

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sodium-dependent phosphate transport protein 2B (Rattus norvegicus) | BDBM50590497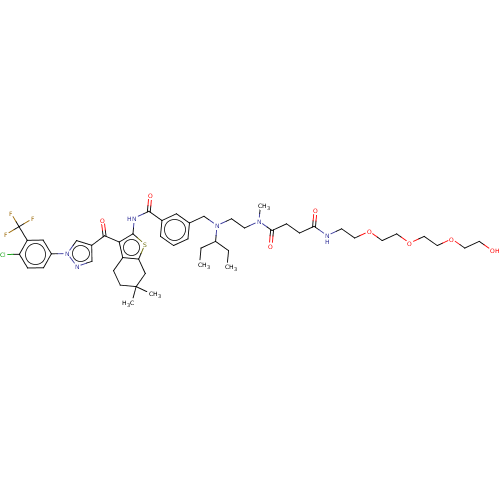 (CHEMBL5182987) | KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128572 BindingDB Entry DOI: 10.7270/Q26977M6 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent phosphate transport protein 2B (Rattus norvegicus) | BDBM50596527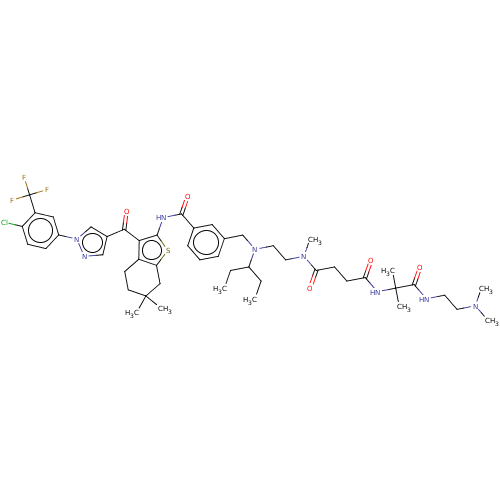 (CHEMBL5195035) | KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128572 BindingDB Entry DOI: 10.7270/Q26977M6 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50503838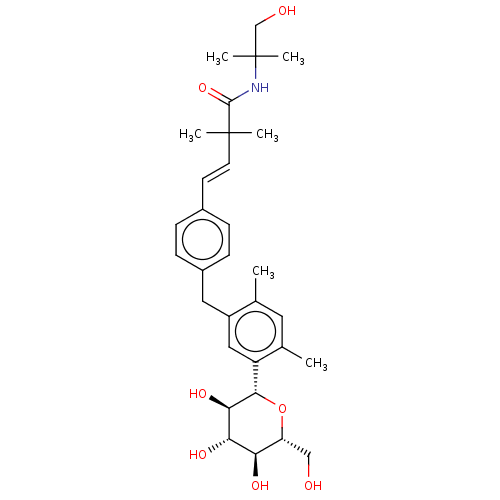 (CHEMBL4450704) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human SGLT2 expressed in CHOK1 cells assessed as reduction in sodium-dependent glucose uptake after 1 hr in presence of [14C]-methyl-al... | Bioorg Med Chem 27: 394-409 (2019) Article DOI: 10.1016/j.bmc.2018.12.015 BindingDB Entry DOI: 10.7270/Q2X63R7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent phosphate transport protein 2B (Rattus norvegicus) | BDBM50600220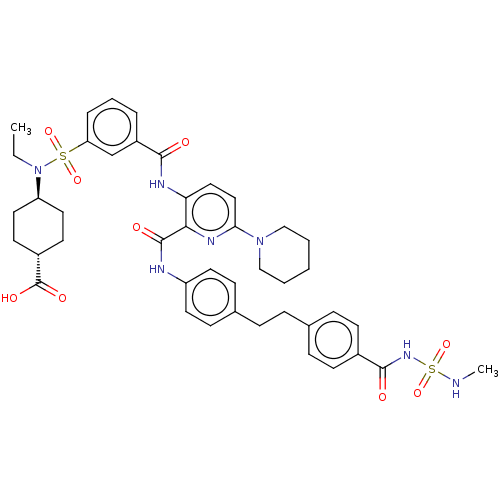 (CHEMBL5177872) | KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128700 BindingDB Entry DOI: 10.7270/Q28W3JB9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242568 (US9422240, 1-378) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242554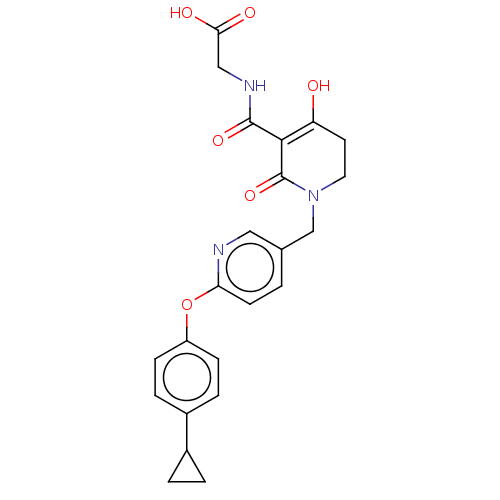 (US9422240, 1-296) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242567 (US9422240, 1-377) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent phosphate transport protein 2B (Homo sapiens (Human)) | BDBM50590497 (CHEMBL5182987) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128572 