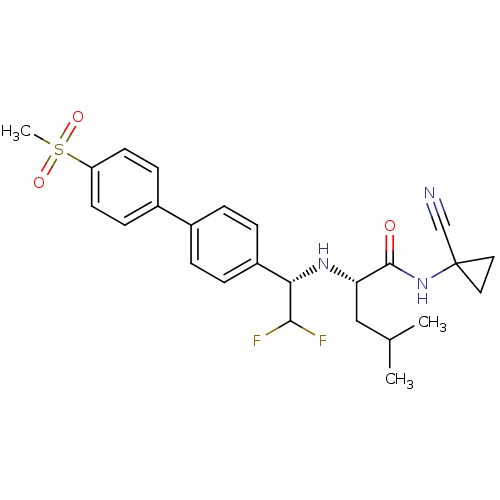
((S)-N-(1-cyanocyclopropyl)-2-((S)-2,2-difluoro-1-(...)Show SMILES CC(C)C[C@H](N[C@H](C(F)F)c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C25H29F2N3O3S/c1-16(2)14-21(24(31)30-25(15-28)12-13-25)29-22(23(26)27)19-6-4-17(5-7-19)18-8-10-20(11-9-18)34(3,32)33/h4-11,16,21-23,29H,12-14H2,1-3H3,(H,30,31)/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 21: 920-3 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.070
BindingDB Entry DOI: 10.7270/Q2Z89CQF |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50331769

((S)-N-((S)-1-cyano-2-(4-cyano-2-fluorophenyl)ethyl...)Show SMILES CC(C)[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C(=O)N[C@@H](Cc1ccc(cc1F)C#N)C#N |r| Show InChI InChI=1S/C30H28F4N4O3S/c1-18(2)27(29(39)37-24(17-36)15-23-5-4-19(16-35)14-26(23)31)38-28(30(32,33)34)22-8-6-20(7-9-22)21-10-12-25(13-11-21)42(3,40)41/h4-14,18,24,27-28,38H,15H2,1-3H3,(H,37,39)/t24-,27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Cat K expressed in Ramos cells |
Bioorg Med Chem Lett 20: 7444-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.015
BindingDB Entry DOI: 10.7270/Q29S1R8D |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50336090
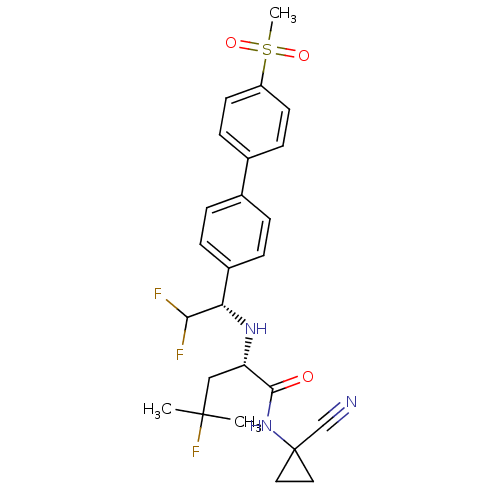
((S)-N-(1-cyanocyclopropyl)-2-((S)-2,2-difluoro-1-(...)Show SMILES CC(C)(F)C[C@H](N[C@H](C(F)F)c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C25H28F3N3O3S/c1-24(2,28)14-20(23(32)31-25(15-29)12-13-25)30-21(22(26)27)18-6-4-16(5-7-18)17-8-10-19(11-9-17)35(3,33)34/h4-11,20-22,30H,12-14H2,1-3H3,(H,31,32)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 21: 920-3 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.070
BindingDB Entry DOI: 10.7270/Q2Z89CQF |
More data for this
Ligand-Target Pair | |
Acyl-CoA desaturase 1
(Rattus norvegicus (Rat)) | BDBM50354682
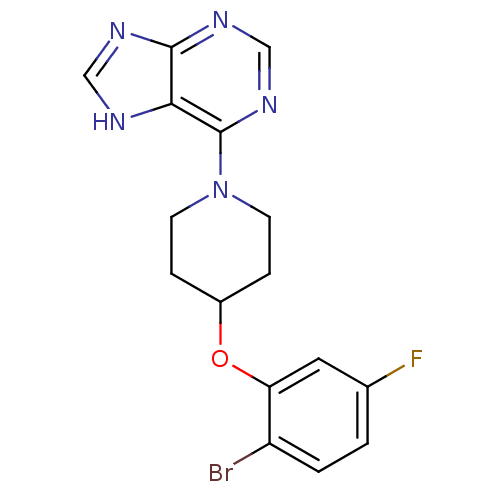
(CHEMBL1834455)Show InChI InChI=1S/C16H15BrFN5O/c17-12-2-1-10(18)7-13(12)24-11-3-5-23(6-4-11)16-14-15(20-8-19-14)21-9-22-16/h1-2,7-9,11H,3-6H2,(H,19,20,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of rat SCD1 in rat liver microsome |
Bioorg Med Chem Lett 21: 5692-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.037
BindingDB Entry DOI: 10.7270/Q2W959K1 |
