

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50165008 ((+)-(R)-2-(alpha-(2-(Diisopropylamino)ethyl)benzyl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka Curated by ChEMBL | Assay Description Displacement of [3H]NMS from muscarinic receptor M1 in Sprague-Dawley rat brain homogenates after 60 mins by liquid scintillation counting method | J Med Chem 61: 4020-4029 (2018) Article DOI: 10.1021/acs.jmedchem.8b00041 BindingDB Entry DOI: 10.7270/Q29Z97H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M5 (RAT) | BDBM50165008 ((+)-(R)-2-(alpha-(2-(Diisopropylamino)ethyl)benzyl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka Curated by ChEMBL | Assay Description Displacement of [3H]NMS from muscarinic receptor M5 in Sprague-Dawley rat brain homogenates after 60 mins by liquid scintillation counting method | J Med Chem 61: 4020-4029 (2018) Article DOI: 10.1021/acs.jmedchem.8b00041 BindingDB Entry DOI: 10.7270/Q29Z97H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50165008 ((+)-(R)-2-(alpha-(2-(Diisopropylamino)ethyl)benzyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka Curated by ChEMBL | Assay Description Displacement of [3H]NMS from muscarinic receptor M2 in Sprague-Dawley rat brain homogenates after 60 mins by liquid scintillation counting method | J Med Chem 61: 4020-4029 (2018) Article DOI: 10.1021/acs.jmedchem.8b00041 BindingDB Entry DOI: 10.7270/Q29Z97H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (RAT) | BDBM50165008 ((+)-(R)-2-(alpha-(2-(Diisopropylamino)ethyl)benzyl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka Curated by ChEMBL | Assay Description Displacement of [3H]NMS from muscarinic receptor M4 in Sprague-Dawley rat brain homogenates after 60 mins by liquid scintillation counting method | J Med Chem 61: 4020-4029 (2018) Article DOI: 10.1021/acs.jmedchem.8b00041 BindingDB Entry DOI: 