

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50368723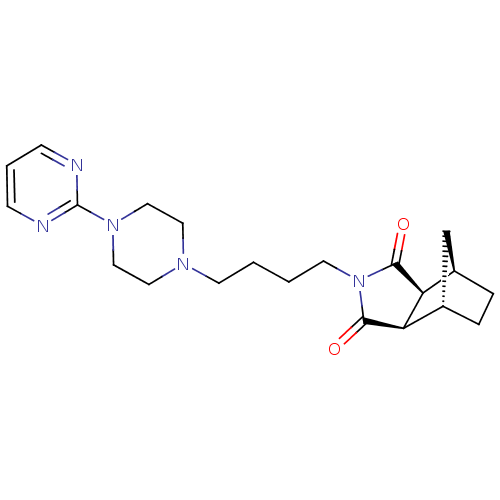 (Metanopirone | Sediel | TANDOSPIRONE HYDROCHLORIDE...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PubMed | 0.00270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Chemical Industry Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity by measuring displacement of [3H]8-OH-DPAT from 5-hydroxytryptamine 1A receptor in rat hippocampus | J Med Chem 36: 3526-32 (1994) BindingDB Entry DOI: 10.7270/Q2D21Z74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50001859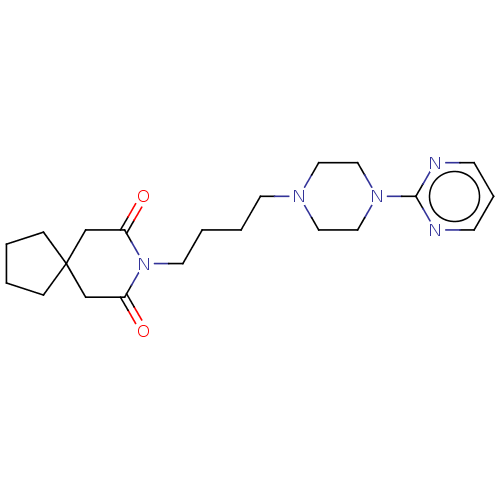 ((buspirone) 8-[4-(4-Pyrimidin-2-yl-piperazin-1-yl)...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Chemical Industry Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity by measuring displacement of [3H]8-OH-DPAT from 5-hydroxytryptamine 1A receptor in rat hippocampus | J Med Chem 36: 3526-32 (1994) BindingDB Entry DOI: 10.7270/Q2D21Z74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50157339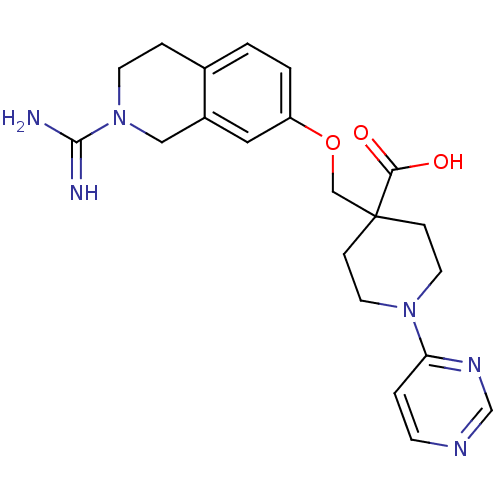 (4-(2-Carbamimidoyl-1,2,3,4-tetrahydro-isoquinolin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human Coagulation factor X | J Med Chem 48: 3586-604 (2005) Article DOI: 10.1021/jm058160e BindingDB Entry DOI: 10.7270/Q2CR5V36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50368723 (Metanopirone | Sediel | TANDOSPIRONE HYDROCHLORIDE...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Chemical Industry Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity for DA2 receptor | J Med Chem 36: 3526-32 (1994) BindingDB Entry DOI: 10.7270/Q2D21Z74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50068488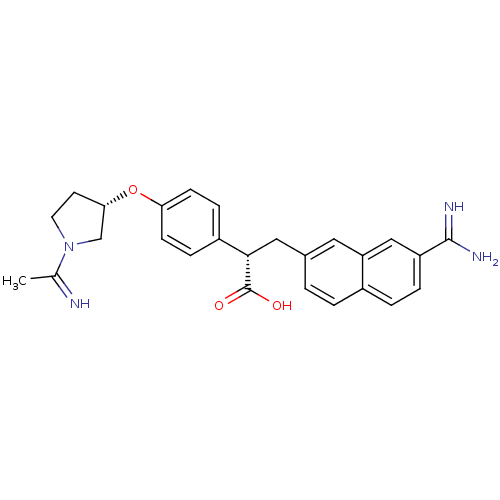 ((S)-3-(7-Carbamimidoyl-naphthalen-2-yl)-2-{4-[(S)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human Coagulation factor X | J Med Chem 48: 3586-604 (2005) Article DOI: 10.1021/jm058160e BindingDB Entry DOI: 10.7270/Q2CR5V36 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50368731 (CHEMBL1203185) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Chemical Industry Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity for 5-hydroxytryptamine 1A receptor by use