 Found 421 hits with Last Name = 'johnson' and Initial = 'mc'
Found 421 hits with Last Name = 'johnson' and Initial = 'mc' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase PAK 4
(Homo sapiens (Human)) | BDBM50389112
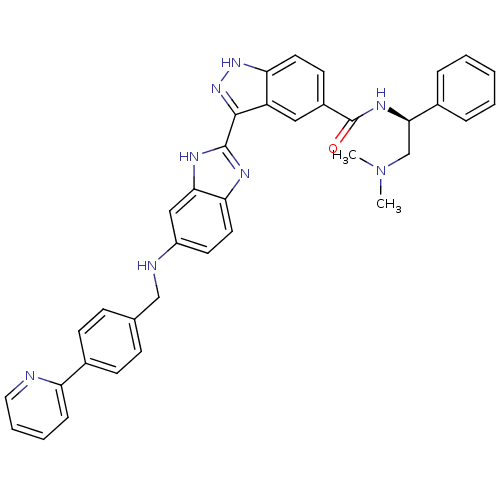
(CHEMBL2064556)Show SMILES CN(C)C[C@@H](NC(=O)c1ccc2[nH]nc(-c3nc4ccc(NCc5ccc(cc5)-c5ccccn5)cc4[nH]3)c2c1)c1ccccc1 |r| Show InChI InChI=1S/C37H34N8O/c1-45(2)23-34(25-8-4-3-5-9-25)42-37(46)27-15-17-31-29(20-27)35(44-43-31)36-40-32-18-16-28(21-33(32)41-36)39-22-24-11-13-26(14-12-24)30-10-6-7-19-38-30/h3-21,34,39H,22-23H2,1-2H3,(H,40,41)(H,42,46)(H,43,44)/t34-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PAK4 |
J Med Chem 55: 4728-39 (2012)
Article DOI: 10.1021/jm300204j
BindingDB Entry DOI: 10.7270/Q2BZ673W |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50389111
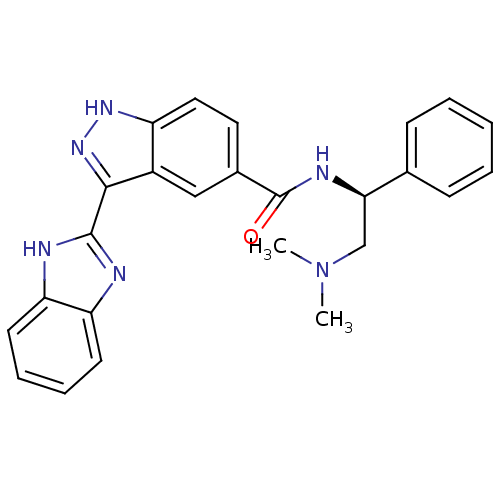
(CHEMBL2064555)Show SMILES CN(C)C[C@@H](NC(=O)c1ccc2[nH]nc(-c3nc4ccccc4[nH]3)c2c1)c1ccccc1 |r| Show InChI InChI=1S/C25H24N6O/c1-31(2)15-22(16-8-4-3-5-9-16)28-25(32)17-12-13-19-18(14-17)23(30-29-19)24-26-20-10-6-7-11-21(20)27-24/h3-14,22H,15H2,1-2H3,(H,26,27)(H,28,32)(H,29,30)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 |
J Med Chem 55: 4728-39 (2012)
Article DOI: 10.1021/jm300204j
BindingDB Entry DOI: 10.7270/Q2BZ673W |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 4
(Homo sapiens (Human)) | BDBM50389111

(CHEMBL2064555)Show SMILES CN(C)C[C@@H](NC(=O)c1ccc2[nH]nc(-c3nc4ccccc4[nH]3)c2c1)c1ccccc1 |r| Show InChI InChI=1S/C25H24N6O/c1-31(2)15-22(16-8-4-3-5-9-16)28-25(32)17-12-13-19-18(14-17)23(30-29-19)24-26-20-10-6-7-11-21(20)27-24/h3-14,22H,15H2,1-2H3,(H,26,27)(H,28,32)(H,29,30)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PAK4 |
J Med Chem 55: 4728-39 (2012)
Article DOI: 10.1021/jm300204j
BindingDB Entry DOI: 10.7270/Q2BZ673W |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM34012
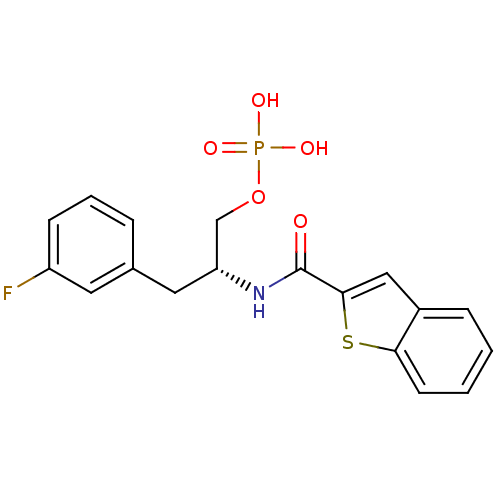
(3-fluorophenylalanine derivative, 21b)Show SMILES OP(O)(=O)OC[C@@H](Cc1cccc(F)c1)NC(=O)c1cc2ccccc2s1 |r| Show InChI InChI=1S/C18H17FNO5PS/c19-14-6-3-4-12(8-14)9-15(11-25-26(22,23)24)20-18(21)17-10-13-5-1-2-7-16(13)27-17/h1-8,10,15H,9,11H2,(H,20,21)(H2,22,23,24)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6 | -45.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 15 |
Pfizer
| Assay Description
The cis/trans conversion of cis-Suc-AEPFpNA peptide by the PPIase led to the cleavage of para nitroaniline by subtilisin which was monitored at 390 n... |
Bioorg Med Chem Lett 19: 5613-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.034
BindingDB Entry DOI: 10.7270/Q2XD100H |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50314714