10.7270/Q29Z97H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50165008 ((+)-(R)-2-(alpha-(2-(Diisopropylamino)ethyl)benzyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka Curated by ChEMBL | Assay Description Displacement of [3H]NMS from muscarinic receptor M3 in Sprague-Dawley rat brain homogenates after 60 mins by liquid scintillation counting method | J Med Chem 61: 4020-4029 (2018) Article DOI: 10.1021/acs.jmedchem.8b00041 BindingDB Entry DOI: 10.7270/Q29Z97H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50374004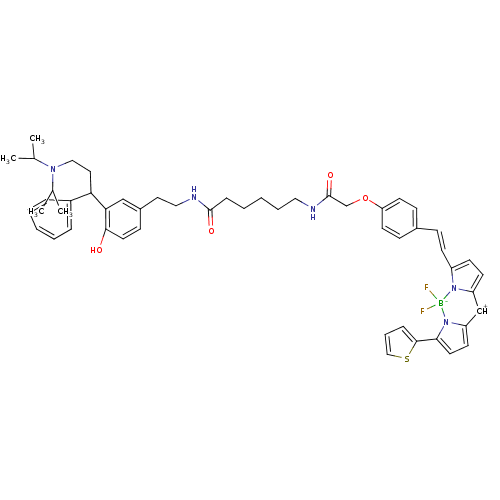 (CHEMBL271108) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka Curated by ChEMBL | Assay Description Displacement of [3H]NMS from muscarinic receptor M1 in Sprague-Dawley rat brain homogenates after 60 mins by liquid scintillation counting method | J Med Chem 61: 4020-4029 (2018) Article DOI: 10.1021/acs.jmedchem.8b00041 BindingDB Entry DOI: 10.7270/Q29Z97H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (RAT) | BDBM50374004 (CHEMBL271108) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka Curated by ChEMBL | Assay Description Displacement of [3H]NMS from muscarinic receptor M4 in Sprague-Dawley rat brain homogenates after 60 mins by liquid scintillation counting method | J Med Chem 61: 4020-4029 (2018) Article DOI: 10.1021/acs.jmedchem.8b00041 BindingDB Entry DOI: 10.7270/Q29Z97H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50374004 (CHEMBL271108) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka Curated by ChEMBL | Assay Description Displacement of [3H]NMS from muscarinic receptor M2 in Sprague-Dawley rat brain homogenates after 60 mins by liquid scintillation counting method | J Med Chem 61: 4020-4029 (2018) Article DOI: 10.1021/acs.jmedchem.8b00041 BindingDB Entry DOI: 10.7270/Q29Z97H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50374004 (CHEMBL271108) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka Curated by ChEMBL | Assay Description Displacement of [3H]NMS from muscarinic receptor M3 in Sprague-Dawley rat brain homogenates after 60 mins by liquid scintillation counting method | J Med Chem 61: 4020-4029 (2018) Article DOI: 10.1021/acs.jmedchem.8b00041 BindingDB Entry DOI: 10.7270/Q29Z97H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M5 (RAT) | BDBM50374004 (CHEMBL271108) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka Curated by ChEMBL | Assay Description Displacement of [3H]NMS from muscarinic receptor M5 in Sprague-Dawley rat brain homogenates after 60 mins by liquid scintillation counting method | J Med Chem 61: 4020-4029 (2018) Article DOI: 10.1021/acs.jmedchem.8b00041 BindingDB Entry DOI: 10.7270/Q29Z97H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM50569664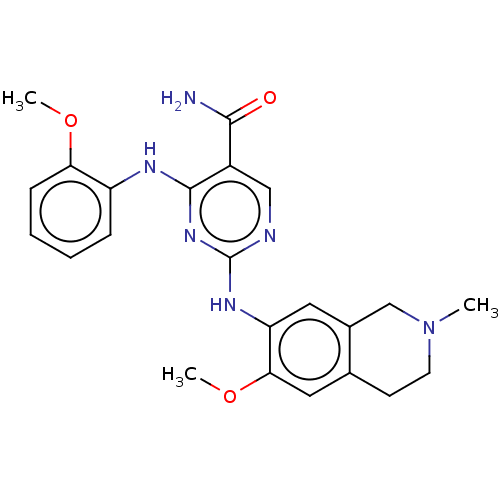 (CHEMBL4859451) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00096 BindingDB Entry DOI: 10.7270/Q2B2802B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM50569663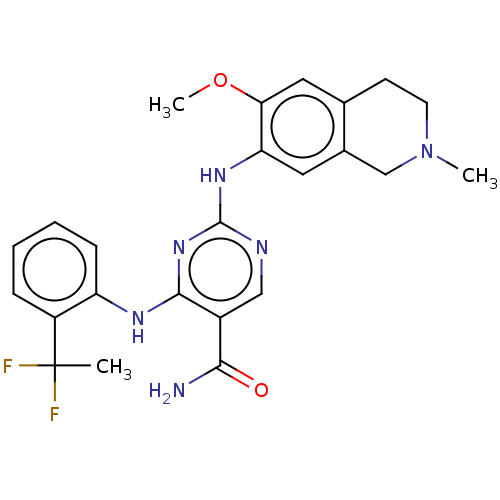 (CHEMBL4870155) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00096 BindingDB Entry DOI: 10.7270/Q2B2802B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM50569665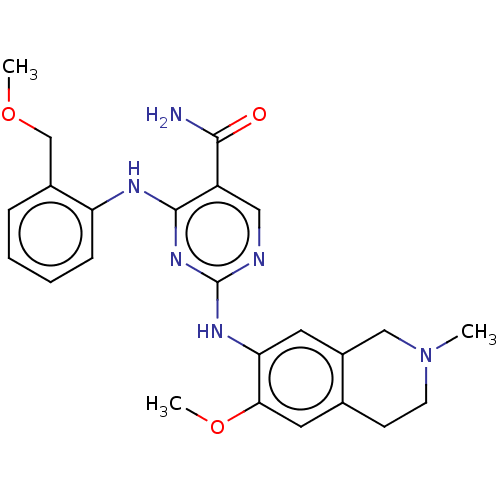 (CHEMBL4864568) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00096 BindingDB Entry DOI: 10.7270/Q2B2802B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM50569667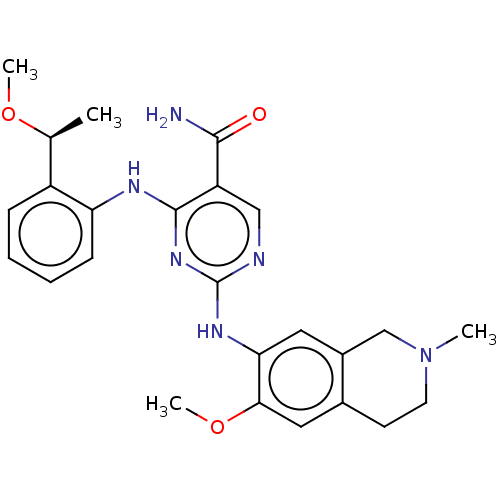 (CHEMBL4848845) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00096 BindingDB Entry DOI: 10.7270/Q2B2802B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM50569669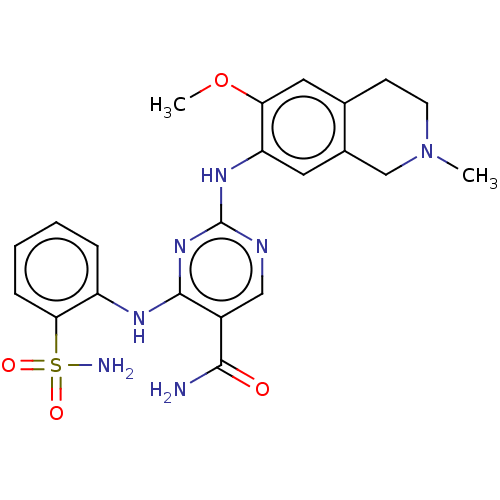 (CHEMBL4866139) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00096 BindingDB Entry DOI: 