of [3H]8-OH-DPAT in male rat | J Med Chem 36: 3526-32 (1994) BindingDB Entry DOI: 10.7270/Q2D21Z74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50001859 ((buspirone) 8-[4-(4-Pyrimidin-2-yl-piperazin-1-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Chemical Industry Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity for DA2 receptor | J Med Chem 36: 3526-32 (1994) BindingDB Entry DOI: 10.7270/Q2D21Z74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50288762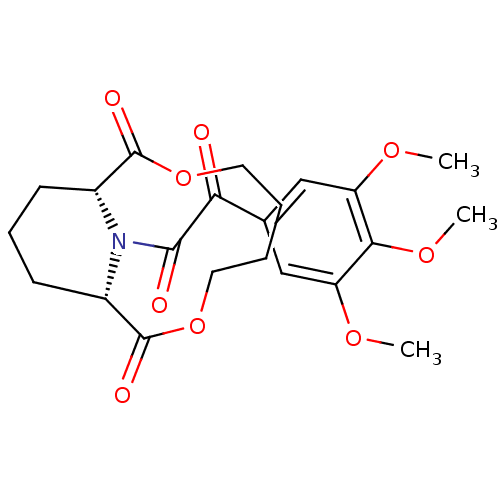 ((1S,10R)-14-[2-Oxo-2-(3,4,5-trimethoxy-phenyl)-ace...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human FKBP-12 rotamase | Bioorg Med Chem Lett 6: 385-390 (1996) Article DOI: 10.1016/0960-894X(96)00032-7 BindingDB Entry DOI: 10.7270/Q2862GFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1/Trypsin-2 (Homo sapiens (Human)) | BDBM50068488 ((S)-3-(7-Carbamimidoyl-naphthalen-2-yl)-2-{4-[(S)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human Trypsin | J Med Chem 48: 3586-604 (2005) Article DOI: 10.1021/jm058160e BindingDB Entry DOI: 10.7270/Q2CR5V36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50288763 ((1S,9R)-5-Benzyloxymethyl-13-[2-oxo-2-(3,4,5-trime...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human FKBP-12 rotamase | Bioorg Med Chem Lett 6: 385-390 (1996) Article DOI: 10.1016/0960-894X(96)00032-7 BindingDB Entry DOI: 10.7270/Q2862GFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50288765 ((1S,9R)-5-(tert-Butyl-dimethyl-silanyloxymethyl)-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human FKBP-12 rotamase | Bioorg Med Chem Lett 6: 385-390 (1996) Article DOI: 10.1016/0960-894X(96)00032-7 BindingDB Entry DOI: 10.7270/Q2862GFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50288764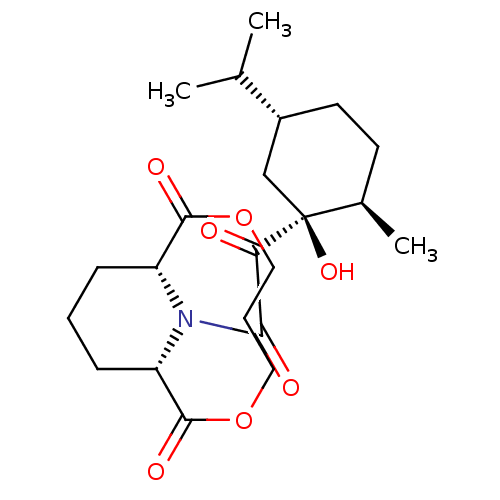 ((1S,9R)-13-[2-((1S,2R,5R)-1-Hydroxy-5-isopropyl-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human FKBP-12 rotamase | Bioorg Med Chem Lett 6: 385-390 (1996) Article DOI: 10.1016/0960-894X(96)00032-7 BindingDB Entry DOI: 10.7270/Q2862GFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50288768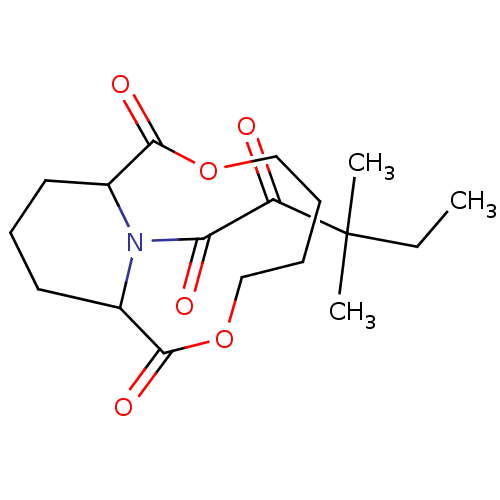 ((1R,10S)-14-(3,3-Dimethyl-2-oxo-pentanoyl)-3,8-dio...