((R)-N-(1-(3-fluorophenyl)-3-hydroxypropan-2-yl)ben...)Show SMILES OC[C@@H](Cc1cccc(F)c1)NC(=O)c1cc2ccccc2s1 |r| Show InChI InChI=1S/C18H16FNO2S/c19-14-6-3-4-12(8-14)9-15(11-21)20-18(22)17-10-13-5-1-2-7-16(13)23-17/h1-8,10,15,21H,9,11H2,(H,20,22)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of WFYpSPFLE from human Pin1 catalytic domain after 10-20 mins by fluorescence polarization assay |
Bioorg Med Chem Lett 20: 2210-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.033
BindingDB Entry DOI: 10.7270/Q2GX4BPW |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM34011
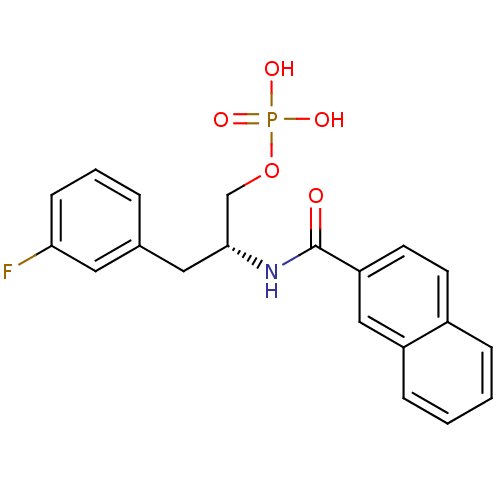
(3-fluorophenylalanine derivative, 21a)Show SMILES OP(O)(=O)OC[C@@H](Cc1cccc(F)c1)NC(=O)c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C20H19FNO5P/c21-18-7-3-4-14(10-18)11-19(13-27-28(24,25)26)22-20(23)17-9-8-15-5-1-2-6-16(15)12-17/h1-10,12,19H,11,13H2,(H,22,23)(H2,24,25,26)/t19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | -44.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 15 |
Pfizer
| Assay Description
The cis/trans conversion of cis-Suc-AEPFpNA peptide by the PPIase led to the cleavage of para nitroaniline by subtilisin which was monitored at 390 n... |
Bioorg Med Chem Lett 19: 5613-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.034
BindingDB Entry DOI: 10.7270/Q2XD100H |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50056217
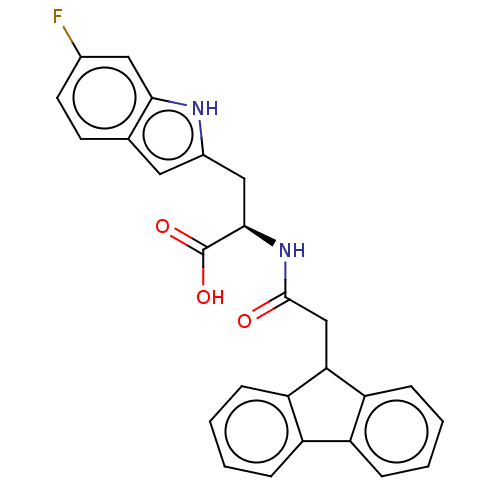
(CHEMBL3353369)Show SMILES OC(=O)[C@@H](Cc1cc2ccc(F)cc2[nH]1)NC(=O)CC1c2ccccc2-c2ccccc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pin1 (unknown origin) |
Bioorg Med Chem Lett 24: 4187-91 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.044
BindingDB Entry DOI: 10.7270/Q2QJ7JX1 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 4
(Homo sapiens (Human)) | BDBM50389116
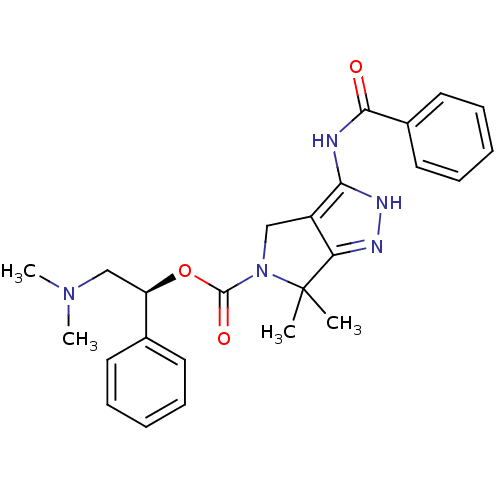
(CHEMBL2064561)Show SMILES CN(C)C[C@@H](OC(=O)N1Cc2c(NC(=O)c3ccccc3)[nH]nc2C1(C)C)c1ccccc1 |r| Show InChI InChI=1S/C25H29N5O3/c1-25(2)21-19(22(28-27-21)26-23(31)18-13-9-6-10-14-18)15-30(25)24(32)33-20(16-29(3)4)17-11-7-5-8-12-17/h5-14,20H,15-16H2,1-4H3,(H2,26,27,28,31)/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PAK4 |
J Med Chem 55: 4728-39 (2012)
Article DOI: 10.1021/jm300204j
BindingDB Entry DOI: 10.7270/Q2BZ673W |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 4
(Homo sapiens (Human)) | BDBM50389117
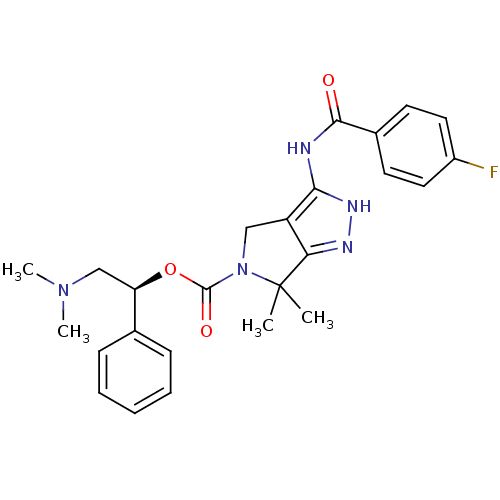
(CHEMBL2064562)Show SMILES CN(C)C[C@@H](OC(=O)N1Cc2c(NC(=O)c3ccc(F)cc3)[nH]nc2C1(C)C)c1ccccc1 |r| Show InChI InChI=1S/C25H28FN5O3/c1-25(2)21-19(22(29-28-21)27-23(32)17-10-12-18(26)13-11-17)14-31(25)24(33)34-20(15-30(3)4)16-8-6-5-7-9-16/h5-13,20H,14-15H2,1-4H3,(H2,27,28,29,32)/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PAK4 |
J Med Chem 55: 4728-39 (2012)
Article DOI: 10.1021/jm300204j
BindingDB Entry DOI: 10.7270/Q2BZ673W |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 4
(Homo sapiens (Human)) | BDBM50389113
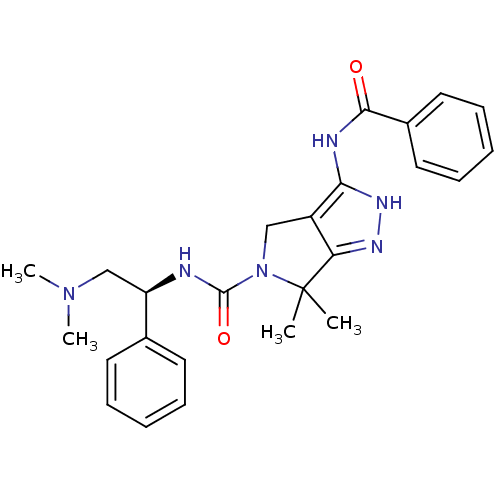
(CHEMBL2064558)Show SMILES CN(C)C[C@@H](NC(=O)N1Cc2c(NC(=O)c3ccccc3)[nH]nc2C1(C)C)c1ccccc1 |r| Show InChI InChI=1S/C25H30N6O2/c1-25(2)21-19(22(29-28-21)27-23(32)18-13-9-6-10-14-18)15-31(25)24(33)26-20(16-30(3)4)17-11-7-5-8-12-17/h5-14,20H,15-16H2,1-4H3,(H,26,33)(H2,27,28,29,32)/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PAK4 |
J Med Chem 55: 4728-39 (2012)
Article DOI: 10.1021/jm300204j
BindingDB Entry DOI: 10.7270/Q2BZ673W |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 4
(Homo sapiens (Human)) | BDBM50389120

