

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283143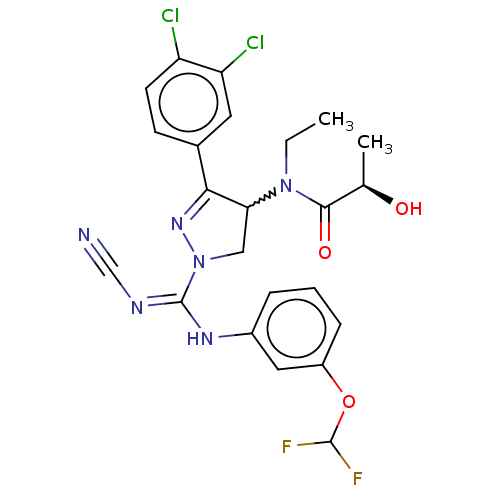 ((2R)—N-[1-{N′-cyano-N-[3-(difluorometho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.64 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description For the detection of SMYD2 cellular methylation activity an In Cell Western (ICW) assay was established. This assay allows rapid processing of multip... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283194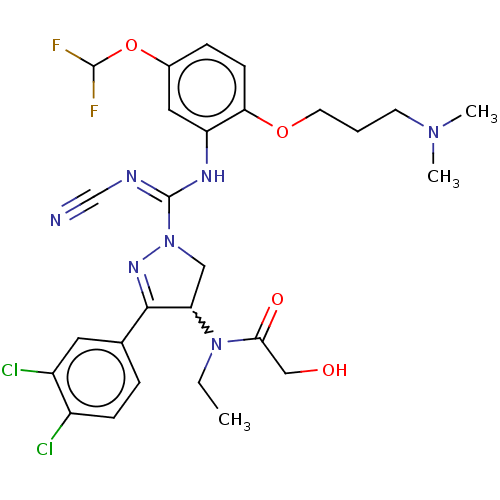 (Rac-N-[1-(N′-cyano-N-{5-(difluoromethoxy)-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.55 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283173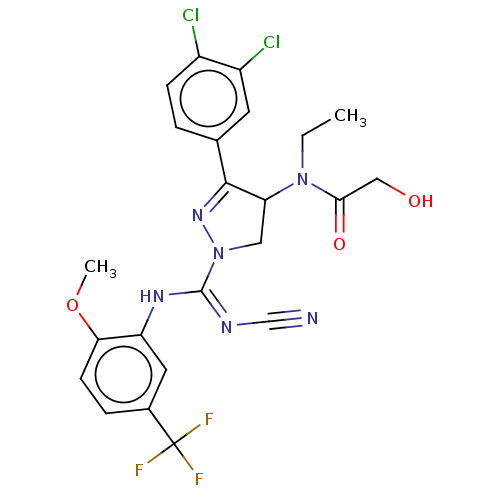 (N-[1-{N′-cyano-N-[2-methoxy-5-(trifluorometh...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.65 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor [E709Y] (Homo sapiens (Human)) | BDBM245167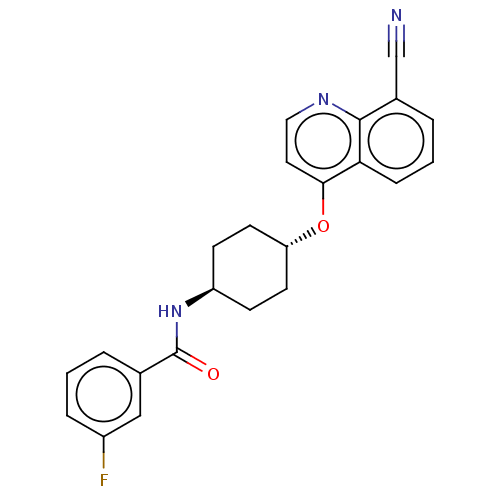 (US9428460, 23) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | 37 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description PC-3 cells (Kaighn et al., Invest. Urol. 17: 16-23, 1979) were plated out at a density of 10000 cells per well of a 96-well cell culture plate in RMP... | US Patent US9428460 (2016) BindingDB Entry DOI: 10.7270/Q25719Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283196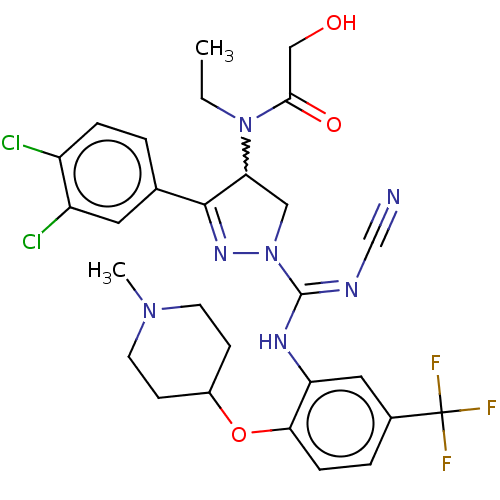 (Rac-N-[1-(N′-cyano-N-{2-[(1-methylpiperidin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.09 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283172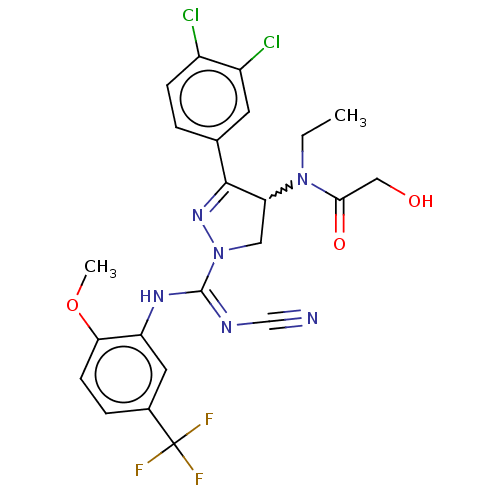 (Rac-N-[1-{N′-cyano-N-[2-methoxy-5-(trifluoro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.98 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283169 (Rac-N-[1-{N′-cyano-N-[5-(difluoromethoxy)-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12.5 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283143 ((2R)—N-[1-{N′-cyano-N-[3-(difluorometho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283139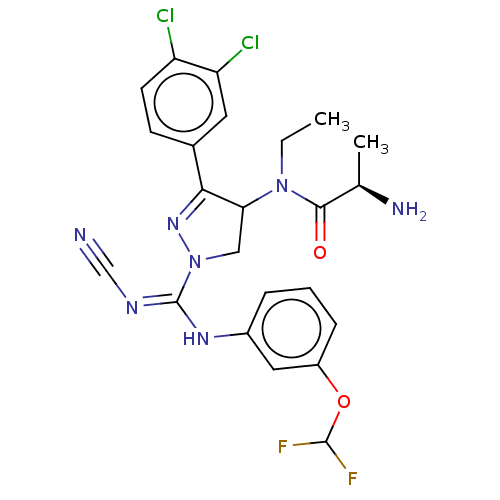 (N-[1-{N′-cyano-N-[3-(difluoromethoxy)phenyl]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description For the detection of SMYD2 cellular methylation activity an In Cell Western (ICW) assay was established. This assay allows rapid processing of multip... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283139 (N-[1-{N′-cyano-N-[3-(difluoromethoxy)phenyl]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13.5 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor [E709Y] (Homo sapiens (Human)) | BDBM245168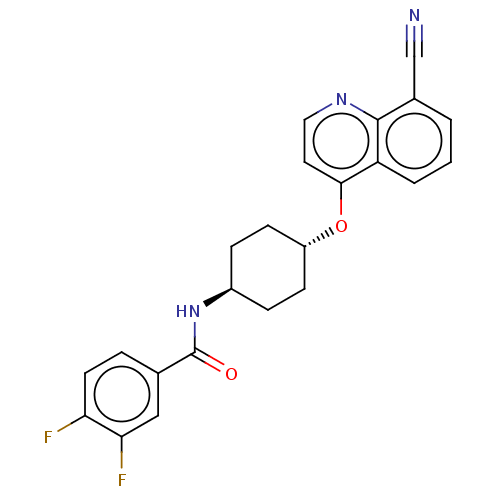 (US9428460, 24) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | 37 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description PC-3 cells (Kaighn et al., Invest. Urol. 17: 16-23, 1979) were plated out at a density of 10000 cells per well of a 96-well cell culture plate in RMP... | US Patent US9428460 (2016) BindingDB Entry DOI: 10.7270/Q25719Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283132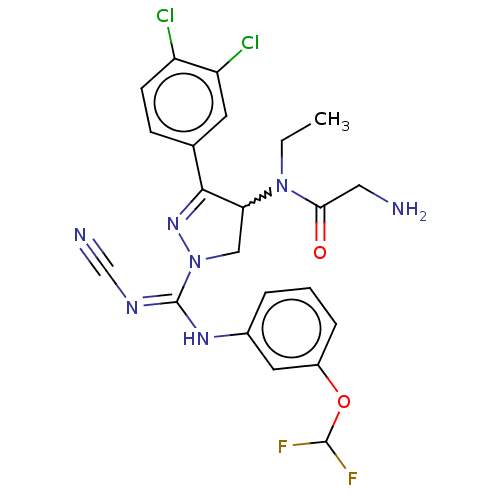 (Rac-N-[1-{N′-cyano-N-[3-(difluoromethoxy)phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283195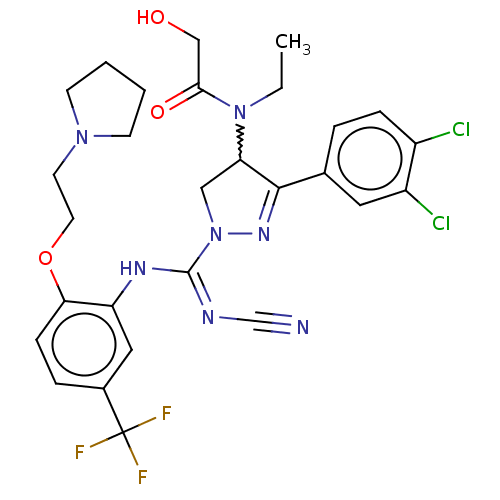 (Rac-N-[r-(N′-cyano-N-[2-[2-(pyrrolidin-1-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15.8 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM245170 (US9428460, 26) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | 37 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description For determining the androgen receptor-dependent transcription, a cellular assay system was