

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Heparanase (Homo sapiens (Human)) | BDBM50388329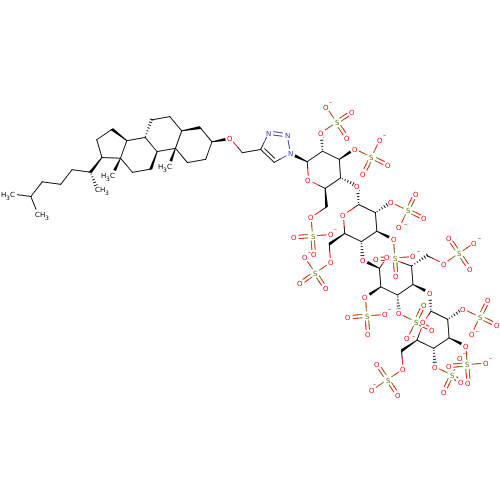 (CHEMBL2059500) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant heparanase after 2 to 24 hrs by WST1 dye based fondaparinux assay | J Med Chem 55: 3804-13 (2012) Article DOI: 10.1021/jm201708h BindingDB Entry DOI: 10.7270/Q2G161WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50388343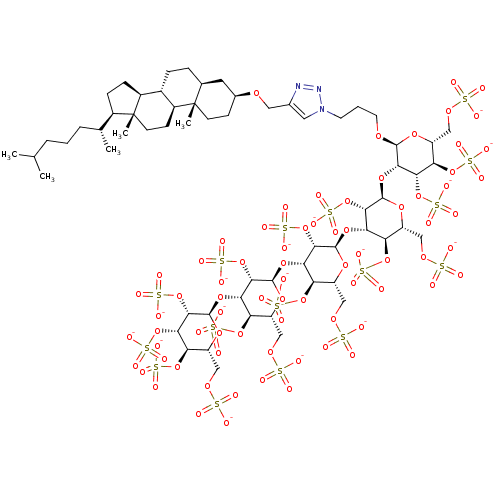 (CHEMBL2059243) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant heparanase after 2 to 24 hrs by WST1 dye based fondaparinux assay | J Med Chem 55: 3804-13 (2012) Article DOI: 10.1021/jm201708h BindingDB Entry DOI: 10.7270/Q2G161WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50388341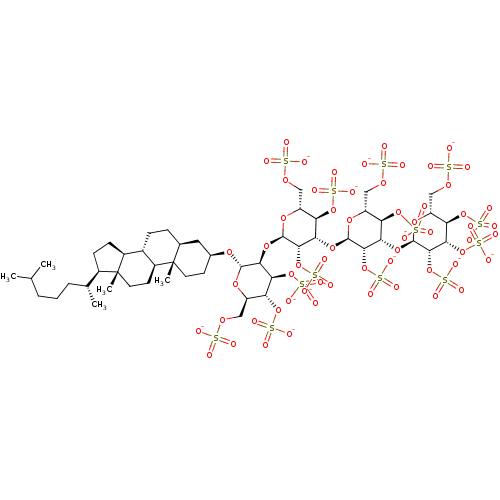 (CHEMBL2059241) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant heparanase after 2 to 24 hrs by WST1 dye based fondaparinux assay | J Med Chem 55: 3804-13 (2012) Article DOI: 10.1021/jm201708h BindingDB Entry DOI: 10.7270/Q2G161WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50388342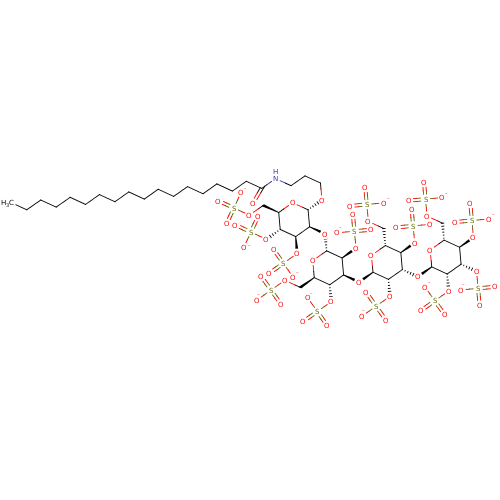 (CHEMBL2059242) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant heparanase after 2 to 24 hrs by WST1 dye based fondaparinux assay | J Med Chem 55: 3804-13 (2012) Article DOI: 10.1021/jm201708h BindingDB Entry DOI: 10.7270/Q2G161WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50388331 (CHEMBL2059499) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant heparanase after 2 to 24 hrs by WST1 dye based fondaparinux assay | J Med Chem 55: 3804-13 (2012) Article DOI: 10.1021/jm201708h BindingDB Entry DOI: 10.7270/Q2G161WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50388345 (CHEMBL2059245) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant heparanase after 2 to 24 hrs by WST1 dye based fondaparinux assay | J Med Chem 55: 3804-13 (2012) Article DOI: 10.1021/jm201708h BindingDB Entry DOI: 10.7270/Q2G161WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50378647 (CHEMBL1627122 | PI-88) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant heparanase after 2 to 24 hrs by WST1 dye based fondaparinux assay | J Med Chem 55: 3804-13 (2012) Article DOI: 10.1021/jm201708h BindingDB Entry DOI: 10.7270/Q2G161WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50388332 (CHEMBL2059498) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant heparanase after 2 to 24 hrs by WST1 dye based fondaparinux assay | J Med Chem 55: 3804-13 (2012) Article DOI: 10.1021/jm201708h BindingDB Entry DOI: 10.7270/Q2G161WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50388346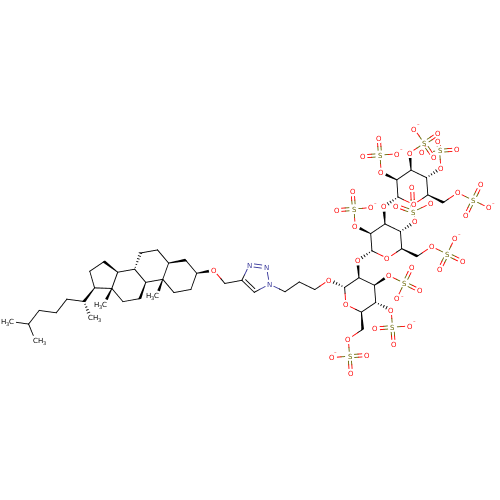 (CHEMBL2059246) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant heparanase after 2 to 24 hrs by WST1 dye based fondaparinux assay | J Med Chem 55: 3804-13 (2012) Article DOI: 10.1021/jm201708h BindingDB Entry DOI: 10.7270/Q2G161WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50388335 (CHEMBL2059503) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant heparanase after 2 to 24 hrs by WST1 dye based fondaparinux assay | J Med Chem 55: 3804-13 (2012) Article DOI: 10.1021/jm201708h BindingDB Entry DOI: 10.7270/Q2G161WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50388336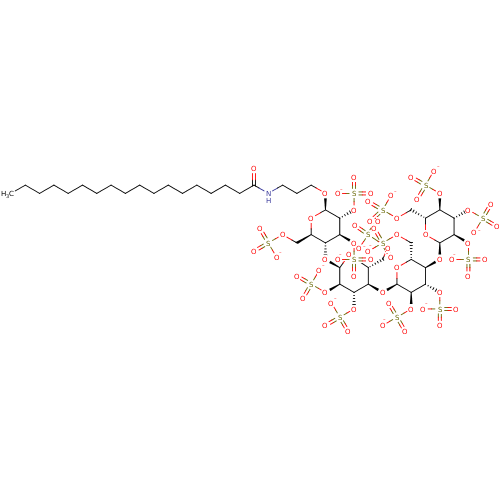 (CHEMBL2059504) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant heparanase after 2 to 24 hrs by WST1 dye based fondaparinux assay | J Med Chem 55: 3804-13 (2012) Article DOI: 10.1021/jm201708h BindingDB Entry DOI: 10.7270/Q2G161WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50388330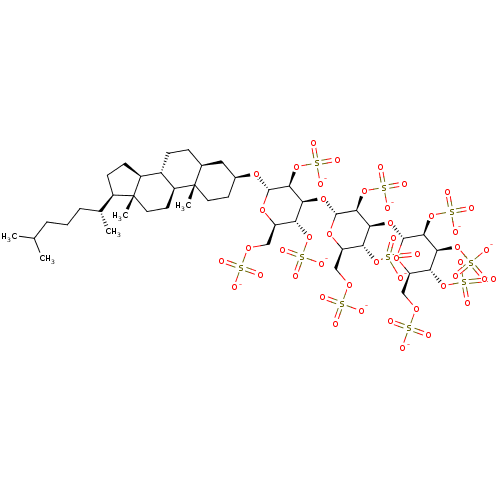 (CHEMBL2059247) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 10.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant heparanase after 2 to 24 hrs by WST1 dye based fondaparinux assay | J Med Chem 55: 3804-13 (2012) Article DOI: 10.1021/jm201708h BindingDB Entry DOI: 10.7270/Q2G161WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50388328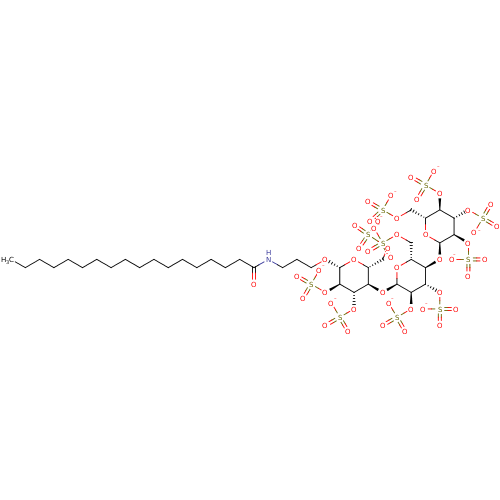 (CHEMBL2059505) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 11.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant heparanase after 2 to 24 hrs by WST1 dye based fondaparinux assay | J Med Chem 55: 3804-13 (2012) Article DOI: 10.1021/jm201708h BindingDB Entry DOI: 10.7270/Q2G161WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50388334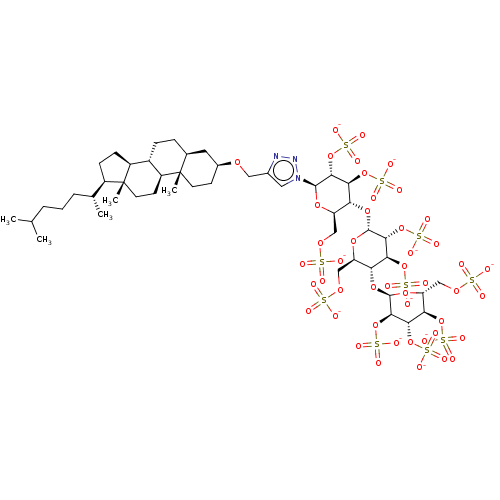 (CHEMBL2059501) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant heparanase after 2 to 24 hrs by WST1 dye based fondaparinux assay | J Med Chem 55: 3804-13 (2012) Article DOI: 10.1021/jm201708h BindingDB Entry