10.7270/Q2B2802B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM50569672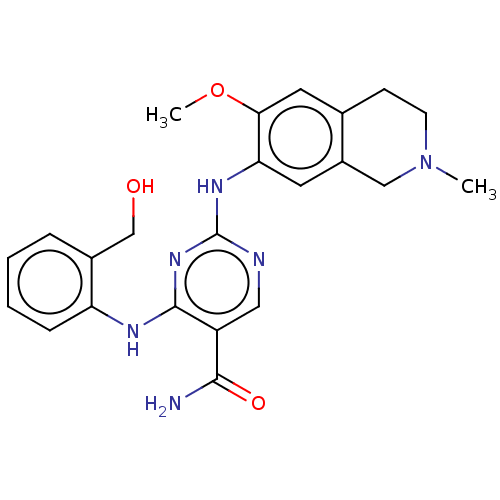 (CHEMBL4865305) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00096 BindingDB Entry DOI: 10.7270/Q2B2802B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM50569666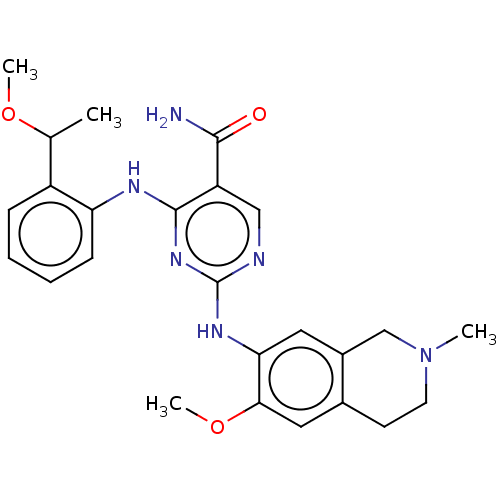 (CHEMBL4855158) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0670 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00096 BindingDB Entry DOI: 10.7270/Q2B2802B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM50569657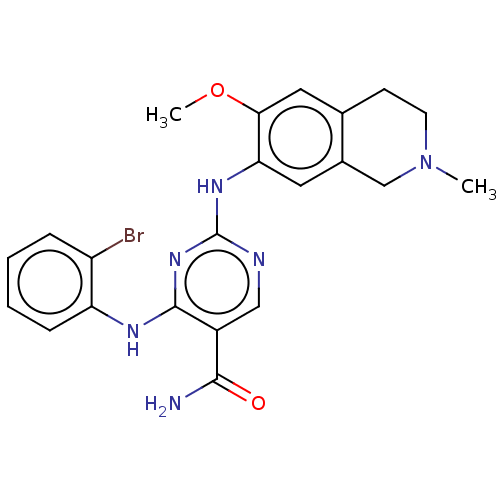 (CHEMBL4863492) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.0940 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00096 BindingDB Entry DOI: 10.7270/Q2B2802B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acyl-CoA desaturase 1 (Mus musculus) | BDBM50296527 (CHEMBL552269 | N-(5-(3,5-difluorobenzyl)thiazol-2-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of SCD1 in mouse microsomes assessed as conversion of [14C]stearate to [14C]oleate after 60 mins | Bioorg Med Chem Lett 19: 4151-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.119 BindingDB Entry DOI: 10.7270/Q21836H1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM50569661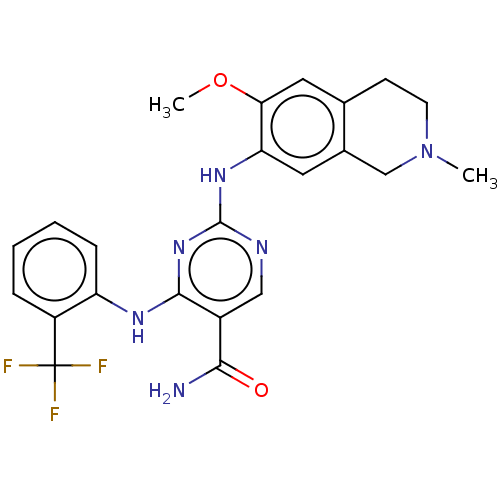 (CHEMBL4872743) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00096 BindingDB Entry DOI: 10.7270/Q2B2802B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM50569659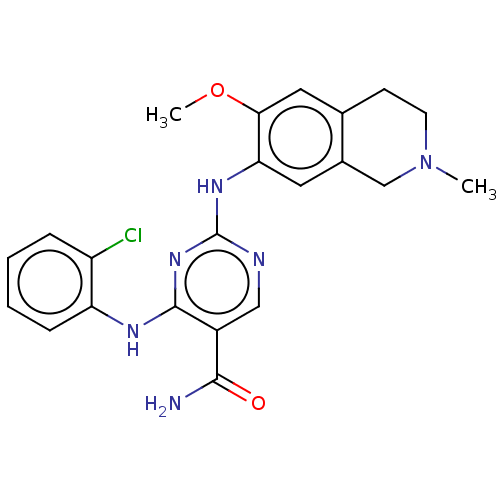 (CHEMBL4866616) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00096 BindingDB Entry DOI: 10.7270/Q2B2802B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM50569671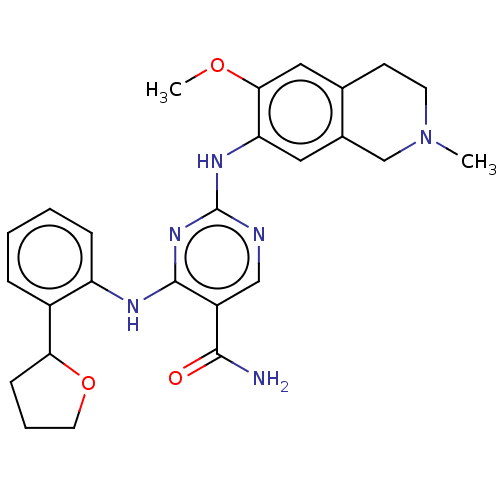 (CHEMBL4860645) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00096 BindingDB Entry DOI: 10.7270/Q2B2802B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM552514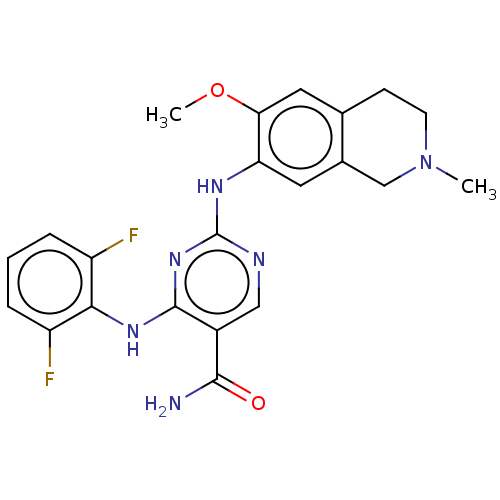 (2-((2-Methoxy-4-(4-methylpiperazine-1-carbonyl)phe...