More data for this
Ligand-Target Pair | |
Acyl-CoA desaturase 1
(Rattus norvegicus (Rat)) | BDBM50354672
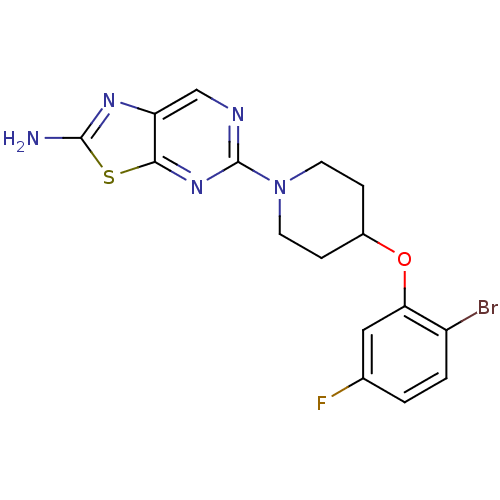
(CHEMBL1834453)Show InChI InChI=1S/C16H15BrFN5OS/c17-11-2-1-9(18)7-13(11)24-10-3-5-23(6-4-10)16-20-8-12-14(22-16)25-15(19)21-12/h1-2,7-8,10H,3-6H2,(H2,19,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of rat SCD1 in rat liver microsome |
Bioorg Med Chem Lett 21: 5692-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.037
BindingDB Entry DOI: 10.7270/Q2W959K1 |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50331787
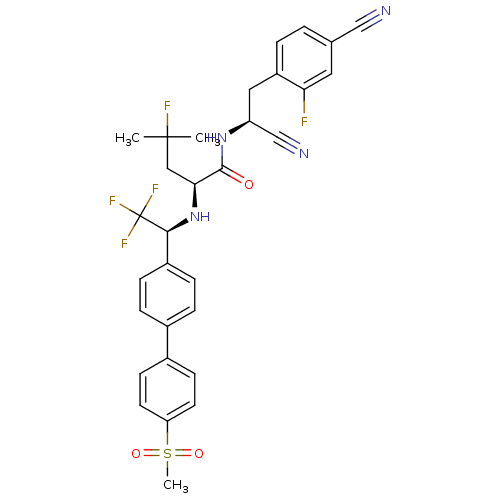
((S)-N-((S)-1-cyano-2-(4-cyano-2-fluorophenyl)ethyl...)Show SMILES CC(C)(F)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C(=O)N[C@@H](Cc1ccc(cc1F)C#N)C#N |r| Show InChI InChI=1S/C31H29F5N4O3S/c1-30(2,33)16-27(29(41)39-24(18-38)15-23-5-4-19(17-37)14-26(23)32)40-28(31(34,35)36)22-8-6-20(7-9-22)21-10-12-25(13-11-21)44(3,42)43/h4-14,24,27-28,40H,15-16H2,1-3H3,(H,39,41)/t24-,27-,28-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of trypanosoma cruzi Cruzipain |
Bioorg Med Chem Lett 20: 7444-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.015
BindingDB Entry DOI: 10.7270/Q29S1R8D |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50336093

((2S)-N-(1-cyanocyclopropyl)-4-methyl-2-((1S)-2,2,2...)Show SMILES CC(C)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)C(O)C(F)(F)F)C(F)(F)F)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C26H27F6N3O2/c1-15(2)13-20(23(37)35-24(14-33)11-12-24)34-21(25(27,28)29)18-7-3-16(4-8-18)17-5-9-19(10-6-17)22(36)26(30,31)32/h3-10,15,20-22,34,36H,11-13H2,1-2H3,(H,35,37)/t20-,21-,22?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 21: 920-3 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.070
BindingDB Entry DOI: 10.7270/Q2Z89CQF |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM19518

((2S)-N-(cyanomethyl)-4-methyl-2-{[(1S)-2,2,2-trifl...)Show SMILES CC(C)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)N1CCNCC1)C(F)(F)F)C(=O)NCC#N |r| Show InChI InChI=1S/C26H32F3N5O/c1-18(2)17-23(25(35)32-12-11-30)33-24(26(27,28)29)21-5-3-19(4-6-21)20-7-9-22(10-8-20)34-15-13-31-14-16-34/h3-10,18,23-24,31,33H,12-17H2,1-2H3,(H,32,35)/t23-,24-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of trypanosoma cruzi Cruzipain |