used, consisting of PC-3 cells (Kaighn et al., Invest. Uro... | US Patent US9428460 (2016) BindingDB Entry DOI: 10.7270/Q25719Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283184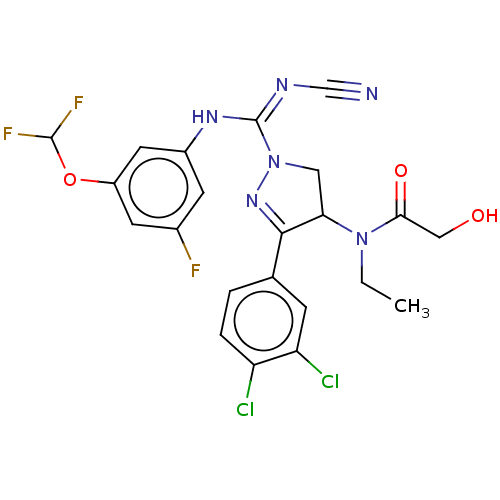 (N-[1-{N′-cyano-N-[3-(difluoromethoxy)-5-fluo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19.2 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM245146 (US9428460, 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | 37 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description For determining the androgen receptor-dependent transcription, a cellular assay system was used, consisting of PC-3 cells (Kaighn et al., Invest. Uro... | US Patent US9428460 (2016) BindingDB Entry DOI: 10.7270/Q25719Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283172 (Rac-N-[1-{N′-cyano-N-[2-methoxy-5-(trifluoro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 21.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description For the detection of SMYD2 cellular methylation activity an In Cell Western (ICW) assay was established. This assay allows rapid processing of multip... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283205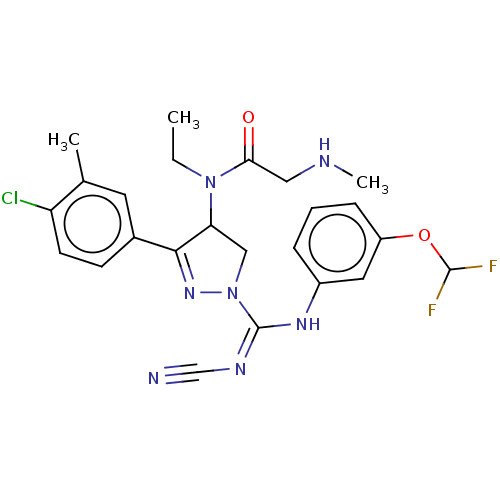 (N-[3-(4-Chloro-3-methylphenyl)-1-{N′-cyano-N...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 22.9 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283173 (N-[1-{N′-cyano-N-[2-methoxy-5-(trifluorometh...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 23.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description For the detection of SMYD2 cellular methylation activity an In Cell Western (ICW) assay was established. This assay allows rapid processing of multip... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283197 (Rac-N-[1-(N′-cyano-N-{2-[(1-methylpiperidin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 26.1 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283169 (Rac-N-[1-{N′-cyano-N-[5-(difluoromethoxy)-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 27.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description For the detection of SMYD2 cellular methylation activity an In Cell Western (ICW) assay was established. This assay allows rapid processing of multip... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM50181508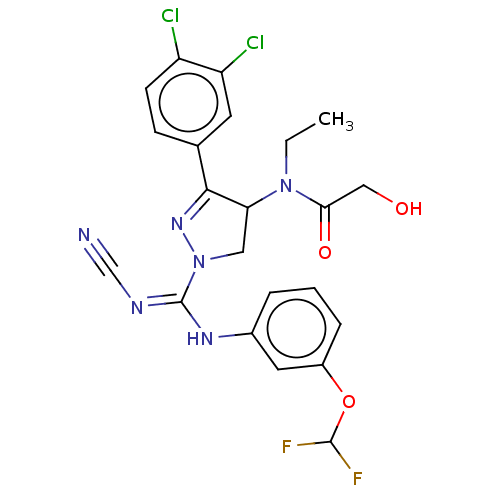 (CHEMBL3818083 | US10023539, Example 4.2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 28.2 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM50181508 (CHEMBL3818083 | US10023539, Example 4.2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 28.2 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283170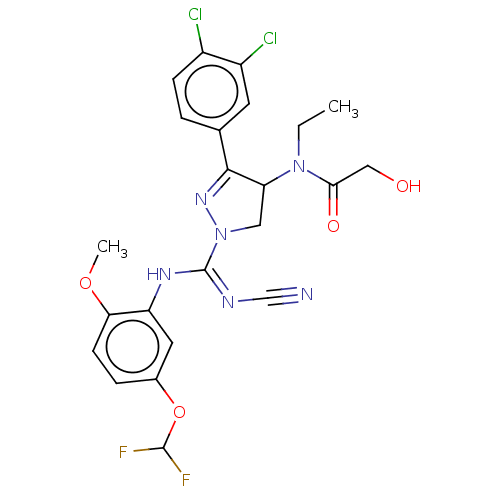 (N-[1-{N′-cyano-N-[5-(difluoromethoxy)-2-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 28.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description For the detection of SMYD2 cellular methylation activity an In Cell Western (ICW) assay was established. This assay allows rapid processing of multip... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor [W742C] (Homo sapiens (Human)) | BDBM245167 (US9428460, 23) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | 37 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description PC-3 cells (Kaighn et al., Invest. Urol. 17: 16-23, 1979) were plated out at a density of 10000 cells per well of a 96-well cell culture plate in RMP... | US Patent US9428460 (2016) BindingDB Entry DOI: 10.7270/Q25719Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283170 (N-[1-{N′-cyano-N-[5-(difluoromethoxy)-2-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283189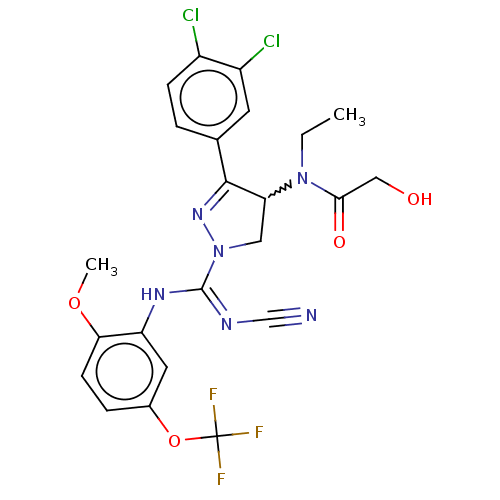 (Rac-N-[1-{N′-cyano-N-[2-methoxy-5-(trifluoro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 30.6 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM245163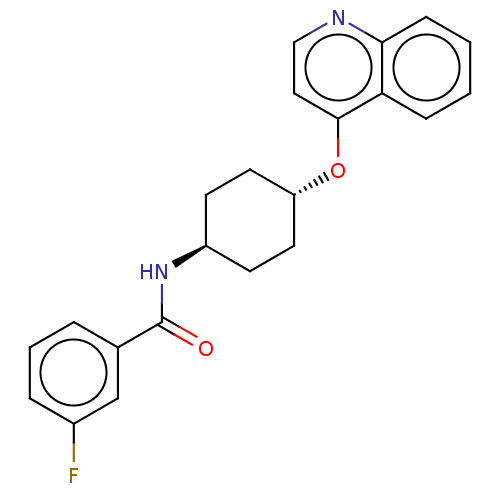 (US9428460, 19) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | 37 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description For determining the androgen receptor-dependent transcription, a cellular assay system was used, consisting of PC-3 cells (Kaighn et al., Invest. Uro... | US Patent US9428460 (2016) BindingDB Entry DOI: 10.7270/Q25719Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283208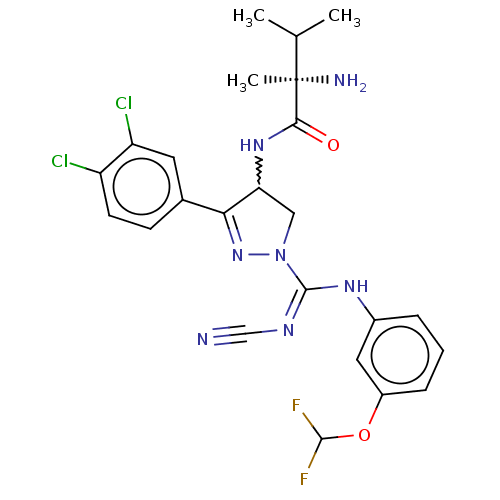 (N-[1-{N′-cyano-N-[3-(difluoromethoxy)phenyl]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 32 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283209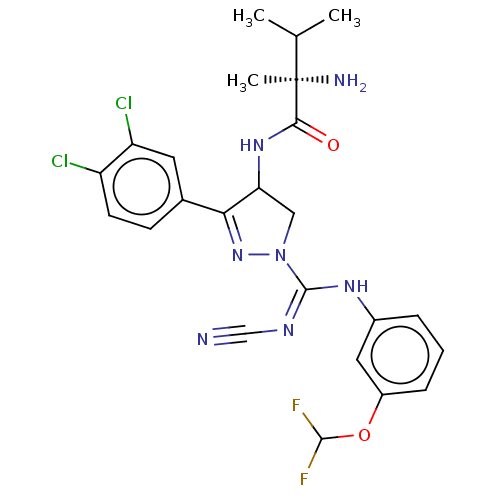 (N-[1-{N′-cyano-N-[3-(difluoromethoxy)phenyl]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 35.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description For the detection of SMYD2 cellular methylation activity an In Cell Western (ICW) assay was established. This assay allows rapid processing of multip... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283208 (N-[1-{N′-cyano-N-[3-(difluoromethoxy)phenyl]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 37.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description For the detection of SMYD2 cellular methylation activity an In Cell Western (ICW) assay was established. This assay allows rapid processing of multip... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283209 (N-[1-{N′-cyano-N-[3-(difluoromethoxy)phenyl]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 37.5 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM50180974 (CHEMBL3818092 | US10023539, Example 33.2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 37.6 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM245174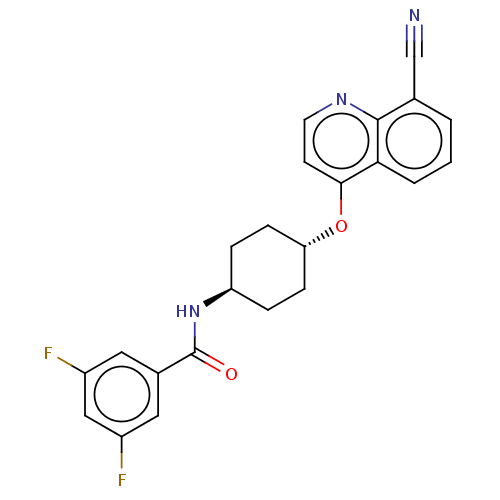 (US9428460, 30) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | 37 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description For determining the androgen receptor-dependent transcription, a cellular assay system was used, consisting of PC-3 cells (Kaighn et al., Invest. Uro... | US Patent US9428460 (2016) BindingDB Entry DOI: 10.7270/Q25719Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283204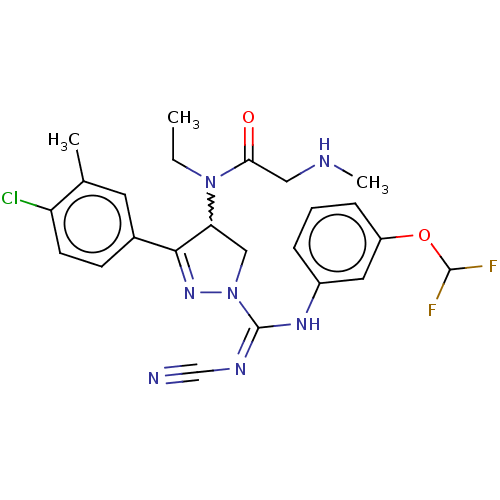 (US10023539, Example 36 | rac-N-[3-(4-Chloro-3-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 40.7 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283150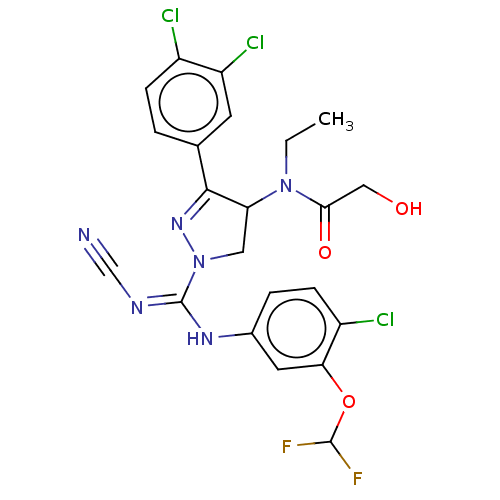 (N-[1-{N-[4-chloro-3-(difluoromethoxy)phenyl]-NR...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 44.1 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM245147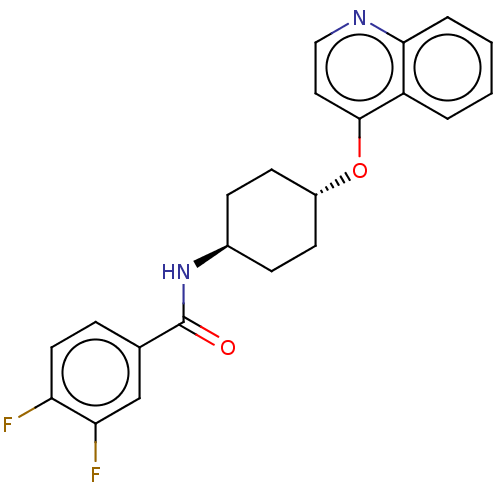 (US9428460, 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | 37 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description For determining the androgen receptor-dependent transcription, a cellular assay system was used, consisting of PC-3 cells (Kaighn et al., Invest. Uro... | US Patent US9428460 (2016) BindingDB Entry DOI: 10.7270/Q25719Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM245172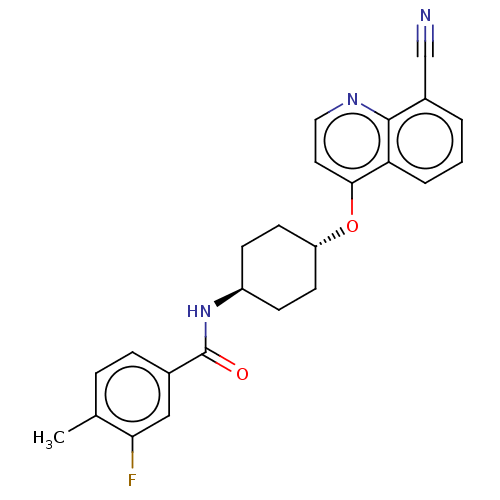 (US9428460, 28) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | 37 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description For determining the androgen receptor-dependent transcription, a cellular assay system was used, consisting of PC-3 cells (Kaighn et al., Invest. Uro... | US Patent US9428460 (2016) BindingDB Entry DOI: 10.7270/Q25719Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM50181508 (CHEMBL3818083 | US10023539, Example 4.2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 46.