 Found 87 hits with Last Name = 'joyner' and Initial = 'ss'
Found 87 hits with Last Name = 'joyner' and Initial = 'ss' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dihydrofolate reductase
(Candida albicans) | BDBM50049912

(7-(1,1-Dimethyl-propyl)-8-methyl-7H-pyrrolo[3,2-f]...)Show InChI InChI=1S/C16H21N5/c1-5-16(3,4)21-9(2)8-10-12(21)7-6-11-13(10)14(17)20-15(18)19-11/h6-8H,5H2,1-4H3,(H4,17,18,19,20) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.00710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18224

(6-[(2,5-dimethoxyphenyl)methyl]-5-methylpyrido[2,3...)Show InChI InChI=1S/C17H19N5O2/c1-9-11(6-10-7-12(23-2)4-5-13(10)24-3)8-20-16-14(9)15(18)21-17(19)22-16/h4-5,7-8H,6H2,1-3H3,(H4,18,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Candida albicans) | BDBM50049905
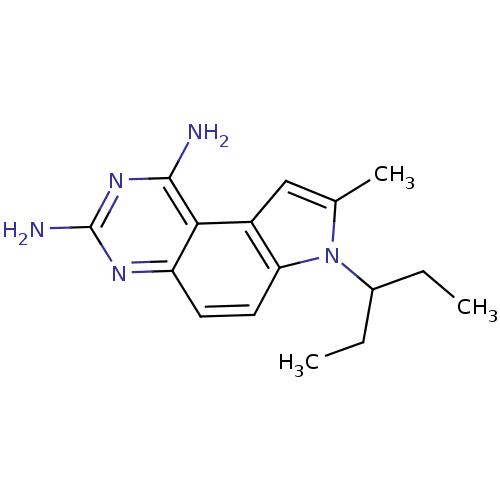
(7-(1-Ethyl-propyl)-8-methyl-7H-pyrrolo[3,2-f]quina...)Show InChI InChI=1S/C16H21N5/c1-4-10(5-2)21-9(3)8-11-13(21)7-6-12-14(11)15(17)20-16(18)19-12/h6-8,10H,4-5H2,1-3H3,(H4,17,18,19,20) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049906
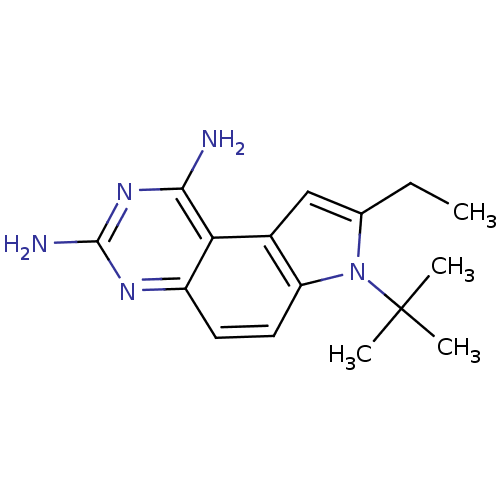
(7-tert-Butyl-8-ethyl-7H-pyrrolo[3,2-f]quinazoline-...)Show InChI InChI=1S/C16H21N5/c1-5-9-8-10-12(21(9)16(2,3)4)7-6-11-13(10)14(17)20-15(18)19-11/h6-8H,5H2,1-4H3,(H4,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049901
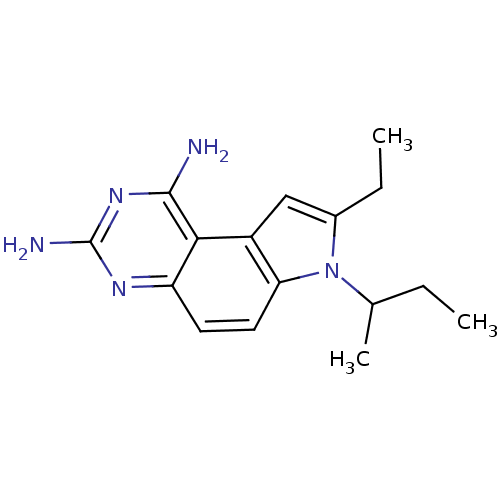
(7-sec-Butyl-8-ethyl-7H-pyrrolo[3,2-f]quinazoline-1...)Show InChI InChI=1S/C16H21N5/c1-4-9(3)21-10(5-2)8-11-13(21)7-6-12-14(11)15(17)20-16(18)19-12/h6-9H,4-5H2,1-3H3,(H4,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049907

(7-(1-Ethyl-propyl)-8-isopropyl-7H-pyrrolo[3,2-f]qu...)Show InChI InChI=1S/C18H25N5/c1-5-11(6-2)23-14-8-7-13-16(17(19)22-18(20)21-13)12(14)9-15(23)10(3)4/h7-11H,5-6H2,1-4H3,(H4,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Candida albicans) | BDBM50049911

(8-tert-Butyl-7H-pyrrolo[3,2-f]quinazoline-1,3-diam...)Show InChI InChI=1S/C14H17N5/c1-14(2,3)10-6-7-8(17-10)4-5-9-11(7)12(15)19-13(16)18-9/h4-6,17H,1-3H3,(H4,15,16,18,19) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Candida albicans) | BDBM50049898