Bioorg Med Chem Lett 20: 7444-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.015
BindingDB Entry DOI: 10.7270/Q29S1R8D |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50336092
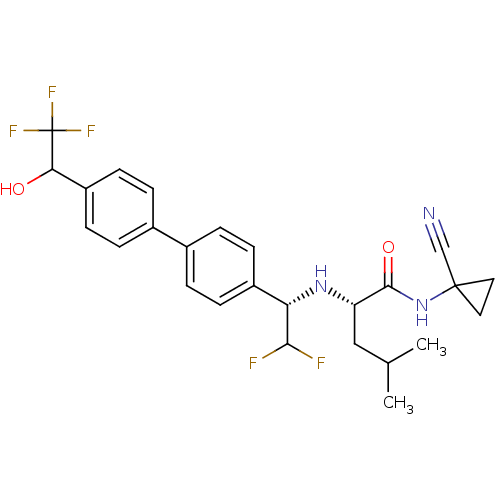
((2S)-N-(1-cyanocyclopropyl)-2-((1S)-2,2-difluoro-1...)Show SMILES CC(C)C[C@H](N[C@H](C(F)F)c1ccc(cc1)-c1ccc(cc1)C(O)C(F)(F)F)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C26H28F5N3O2/c1-15(2)13-20(24(36)34-25(14-32)11-12-25)33-21(23(27)28)18-7-3-16(4-8-18)17-5-9-19(10-6-17)22(35)26(29,30)31/h3-10,15,20-23,33,35H,11-13H2,1-2H3,(H,34,36)/t20-,21-,22?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 21: 920-3 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.070
BindingDB Entry DOI: 10.7270/Q2Z89CQF |
More data for this
Ligand-Target Pair | |
Acyl-CoA desaturase 1
(Rattus norvegicus (Rat)) | BDBM50354677
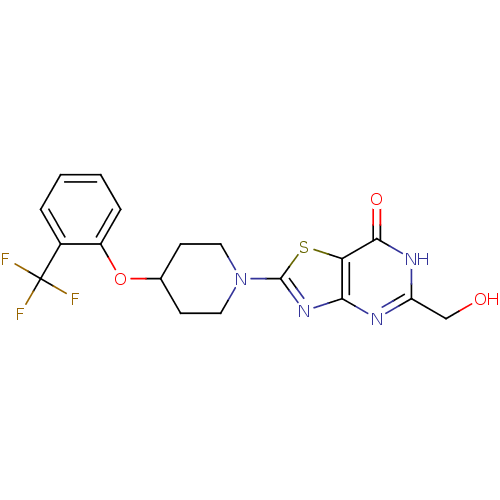
(CHEMBL1834443)Show SMILES OCc1nc2nc(sc2c(=O)[nH]1)N1CCC(CC1)Oc1ccccc1C(F)(F)F Show InChI InChI=1S/C18H17F3N4O3S/c19-18(20,21)11-3-1-2-4-12(11)28-10-5-7-25(8-6-10)17-24-15-14(29-17)16(27)23-13(9-26)22-15/h1-4,10,26H,5-9H2,(H,22,23,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of rat SCD1 in rat liver microsome |
Bioorg Med Chem Lett 21: 5692-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.037
BindingDB Entry DOI: 10.7270/Q2W959K1 |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50331778
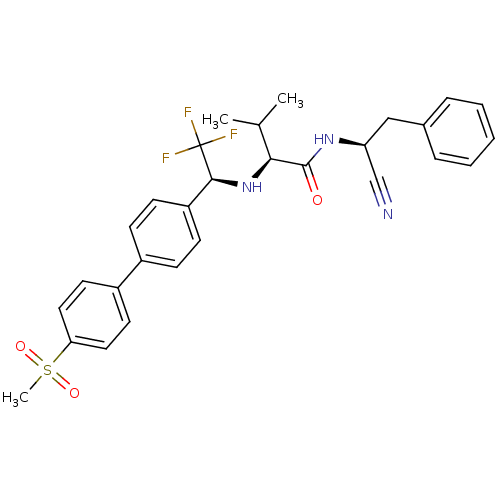
((S)-N-((S)-1-cyano-2-phenylethyl)-3-methyl-2-((S)-...)Show SMILES CC(C)[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C(=O)N[C@@H](Cc1ccccc1)C#N |r| Show InChI InChI=1S/C29H30F3N3O3S/c1-19(2)26(28(36)34-24(18-33)17-20-7-5-4-6-8-20)35-27(29(30,31)32)23-11-9-21(10-12-23)22-13-15-25(16-14-22)39(3,37)38/h4-16,19,24,26-27,35H,17H2,1-3H3,(H,34,36)/t24-,26-,27-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of trypanosoma cruzi Cruzipain |
Bioorg Med Chem Lett 20: 7444-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.015
BindingDB Entry DOI: 10.7270/Q29S1R8D |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50331768