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human FKBP-12 rotamase | Bioorg Med Chem Lett 6: 385-390 (1996) Article DOI: 10.1016/0960-894X(96)00032-7 BindingDB Entry DOI: 10.7270/Q2862GFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50288767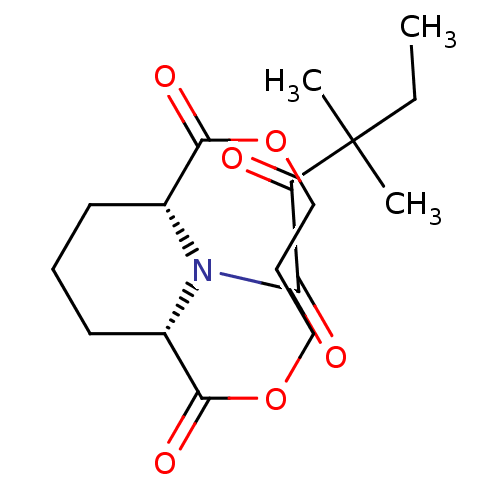 ((1R,9S)-13-(3,3-Dimethyl-2-oxo-pentanoyl)-3,7-diox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human FKBP-12 rotamase | Bioorg Med Chem Lett 6: 385-390 (1996) Article DOI: 10.1016/0960-894X(96)00032-7 BindingDB Entry DOI: 10.7270/Q2862GFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1/Trypsin-2 (Homo sapiens (Human)) | BDBM50157339 (4-(2-Carbamimidoyl-1,2,3,4-tetrahydro-isoquinolin-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human Trypsin | J Med Chem 48: 3586-604 (2005) Article DOI: 10.1021/jm058160e BindingDB Entry DOI: 10.7270/Q2CR5V36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50157341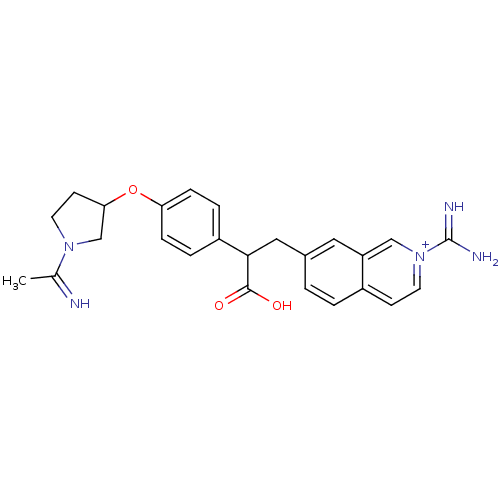 (3-(2-Carbamimidoyl-isoquinolin-7-yl)-2-{4-[1-(1-im...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Mean inhibitory concentration against plasmin; n=3 | Bioorg Med Chem Lett 15: 185-9 (2004) Article DOI: 10.1016/j.bmcl.2004.10.033 BindingDB Entry DOI: 10.7270/Q26Q1Z14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50068488 ((S)-3-(7-Carbamimidoyl-naphthalen-2-yl)-2-{4-[(S)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human Plasmin | J Med Chem 48: 3586-604 (2005) Article DOI: 10.1021/jm058160e BindingDB Entry DOI: 10.7270/Q2CR5V36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50288766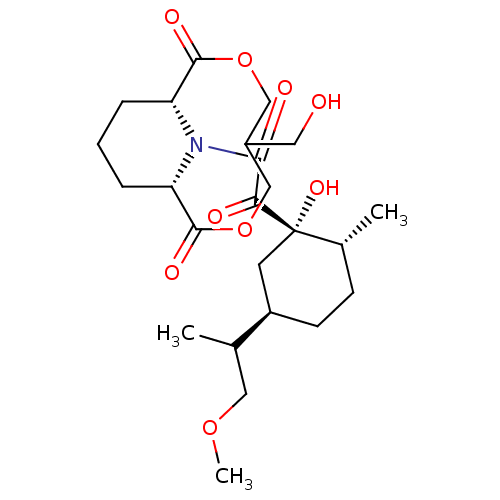 ((1S,9R)-13-{2-[(1S,2R,5R)-1-Hydroxy-5-(2-methoxy-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human FKBP-12 rotamase | Bioorg Med Chem Lett 6: 385-390 (1996) Article DOI: 10.1016/0960-894X(96)00032-7 BindingDB Entry DOI: 10.7270/Q2862GFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50157335 (4-(2-Carbamimidoyl-1,2,3,4-tetrahydro-isoquinolin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Mean inhibitory concentration against plasmin; n=3 | Bioorg Med Chem Lett 15: 185-9 (2004) Article DOI: 10.1016/j.bmcl.2004.10.033 BindingDB Entry DOI: 