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description For the detection of SMYD2 cellular methylation activity an In Cell Western (ICW) assay was established. This assay allows rapid processing of multip... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283159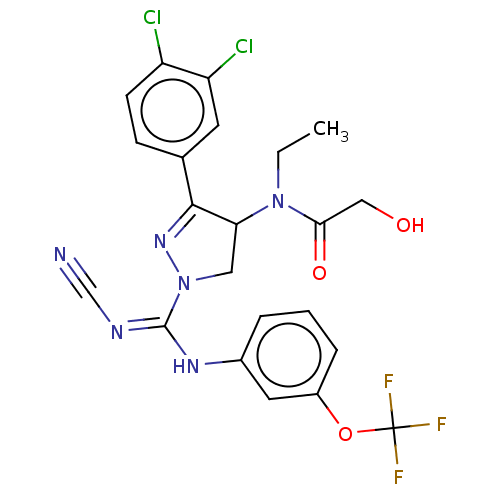 (N-[1-{N′-cyano-N-[3-(trifluoromethoxy)phenyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM245167 (US9428460, 23) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | 37 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description For determining the androgen receptor-dependent transcription, a cellular assay system was used, consisting of PC-3 cells (Kaighn et al., Invest. Uro... | US Patent US9428460 (2016) BindingDB Entry DOI: 10.7270/Q25719Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283179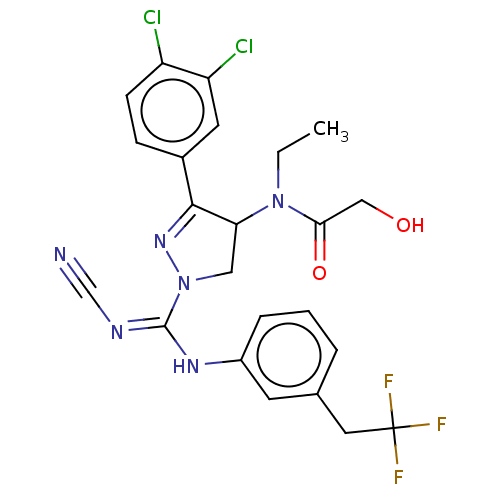 (N-[1-{N′-cyano-N-[3-(2,2,2-trifluoroethyl)ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 53 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283156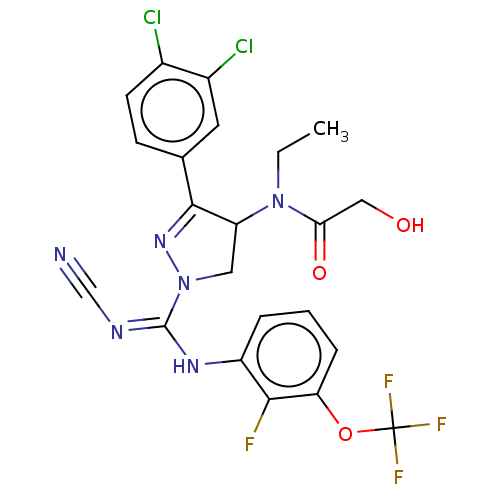 (N-[1-{N′-cyano-N-[2-fluoro-3-(trifluorometho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 54.1 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor [E709Y] (Homo sapiens (Human)) | BDBM245153 (US9428460, 9) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | 37 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description PC-3 cells (Kaighn et al., Invest. Urol. 17: 16-23, 1979) were plated out at a density of 10000 cells per well of a 96-well cell culture plate in RMP... | US Patent US9428460 (2016) BindingDB Entry DOI: 10.7270/Q25719Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283168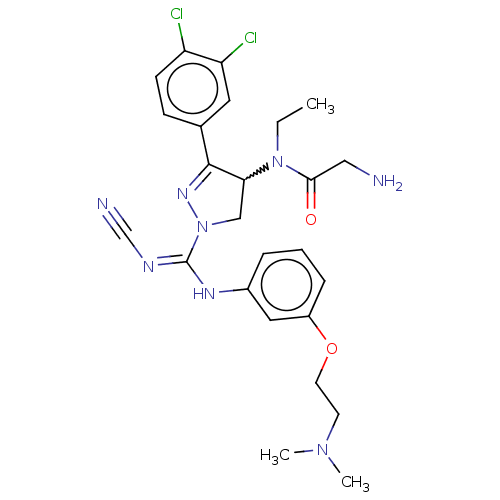 (Rac-N-[1-(N′-cyano-N-{3-[2-(dimethylamino)et...