BindingDB Entry DOI: 10.7270/Q26977M6 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50503827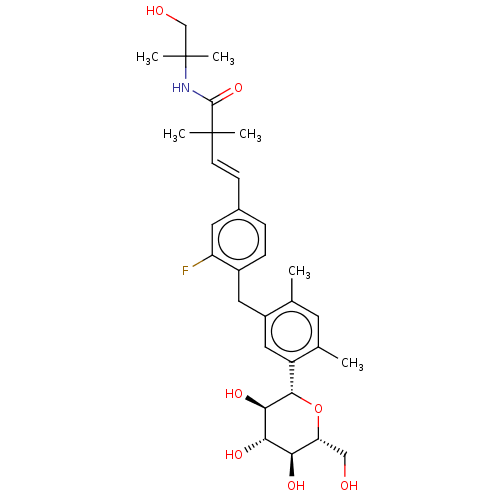 (CHEMBL4439652) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human SGLT2 expressed in CHOK1 cells assessed as reduction in sodium-dependent glucose uptake after 1 hr in presence of [14C]-methyl-al... | Bioorg Med Chem 27: 394-409 (2019) Article DOI: 10.1016/j.bmc.2018.12.015 BindingDB Entry DOI: 10.7270/Q2X63R7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50503834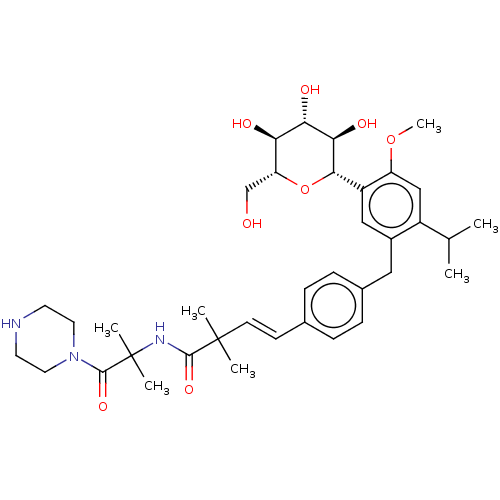 (CHEMBL4591009) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human SGLT2 expressed in CHOK1 cells assessed as reduction in sodium-dependent glucose uptake after 1 hr in presence of [14C]-methyl-al... | Bioorg Med Chem 27: 394-409 (2019) Article DOI: 10.1016/j.bmc.2018.12.015 BindingDB Entry DOI: 10.7270/Q2X63R7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242563 (US9422240, 1-366) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242526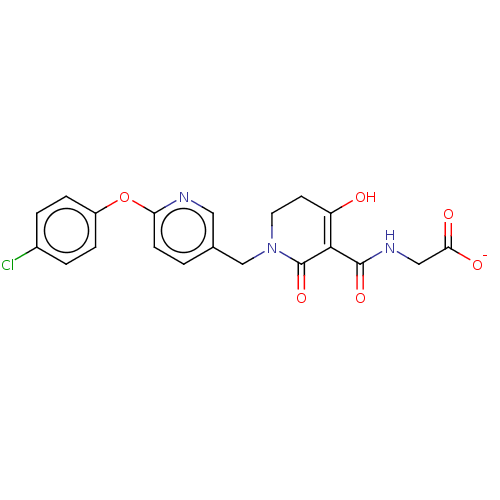 (US9422240, 1-4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50503822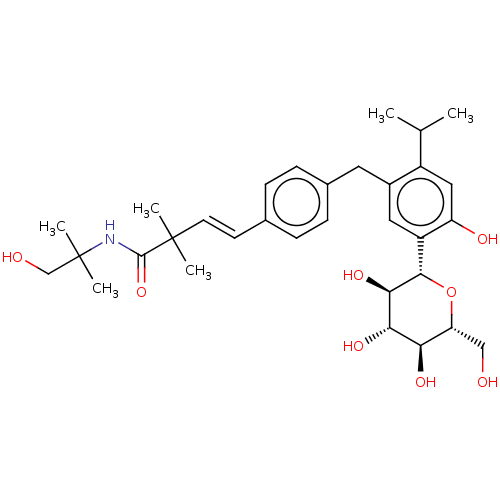 (CHEMBL4460788) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human SGLT2 expressed in CHOK1 cells assessed as reduction in sodium-dependent glucose uptake after 1 hr in presence of [14C]-methyl-al... | Bioorg Med Chem 27: 394-409 (2019) Article DOI: 10.1016/j.bmc.2018.12.015 BindingDB Entry DOI: 10.7270/Q2X63R7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50503841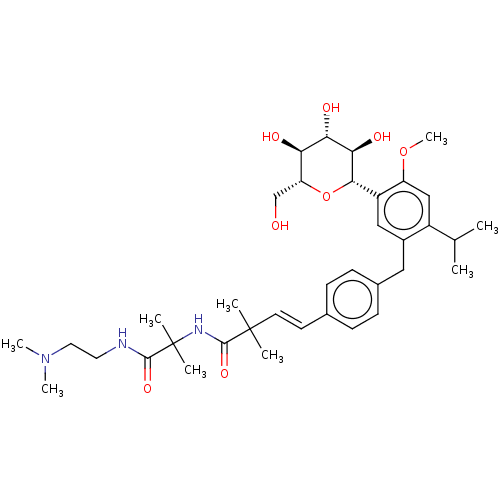 (CHEMBL4554040) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human SGLT2 expressed in CHOK1 cells assessed as reduction in sodium-dependent glucose uptake