DOI: 10.7270/Q2G161WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50388333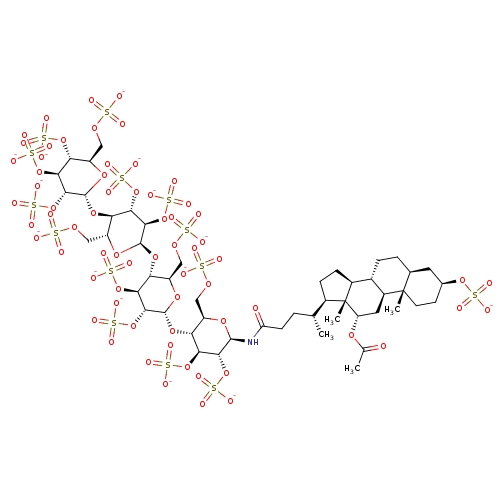 (CHEMBL2059502) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant heparanase after 2 to 24 hrs by WST1 dye based fondaparinux assay | J Med Chem 55: 3804-13 (2012) Article DOI: 10.1021/jm201708h BindingDB Entry DOI: 10.7270/Q2G161WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50388344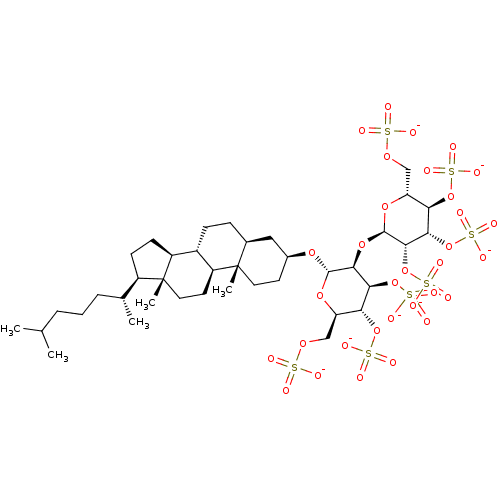 (CHEMBL2059244) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 22.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant heparanase after 2 to 24 hrs by WST1 dye based fondaparinux assay | J Med Chem 55: 3804-13 (2012) Article DOI: 10.1021/jm201708h BindingDB Entry DOI: 10.7270/Q2G161WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50388340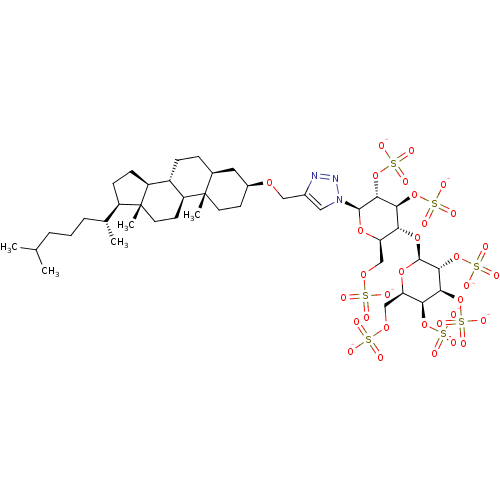 (CHEMBL2059510) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant heparanase after 2 to 24 hrs by WST1 dye based fondaparinux assay | J Med Chem 55: 3804-13 (2012) Article DOI: 10.1021/jm201708h BindingDB Entry DOI: 10.7270/Q2G161WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50388338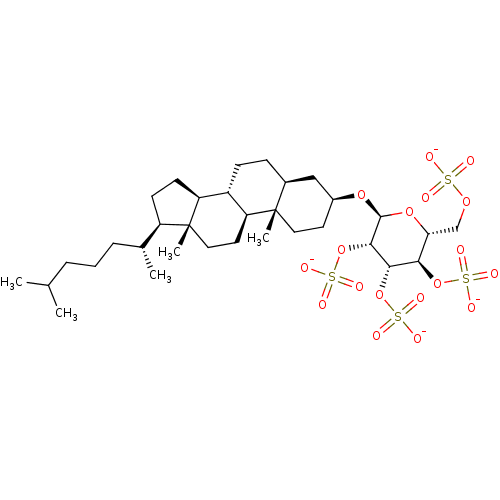 (CHEMBL2059508) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 111 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant heparanase after 2 to 24 hrs by WST1 dye based fondaparinux assay | J Med Chem 55: 3804-13 (2012) Article DOI: 10.1021/jm201708h BindingDB Entry DOI: 10.7270/Q2G161WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50375292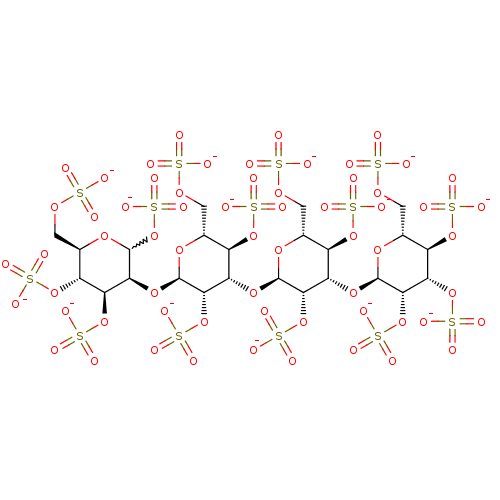 (CHEMBL407200) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited Curated by ChEMBL | Assay Description Inhibition of human platelet heparanase by competitive binding assay | Bioorg Med Chem 16: 699-709 (2008) Article DOI: 10.1016/j.bmc.2007.10.044 BindingDB Entry DOI: 10.7270/Q2SQ918D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50375290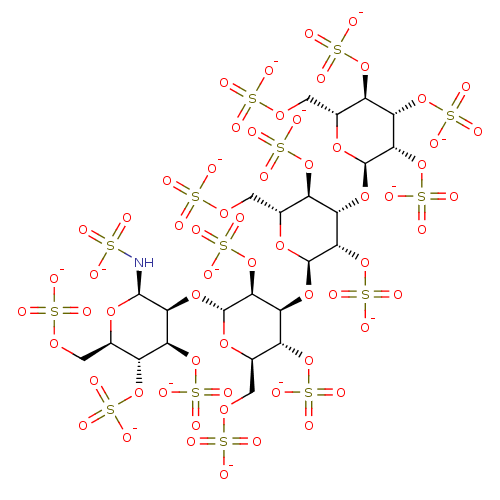 (CHEMBL279625) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited Curated by ChEMBL | Assay Description Inhibition of human platelet heparanase by competitive binding assay | Bioorg Med Chem 16: 699-709 (2008) Article DOI: 10.1016/j.bmc.2007.10.044 BindingDB Entry DOI: 10.7270/Q2SQ918D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50375291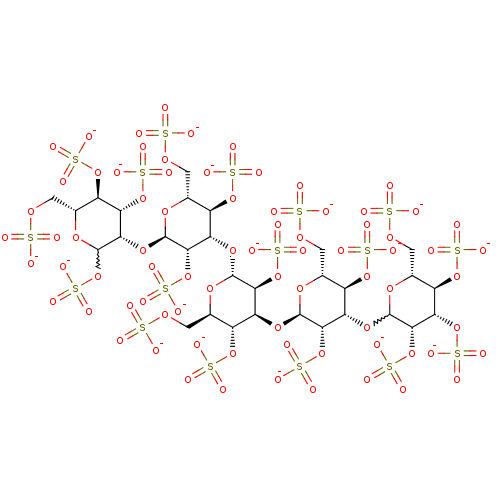 (CHEMBL439118) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited Curated by ChEMBL | Assay Description Inhibition of human platelet heparanase by competitive binding assay | Bioorg Med Chem 16: 699-709 (2008) Article DOI: 10.1016/j.bmc.2007.10.044 BindingDB Entry DOI: 10.7270/Q2SQ918D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50375287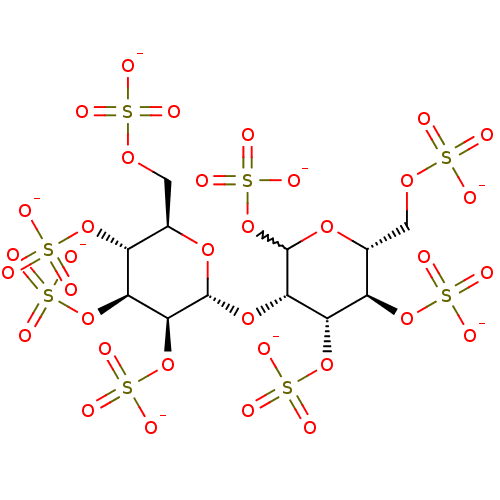 (CHEMBL260220) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited Curated by ChEMBL | Assay Description Inhibition of human platelet heparanase by competitive binding assay | Bioorg Med Chem 16: 699-709 (2008) Article DOI: 10.1016/j.bmc.2007.10.044 BindingDB Entry DOI: 10.7270/Q2SQ918D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50375287 (CHEMBL260220) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 6.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited Curated by ChEMBL | Assay Description Inhibition of human platelet heparanase by uncompetitive binding assay | Bioorg Med Chem 16: 699-709 (2008) Article DOI: 10.1016/j.bmc.2007.10.044 BindingDB Entry DOI: 10.7270/Q2SQ918D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1A (Homo sapiens (Human)) | BDBM528351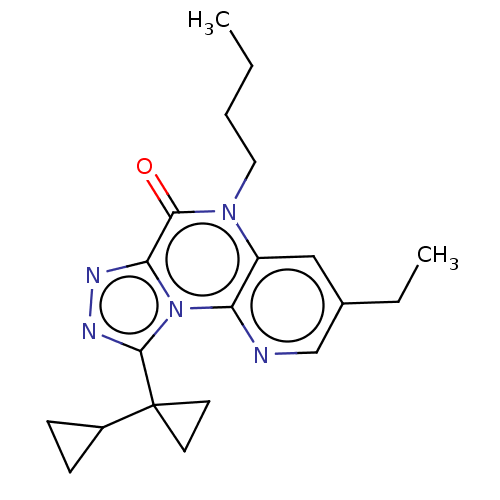 (9-([1,1'- Bi(cyclopropan)]-1- yl)-5-butyl-3- ethyl...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.214 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description PDE1B, PDE1A, and PDE1C are cloned and purified following standard protein generation procedures. The assay buffer is prepared to give a final concen... | Citation and Details BindingDB Entry DOI: 10.7270/Q27947WZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1A (Homo sapiens (Human)) | BDBM528352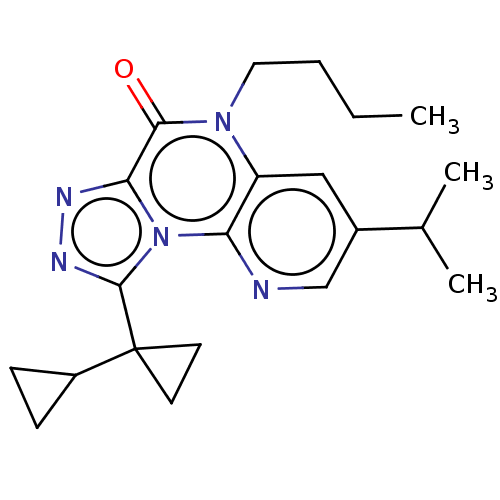 (9-([1,1'-bi(cyclopropan)]-1-yl)-5-butyl-3-isopropy...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description PDE1B, PDE1A, and PDE1C are cloned and purified following standard protein generation procedures. The assay buffer is prepared to give a final concen... | Citation and Details BindingDB Entry DOI: 10.7270/Q27947WZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50119367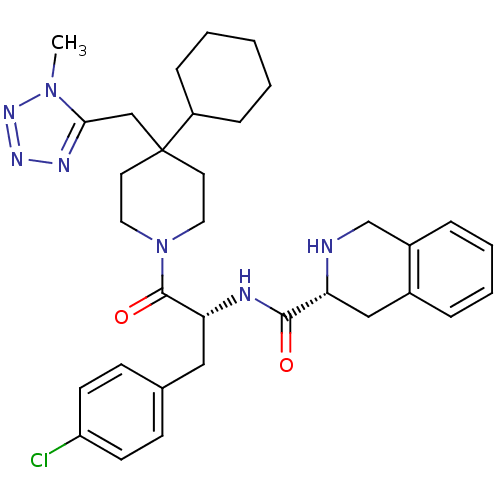 ((3R)-N-[(2R)-3-(4-chlorophenyl)-1-{4-cyclohexyl-4-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Evaluated for binding affinity against Melanocortin-4 receptor by displacing [125I]-NDP-alpha-MSH radioligand expressed in CHO cells | J Med Chem 45: 4589-93 (2002) BindingDB Entry DOI: 10.7270/Q2GT5MH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1A (Homo sapiens (Human)) | BDBM528349 (9-([1,1'- Bi(cyclopropan)]-1- yl)-5-butyl-2,3- dim...