(CHEMBL2064565)Show SMILES CN(C)C[C@@H](OC(=O)N1Cc2c(NC(=O)c3ccccn3)[nH]nc2C1(C)C)c1ccccc1 |r| Show InChI InChI=1S/C24H28N6O3/c1-24(2)20-17(21(28-27-20)26-22(31)18-12-8-9-13-25-18)14-30(24)23(32)33-19(15-29(3)4)16-10-6-5-7-11-16/h5-13,19H,14-15H2,1-4H3,(H2,26,27,28,31)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PAK4 |
J Med Chem 55: 4728-39 (2012)
Article DOI: 10.1021/jm300204j
BindingDB Entry DOI: 10.7270/Q2BZ673W |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM34013
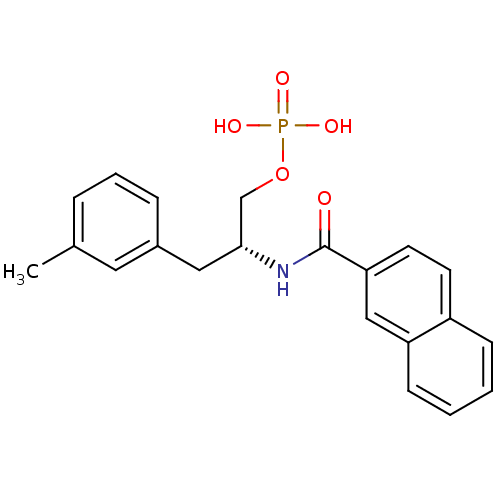
(3-methylphenylalanine derivative, 22a)Show SMILES Cc1cccc(C[C@H](COP(O)(O)=O)NC(=O)c2ccc3ccccc3c2)c1 |r| Show InChI InChI=1S/C21H22NO5P/c1-15-5-4-6-16(11-15)12-20(14-27-28(24,25)26)22-21(23)19-10-9-17-7-2-3-8-18(17)13-19/h2-11,13,20H,12,14H2,1H3,(H,22,23)(H2,24,25,26)/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 32 | -41.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 15 |
Pfizer
| Assay Description
The cis/trans conversion of cis-Suc-AEPFpNA peptide by the PPIase led to the cleavage of para nitroaniline by subtilisin which was monitored at 390 n... |
Bioorg Med Chem Lett 19: 5613-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.034
BindingDB Entry DOI: 10.7270/Q2XD100H |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM34014
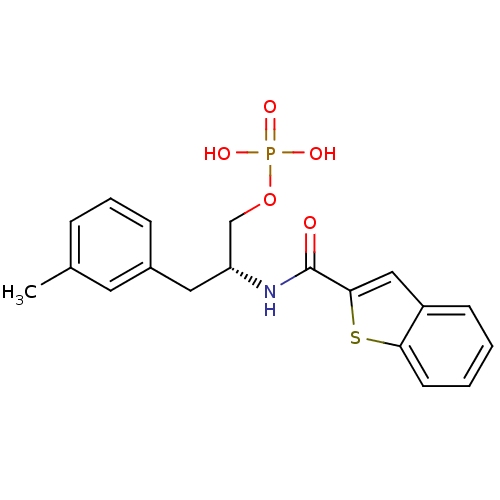
(3-methylphenylalanine derivative, 22b)Show SMILES Cc1cccc(C[C@H](COP(O)(O)=O)NC(=O)c2cc3ccccc3s2)c1 |r| Show InChI InChI=1S/C19H20NO5PS/c1-13-5-4-6-14(9-13)10-16(12-25-26(22,23)24)20-19(21)18-11-15-7-2-3-8-17(15)27-18/h2-9,11,16H,10,12H2,1H3,(H,20,21)(H2,22,23,24)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 57 | -40.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 15 |
Pfizer
| Assay Description
The cis/trans conversion of cis-Suc-AEPFpNA peptide by the PPIase led to the cleavage of para nitroaniline by subtilisin which was monitored at 390 n... |
Bioorg Med Chem Lett 19: 5613-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.034
BindingDB Entry DOI: 10.7270/Q2XD100H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase PAK 4
(Homo sapiens (Human)) | BDBM50389109
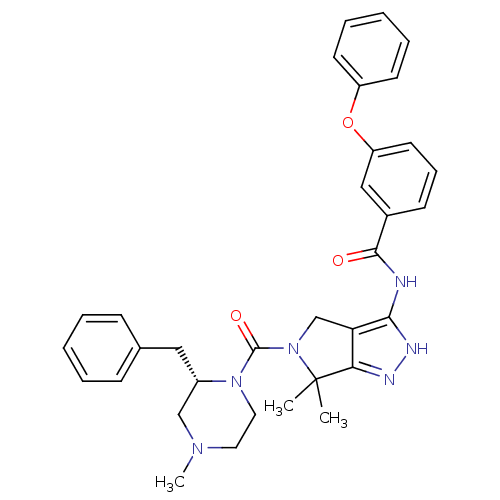
(CHEMBL2064557)Show SMILES CN1CCN([C@@H](Cc2ccccc2)C1)C(=O)N1Cc2c(NC(=O)c3cccc(Oc4ccccc4)c3)[nH]nc2C1(C)C |r| Show InChI InChI=1S/C33H36N6O3/c1-33(2)29-28(22-39(33)32(41)38-18-17-37(3)21-25(38)19-23-11-6-4-7-12-23)30(36-35-29)34-31(40)24-13-10-16-27(20-24)42-26-14-8-5-9-15-26/h4-16,20,25H,17-19,21-22H2,1-3H3,(H2,34,35,36,40)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PAK4 |
J Med Chem 55: 4728-39 (2012)
Article DOI: 10.1021/jm300204j
BindingDB Entry DOI: 10.7270/Q2BZ673W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase PAK 4
(Homo sapiens (Human)) | BDBM50389119
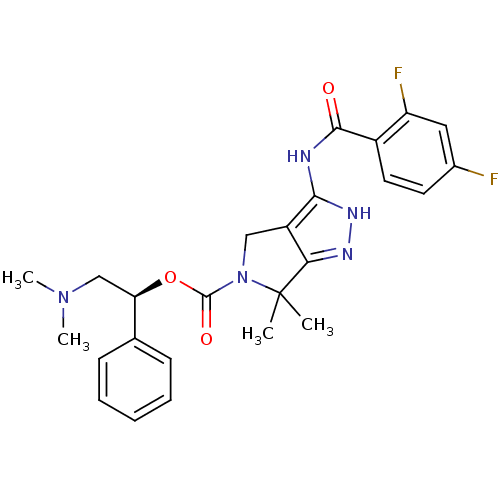
(CHEMBL2064564)Show SMILES CN(C)C[C@@H](OC(=O)N1Cc2c(NC(=O)c3ccc(F)cc3F)[nH]nc2C1(C)C)c1ccccc1 |r| Show InChI InChI=1S/C25H27F2N5O3/c1-25(2)21-18(22(30-29-21)28-23(33)17-11-10-16(26)12-19(17)27)13-32(25)24(34)35-20(14-31(3)4)15-8-6-5-7-9-15/h5-12,20H,13-14H2,1-4H3,(H2,28,29,30,33)/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PAK4 |
J Med Chem 55: 4728-39 (2012)
Article DOI: 10.1021/jm300204j
BindingDB Entry DOI: 10.7270/Q2BZ673W |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50056216