(8-Isopropyl-7H-pyrrolo[3,2-f]quinazoline-1,3-diami...)Show InChI InChI=1S/C13H15N5/c1-6(2)10-5-7-8(16-10)3-4-9-11(7)12(14)18-13(15)17-9/h3-6,16H,1-2H3,(H4,14,15,17,18) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049897

(8-Ethyl-7-(1-ethyl-propyl)-7H-pyrrolo[3,2-f]quinaz...)Show InChI InChI=1S/C17H23N5/c1-4-10(5-2)22-11(6-3)9-12-14(22)8-7-13-15(12)16(18)21-17(19)20-13/h7-10H,4-6H2,1-3H3,(H4,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049909

(8-tert-Butyl-7-isopropyl-7H-pyrrolo[3,2-f]quinazol...)Show InChI InChI=1S/C17H23N5/c1-9(2)22-12-7-6-11-14(15(18)21-16(19)20-11)10(12)8-13(22)17(3,4)5/h6-9H,1-5H3,(H4,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Candida albicans) | BDBM50049899
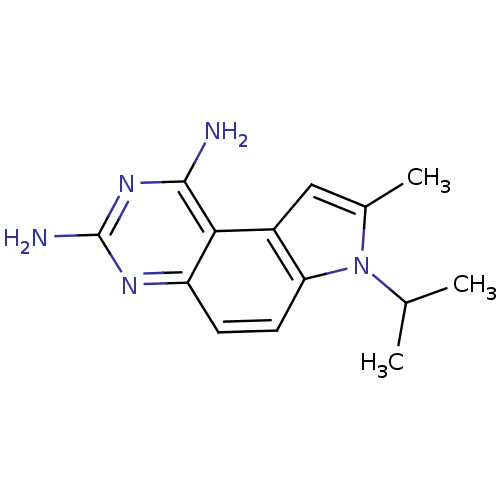
(7-Isopropyl-8-methyl-7H-pyrrolo[3,2-f]quinazoline-...)Show InChI InChI=1S/C14H17N5/c1-7(2)19-8(3)6-9-11(19)5-4-10-12(9)13(15)18-14(16)17-10/h4-7H,1-3H3,(H4,15,16,17,18) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Candida albicans) | BDBM18043
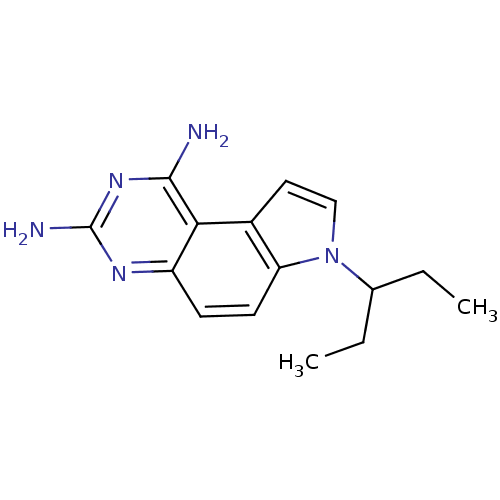
(1,3-DIAMINO-7-(1-ETHYEPROPYE)-7H-PYRRALO-[3,2-F]QU...)Show InChI InChI=1S/C15H19N5/c1-3-9(4-2)20-8-7-10-12(20)6-5-11-13(10)14(16)19-15(17)18-11/h5-9H,3-4H2,1-2H3,(H4,16,17,18,19) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Candida albicans) | BDBM50049914

(7-(3,4,5-Trimethoxy-benzyl)-7H-pyrrolo[3,2-f]quina...)Show SMILES COc1cc(Cn2ccc3c2ccc2nc(N)nc(N)c32)cc(OC)c1OC Show InChI InChI=1S/C20H21N5O3/c1-26-15-8-11(9-16(27-2)18(15)28-3)10-25-7-6-12-14(25)5-4-13-17(12)19(21)24-20(22)23-13/h4-9H,10H2,1-3H3,(H4,21,22,23,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049905

(7-(1-Ethyl-propyl)-8-methyl-7H-pyrrolo[3,2-f]quina...)Show InChI InChI=1S/C16H21N5/c1-4-10(5-2)21-9(3)8-11-13(21)7-6-12-14(11)15(17)20-16(18)19-12/h6-8,10H,4-5H2,1-3H3,(H4,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Candida albicans) | BDBM50049913
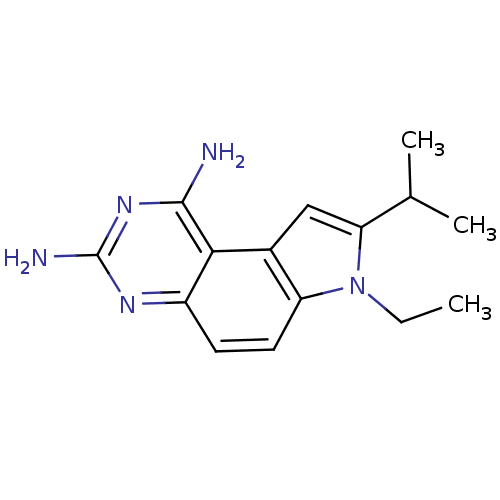
(7-Ethyl-8-isopropyl-7H-pyrrolo[3,2-f]quinazoline-1...)Show InChI InChI=1S/C15H19N5/c1-4-20-11-6-5-10-13(14(16)19-15(17)18-10)9(11)7-12(20)8(2)3/h5-8H,4H2,1-3H3,(H4,16,17,18,19) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Candida albicans) | BDBM50049902