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.343 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description PDE1B, PDE1A, and PDE1C are cloned and purified following standard protein generation procedures. The assay buffer is prepared to give a final concen... | Citation and Details BindingDB Entry DOI: 10.7270/Q27947WZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1A (Homo sapiens (Human)) | BDBM528331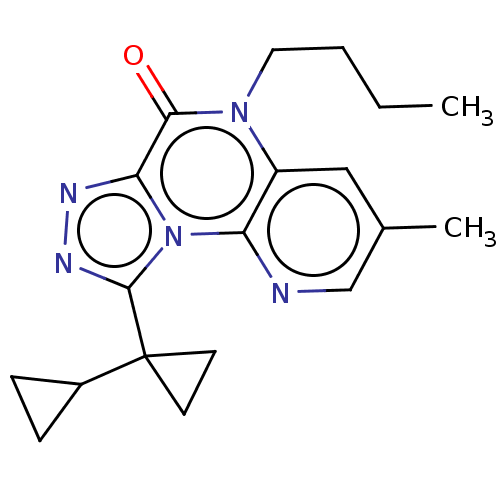 (9-([1,1'-bi(cyclopropan)]-1-yl)-5-butyl-3-methylpy...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.366 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description PDE1B, PDE1A, and PDE1C are cloned and purified following standard protein generation procedures. The assay buffer is prepared to give a final concen... | Citation and Details BindingDB Entry DOI: 10.7270/Q27947WZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1C (Homo sapiens (Human)) | BDBM528352 (9-([1,1'-bi(cyclopropan)]-1-yl)-5-butyl-3-isopropy...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.396 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description PDE1B, PDE1A, and PDE1C are cloned and purified following standard protein generation procedures. The assay buffer is prepared to give a final concen... | Citation and Details BindingDB Entry DOI: 10.7270/Q27947WZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1C (Homo sapiens (Human)) | BDBM528349 (9-([1,1'- Bi(cyclopropan)]-1- yl)-5-butyl-2,3- dim...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description PDE1B, PDE1A, and PDE1C are cloned and purified following standard protein generation procedures. The assay buffer is prepared to give a final concen... | Citation and Details BindingDB Entry DOI: 10.7270/Q27947WZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1C (Homo sapiens (Human)) | BDBM528331 (9-([1,1'-bi(cyclopropan)]-1-yl)-5-butyl-3-methylpy...