10.7270/Q26Q1Z14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50157339 (4-(2-Carbamimidoyl-1,2,3,4-tetrahydro-isoquinolin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human Plasmin | J Med Chem 48: 3586-604 (2005) Article DOI: 10.1021/jm058160e BindingDB Entry DOI: 10.7270/Q2CR5V36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50157339 (4-(2-Carbamimidoyl-1,2,3,4-tetrahydro-isoquinolin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human Coagulation factor II (thrombin) | J Med Chem 48: 3586-604 (2005) Article DOI: 10.1021/jm058160e BindingDB Entry DOI: 10.7270/Q2CR5V36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50068488 ((S)-3-(7-Carbamimidoyl-naphthalen-2-yl)-2-{4-[(S)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human Coagulation factor II (thrombin) | J Med Chem 48: 3586-604 (2005) Article DOI: 10.1021/jm058160e BindingDB Entry DOI: 10.7270/Q2CR5V36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50288769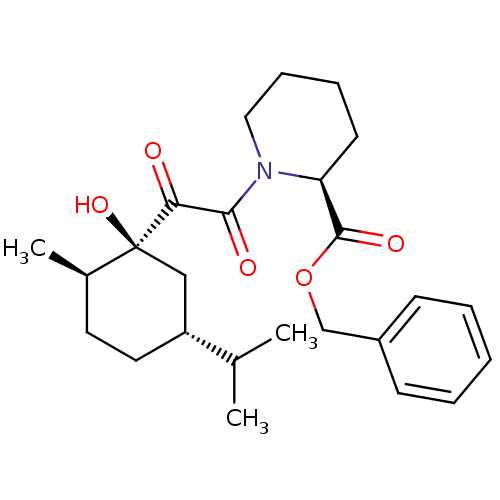 ((S)-1-[2-((1S,2R,5R)-1-Hydroxy-5-isopropyl-2-methy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human FKBP-12 rotamase | Bioorg Med Chem Lett 6: 385-390 (1996) Article DOI: 10.1016/0960-894X(96)00032-7 BindingDB Entry DOI: 10.7270/Q2862GFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50409938 (CHEMBL2094062) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human Coagulation factor X | Bioorg Med Chem Lett 14: 4281-6 (2004) Article DOI: 10.1016/j.bmcl.2004.05.092 BindingDB Entry DOI: 10.7270/Q22808C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50150502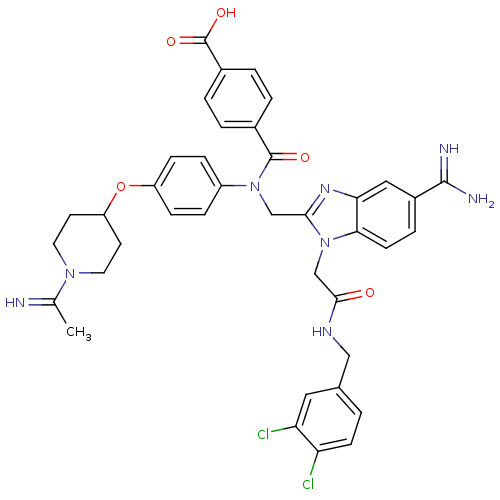 (CHEMBL537184 | N-{5-Carbamimidoyl-1-[(3,4-dichloro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human Coagulation factor X | Bioorg Med Chem Lett 14: 4281-6 (2004) Article DOI: 10.1016/j.bmcl.2004.05.092 BindingDB Entry DOI: 10.7270/Q22808C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50150499 (CHEMBL536722 | N-(5-Carbamimidoyl-1-cyclohexylcarb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human Coagulation factor X | Bioorg Med Chem Lett 14: 4281-6 (2004) Article DOI: 10.1016/j.bmcl.2004.05.092 BindingDB Entry DOI: 10.7270/Q22808C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50150496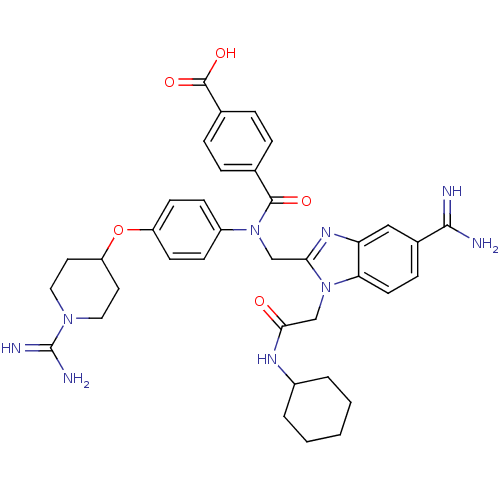 (CHEMBL536490 | N-(5-Carbamimidoyl-1-cyclohexylcarb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human Coagulation factor X | Bioorg Med Chem Lett 14: 4281-6 (2004) Article DOI: 10.1016/j.bmcl.2004.05.092 BindingDB Entry DOI: 10.7270/Q22808C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50368724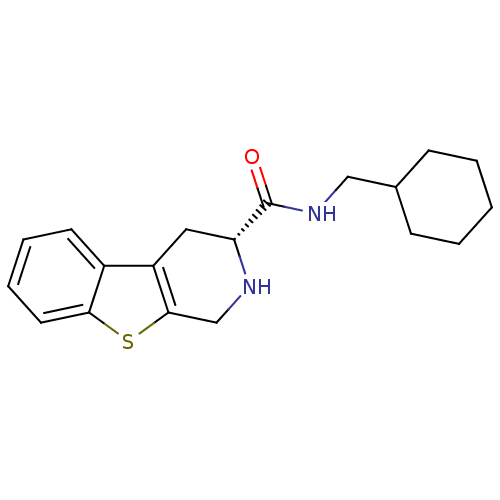 (CHEMBL1203171) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Chemical Industry Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity by measuring displacement of [3H]-8-OH-DPAT from 5-hydroxytryptamine 1A receptor in rat hippocampus | J Med Chem 36: 3526-32 (1994) BindingDB Entry DOI: 10.7270/Q2D21Z74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50368736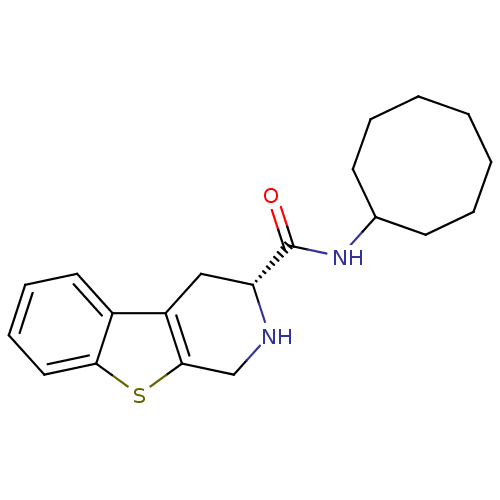 (CHEMBL1203188) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Chemical Industry Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity by measuring displacement of [3H]-8-OH-DPAT from 5-hydroxytryptamine 1A receptor in rat hippocampus | J Med Chem 36: 3526-32 (1994) BindingDB Entry DOI: 10.7270/Q2D21Z74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50157335 (4-(2-Carbamimidoyl-1,2,3,4-tetrahydro-isoquinolin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human Coagulation factor X | J Med Chem 48: 3586-604 (2005) Article DOI: 10.1021/jm058160e BindingDB Entry DOI: 10.7270/Q2CR5V36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50368720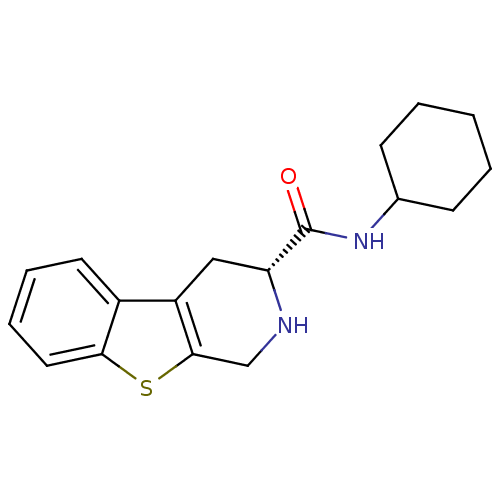 (CHEMBL1203158) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Chemical Industry Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity by measuring displacement of [3H]-8-OH-DPAT from 5-hydroxytryptamine 1A receptor in rat hippocampus | J Med Chem 36: 3526-32 (1994) BindingDB Entry DOI: 10.7270/Q2D21Z74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50368715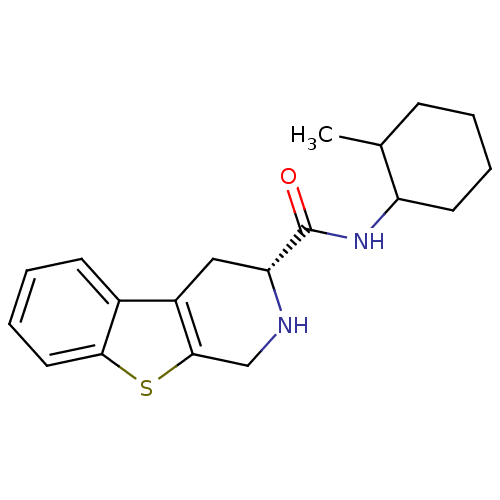 (CHEMBL1203194) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Chemical Industry Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity by measuring displacement of [3H]-8-OH-DPAT from 5-hydroxytryptamine 1A receptor in rat hippocampus | J Med Chem 36: 3526-32 (1994) BindingDB Entry