after 1 hr in presence of [14C]-methyl-al... | Bioorg Med Chem 27: 394-409 (2019) Article DOI: 10.1016/j.bmc.2018.12.015 BindingDB Entry DOI: 10.7270/Q2X63R7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242577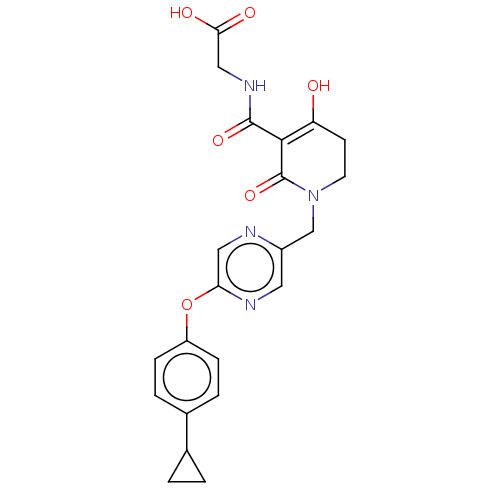 (US9422240, 1-443) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 21 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM50503835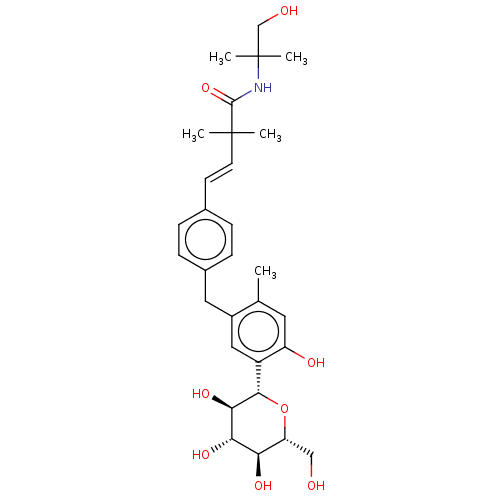 (CHEMBL4524341) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human SGLT1 expressed in CHOK1 cells assessed as reduction in sodium-dependent glucose uptake after 30 mins in presence of [14C]-methyl... | Bioorg Med Chem 27: 394-409 (2019) Article DOI: 10.1016/j.bmc.2018.12.015 BindingDB Entry DOI: 10.7270/Q2X63R7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent phosphate transport protein 2B (Homo sapiens (Human)) | BDBM50600220 (CHEMBL5177872) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128700 BindingDB Entry DOI: 10.7270/Q28W3JB9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242566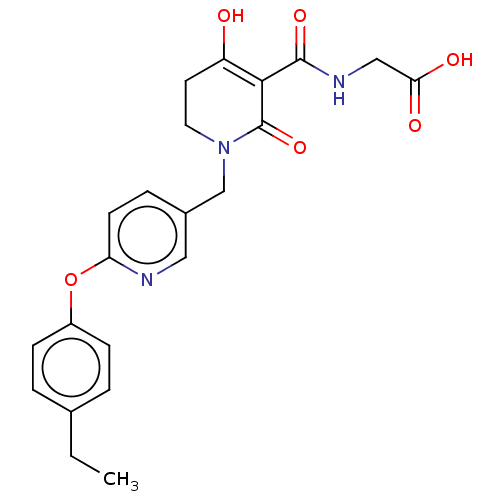 (US9422240, 1-376) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 22 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM50503838 (CHEMBL4450704) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human SGLT1 expressed in CHOK1 cells assessed as reduction in sodium-dependent glucose uptake after 30 mins in presence of [14C]-methyl... | Bioorg Med Chem 27: 394-409 (2019) Article DOI: 10.1016/j.bmc.2018.12.015 BindingDB Entry DOI: 10.7270/Q2X63R7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM50503822 (CHEMBL4460788) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human SGLT1 expressed in CHOK1 cells assessed as reduction in sodium-dependent