(CHEMBL3353368)Show SMILES OC(=O)[C@@H](Cc1cc2ccc(F)cc2[nH]1)NC(=O)CCc1c(Cl)cccc1Cl |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pin1 (unknown origin) |
Bioorg Med Chem Lett 24: 4187-91 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.044
BindingDB Entry DOI: 10.7270/Q2QJ7JX1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50056208

(CHEMBL3322220)Show SMILES OC(=O)[C@@H](Cc1nc2cc(F)ccc2[nH]1)NC(=O)c1cc2ccccc2s1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pin1 (unknown origin) |
Bioorg Med Chem Lett 24: 4187-91 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.044
BindingDB Entry DOI: 10.7270/Q2QJ7JX1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM34015
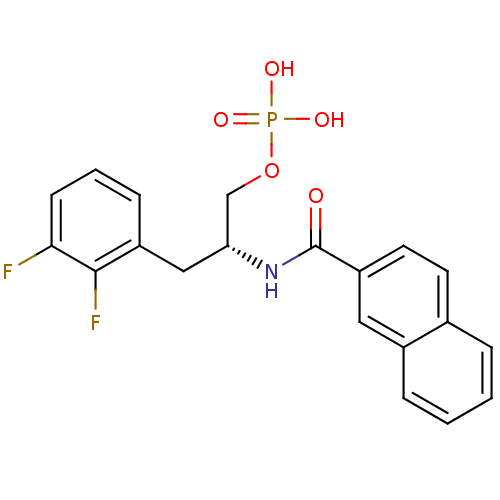
(2,3-difluorophenylalanine derivative, 23a)Show SMILES OP(O)(=O)OC[C@@H](Cc1cccc(F)c1F)NC(=O)c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C20H18F2NO5P/c21-18-7-3-6-15(19(18)22)11-17(12-28-29(25,26)27)23-20(24)16-9-8-13-4-1-2-5-14(13)10-16/h1-10,17H,11-12H2,(H,23,24)(H2,25,26,27)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 78 | -39.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 15 |
Pfizer
| Assay Description
The cis/trans conversion of cis-Suc-AEPFpNA peptide by the PPIase led to the cleavage of para nitroaniline by subtilisin which was monitored at 390 n... |
Bioorg Med Chem Lett 19: 5613-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.034
BindingDB Entry DOI: 10.7270/Q2XD100H |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50056214
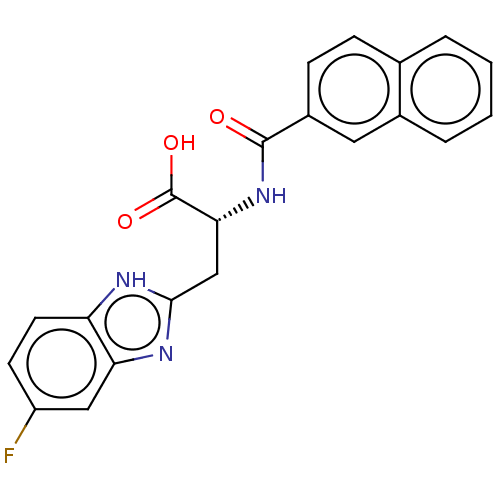
(CHEMBL3322219)Show SMILES OC(=O)[C@@H](Cc1nc2cc(F)ccc2[nH]1)NC(=O)c1ccc2ccccc2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pin1 (unknown origin) |
Bioorg Med Chem Lett 24: 4187-91 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.044
BindingDB Entry DOI: 10.7270/Q2QJ7JX1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM34016
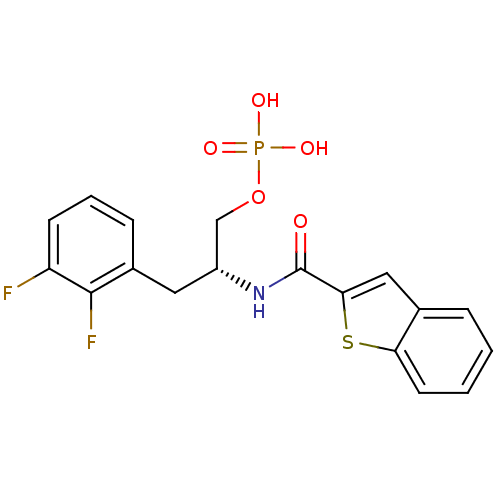
(2,3-difluorophenylalanine derivative, 23b)Show SMILES OP(O)(=O)OC[C@@H](Cc1cccc(F)c1F)NC(=O)c1cc2ccccc2s1 |r| Show InChI InChI=1S/C18H16F2NO5PS/c19-14-6-3-5-12(17(14)20)8-13(10-26-27(23,24)25)21-18(22)16-9-11-4-1-2-7-15(11)28-16/h1-7,9,13H,8,10H2,(H,21,22)(H2,23,24,25)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 89 | -38.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 15 |
Pfizer
| Assay Description
The cis/trans conversion of cis-Suc-AEPFpNA peptide by the PPIase led to the cleavage of para nitroaniline by subtilisin which was monitored at 390 n... |
Bioorg Med Chem Lett 19: 5613-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.034
BindingDB Entry DOI: 10.7270/Q2XD100H |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 4
(Homo sapiens (Human)) | BDBM50389118
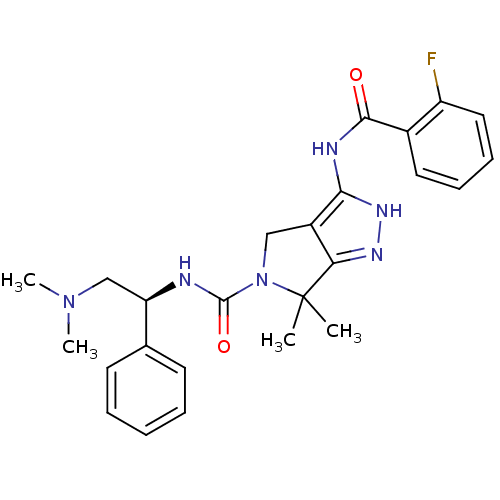
(CHEMBL2064563)Show SMILES CN(C)C[C@@H](NC(=O)N1Cc2c(NC(=O)c3ccccc3F)[nH]nc2C1(C)C)c1ccccc1 |r| Show InChI InChI=1S/C25H29FN6O2/c1-25(2)21-18(22(30-29-21)28-23(33)17-12-8-9-13-19(17)26)14-32(25)24(34)27-20(15-31(3)4)16-10-6-5-7-11-16/h5-13,20H,14-15H2,1-4H3,(H,27,34)(H2,28,29,30,33)/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PAK4 |
J Med Chem 55: 4728-39 (2012)
Article DOI: 10.1021/jm300204j
BindingDB Entry DOI: 10.7270/Q2BZ673W |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM34009
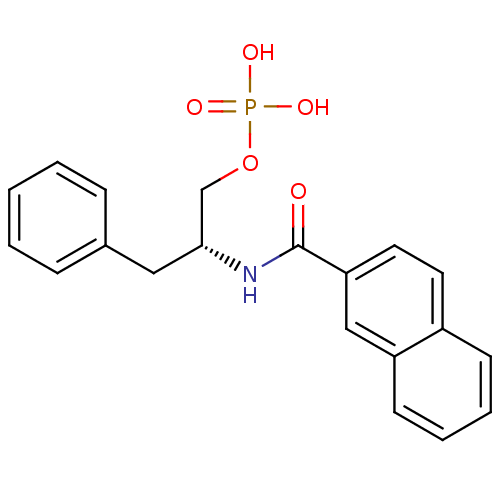
(naphthalene carboxamide, 18a)Show SMILES OP(O)(=O)OC[C@@H](Cc1ccccc1)NC(=O)c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C20H20NO5P/c22-20(18-11-10-16-8-4-5-9-17(16)13-18)21-19(14-26-27(23,24)25)12-15-6-2-1-3-7-15/h1-11,13,19H,12,14H2,(H,21,22)(H2,23,24,25)/t19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 100 | -38.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 15 |
Pfizer
| Assay Description
The cis/trans conversion of cis-Suc-AEPFpNA peptide by the PPIase led to the cleavage of para nitroaniline by subtilisin which was monitored at 390 n... |
Bioorg Med Chem Lett 19: 5613-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.034
BindingDB Entry DOI: 10.7270/Q2XD100H |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50056211
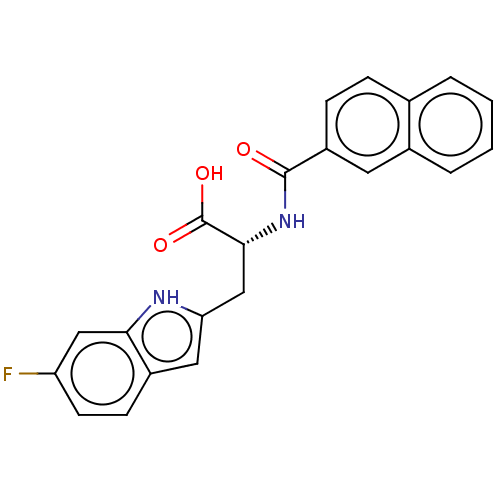
(CHEMBL3322225)Show SMILES OC(=O)[C@@H](Cc1cc2ccc(F)cc2[nH]1)NC(=O)c1ccc2ccccc2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pin1 (unknown origin) |
Bioorg Med Chem Lett 24: 4187-91 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.044
BindingDB Entry DOI: 10.7270/Q2QJ7JX1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50056204
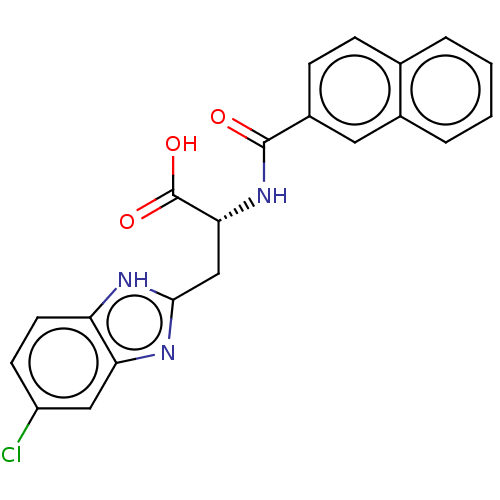
(CHEMBL3322215)Show SMILES OC(=O)[C@@H](Cc1nc2cc(Cl)ccc2[nH]1)NC(=O)c1ccc2ccccc2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 173 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pin1 (unknown origin) |
Bioorg Med Chem Lett 24: 4187-91 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.044
BindingDB Entry DOI: 10.7270/Q2QJ7JX1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM34010