DOI: 10.7270/Q2D21Z74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50368728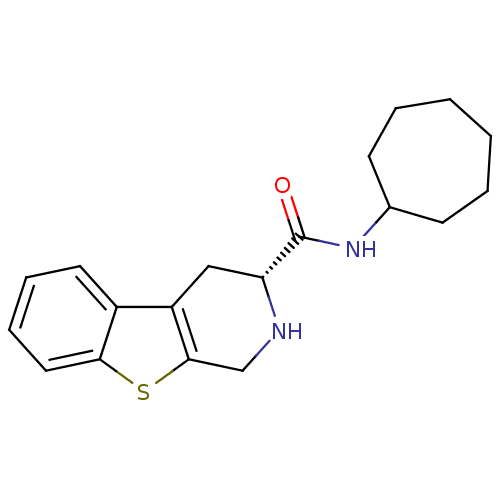 (CHEMBL1203179) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Chemical Industry Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity by measuring displacement of [3H]-8-OH-DPAT from 5-hydroxytryptamine 1A receptor in rat hippocampus | J Med Chem 36: 3526-32 (1994) BindingDB Entry DOI: 10.7270/Q2D21Z74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50150492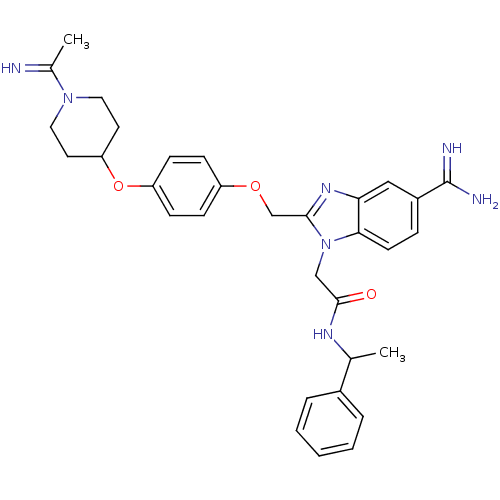 (2-(5-Carbamimidoyl-2-{4-[1-(1-imino-ethyl)-piperid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human Coagulation factor X | Bioorg Med Chem Lett 14: 4281-6 (2004) Article DOI: 10.1016/j.bmcl.2004.05.092 BindingDB Entry DOI: 10.7270/Q22808C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50150488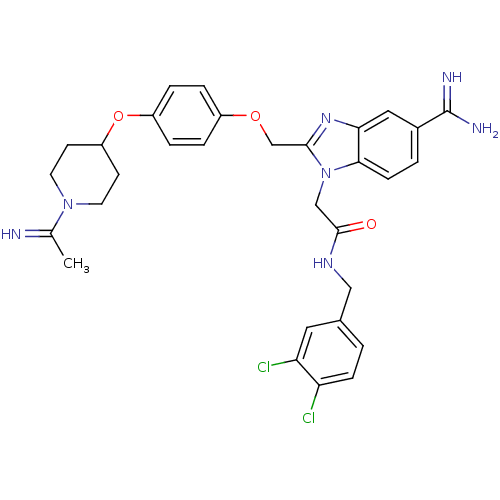 (2-(5-Carbamimidoyl-2-{4-[1-(1-imino-ethyl)-piperid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human Coagulation factor X | Bioorg Med Chem Lett 14: 4281-6 (2004) Article DOI: 10.1016/j.bmcl.2004.05.092 BindingDB Entry DOI: 10.7270/Q22808C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50368716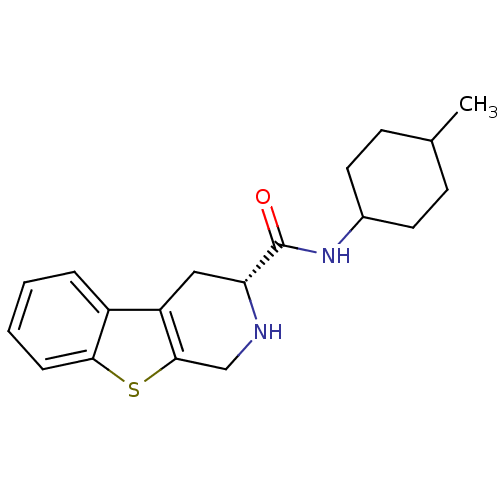 (CHEMBL1203193) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Chemical Industry Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity by measuring displacement of [3H]-8-OH-DPAT from 5-hydroxytryptamine 1A receptor in rat hippocampus | J Med Chem 36: 3526-32 (1994) BindingDB Entry DOI: 10.7270/Q2D21Z74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50150495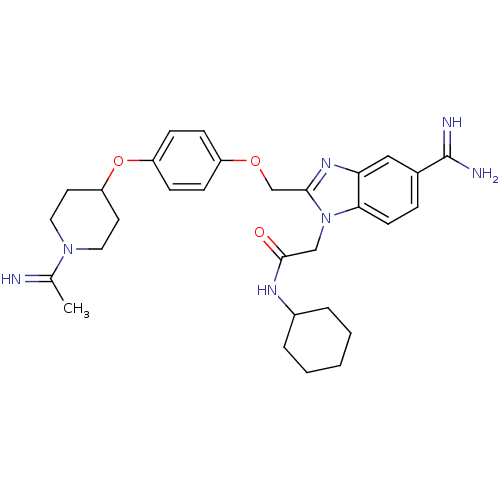 (2-(5-Carbamimidoyl-2-{4-[1-(1-imino-ethyl)-piperid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human Coagulation factor X | Bioorg Med Chem Lett 14: 4281-6 (2004) Article DOI: 10.1016/j.bmcl.2004.05.092 BindingDB Entry DOI: 10.7270/Q22808C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50368717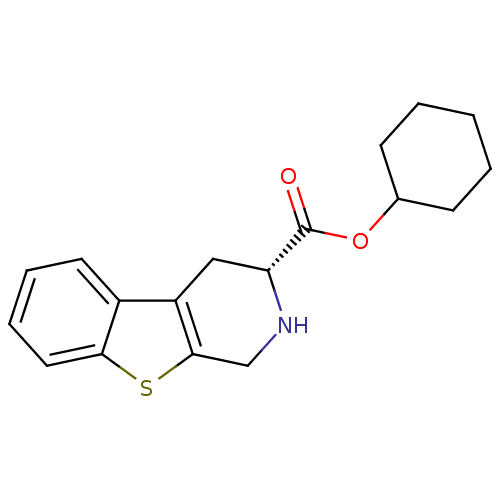 (CHEMBL1203173) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Chemical Industry Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity by measuring displacement of [3H]-8-OH-DPAT from 5-hydroxytryptamine 1A