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.406 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description PDE1B, PDE1A, and PDE1C are cloned and purified following standard protein generation procedures. The assay buffer is prepared to give a final concen... | Citation and Details BindingDB Entry DOI: 10.7270/Q27947WZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1C (Homo sapiens (Human)) | BDBM528351 (9-([1,1'- Bi(cyclopropan)]-1- yl)-5-butyl-3- ethyl...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.419 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description PDE1B, PDE1A, and PDE1C are cloned and purified following standard protein generation procedures. The assay buffer is prepared to give a final concen... | Citation and Details BindingDB Entry DOI: 10.7270/Q27947WZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50119371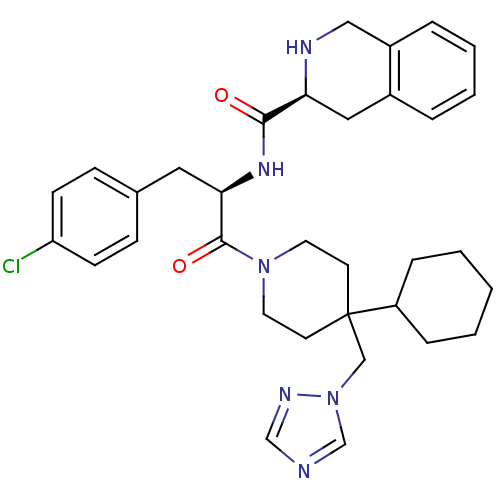 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Evaluated for binding affinity against Melanocortin-4 receptor by displacing [125I]-NDP-alpha-MSH radioligand expressed in CHO cells | J Med Chem 45: 4589-93 (2002) BindingDB Entry DOI: 10.7270/Q2GT5MH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM17744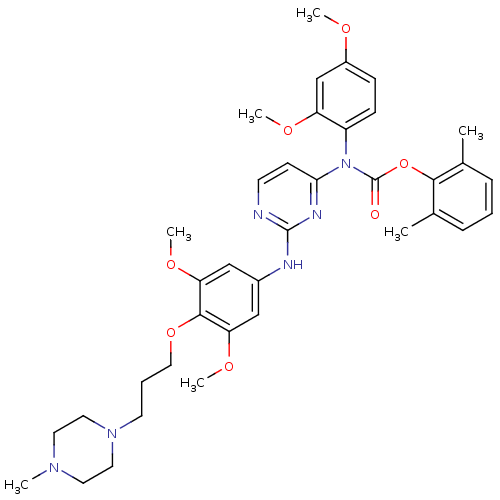 (2,6-dimethylphenyl N-[2-({3,5-dimethoxy-4-[3-(4-me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... | J Med Chem 49: 4981-91 (2006) Article DOI: 10.1021/jm060435i BindingDB Entry DOI: 10.7270/Q2SB440C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Melanocortin receptor 4 (Rattus norvegicus) | BDBM50119368 ((R)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Evaluated for binding affinity against rat Melanocortin-4 receptor (rMC4R) by displacing [125I]NDP-alpha-MSH radioligand expressed in CHO cells | J Med Chem 45: 4589-93 (2002) BindingDB Entry DOI: 10.7270/Q2GT5MH9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1A (Homo sapiens (Human)) | BDBM528355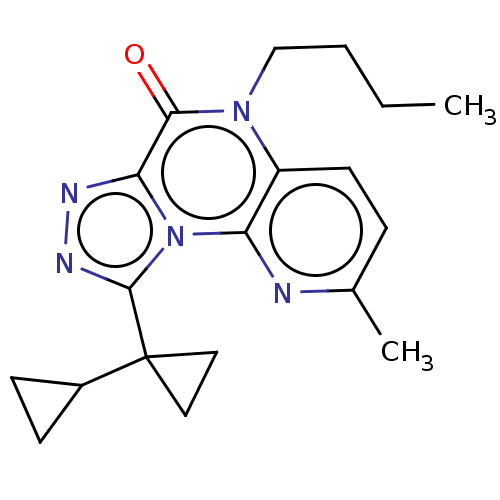 (9-([1,1'-bi(cyclopropan)]-1-yl)-5-butyl-2-methylpy...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.816 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description PDE1B, PDE1A, and PDE1C are cloned and purified following standard protein generation procedures. The assay buffer is prepared to give a final concen... | Citation and Details BindingDB Entry DOI: 10.7270/Q27947WZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1C (Homo sapiens (Human)) | BDBM528353 (9-([1,1'-bi(cyclopropan)]-1-yl)-5-butyl-3-((2-(tri...