((S)-N-((S)-1-cyano-2-(4-cyano-2-fluorophenyl)ethyl...)Show SMILES CC(C)[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)[C@@H](O)C(F)F)C(F)(F)F)C(=O)N[C@@H](Cc1ccc(cc1F)C#N)C#N |r| Show InChI InChI=1S/C31H28F6N4O2/c1-17(2)26(30(43)40-24(16-39)14-23-4-3-18(15-38)13-25(23)32)41-28(31(35,36)37)22-11-7-20(8-12-22)19-5-9-21(10-6-19)27(42)29(33)34/h3-13,17,24,26-29,41-42H,14H2,1-2H3,(H,40,43)/t24-,26-,27+,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Cat K expressed in Ramos cells |
Bioorg Med Chem Lett 20: 7444-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.015
BindingDB Entry DOI: 10.7270/Q29S1R8D |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50306312

((S)-N-(1-cyanocyclopropyl)-4-fluoro-4-methyl-2-((S...)Show SMILES CC(C)(F)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(nc1)S(C)(=O)=O)C(F)(F)F)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C24H26F4N4O3S/c1-22(2,25)12-18(21(33)32-23(14-29)10-11-23)31-20(24(26,27)28)16-6-4-15(5-7-16)17-8-9-19(30-13-17)36(3,34)35/h4-9,13,18,20,31H,10-12H2,1-3H3,(H,32,33)/t18-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 20: 887-92 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.083
BindingDB Entry DOI: 10.7270/Q27D2V75 |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50331770
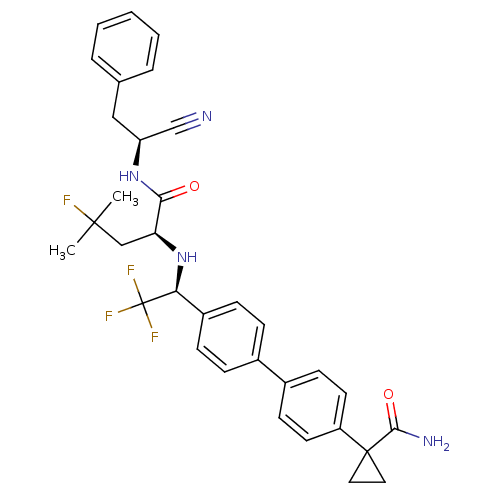
(1-(4'-((S)-1-((S)-1-((S)-1-cyano-2-phenylethylamin...)Show SMILES CC(C)(F)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)C1(CC1)C(N)=O)C(F)(F)F)C(=O)N[C@@H](Cc1ccccc1)C#N |r| Show InChI InChI=1S/C33H34F4N4O2/c1-31(2,34)19-27(29(42)40-26(20-38)18-21-6-4-3-5-7-21)41-28(33(35,36)37)24-10-8-22(9-11-24)23-12-14-25(15-13-23)32(16-17-32)30(39)43/h3-15,26-28,41H,16-19H2,1-2H3,(H2,39,43)(H,40,42)/t26-,27-,28-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of trypanosoma cruzi Cruzipain |
Bioorg Med Chem Lett 20: 7444-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.015
BindingDB Entry DOI: 10.7270/Q29S1R8D |
More data for this
Ligand-Target Pair | |
Acyl-CoA desaturase 1
(Mus musculus) | BDBM50362592
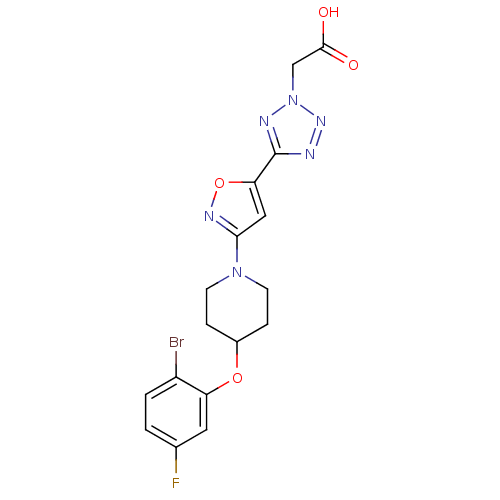
(CHEMBL1938870)Show SMILES OC(=O)Cn1nnc(n1)-c1cc(no1)N1CCC(CC1)Oc1cc(F)ccc1Br Show InChI InChI=1S/C17H16BrFN6O4/c18-12-2-1-10(19)7-13(12)28-11-3-5-24(6-4-11)15-8-14(29-22-15)17-20-23-25(21-17)9-16(26)27/h1-2,7-8,11H,3-6,9H2,(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of mouse SCD-1 |