(benzothiophene carboxamide, 18b)Show SMILES OP(O)(=O)OC[C@@H](Cc1ccccc1)NC(=O)c1cc2ccccc2s1 |r| Show InChI InChI=1S/C18H18NO5PS/c20-18(17-11-14-8-4-5-9-16(14)26-17)19-15(12-24-25(21,22)23)10-13-6-2-1-3-7-13/h1-9,11,15H,10,12H2,(H,19,20)(H2,21,22,23)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 179 | -37.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 15 |
Pfizer
| Assay Description
The cis/trans conversion of cis-Suc-AEPFpNA peptide by the PPIase led to the cleavage of para nitroaniline by subtilisin which was monitored at 390 n... |
Bioorg Med Chem Lett 19: 5613-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.034
BindingDB Entry DOI: 10.7270/Q2XD100H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50314715

((R)-N-(1-hydroxy-3-phenylpropan-2-yl)benzo[b]thiop...)Show InChI InChI=1S/C18H17NO2S/c20-12-15(10-13-6-2-1-3-7-13)19-18(21)17-11-14-8-4-5-9-16(14)22-17/h1-9,11,15,20H,10,12H2,(H,19,21)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 179 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of WFYpSPFLE from human Pin1 catalytic domain after 10-20 mins by fluorescence polarization assay |
Bioorg Med Chem Lett 20: 2210-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.033
BindingDB Entry DOI: 10.7270/Q2GX4BPW |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50056205
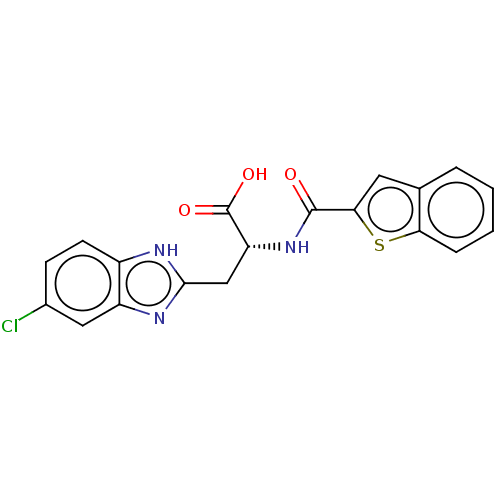
(CHEMBL3322216)Show SMILES OC(=O)[C@@H](Cc1nc2cc(Cl)ccc2[nH]1)NC(=O)c1cc2ccccc2s1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 189 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pin1 (unknown origin) |
Bioorg Med Chem Lett 24: 4187-91 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.044
BindingDB Entry DOI: 10.7270/Q2QJ7JX1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50056219