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00096 BindingDB Entry DOI: 10.7270/Q2B2802B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Mus musculus) | BDBM50296528 (CHEMBL557445 | N-(5-(4-fluoro-3-(trifluoromethyl)b...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of SCD1 in mouse microsomes assessed as conversion of [14C]stearate to [14C]oleate after 60 mins | Bioorg Med Chem Lett 19: 4151-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.119 BindingDB Entry DOI: 10.7270/Q21836H1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50296295 (CHEMBL552173 | N-(5-(3,5-bis(trifluoromethyl)benzy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of SCD1 in human microsomes assessed as conversion of [14C]stearate to [14C]oleate after 60 mins | Bioorg Med Chem Lett 19: 4151-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.119 BindingDB Entry DOI: 10.7270/Q21836H1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM50569660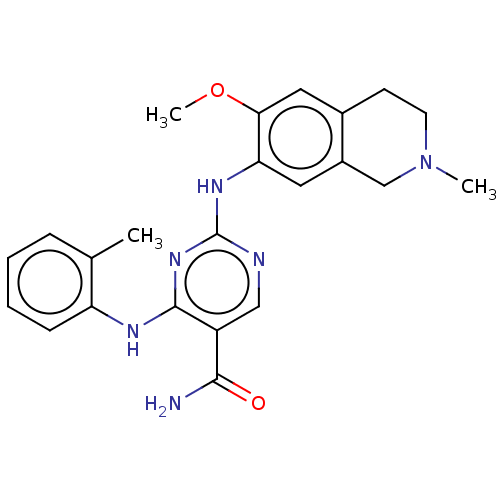 (CHEMBL4849567) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00096 BindingDB Entry DOI: 10.7270/Q2B2802B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM50569668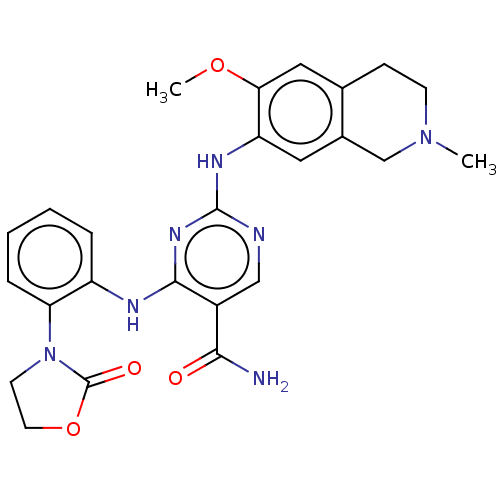 (CHEMBL4857184) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00096 BindingDB Entry DOI: 10.7270/Q2B2802B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM552521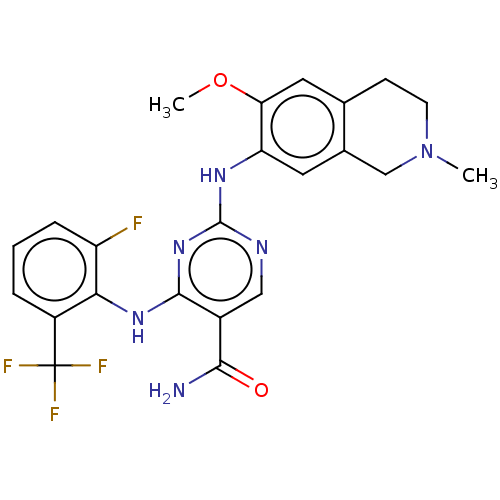 (WO2022098809, Example 4-8) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00096 BindingDB Entry DOI: 10.7270/Q2B2802B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM50569662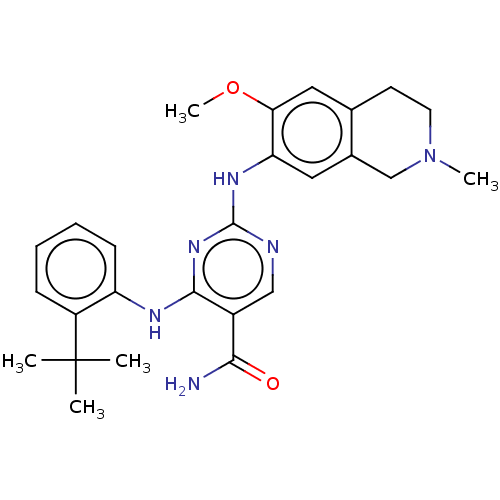 (CHEMBL4874375) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00096 BindingDB Entry DOI: 10.7270/Q2B2802B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM50569656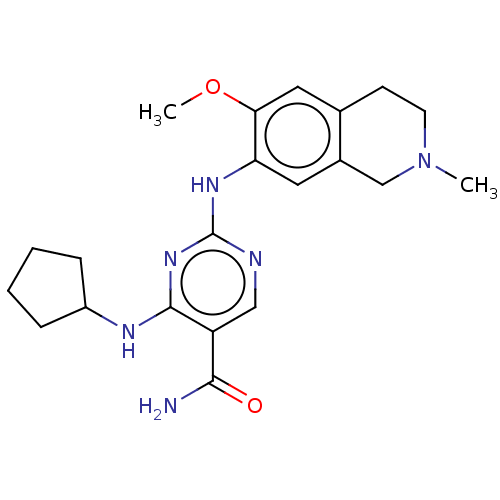 (CHEMBL4871382) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00096 BindingDB Entry DOI: 10.7270/Q2B2802B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM552516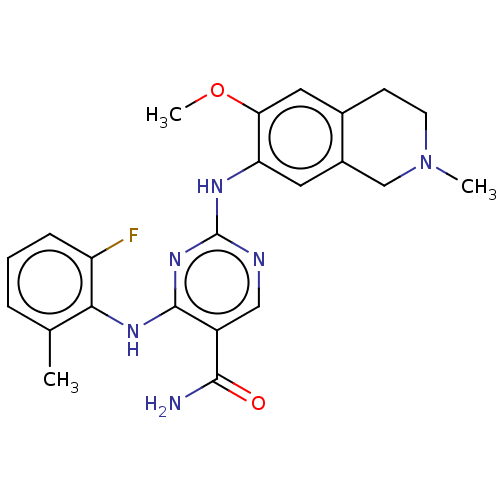 (WO2022098809, Example 4-3 | WO2022098809, Example ...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00096 BindingDB Entry DOI: 10.7270/Q2B2802B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM50569675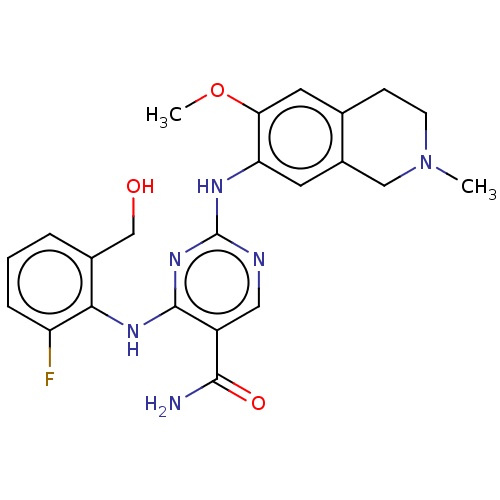 (CHEMBL4859773) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00096 BindingDB Entry DOI: 10.7270/Q2B2802B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM50569658 (CHEMBL4851277) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00096 BindingDB Entry DOI: 10.7270/Q2B2802B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Mus musculus) | BDBM50296295 (CHEMBL552173 | N-(5-(3,5-bis(trifluoromethyl)benzy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of SCD1 in mouse microsomes assessed as conversion of [14C]stearate to [14C]oleate after 60 mins | Bioorg Med Chem Lett 19: 4151-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.119 BindingDB Entry DOI: 10.7270/Q21836H1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50296528 (CHEMBL557445 | N-(5-(4-fluoro-3-(trifluoromethyl)b...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of SCD1 in human microsomes assessed as conversion of [14C]stearate to [14C]oleate after 60 mins | Bioorg Med Chem Lett 19: 4151-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.119 BindingDB Entry DOI: 10.7270/Q21836H1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM50569670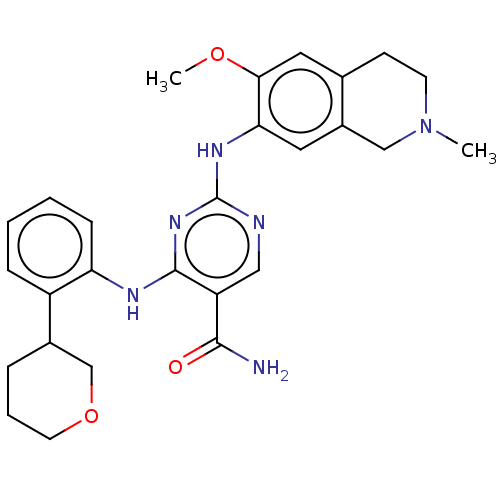 (CHEMBL4868682) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00096 BindingDB Entry DOI: 10.7270/Q2B2802B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Mus