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.928 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description PDE1B, PDE1A, and PDE1C are cloned and purified following standard protein generation procedures. The assay buffer is prepared to give a final concen... | Citation and Details BindingDB Entry DOI: 10.7270/Q27947WZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1A (Homo sapiens (Human)) | BDBM528353 (9-([1,1'-bi(cyclopropan)]-1-yl)-5-butyl-3-((2-(tri...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.996 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description PDE1B, PDE1A, and PDE1C are cloned and purified following standard protein generation procedures. The assay buffer is prepared to give a final concen... | Citation and Details BindingDB Entry DOI: 10.7270/Q27947WZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM17734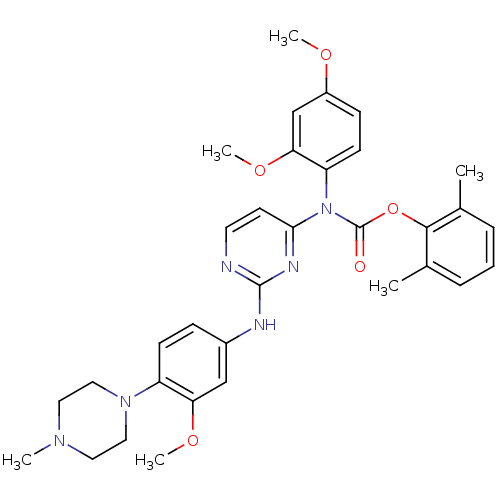 (2,6-dimethylphenyl N-(2,4-dimethoxyphenyl)-N-(2-{[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... | J Med Chem 49: 4981-91 (2006) Article DOI: 10.1021/jm060435i BindingDB Entry DOI: 10.7270/Q2SB440C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092228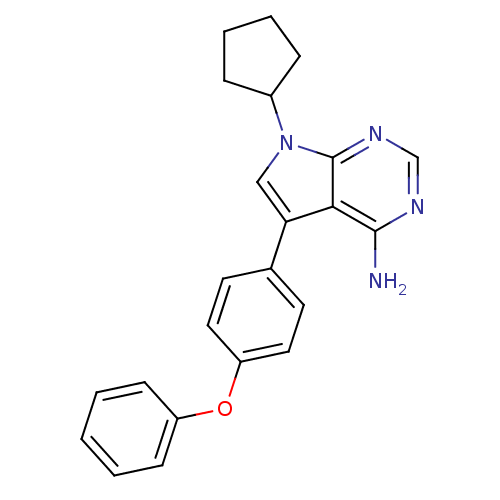 (4-Amino-5-(4-phenoxyphenyl)-7H-pyrrolo[2,3-d]pyrim...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase at 5 uM ATP | Bioorg Med Chem Lett 10: 2167-70 (2001) BindingDB Entry DOI: 10.7270/Q2J38RTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM17736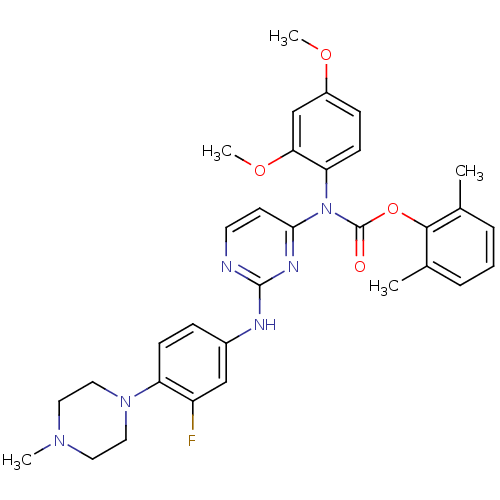 (2,6-dimethylphenyl N-(2,4-dimethoxyphenyl)-N-(2-{[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... | J Med Chem 49: 4981-91 (2006) Article DOI: 10.1021/jm060435i BindingDB Entry DOI: 10.7270/Q2SB440C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM17737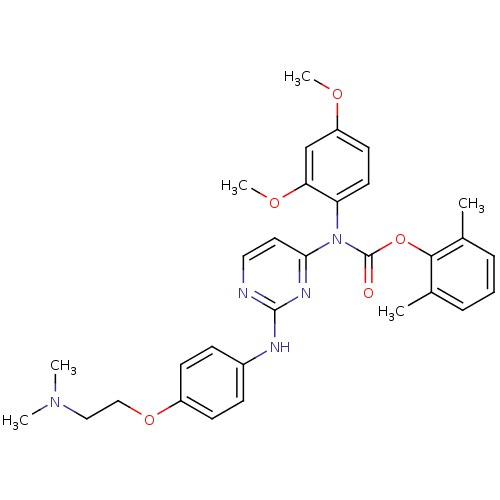 (2,6-dimethylphenyl N-(2,4-dimethoxyphenyl)-N-[2-({...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... | J Med Chem 49: 4981-91 (2006) Article DOI: 10.1021/jm060435i BindingDB Entry DOI: 10.7270/Q2SB440C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM17738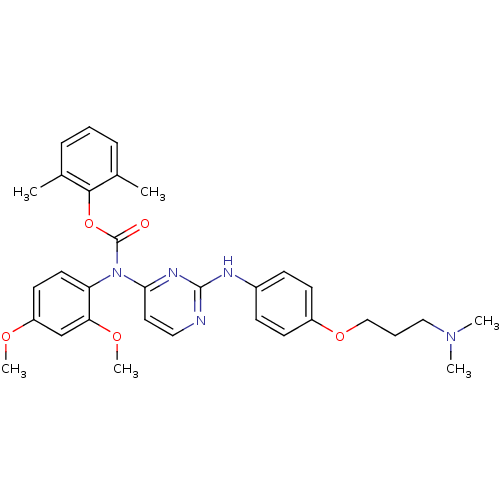 (2,6-dimethylphenyl N-(2,4-dimethoxyphenyl)-N-[2-({...