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 59.5 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM245173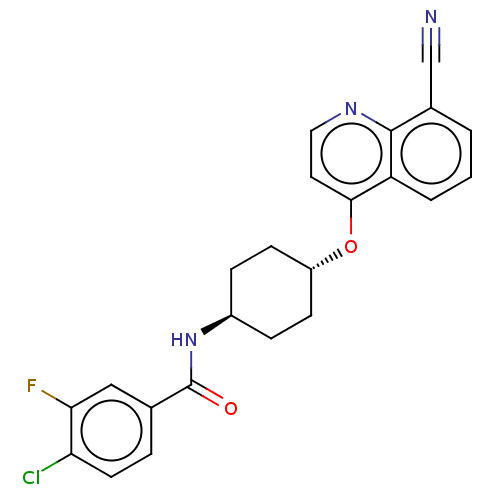 (US9428460, 29) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | 37 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description For determining the androgen receptor-dependent transcription, a cellular assay system was used, consisting of PC-3 cells (Kaighn et al., Invest. Uro... | US Patent US9428460 (2016) BindingDB Entry DOI: 10.7270/Q25719Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283183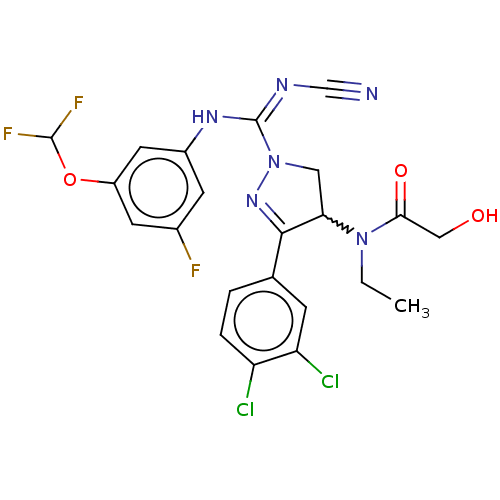 (Rac-N-[1-{N′-cyano-N-[3-(difluoromethoxy)-5-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 63.1 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283181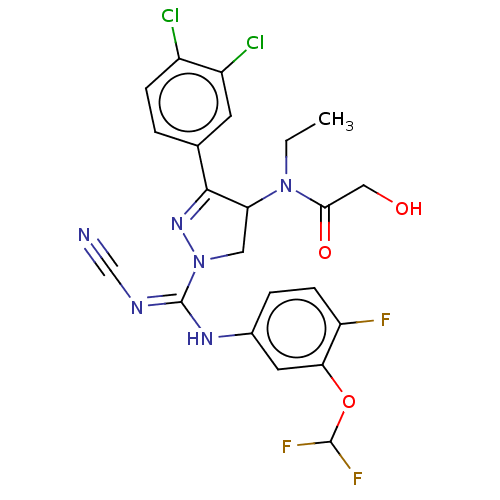 (Rac-N-[1-{N′-cyano-N-[3-(difluoromethoxy)-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 64.8 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM245171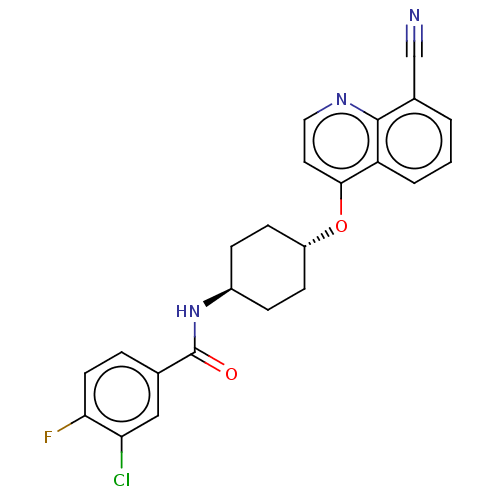 (US9428460, 27) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | 37 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description For determining the androgen receptor-dependent transcription, a cellular assay system was used, consisting of PC-3 cells (Kaighn et al., Invest. Uro... | US Patent US9428460 (2016) BindingDB Entry DOI: 10.7270/Q25719Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283182 (Rac-N-[1-{N′-cyano-N-[3-(difluoromethoxy)-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 67.4 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 147 total ) | Next | Last >> |