receptor in rat hippocampus | J Med Chem 36: 3526-32 (1994) BindingDB Entry DOI: 10.7270/Q2D21Z74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50368735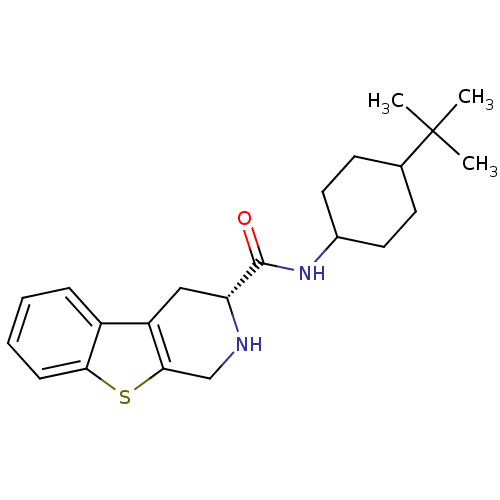 (CHEMBL1203192) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Chemical Industry Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity by measuring displacement of [3H]-8-OH-DPAT from 5-hydroxytryptamine 1A receptor in rat hippocampus | J Med Chem 36: 3526-32 (1994) BindingDB Entry DOI: 10.7270/Q2D21Z74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50368731 (CHEMBL1203185) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Chemical Industry Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity by measuring displacement of [3H]-8-OH-DPAT from 5-hydroxytryptamine 1A receptor in rat hippocampus | J Med Chem 36: 3526-32 (1994) BindingDB Entry DOI: 10.7270/Q2D21Z74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50368726 (CHEMBL1203156) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Chemical Industry Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity by measuring displacement of [3H]-8-OH-DPAT from 5-hydroxytryptamine 1A receptor in rat hippocampus | J Med Chem 36: 3526-32 (1994) BindingDB Entry DOI: 10.7270/Q2D21Z74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50157342 (7-(3,4,5,6-Tetrahydro-2H-[1,4'']bipyridinyl-4-ylme...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human Coagulation factor X | J Med Chem 48: 3586-604 (2005) Article DOI: 10.1021/jm058160e BindingDB Entry DOI: 10.7270/Q2CR5V36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50368721 (CHEMBL1203199) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Chemical Industry Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity by measuring displacement of [3H]-8-OH-DPAT from 5-hydroxytryptamine 1A receptor in rat hippocampus | J Med Chem 36: 3526-32 (1994) BindingDB Entry DOI: 10.7270/Q2D21Z74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50041218 (3-(7-Carbamimidoyl-naphthalen-2-yl)-2-{4-[(S)-1-(1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human Coagulation factor X | Bioorg Med Chem Lett 14: 4281-6 (2004) Article DOI: 10.1016/j.bmcl.2004.05.092 BindingDB Entry DOI: 