Bioorg Med Chem Lett 22: 980-4 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.002
BindingDB Entry DOI: 10.7270/Q2833SHH |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50331767

(1-(4'-((S)-1-((S)-1-((S)-1-cyano-2-(4-cyano-2-fluo...)Show SMILES CC(C)[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)C1(CC1)C(N)=O)C(F)(F)F)C(=O)N[C@@H](Cc1ccc(cc1F)C#N)C#N |r| Show InChI InChI=1S/C33H31F4N5O2/c1-19(2)28(30(43)41-26(18-39)16-24-4-3-20(17-38)15-27(24)34)42-29(33(35,36)37)23-7-5-21(6-8-23)22-9-11-25(12-10-22)32(13-14-32)31(40)44/h3-12,15,19,26,28-29,42H,13-14,16H2,1-2H3,(H2,40,44)(H,41,43)/t26-,28-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Cat K expressed in Ramos cells |
Bioorg Med Chem Lett 20: 7444-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.015
BindingDB Entry DOI: 10.7270/Q29S1R8D |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50331771
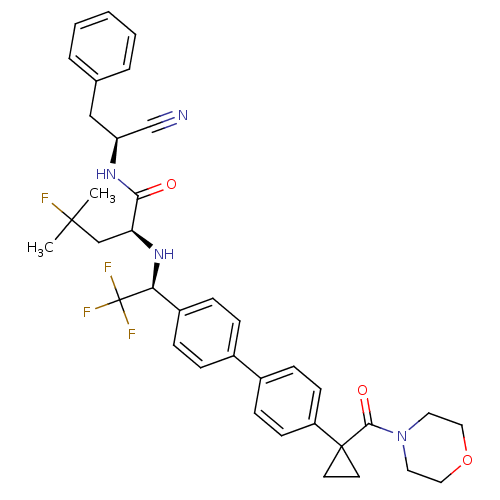
((S)-N-((S)-1-cyano-2-phenylethyl)-4-fluoro-4-methy...)Show SMILES CC(C)(F)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)C1(CC1)C(=O)N1CCOCC1)C(F)(F)F)C(=O)N[C@@H](Cc1ccccc1)C#N |r| Show InChI InChI=1S/C37H40F4N4O3/c1-35(2,38)23-31(33(46)43-30(24-42)22-25-6-4-3-5-7-25)44-32(37(39,40)41)28-10-8-26(9-11-28)27-12-14-29(15-13-27)36(16-17-36)34(47)45-18-20-48-21-19-45/h3-15,30-32,44H,16-23H2,1-2H3,(H,43,46)/t30-,31-,32-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of trypanosoma cruzi Cruzipain |
Bioorg Med Chem Lett 20: 7444-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.015
BindingDB Entry DOI: 10.7270/Q29S1R8D |
More data for this
Ligand-Target Pair | |
Acyl-CoA desaturase 1
(Rattus norvegicus (Rat)) | BDBM50354671

(CHEMBL1834452)Show InChI InChI=1S/C16H15BrFN5O/c17-12-2-1-10(18)7-14(12)24-11-3-5-23(6-4-11)16-19-8-13-15(22-16)21-9-20-13/h1-2,7-9,11H,3-6H2,(H,19,20,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of rat SCD1 in rat liver microsome |
Bioorg Med Chem Lett 21: 5692-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.037
BindingDB Entry DOI: 10.7270/Q2W959K1 |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50203364