(CHEMBL3322230)Show SMILES OC(=O)C1(Cc2cc3ccc(F)cc3[nH]2)CSC(CCc2c(Cl)cccc2Cl)=N1 |c:30| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 196 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pin1 (unknown origin) |
Bioorg Med Chem Lett 24: 4187-91 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.044
BindingDB Entry DOI: 10.7270/Q2QJ7JX1 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 4
(Homo sapiens (Human)) | BDBM50389115
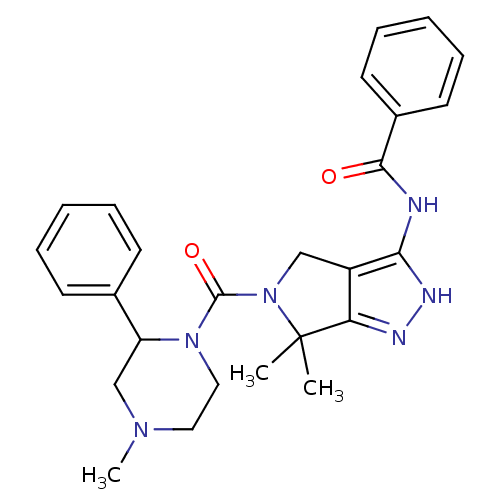
(CHEMBL2064560)Show SMILES CN1CCN(C(C1)c1ccccc1)C(=O)N1Cc2c(NC(=O)c3ccccc3)[nH]nc2C1(C)C Show InChI InChI=1S/C26H30N6O2/c1-26(2)22-20(23(29-28-22)27-24(33)19-12-8-5-9-13-19)16-32(26)25(34)31-15-14-30(3)17-21(31)18-10-6-4-7-11-18/h4-13,21H,14-17H2,1-3H3,(H2,27,28,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 207 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PAK4 |
J Med Chem 55: 4728-39 (2012)
Article DOI: 10.1021/jm300204j
BindingDB Entry DOI: 10.7270/Q2BZ673W |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50056220
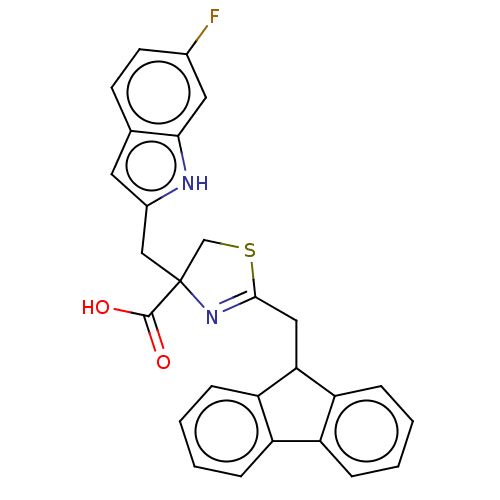
(CHEMBL3322295)Show SMILES OC(=O)C1(Cc2cc3ccc(F)cc3[nH]2)CSC(CC2c3ccccc3-c3ccccc23)=N1 |c:36| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pin1 (unknown origin) |
Bioorg Med Chem Lett 24: 4187-91 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.044
BindingDB Entry DOI: 10.7270/Q2QJ7JX1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50056215

(CHEMBL3353367)Show SMILES OC(=O)[C@@H](Cc1cc2ccc(F)cc2[nH]1)NC(=O)Cc1cccc2ccccc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pin1 (unknown origin) |
Bioorg Med Chem Lett 24: 4187-91 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.044
BindingDB Entry DOI: 10.7270/Q2QJ7JX1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50056207

(CHEMBL3322218)Show SMILES Cc1ccc2[nH]c(C[C@@H](NC(=O)c3cc4ccccc4s3)C(O)=O)nc2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 424 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pin1 (unknown origin) |
Bioorg Med Chem Lett 24: 4187-91 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.044
BindingDB Entry DOI: 10.7270/Q2QJ7JX1 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 4
(Homo sapiens (Human)) | BDBM50389114
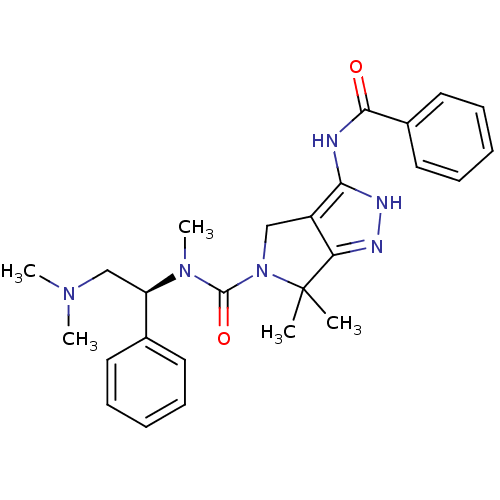
(CHEMBL2064559)Show SMILES CN(C)C[C@@H](N(C)C(=O)N1Cc2c(NC(=O)c3ccccc3)[nH]nc2C1(C)C)c1ccccc1 |r| Show InChI InChI=1S/C26H32N6O2/c1-26(2)22-20(23(29-28-22)27-24(33)19-14-10-7-11-15-19)16-32(26)25(34)31(5)21(17-30(3)4)18-12-8-6-9-13-18/h6-15,21H,16-17H2,1-5H3,(H2,27,28,29,33)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 512 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PAK4 |
J Med Chem 55: 4728-39 (2012)
Article DOI: 10.1021/jm300204j
BindingDB Entry DOI: 10.7270/Q2BZ673W |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM34008

(amide, 17c)Show SMILES OP(O)(=O)OC[C@@H](Cc1ccccc1)NC(=O)c1ccccc1 |r| Show InChI InChI=1S/C16H18NO5P/c18-16(14-9-5-2-6-10-14)17-15(12-22-23(19,20)21)11-13-7-3-1-4-8-13/h1-10,15H,11-12H2,(H,17,18)(H2,19,20,21)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 525 | -34.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 15 |
Pfizer
| Assay Description
The cis/trans conversion of cis-Suc-AEPFpNA peptide by the PPIase led to the cleavage of para nitroaniline by subtilisin which was monitored at 390 n... |
Bioorg Med Chem Lett 19: 5613-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.034
BindingDB Entry DOI: 10.7270/Q2XD100H |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50056203

(CHEMBL3322296)Show SMILES OC(=O)C1(Cc2cc3ccc(F)cc3[nH]2)CSC(\C=C\c2c(Cl)cccc2Cl)=N1 |c:30| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pin1 (unknown origin) |
Bioorg Med Chem Lett 24: 4187-91 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.044
BindingDB Entry DOI: 10.7270/Q2QJ7JX1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50056206

(CHEMBL3322217)Show SMILES Cc1ccc2[nH]c(C[C@@H](NC(=O)c3ccc4ccccc4c3)C(O)=O)nc2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pin1 (unknown origin) |
Bioorg Med Chem Lett 24: 4187-91 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.044
BindingDB Entry DOI: 10.7270/Q2QJ7JX1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM34005
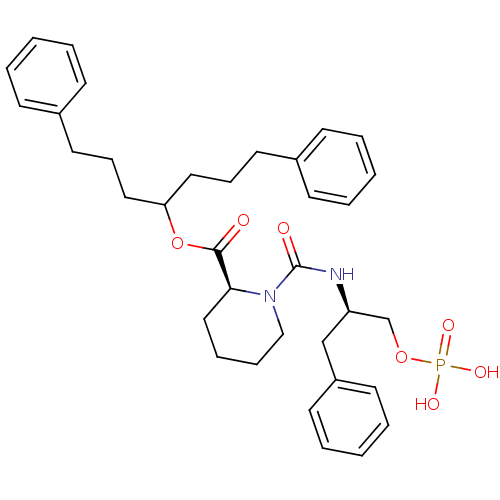
(pipecolate deriv., 12b)Show SMILES OP(O)(=O)OC[C@@H](Cc1ccccc1)NC(=O)N1CCCC[C@H]1C(=O)OC(CCCc1ccccc1)CCCc1ccccc1 |r| Show InChI InChI=1S/C35H45N2O7P/c38-34(44-32(22-12-20-28-14-4-1-5-15-28)23-13-21-29-16-6-2-7-17-29)33-24-10-11-25-37(33)35(39)36-31(27-43-45(40,41)42)26-30-18-8-3-9-19-30/h1-9,14-19,31-33H,10-13,20-27H2,(H,36,39)(H2,40,41,42)/t31-,33+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 800 | -33.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 15 |
Pfizer
| Assay Description
The cis/trans conversion of cis-Suc-AEPFpNA peptide by the PPIase led to the cleavage of para nitroaniline by subtilisin which was monitored at 390 n... |
Bioorg Med Chem Lett 19: 5613-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.034
BindingDB Entry DOI: 10.7270/Q2XD100H |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50314706