(7,8-Diethyl-7H-pyrrolo[3,2-f]quinazoline-1,3-diami...)Show InChI InChI=1S/C14H17N5/c1-3-8-7-9-11(19(8)4-2)6-5-10-12(9)13(15)18-14(16)17-10/h5-7H,3-4H2,1-2H3,(H4,15,16,17,18) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Candida albicans) | BDBM50049896
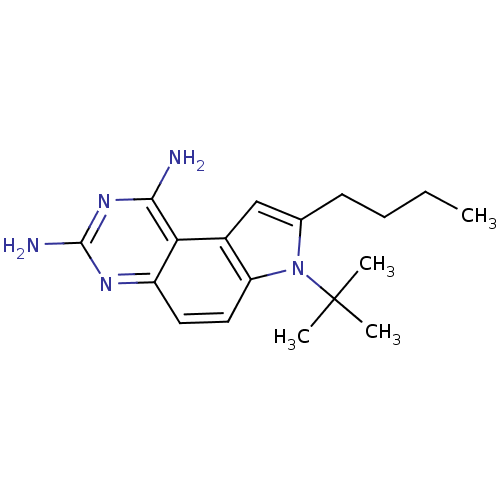
(8-Butyl-7-tert-butyl-7H-pyrrolo[3,2-f]quinazoline-...)Show InChI InChI=1S/C18H25N5/c1-5-6-7-11-10-12-14(23(11)18(2,3)4)9-8-13-15(12)16(19)22-17(20)21-13/h8-10H,5-7H2,1-4H3,(H4,19,20,21,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049912

(7-(1,1-Dimethyl-propyl)-8-methyl-7H-pyrrolo[3,2-f]...)Show InChI InChI=1S/C16H21N5/c1-5-16(3,4)21-9(2)8-10-12(21)7-6-11-13(10)14(17)20-15(18)19-11/h6-8H,5H2,1-4H3,(H4,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049903
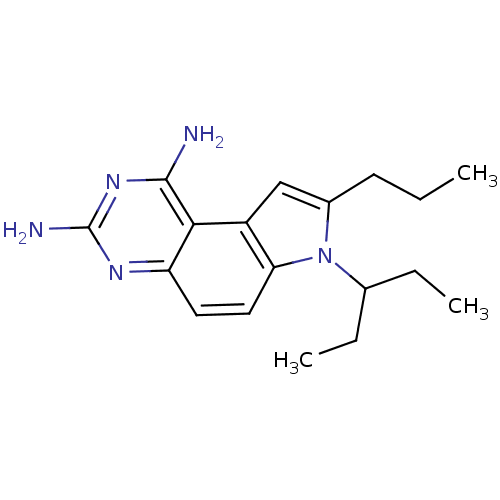
(7-(1-Ethyl-propyl)-8-propyl-7H-pyrrolo[3,2-f]quina...)Show InChI InChI=1S/C18H25N5/c1-4-7-12-10-13-15(23(12)11(5-2)6-3)9-8-14-16(13)17(19)22-18(20)21-14/h8-11H,4-7H2,1-3H3,(H4,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Candida albicans) | BDBM50049910

(7-Ethyl-8-propyl-7H-pyrrolo[3,2-f]quinazoline-1,3-...)Show InChI InChI=1S/C15H19N5/c1-3-5-9-8-10-12(20(9)4-2)7-6-11-13(10)14(16)19-15(17)18-11/h6-8H,3-5H2,1-2H3,(H4,16,17,18,19) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Candida albicans) | BDBM50049915
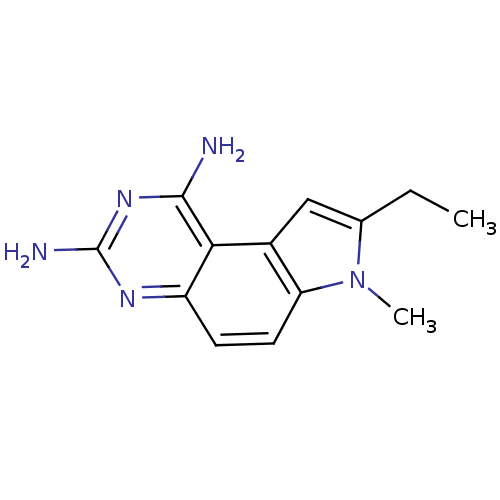
(8-Ethyl-7-methyl-7H-pyrrolo[3,2-f]quinazoline-1,3-...)Show InChI InChI=1S/C13H15N5/c1-3-7-6-8-10(18(7)2)5-4-9-11(8)12(14)17-13(15)16-9/h4-6H,3H2,1-2H3,(H4,14,15,16,17) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18268