glucose uptake after 30 mins in presence of [14C]-methyl... | Bioorg Med Chem 27: 394-409 (2019) Article DOI: 10.1016/j.bmc.2018.12.015 BindingDB Entry DOI: 10.7270/Q2X63R7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242535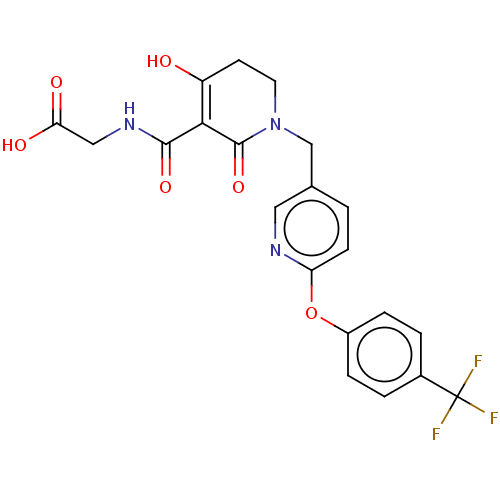 (US9422240, 1-254) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 24 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242542 (US9422240, 1-265 | US9422240, 1-355) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 24 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242575 (US9422240, 1-409) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 25 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242564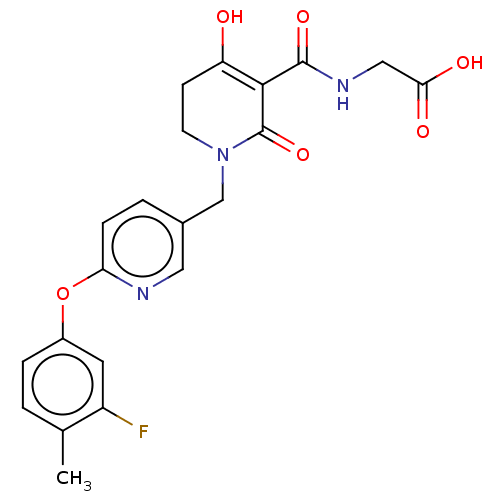 (US9422240, 1-367) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 26 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242571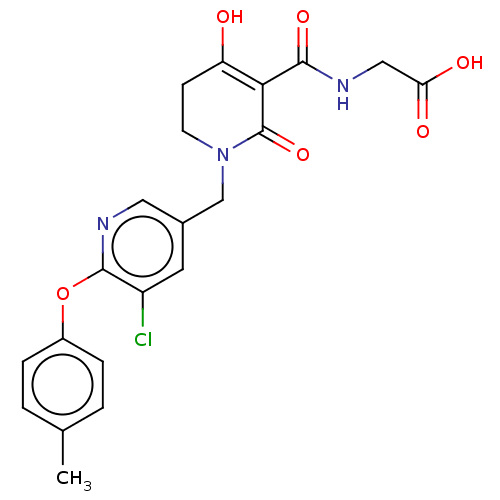 (US9422240, 1-392) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 26 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50503832 (CHEMBL4514344) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human SGLT2 expressed in CHOK1 cells assessed as reduction in sodium-dependent glucose uptake after 1 hr in presence of [14C]-methyl-al... | Bioorg Med Chem 27: 394-409 (2019) Article DOI: 10.1016/j.bmc.2018.12.015 BindingDB Entry DOI: 10.7270/Q2X63R7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM50503837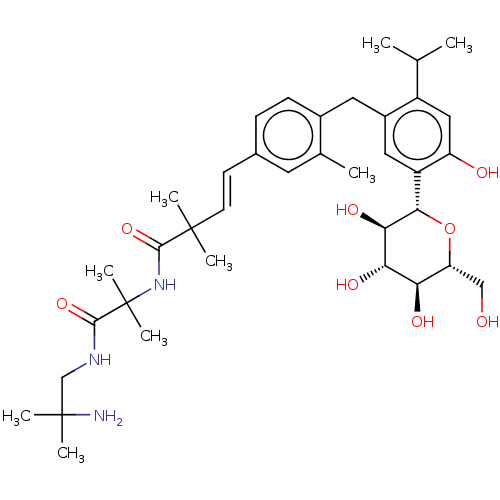 (CHEMBL4516721) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human SGLT1 expressed in CHOK1 cells assessed as reduction in sodium-dependent glucose uptake after 30 mins in presence of [14C]-methyl... | Bioorg Med Chem 27: 394-409 (2019) Article DOI: 10.1016/j.bmc.2018.12.015 