((R)-2-(2-naphthamido)-5-(3-fluorophenyl)pent-4-eno...)Show SMILES OC(=O)[C@@H](C\C=C\c1cccc(F)c1)NC(=O)c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C22H18FNO3/c23-19-9-3-5-15(13-19)6-4-10-20(22(26)27)24-21(25)18-12-11-16-7-1-2-8-17(16)14-18/h1-9,11-14,20H,10H2,(H,24,25)(H,26,27)/b6-4+/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of WFYpSPFLE from human Pin1 catalytic domain after 10-20 mins by fluorescence polarization assay |
Bioorg Med Chem Lett 20: 2210-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.033
BindingDB Entry DOI: 10.7270/Q2GX4BPW |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50056210
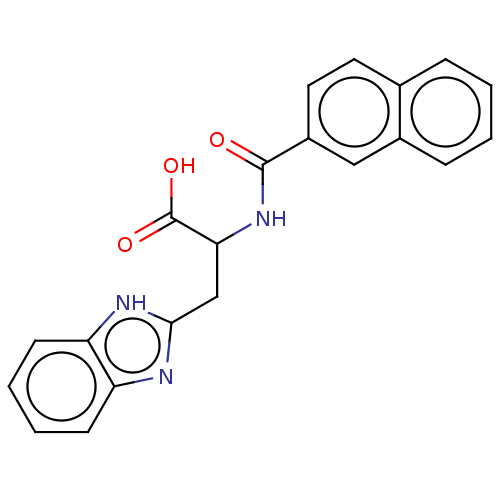
(CHEMBL3322222)Show SMILES OC(=O)C(Cc1nc2ccccc2[nH]1)NC(=O)c1ccc2ccccc2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pin1 (unknown origin) |
Bioorg Med Chem Lett 24: 4187-91 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.044
BindingDB Entry DOI: 10.7270/Q2QJ7JX1 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50389117

(CHEMBL2064562)Show SMILES CN(C)C[C@@H](OC(=O)N1Cc2c(NC(=O)c3ccc(F)cc3)[nH]nc2C1(C)C)c1ccccc1 |r| Show InChI InChI=1S/C25H28FN5O3/c1-25(2)21-19(22(29-28-21)27-23(32)17-10-12-18(26)13-11-17)14-31(25)24(33)34-20(15-30(3)4)16-8-6-5-7-9-16/h5-13,20H,14-15H2,1-4H3,(H2,27,28,29,32)/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 |
J Med Chem 55: 4728-39 (2012)
Article DOI: 10.1021/jm300204j
BindingDB Entry DOI: 10.7270/Q2BZ673W |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50056218

(CHEMBL3322229)Show SMILES OC(=O)C1(Cc2cc3ccc(F)cc3[nH]2)CSC(=N1)c1ccc2ccccc2c1 |c:19| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pin1 (unknown origin) |
Bioorg Med Chem Lett 24: 4187-91 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.044
BindingDB Entry DOI: 10.7270/Q2QJ7JX1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM34003

(pipecolate deriv., 11)Show SMILES OP(O)(=O)OC[C@@H](Cc1ccccc1)NC(=O)N1CCCC[C@H]1C(=O)OCCCCc1ccccc1 |r| Show InChI InChI=1S/C26H35N2O7P/c29-25(34-18-10-8-13-21-11-3-1-4-12-21)24-16-7-9-17-28(24)26(30)27-23(20-35-36(31,32)33)19-22-14-5-2-6-15-22/h1-6,11-12,14-15,23-24H,7-10,13,16-20H2,(H,27,30)(H2,31,32,33)/t23-,24+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70E+3 | -31.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 15 |
Pfizer
| Assay Description
The cis/trans conversion of cis-Suc-AEPFpNA peptide by the PPIase led to the cleavage of para nitroaniline by subtilisin which was monitored at 390 n... |
Bioorg Med Chem Lett 19: 5613-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.034
BindingDB Entry DOI: 10.7270/Q2XD100H |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50314705
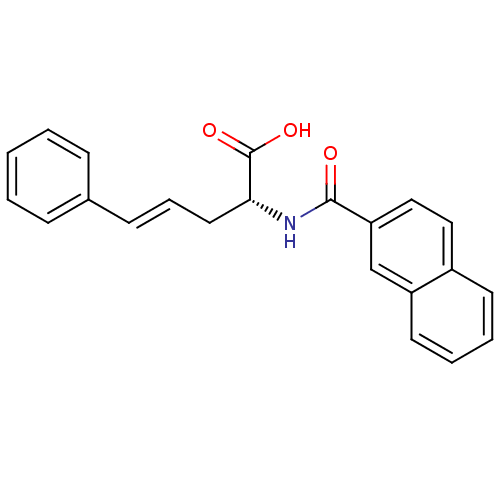
((2R,4E)-2-[(naphthalen-2-ylcarbonyl)amino]-5-pheny...)Show SMILES OC(=O)[C@@H](C\C=C\c1ccccc1)NC(=O)c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C22H19NO3/c24-21(19-14-13-17-10-4-5-11-18(17)15-19)23-20(22(25)26)12-6-9-16-7-2-1-3-8-16/h1-11,13-15,20H,12H2,(H,23,24)(H,25,26)/b9-6+/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of WFYpSPFLE from human Pin1 catalytic domain after 10-20 mins by fluorescence polarization assay |
Bioorg Med Chem Lett 20: 2210-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.033
BindingDB Entry DOI: 10.7270/Q2GX4BPW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50389113