(5-methyl-6-{[(3,4,5-trimethoxyphenyl)amino]methyl}...)Show InChI InChI=1S/C19H23N5O3/c1-10-11(5-6-13-16(10)18(20)24-19(21)23-13)9-22-12-7-14(25-2)17(27-4)15(8-12)26-3/h5-8,22H,9H2,1-4H3,(H4,20,21,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Candida albicans) | BDBM18224

(6-[(2,5-dimethoxyphenyl)methyl]-5-methylpyrido[2,3...)Show InChI InChI=1S/C17H19N5O2/c1-9-11(6-10-7-12(23-2)4-5-13(10)24-3)8-20-16-14(9)15(18)21-17(19)22-16/h4-5,7-8H,6H2,1-3H3,(H4,18,19,20,21,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Candida albicans) | BDBM18268

(5-methyl-6-{[(3,4,5-trimethoxyphenyl)amino]methyl}...)Show InChI InChI=1S/C19H23N5O3/c1-10-11(5-6-13-16(10)18(20)24-19(21)23-13)9-22-12-7-14(25-2)17(27-4)15(8-12)26-3/h5-8,22H,9H2,1-4H3,(H4,20,21,23,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Candida albicans) | BDBM50049908
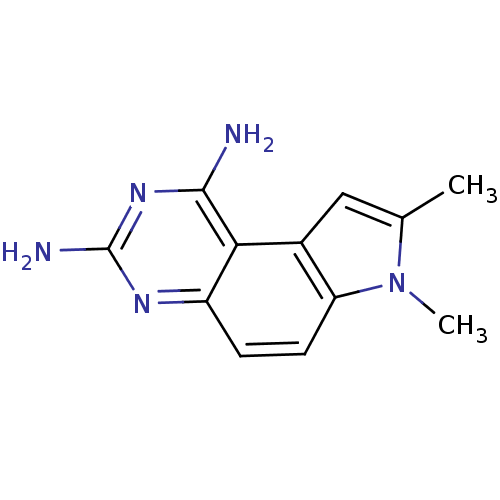
(7,8-Dimethyl-7H-pyrrolo[3,2-f]quinazoline-1,3-diam...)Show InChI InChI=1S/C12H13N5/c1-6-5-7-9(17(6)2)4-3-8-10(7)11(13)16-12(14)15-8/h3-5H,1-2H3,(H4,13,14,15,16) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18043

(1,3-DIAMINO-7-(1-ETHYEPROPYE)-7H-PYRRALO-[3,2-F]QU...)Show InChI InChI=1S/C15H19N5/c1-3-9(4-2)20-8-7-10-12(20)6-5-11-13(10)14(16)19-15(17)18-11/h5-9H,3-4H2,1-2H3,(H4,16,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049914

(7-(3,4,5-Trimethoxy-benzyl)-7H-pyrrolo[3,2-f]quina...)Show SMILES COc1cc(Cn2ccc3c2ccc2nc(N)nc(N)c32)cc(OC)c1OC Show InChI InChI=1S/C20H21N5O3/c1-26-15-8-11(9-16(27-2)18(15)28-3)10-25-7-6-12-14(25)5-4-13-17(12)19(21)24-20(22)23-13/h4-9H,10H2,1-3H3,(H4,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049899

(7-Isopropyl-8-methyl-7H-pyrrolo[3,2-f]quinazoline-...)Show InChI InChI=1S/C14H17N5/c1-7(2)19-8(3)6-9-11(19)5-4-10-12(9)13(15)18-14(16)17-10/h4-7H,1-3H3,(H4,15,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049898

(8-Isopropyl-7H-pyrrolo[3,2-f]quinazoline-1,3-diami...)Show InChI InChI=1S/C13H15N5/c1-6(2)10-5-7-8(16-10)3-4-9-11(7)12(14)18-13(15)17-9/h3-6,16H,1-2H3,(H4,14,15,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049902

(7,8-Diethyl-7H-pyrrolo[3,2-f]quinazoline-1,3-diami...)Show InChI InChI=1S/C14H17N5/c1-3-8-7-9-11(19(8)4-2)6-5-10-12(9)13(15)18-14(16)17-10/h5-7H,3-4H2,1-2H3,(H4,15,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Candida albicans) | BDBM50049904
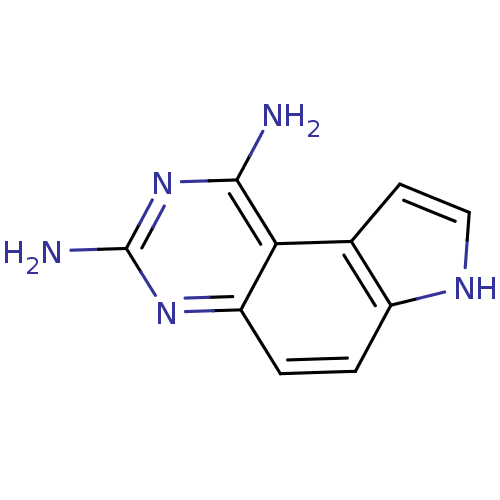
(7H-Pyrrolo[3,2-f]quinazoline-1,3-diamine | CHEMBL3...)Show InChI InChI=1S/C10H9N5/c11-9-8-5-3-4-13-6(5)1-2-7(8)14-10(12)15-9/h1-4,13H,(H4,11,12,14,15) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049910