BindingDB Entry DOI: 10.7270/Q2X63R7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50503823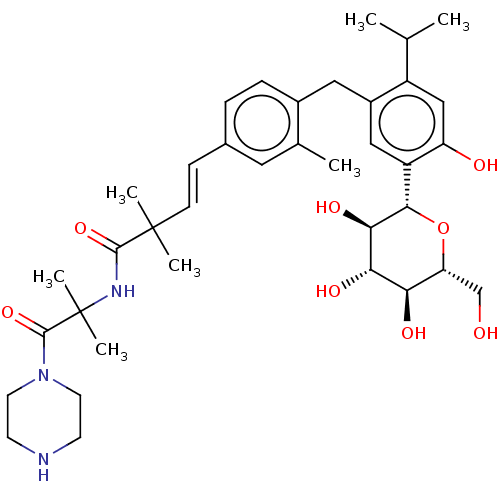 (CHEMBL4452698) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human SGLT2 expressed in CHOK1 cells assessed as reduction in sodium-dependent glucose uptake after 1 hr in presence of [14C]-methyl-al... | Bioorg Med Chem 27: 394-409 (2019) Article DOI: 10.1016/j.bmc.2018.12.015 BindingDB Entry DOI: 10.7270/Q2X63R7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent phosphate transport protein 2B (Rattus norvegicus) | BDBM50594205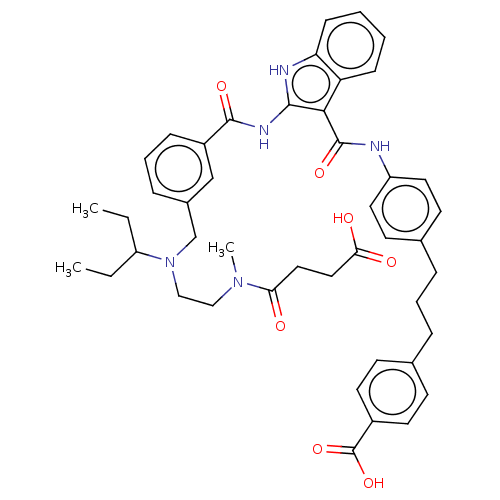 (CHEMBL5192917) | KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116783 BindingDB Entry DOI: 10.7270/Q2QF8XV1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM50503841 (CHEMBL4554040) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human SGLT1 expressed in CHOK1 cells assessed as reduction in sodium-dependent glucose uptake after 30 mins in presence of [14C]-methyl... | Bioorg Med Chem 27: 394-409 (2019) Article DOI: 10.1016/j.bmc.2018.12.015 BindingDB Entry DOI: 10.7270/Q2X63R7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM50503823 (CHEMBL4452698) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human SGLT1 expressed in CHOK1 cells assessed as reduction in sodium-dependent glucose uptake after 30 mins in presence of [14C]-methyl... | Bioorg Med Chem 27: 394-409 (2019) Article DOI: 10.1016/j.bmc.2018.12.015 BindingDB Entry DOI: 10.7270/Q2X63R7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242572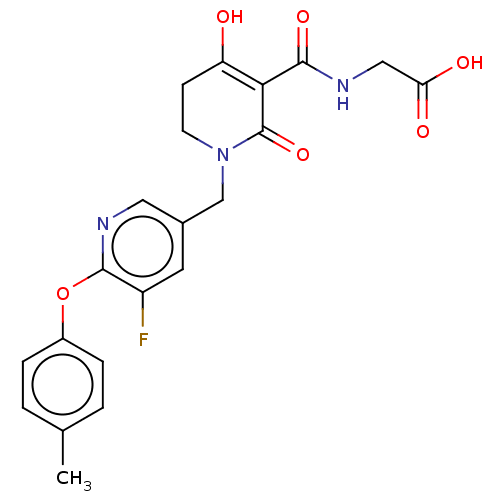 (US9422240, 1-393) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 32 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM50503825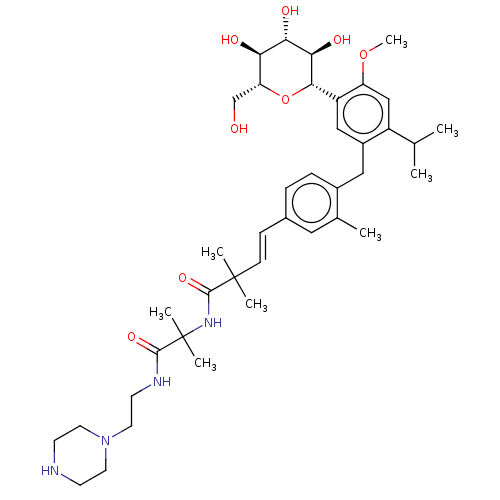 (CHEMBL4564494) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human SGLT1 expressed in CHOK1 cells assessed as reduction in sodium-dependent glucose uptake after 30 mins in presence of [14C]-methyl... | Bioorg Med Chem 27: 394-409 (2019) Article DOI: 10.1016/j.bmc.2018.12.015 BindingDB Entry DOI: 10.7270/Q2X63R7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM50503827 (CHEMBL4439652) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human SGLT1 expressed in CHOK1 cells assessed as reduction in sodium-dependent glucose uptake after 30 mins in presence of [14C]-methyl... | Bioorg Med Chem 27: 394-409 (2019) Article DOI: 10.1016/j.bmc.2018.12.015 BindingDB Entry DOI: 10.7270/Q2X63R7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50503840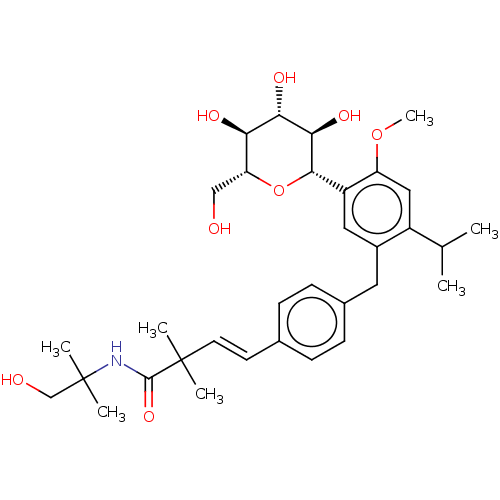 (CHEMBL4551742) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human SGLT2 expressed in CHOK1 cells assessed as reduction in sodium-dependent glucose uptake after 1 hr in presence of [14C]-methyl-al... | Bioorg Med Chem 27: 394-409 (2019) Article DOI: 10.1016/j.bmc.2018.12.015 BindingDB Entry DOI: 10.7270/Q2X63R7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM50503824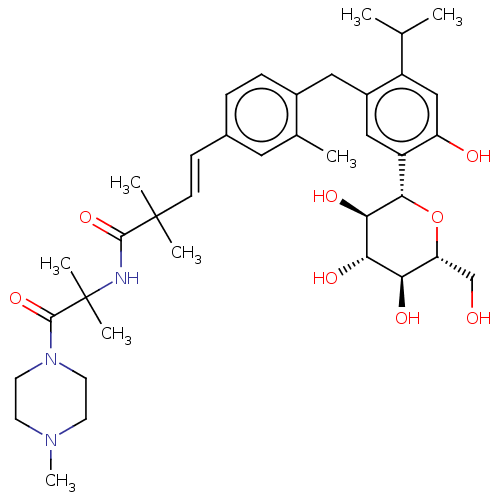 (CHEMBL4457957) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human SGLT1 expressed in CHOK1 cells assessed as reduction in sodium-dependent glucose uptake after 30 mins in presence of [14C]-methyl... | Bioorg Med Chem 27: 394-409 (2019) Article DOI: 10.1016/j.bmc.2018.12.015 BindingDB Entry DOI: 10.7270/Q2X63R7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM50503833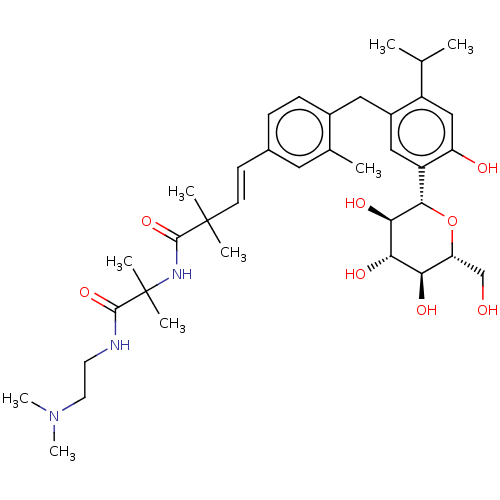 (CHEMBL4472308) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human