(CHEMBL2064558)Show SMILES CN(C)C[C@@H](NC(=O)N1Cc2c(NC(=O)c3ccccc3)[nH]nc2C1(C)C)c1ccccc1 |r| Show InChI InChI=1S/C25H30N6O2/c1-25(2)21-19(22(29-28-21)27-23(32)18-13-9-6-10-14-18)15-31(25)24(33)26-20(16-30(3)4)17-11-7-5-8-12-17/h5-14,20H,15-16H2,1-4H3,(H,26,33)(H2,27,28,29,32)/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 |
J Med Chem 55: 4728-39 (2012)
Article DOI: 10.1021/jm300204j
BindingDB Entry DOI: 10.7270/Q2BZ673W |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50314682
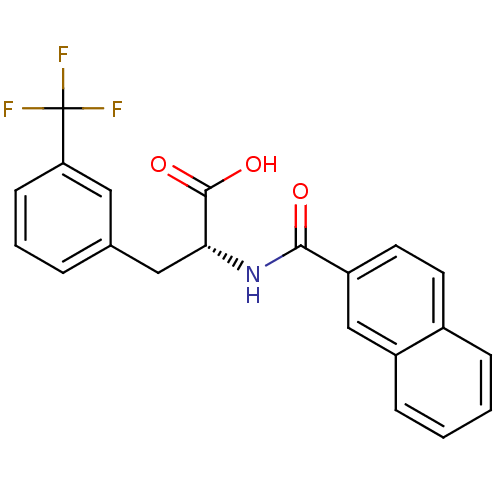
((R)-2-(2-naphthamido)-3-(3-(trifluoromethyl)phenyl...)Show SMILES OC(=O)[C@@H](Cc1cccc(c1)C(F)(F)F)NC(=O)c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C21H16F3NO3/c22-21(23,24)17-7-3-4-13(10-17)11-18(20(27)28)25-19(26)16-9-8-14-5-1-2-6-15(14)12-16/h1-10,12,18H,11H2,(H,25,26)(H,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of WFYpSPFLE from human Pin1 catalytic domain after 10-20 mins by fluorescence polarization assay |
Bioorg Med Chem Lett 20: 2210-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.033
BindingDB Entry DOI: 10.7270/Q2GX4BPW |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50314701
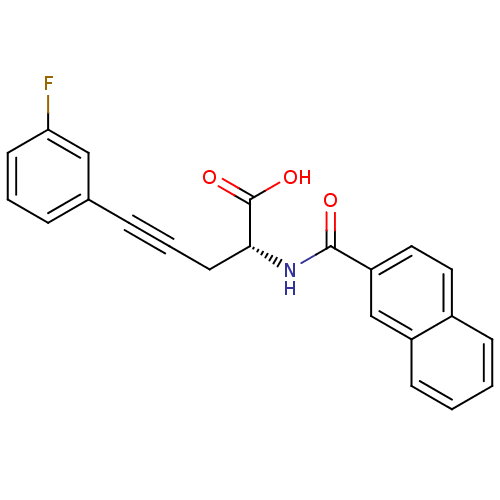
((R)-2-(2-naphthamido)-5-(3-fluorophenyl)pent-4-yno...)Show SMILES OC(=O)[C@@H](CC#Cc1cccc(F)c1)NC(=O)c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C22H16FNO3/c23-19-9-3-5-15(13-19)6-4-10-20(22(26)27)24-21(25)18-12-11-16-7-1-2-8-17(16)14-18/h1-3,5,7-9,11-14,20H,10H2,(H,24,25)(H,26,27)/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.87E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of WFYpSPFLE from human Pin1 catalytic domain after 10-20 mins by fluorescence polarization assay |
Bioorg Med Chem Lett 20: 2210-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.033
BindingDB Entry DOI: 10.7270/Q2GX4BPW |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50056212

(CHEMBL3322224)Show SMILES OC(=O)C(Cc1cc2ccccc2[nH]1)NC(=O)c1ccc2ccccc2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pin1 (unknown origin) |
Bioorg Med Chem Lett 24: 4187-91 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.044
BindingDB Entry DOI: 10.7270/Q2QJ7JX1 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50389116

(CHEMBL2064561)Show SMILES CN(C)C[C@@H](OC(=O)N1Cc2c(NC(=O)c3ccccc3)[nH]nc2C1(C)C)c1ccccc1 |r| Show InChI InChI=1S/C25H29N5O3/c1-25(2)21-19(22(28-27-21)26-23(31)18-13-9-6-10-14-18)15-30(25)24(32)33-20(16-29(3)4)17-11-7-5-8-12-17/h5-14,20H,15-16H2,1-4H3,(H2,26,27,28,31)/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 |
J Med Chem 55: 4728-39 (2012)
Article DOI: 10.1021/jm300204j
BindingDB Entry DOI: 10.7270/Q2BZ673W |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50314708
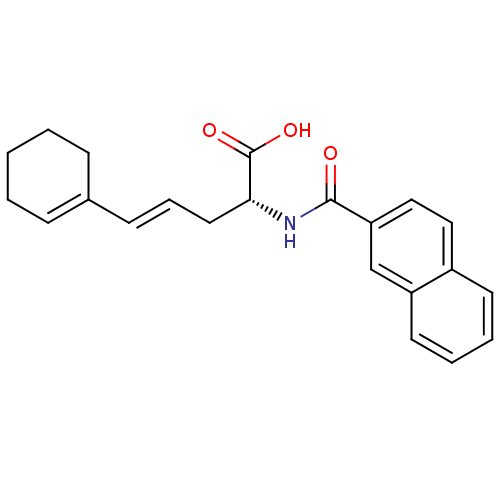
((R)-2-(2-naphthamido)-5-cyclohexenylpent-4-enoic a...)Show SMILES OC(=O)[C@@H](C\C=C\C1=CCCCC1)NC(=O)c1ccc2ccccc2c1 |r,t:7| Show InChI InChI=1S/C22H23NO3/c24-21(19-14-13-17-10-4-5-11-18(17)15-19)23-20(22(25)26)12-6-9-16-7-2-1-3-8-16/h4-7,9-11,13-15,20H,1-3,8,12H2,(H,23,24)(H,25,26)/b9-6+/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of WFYpSPFLE from human Pin1 catalytic domain after 10-20 mins by fluorescence polarization assay |
Bioorg Med Chem Lett 20: 2210-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.033
BindingDB Entry DOI: 10.7270/Q2GX4BPW |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50314685
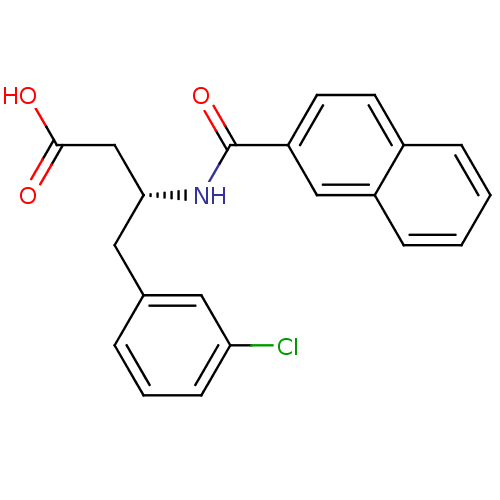
((R)-3-(2-naphthamido)-4-(3-chlorophenyl)butanoic a...)Show SMILES OC(=O)C[C@@H](Cc1cccc(Cl)c1)NC(=O)c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C21H18ClNO3/c22-18-7-3-4-14(10-18)11-19(13-20(24)25)23-21(26)17-9-8-15-5-1-2-6-16(15)12-17/h1-10,12,19H,11,13H2,(H,23,26)(H,24,25)/t19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of WFYpSPFLE from human Pin1 catalytic domain after 10-20 mins by fluorescence polarization assay |
Bioorg Med Chem Lett 20: 2210-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.033
BindingDB Entry DOI: 10.7270/Q2GX4BPW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data