(7-Ethyl-8-propyl-7H-pyrrolo[3,2-f]quinazoline-1,3-...)Show InChI InChI=1S/C15H19N5/c1-3-5-9-8-10-12(20(9)4-2)7-6-11-13(10)14(16)19-15(17)18-11/h6-8H,3-5H2,1-2H3,(H4,16,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Candida albicans) | BDBM50049900
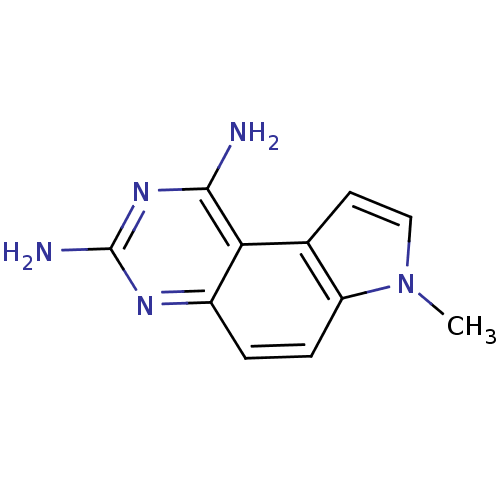
(7-Methyl-7H-pyrrolo[3,2-f]quinazoline-1,3-diamine ...)Show InChI InChI=1S/C11H11N5/c1-16-5-4-6-8(16)3-2-7-9(6)10(12)15-11(13)14-7/h2-5H,1H3,(H4,12,13,14,15) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049915

(8-Ethyl-7-methyl-7H-pyrrolo[3,2-f]quinazoline-1,3-...)Show InChI InChI=1S/C13H15N5/c1-3-7-6-8-10(18(7)2)5-4-9-11(8)12(14)17-13(15)16-9/h4-6H,3H2,1-2H3,(H4,14,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049896

(8-Butyl-7-tert-butyl-7H-pyrrolo[3,2-f]quinazoline-...)Show InChI InChI=1S/C18H25N5/c1-5-6-7-11-10-12-14(23(11)18(2,3)4)9-8-13-15(12)16(19)22-17(20)21-13/h8-10H,5-7H2,1-4H3,(H4,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049908

(7,8-Dimethyl-7H-pyrrolo[3,2-f]quinazoline-1,3-diam...)Show InChI InChI=1S/C12H13N5/c1-6-5-7-9(17(6)2)4-3-8-10(7)11(13)16-12(14)15-8/h3-5H,1-2H3,(H4,13,14,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049900

(7-Methyl-7H-pyrrolo[3,2-f]quinazoline-1,3-diamine ...)Show InChI InChI=1S/C11H11N5/c1-16-5-4-6-8(16)3-2-7-9(6)10(12)15-11(13)14-7/h2-5H,1H3,(H4,12,13,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049904