SGLT1 expressed in CHOK1 cells assessed as reduction in sodium-dependent glucose uptake after 30 mins in presence of [14C]-methyl... | Bioorg Med Chem 27: 394-409 (2019) Article DOI: 10.1016/j.bmc.2018.12.015 BindingDB Entry DOI: 10.7270/Q2X63R7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM50503840 (CHEMBL4551742) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human SGLT1 expressed in CHOK1 cells assessed as reduction in sodium-dependent glucose uptake after 30 mins in presence of [14C]-methyl... | Bioorg Med Chem 27: 394-409 (2019) Article DOI: 10.1016/j.bmc.2018.12.015 BindingDB Entry DOI: 10.7270/Q2X63R7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent phosphate transport protein 2B (Homo sapiens (Human)) | BDBM50600212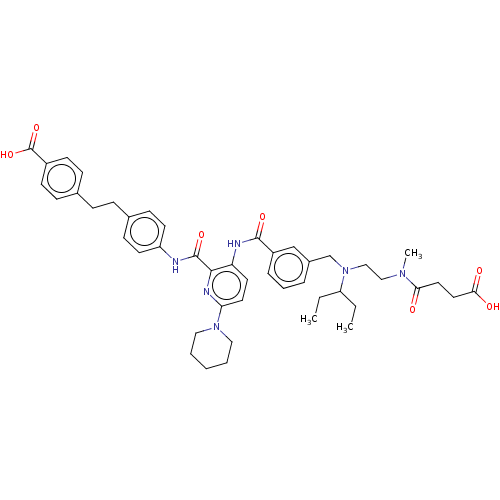 (CHEMBL5177122) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128700 BindingDB Entry DOI: 10.7270/Q28W3JB9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent phosphate transport protein 2B (Homo sapiens (Human)) | BDBM50596515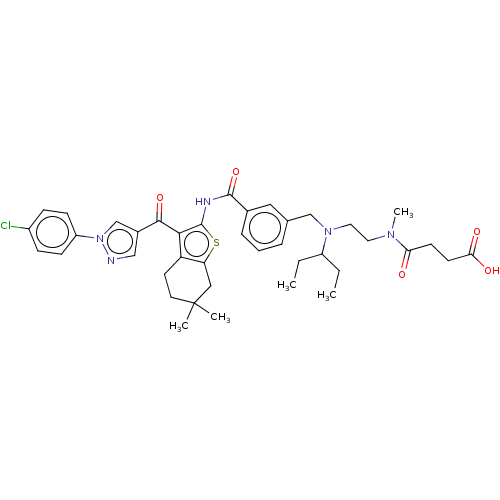 (CHEMBL5204879) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128572 BindingDB Entry DOI: 10.7270/Q26977M6 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM50503834 (CHEMBL4591009) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human SGLT1 expressed in CHOK1 cells assessed as reduction in sodium-dependent glucose uptake after 30 mins in presence of [14C]-methyl... | Bioorg Med Chem 27: 394-409 (2019) Article DOI: 10.1016/j.bmc.2018.12.015 BindingDB Entry DOI: 10.7270/Q2X63R7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent phosphate transport protein 2B (Homo sapiens (Human)) | BDBM50596516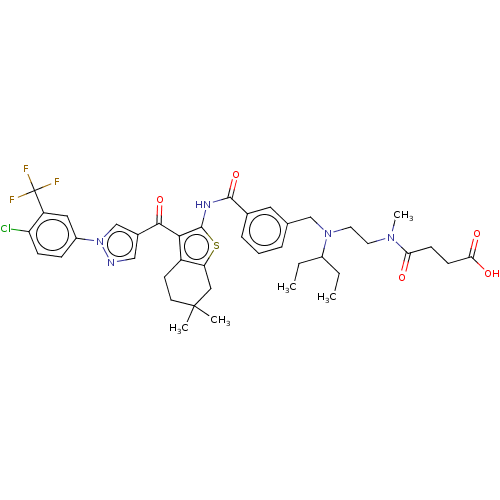 (CHEMBL5192822) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128572 BindingDB Entry DOI: 10.7270/Q26977M6 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242576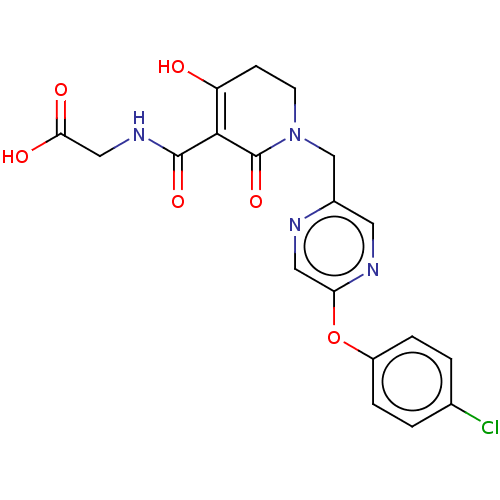 (US9422240, 1-440) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 39 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242528 (US9422240, 1-70) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 39 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent phosphate transport protein 2B (Homo sapiens (Human)) | BDBM50596525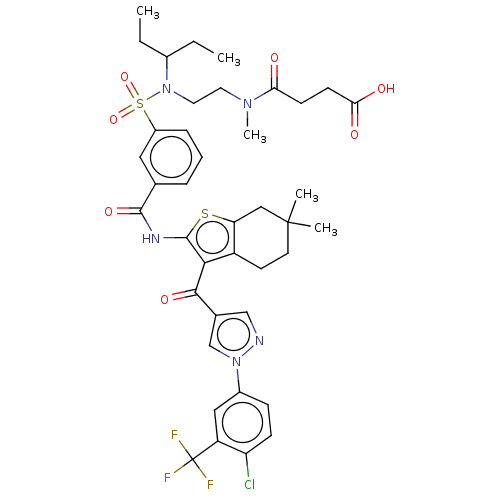 (CHEMBL5177313) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128572 BindingDB Entry DOI: 10.7270/Q26977M6 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242532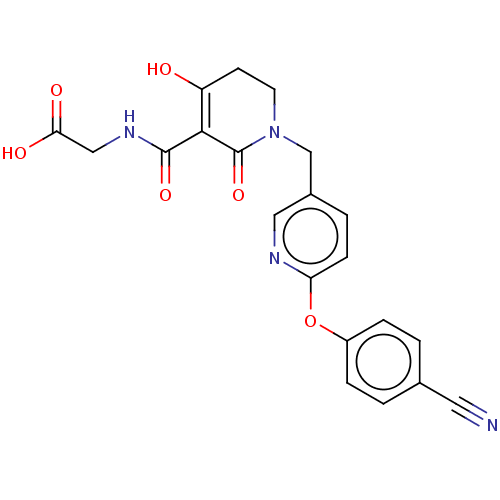 (US9422240, 1-225) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242548 (US9422240, 1-284) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242565 (US9422240, 1-368) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 41 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM50503832 (CHEMBL4514344) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human SGLT1 expressed in CHOK1 cells assessed as reduction in sodium-dependent glucose uptake after 30 mins in presence of [14C]-methyl... | Bioorg Med Chem 27: 394-409 (2019) Article DOI: 10.1016/j.bmc.2018.12.015 BindingDB Entry DOI: 10.7270/Q2X63R7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent phosphate transport protein 2B (Homo sapiens (Human)) | BDBM50596524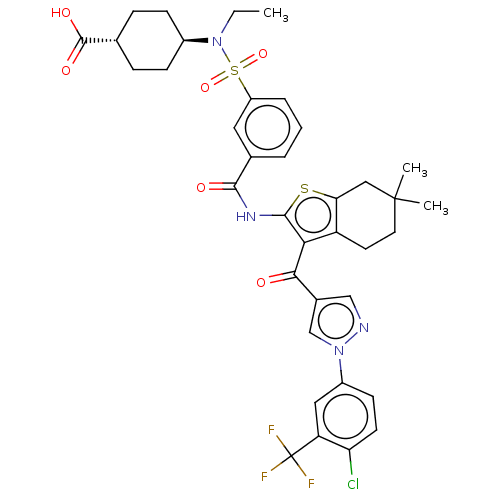 (CHEMBL5188308) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128572 BindingDB Entry DOI: 10.7270/Q26977M6 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 203 total ) | Next | Last >> |