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... | J Med Chem 49: 4981-91 (2006) Article DOI: 10.1021/jm060435i BindingDB Entry DOI: 10.7270/Q2SB440C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM17739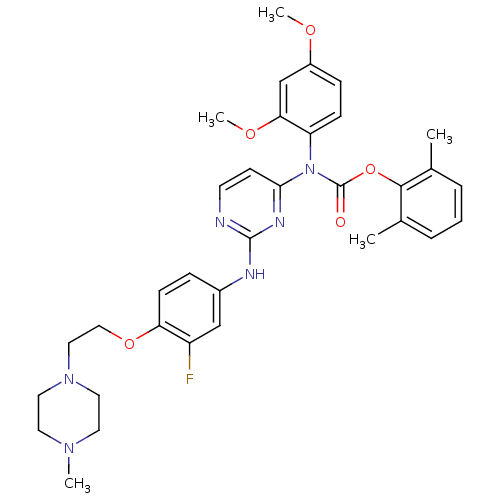 (2,6-dimethylphenyl N-(2,4-dimethoxyphenyl)-N-[2-({...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... | J Med Chem 49: 4981-91 (2006) Article DOI: 10.1021/jm060435i BindingDB Entry DOI: 10.7270/Q2SB440C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM17740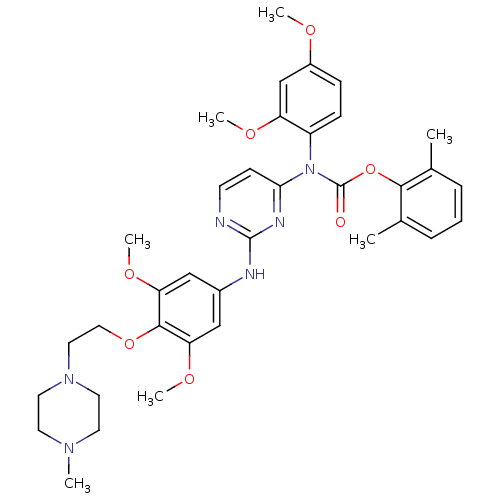 (2,6-dimethylphenyl N-[2-({3,5-dimethoxy-4-[2-(4-me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... | J Med Chem 49: 4981-91 (2006) Article DOI: 10.1021/jm060435i BindingDB Entry DOI: 10.7270/Q2SB440C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM17741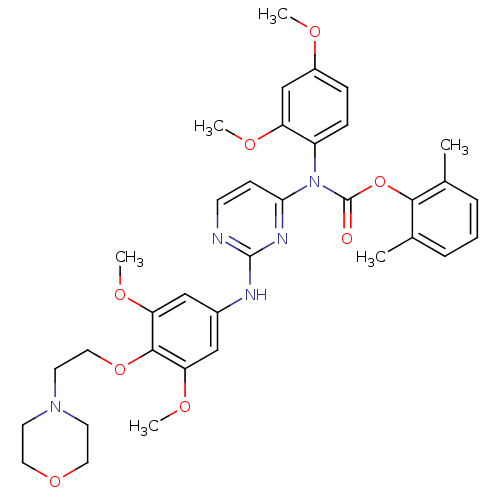 (2,6-dimethylphenyl N-[2-({3,5-dimethoxy-4-[2-(morp...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... | J Med Chem 49: 4981-91 (2006) Article DOI: 10.1021/jm060435i BindingDB Entry DOI: 10.7270/Q2SB440C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM17742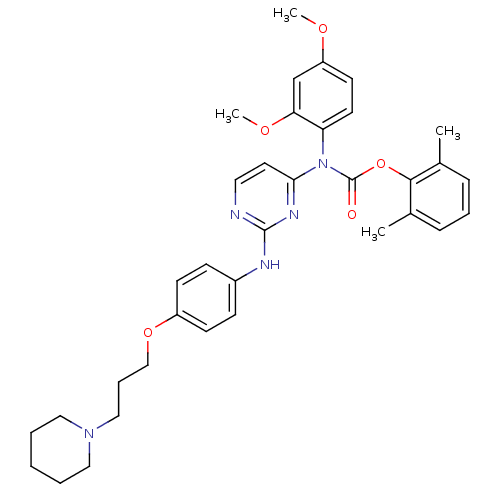 (2,6-dimethylphenyl N-(2,4-dimethoxyphenyl)-N-[2-({...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... | J Med Chem 49: 4981-91 (2006) Article DOI: 10.1021/jm060435i BindingDB Entry DOI: 10.7270/Q2SB440C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM17743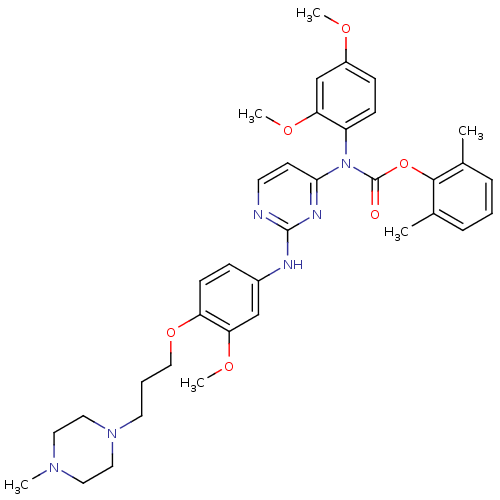 (2,6-dimethylphenyl N-(2,4-dimethoxyphenyl)-N-[2-({...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... | J Med Chem 49: 4981-91 (2006) Article DOI: 10.1021/jm060435i BindingDB Entry DOI: 10.7270/Q2SB440C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM17745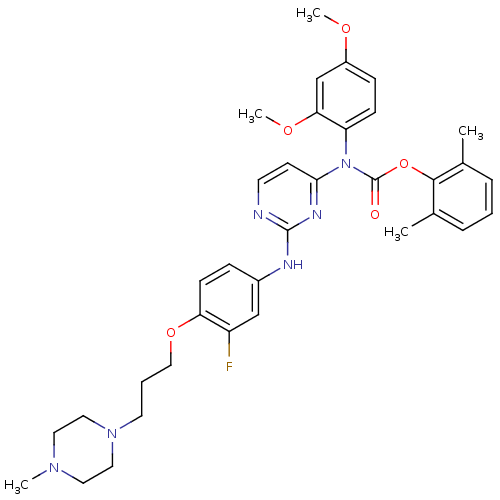 (2,6-dimethylphenyl N-(2,4-dimethoxyphenyl)-N-[2-({...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... | J Med Chem 49: 4981-91 (2006) Article DOI: 10.1021/jm060435i BindingDB Entry DOI: 10.7270/Q2SB440C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092224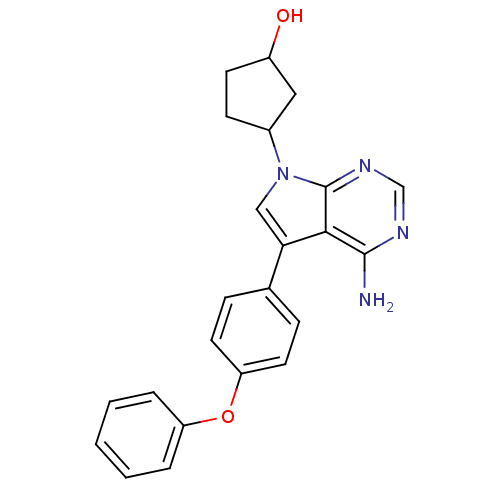 (3-[4-Amino-5-(4-phenoxy-phenyl)-pyrrolo[2,3-d]pyri...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase at 5 uM ATP | Bioorg Med Chem Lett 10: 2167-70 (2001) BindingDB Entry DOI: 10.7270/Q2J38RTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1099 total ) | Next | Last >> |