(7H-Pyrrolo[3,2-f]quinazoline-1,3-diamine | CHEMBL3...)Show InChI InChI=1S/C10H9N5/c11-9-8-5-3-4-13-6(5)1-2-7(8)14-10(12)15-9/h1-4,13H,(H4,11,12,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Uridine phosphorylase 1
(Mus musculus) | BDBM50030972
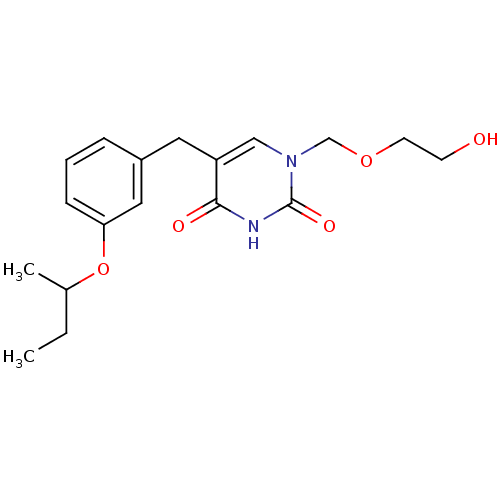
(5-(3-sec-Butoxy-benzyl)-1-(2-hydroxy-ethoxymethyl)...)Show InChI InChI=1S/C18H24N2O5/c1-3-13(2)25-16-6-4-5-14(10-16)9-15-11-20(12-24-8-7-21)18(23)19-17(15)22/h4-6,10-11,13,21H,3,7-9,12H2,1-2H3,(H,19,22,23) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
Inhibition of uridine phosphorylase (UrdPase) from murine liver. |
J Med Chem 38: 3850-6 (1995)
BindingDB Entry DOI: 10.7270/Q2B27T91 |
More data for this
Ligand-Target Pair | |
Uridine phosphorylase 1
(Mus musculus) | BDBM50031005
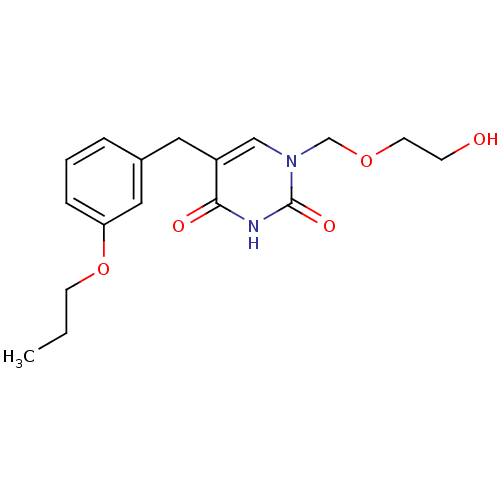
(1-(2-Hydroxy-ethoxymethyl)-5-(3-propoxy-benzyl)-1H...)Show InChI InChI=1S/C17H22N2O5/c1-2-7-24-15-5-3-4-13(10-15)9-14-11-19(12-23-8-6-20)17(22)18-16(14)21/h3-5,10-11,20H,2,6-9,12H2,1H3,(H,18,21,22) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
Inhibition of uridine phosphorylase (UrdPase) from murine liver. |
J Med Chem 38: 3850-6 (1995)
BindingDB Entry DOI: 10.7270/Q2B27T91 |
More data for this
Ligand-Target Pair | |
Uridine phosphorylase 1
(Mus musculus) | BDBM50030974
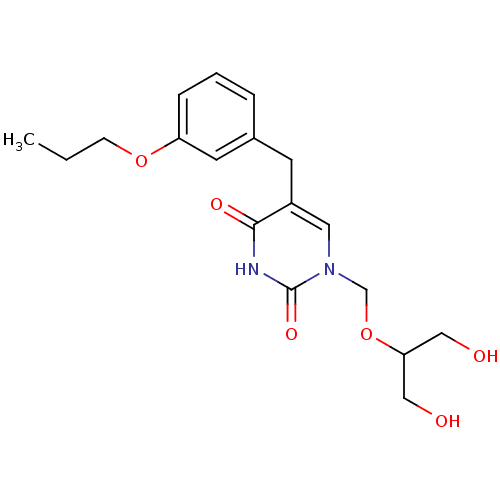
(1-(2-Hydroxy-1-hydroxymethyl-ethoxymethyl)-5-(3-pr...)Show SMILES CCCOc1cccc(Cc2cn(COC(CO)CO)c(=O)[nH]c2=O)c1 Show InChI InChI=1S/C18H24N2O6/c1-2-6-25-15-5-3-4-13(8-15)7-14-9-20(18(24)19-17(14)23)12-26-16(10-21)11-22/h3-5,8-9,16,21-22H,2,6-7,10-12H2,1H3,(H,19,23,24) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
Inhibition of uridine phosphorylase (UrdPase) from murine liver. |
J Med Chem 38: 3850-6 (1995)
BindingDB Entry DOI: 10.7270/Q2B27T91 |
More data for this
Ligand-Target Pair | |
Uridine phosphorylase 1
(Mus musculus) | BDBM50031009