10.7270/Q22808C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50150494 (2-(5-Carbamimidoyl-2-{4-[1-(1-imino-ethyl)-piperid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human Coagulation factor X | Bioorg Med Chem Lett 14: 4281-6 (2004) Article DOI: 10.1016/j.bmcl.2004.05.092 BindingDB Entry DOI: 10.7270/Q22808C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50368732 (CHEMBL1203191) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Chemical Industry Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity by measuring displacement of [3H]-8-OH-DPAT from 5-hydroxytryptamine 1A receptor in rat hippocampus | J Med Chem 36: 3526-32 (1994) BindingDB Entry DOI: 10.7270/Q2D21Z74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50036656 (1,2,3,4-Tetrahydro-benzo[4,5]thieno[2,3-c]pyridine...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Chemical Industry Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity by measuring displacement of [3H]-8-OH-DPAT from 5-hydroxytryptamine 1A receptor in rat hippocampus | J Med Chem 36: 3526-32 (1994) BindingDB Entry DOI: 10.7270/Q2D21Z74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50368719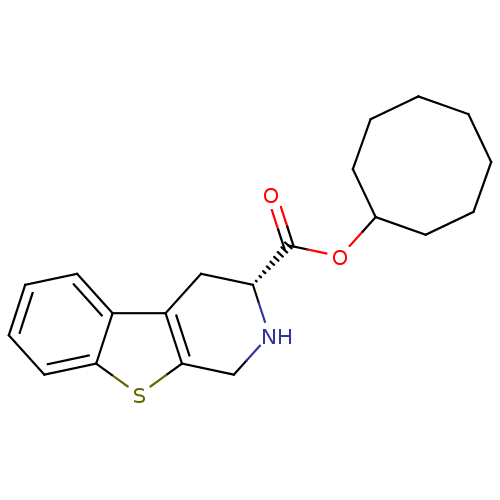 (CHEMBL1203209) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Chemical Industry Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity by measuring displacement of [3H]-8-OH-DPAT from 5-hydroxytryptamine 1A receptor in rat hippocampus | J Med Chem 36: 3526-32 (1994) BindingDB Entry DOI: 10.7270/Q2D21Z74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50157330 (4-(2-Carbamimidoyl-1,2,3,4-tetrahydro-isoquinolin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human Coagulation factor X | J Med Chem 48: 3586-604 (2005) Article DOI: 10.1021/jm058160e BindingDB Entry DOI: 10.7270/Q2CR5V36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50409939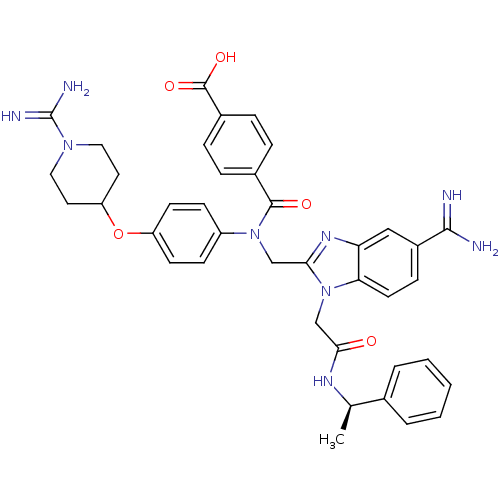 (CHEMBL2093954) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human Coagulation factor X | Bioorg Med Chem Lett 14: 4281-6 (2004) Article DOI: 10.1016/j.bmcl.2004.05.092 BindingDB Entry DOI: 10.7270/Q22808C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 143 total ) | Next | Last >> |