(CHEMBL248310 | N-((S)-3-(2-(3-(1H-tetrazol-5-ylami...)Show SMILES CCCCCC[NH+](C)CC(=O)[C@H](CC(O)=O)NC(=O)C(CC)n1cc(nc(Nc2nnn[nH]2)c1=O)C(C)(C)C |w:19.19,6.6| Show InChI InChI=1S/C25H41N9O5/c1-7-9-10-11-12-33(6)14-18(35)16(13-20(36)37)26-22(38)17(8-2)34-15-19(25(3,4)5)27-21(23(34)39)28-24-29-31-32-30-24/h15-17H,7-14H2,1-6H3,(H,26,38)(H,36,37)(H2,27,28,29,30,31,32)/p+1/t16-,17?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant caspase 1 |
Bioorg Med Chem Lett 17: 1671-4 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.110
BindingDB Entry DOI: 10.7270/Q22R3RBJ |
More data for this
Ligand-Target Pair | |
Cathepsin F
(Homo sapiens (Human)) | BDBM50331776

((S)-4,4-dichloro-N-((S)-1-cyano-2-phenylethyl)-2-(...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1ccc(cc1)[C@H](N[C@@H](CC(Cl)Cl)C(=O)N[C@@H](Cc1ccccc1)C#N)C(F)(F)F |r| Show InChI InChI=1S/C28H26Cl2F3N3O3S/c1-40(38,39)23-13-11-20(12-14-23)19-7-9-21(10-8-19)26(28(31,32)33)36-24(16-25(29)30)27(37)35-22(17-34)15-18-5-3-2-4-6-18/h2-14,22,24-26,36H,15-16H2,1H3,(H,35,37)/t22-,24-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Cat F expressed in rabbit HIG82 cells |
Bioorg Med Chem Lett 20: 7444-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.015
BindingDB Entry DOI: 10.7270/Q29S1R8D |
More data for this
Ligand-Target Pair | |
Stearoyl-CoA desaturase 5
(Homo sapiens (Human)) | BDBM50364012

(CHEMBL1950397)Show SMILES OC(=O)c1cncc(c1)-c1ccc(nn1)N1CCC(CC1)Oc1cc(F)ccc1Br Show InChI InChI=1S/C21H18BrFN4O3/c22-17-2-1-15(23)10-19(17)30-16-5-7-27(8-6-16)20-4-3-18(25-26-20)13-9-14(21(28)29)12-24-11-13/h1-4,9-12,16H,5-8H2,(H,28,29) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human SCD5 |
Bioorg Med Chem Lett 21: 7281-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.040
BindingDB Entry DOI: 10.7270/Q2G44QQZ |
More data for this
Ligand-Target Pair | |
Acyl-CoA desaturase 1
(Rattus norvegicus (Rat)) | BDBM50354674
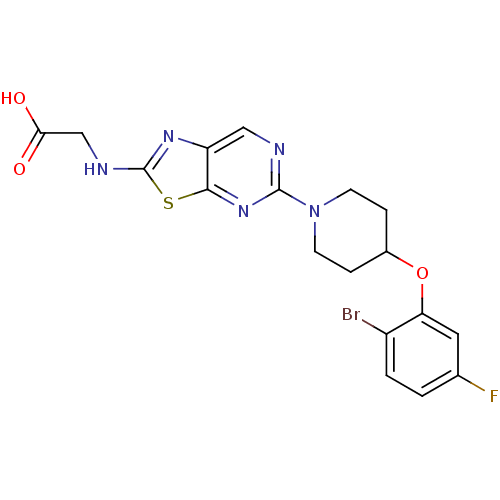
(CHEMBL1834457)Show SMILES OC(=O)CNc1nc2cnc(nc2s1)N1CCC(CC1)Oc1cc(F)ccc1Br Show InChI InChI=1S/C18H17BrFN5O3S/c19-12-2-1-10(20)7-14(12)28-11-3-5-25(6-4-11)17-21-8-13-16(24-17)29-18(23-13)22-9-15(26)27/h1-2,7-8,11H,3-6,9H2,(H,22,23)(H,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of rat SCD1 in rat liver microsome |