(1-(2-Hydroxy-ethoxymethyl)-5-(3-isobutoxy-benzyl)-...)Show InChI InChI=1S/C18H24N2O5/c1-13(2)11-25-16-5-3-4-14(9-16)8-15-10-20(12-24-7-6-21)18(23)19-17(15)22/h3-5,9-10,13,21H,6-8,11-12H2,1-2H3,(H,19,22,23) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
Inhibition of uridine phosphorylase (UrdPase) from murine liver. |
J Med Chem 38: 3850-6 (1995)
BindingDB Entry DOI: 10.7270/Q2B27T91 |
More data for this
Ligand-Target Pair | |
Uridine phosphorylase 1
(Mus musculus) | BDBM50030990
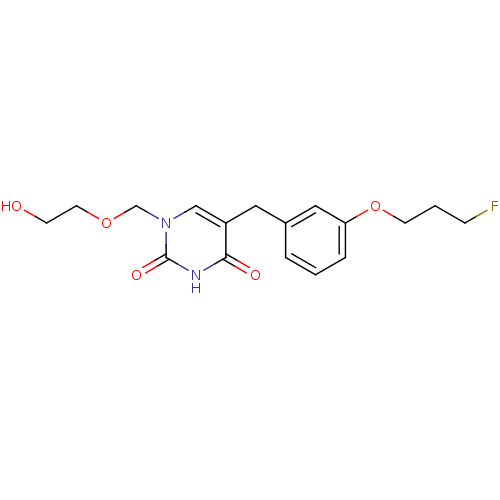
(5-[3-(3-Fluoro-propoxy)-benzyl]-1-(2-hydroxy-ethox...)Show InChI InChI=1S/C17H21FN2O5/c18-5-2-7-25-15-4-1-3-13(10-15)9-14-11-20(12-24-8-6-21)17(23)19-16(14)22/h1,3-4,10-11,21H,2,5-9,12H2,(H,19,22,23) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
Inhibition of uridine phosphorylase (UrdPase) from murine liver. |
J Med Chem 38: 3850-6 (1995)
BindingDB Entry DOI: 10.7270/Q2B27T91 |
More data for this
Ligand-Target Pair | |
Uridine phosphorylase 1
(Mus musculus) | BDBM50030980
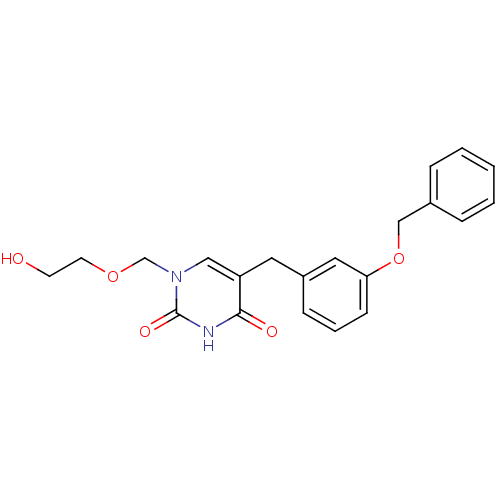
(1-((2-HYDROXYETHOXY)METHYL)-5-(3-(BENZYLOXY)BENZYL...)Show SMILES OCCOCn1cc(Cc2cccc(OCc3ccccc3)c2)c(=O)[nH]c1=O Show InChI InChI=1S/C21H22N2O5/c24-9-10-27-15-23-13-18(20(25)22-21(23)26)11-17-7-4-8-19(12-17)28-14-16-5-2-1-3-6-16/h1-8,12-13,24H,9-11,14-15H2,(H,22,25,26) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
Inhibition of uridine phosphorylase (UrdPase) from murine liver. |
J Med Chem 38: 3850-6 (1995)
BindingDB Entry DOI: 10.7270/Q2B27T91 |
More data for this
Ligand-Target Pair | |
Uridine phosphorylase 1
(Mus musculus) | BDBM50031006
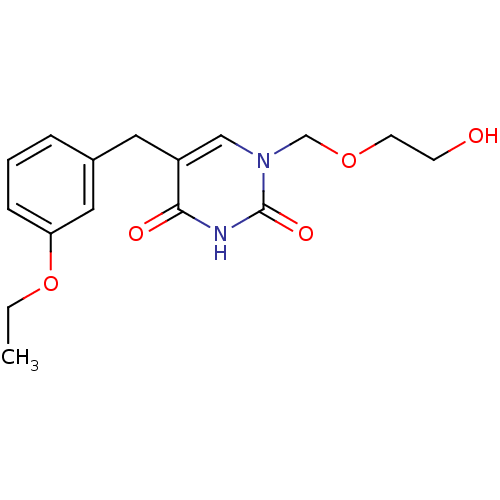
(5-(3-Ethoxy-benzyl)-1-(2-hydroxy-ethoxymethyl)-1H-...)Show InChI InChI=1S/C16H20N2O5/c1-2-23-14-5-3-4-12(9-14)8-13-10-18(11-22-7-6-19)16(21)17-15(13)20/h3-5,9-10,19H,2,6-8,11H2,1H3,(H,17,20,21) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
Inhibition of uridine phosphorylase (UrdPase) from murine liver. |
J Med Chem 38: 3850-6 (1995)
BindingDB Entry DOI: 10.7270/Q2B27T91 |
More data for this
Ligand-Target Pair | |
Uridine phosphorylase 1
(Mus musculus) | BDBM50030999
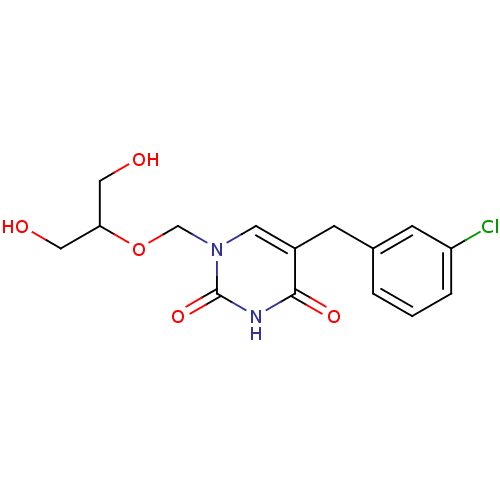
(5-(3-Chloro-benzyl)-1-(2-hydroxy-1-hydroxymethyl-e...)Show InChI InChI=1S/C15H17ClN2O5/c16-12-3-1-2-10(5-12)4-11-6-18(15(22)17-14(11)21)9-23-13(7-19)8-20/h1-3,5-6,13,19-20H,4,7-9H2,(H,17,21,22) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