Bioorg Med Chem Lett 21: 5692-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.037
BindingDB Entry DOI: 10.7270/Q2W959K1 |
More data for this
Ligand-Target Pair | |
Cathepsin F
(Homo sapiens (Human)) | BDBM50331787

((S)-N-((S)-1-cyano-2-(4-cyano-2-fluorophenyl)ethyl...)Show SMILES CC(C)(F)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C(=O)N[C@@H](Cc1ccc(cc1F)C#N)C#N |r| Show InChI InChI=1S/C31H29F5N4O3S/c1-30(2,33)16-27(29(41)39-24(18-38)15-23-5-4-19(17-37)14-26(23)32)40-28(31(34,35)36)22-8-6-20(7-9-22)21-10-12-25(13-11-21)44(3,42)43/h4-14,24,27-28,40H,15-16H2,1-3H3,(H,39,41)/t24-,27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Cat F expressed in rabbit HIG82 cells |
Bioorg Med Chem Lett 20: 7444-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.015
BindingDB Entry DOI: 10.7270/Q29S1R8D |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50253069
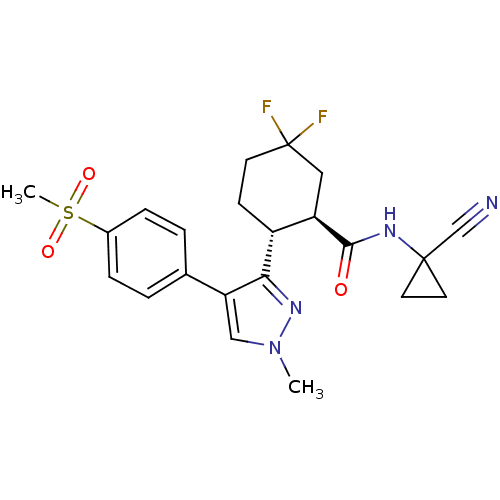
((1R,2R)-N-(1-cyanocyclopropyl)-5,5-difluoro-2-(1-m...)Show SMILES Cn1cc(c(n1)[C@@H]1CCC(F)(F)C[C@H]1C(=O)NC1(CC1)C#N)-c1ccc(cc1)S(C)(=O)=O |r| Show InChI InChI=1S/C22H24F2N4O3S/c1-28-12-18(14-3-5-15(6-4-14)32(2,30)31)19(27-28)16-7-8-22(23,24)11-17(16)20(29)26-21(13-25)9-10-21/h3-6,12,16-17H,7-11H2,1-2H3,(H,26,29)/t16-,17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of rabbit cathepsin K |
J Med Chem 51: 6410-20 (2008)
Article DOI: 10.1021/jm800610j
BindingDB Entry DOI: 10.7270/Q261105F |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM10551

((3S)-5-(benzylsulfanyl)-3-[(2S)-2-[2-(5-bromo-2-me...)Show SMILES COc1ccc(Br)cc1C(O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)CSCc1ccccc1 |r| Show InChI InChI=1S/C26H31BrN2O7S/c1-15(2)23(29-26(35)24(33)18-11-17(27)9-10-21(18)36-3)25(34)28-19(12-22(31)32)20(30)14-37-13-16-7-5-4-6-8-16/h4-11,15,19,23-24,33H,12-14H2,1-3H3,(H,28,34)(H,29,35)(H,31,32)/t19-,23-,24?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Canada & Co.
| Assay Description
The substrate peptides terminating in AMC are processed by caspases with or without inhibitors, and the accumulation of AMC was assessed with a Cytof... |
Bioorg Med Chem Lett 15: 3886-90 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.116
BindingDB Entry DOI: 10.7270/Q2959FS7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data