Inhibition of uridine phosphorylase (UrdPase) from murine liver. |
J Med Chem 38: 3850-6 (1995)
BindingDB Entry DOI: 10.7270/Q2B27T91 |
More data for this
Ligand-Target Pair | |
Uridine phosphorylase 1
(Mus musculus) | BDBM50031007
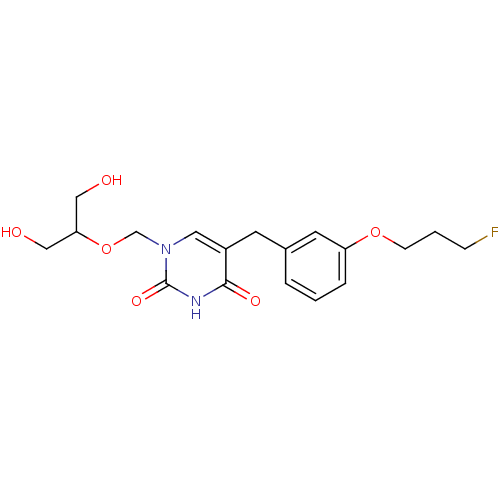
(5-[3-(3-Fluoro-propoxy)-benzyl]-1-(2-hydroxy-1-hyd...)Show SMILES OCC(CO)OCn1cc(Cc2cccc(OCCCF)c2)c(=O)[nH]c1=O Show InChI InChI=1S/C18H23FN2O6/c19-5-2-6-26-15-4-1-3-13(8-15)7-14-9-21(18(25)20-17(14)24)12-27-16(10-22)11-23/h1,3-4,8-9,16,22-23H,2,5-7,10-12H2,(H,20,24,25) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
Inhibition of uridine phosphorylase (UrdPase) from murine liver. |
J Med Chem 38: 3850-6 (1995)
BindingDB Entry DOI: 10.7270/Q2B27T91 |
More data for this
Ligand-Target Pair | |
Uridine phosphorylase 1
(Mus musculus) | BDBM50030983
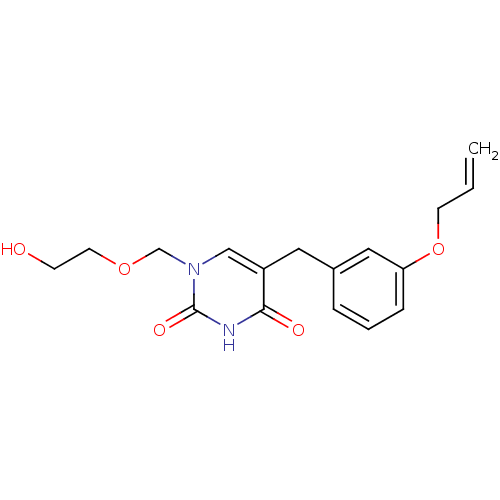
(5-(3-Allyloxy-benzyl)-1-(2-hydroxy-ethoxymethyl)-1...)Show InChI InChI=1S/C17H20N2O5/c1-2-7-24-15-5-3-4-13(10-15)9-14-11-19(12-23-8-6-20)17(22)18-16(14)21/h2-5,10-11,20H,1,6-9,12H2,(H,18,21,22) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
Inhibition of uridine phosphorylase (UrdPase) from murine liver. |
J Med Chem 38: 3850-6 (1995)
BindingDB Entry DOI: 10.7270/Q2B27T91 |
More data for this
Ligand-Target Pair | |
Uridine phosphorylase 1
(Mus musculus) | BDBM50030995
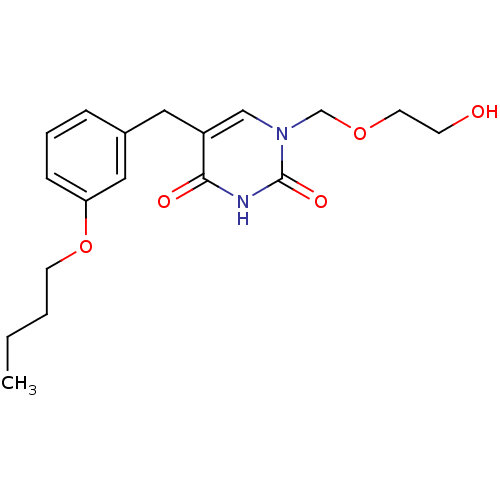
(5-(3-Butoxy-benzyl)-1-(2-hydroxy-ethoxymethyl)-1H-...)Show InChI InChI=1S/C18H24N2O5/c1-2-3-8-25-16-6-4-5-14(11-16)10-15-12-20(13-24-9-7-21)18(23)19-17(15)22/h4-6,11-12,21H,2-3,7-10,13H2,1H3,(H,19,22,23) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
Inhibition of uridine phosphorylase (UrdPase) from murine liver. |
J Med Chem 38: 3850-6 (1995)
BindingDB Entry DOI: 10.7270/Q2B27T91 |
More data for this
Ligand-Target Pair | |
Uridine phosphorylase 1
(Mus musculus) | BDBM50031004
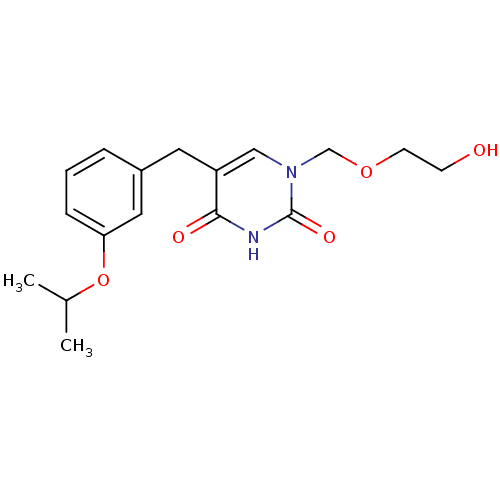
(1-(2-Hydroxy-ethoxymethyl)-5-(3-isopropoxy-benzyl)...)Show InChI InChI=1S/C17H22N2O5/c1-12(2)24-15-5-3-4-13(9-15)8-14-10-19(11-23-7-6-20)17(22)18-16(14)21/h3-5,9-10,12,20H,6-8,11H2,1-2H3,(H,18,21,22) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
Inhibition of uridine phosphorylase (UrdPase) from murine liver. |
J Med Chem 38: 3850-6 (1995)
BindingDB Entry DOI: 10.7270/Q2B27T91 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data