musculus) | BDBM50296526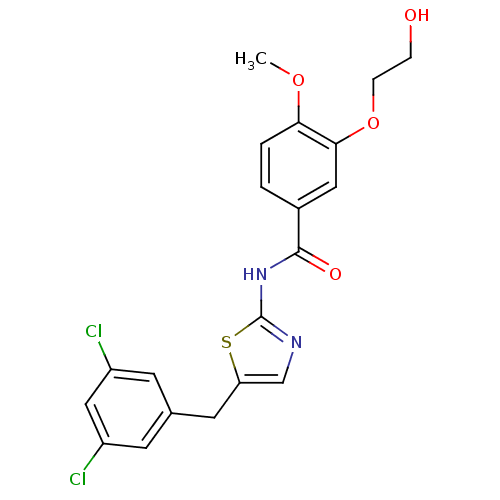 (CHEMBL552126 | N-(5-(3,5-dichlorobenzyl)thiazol-2-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of SCD1 in mouse microsomes assessed as conversion of [14C]stearate to [14C]oleate after 60 mins | Bioorg Med Chem Lett 19: 4151-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.119 BindingDB Entry DOI: 10.7270/Q21836H1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Mus musculus) | BDBM50296531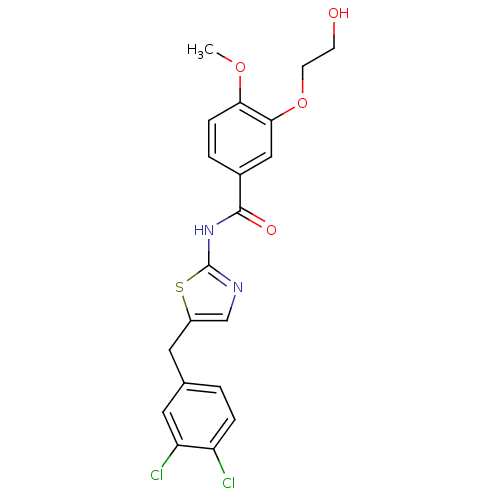 (CHEMBL550800 | N-(5-(3,4-dichlorobenzyl)thiazol-2-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of SCD1 in mouse microsomes assessed as conversion of [14C]stearate to [14C]oleate after 60 mins | Bioorg Med Chem Lett 19: 4151-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.119 BindingDB Entry DOI: 10.7270/Q21836H1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM552551 (2-((6-Methoxy-2-methyl-1,2,3,4-tetrahydroisoquinol...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00096 BindingDB Entry DOI: 10.7270/Q2B2802B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50296531 (CHEMBL550800 | N-(5-(3,4-dichlorobenzyl)thiazol-2-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of SCD1 in human microsomes assessed as conversion of [14C]stearate to [14C]oleate after 60 mins | Bioorg Med Chem Lett 19: 4151-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.119 BindingDB Entry DOI: 10.7270/Q21836H1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50296526 (CHEMBL552126 | N-(5-(3,5-dichlorobenzyl)thiazol-2-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of SCD1 in human microsomes assessed as conversion of [14C]stearate to [14C]oleate after 60 mins | Bioorg Med Chem Lett 19: 4151-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.119 BindingDB Entry DOI: 10.7270/Q21836H1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Mus musculus) | BDBM50296522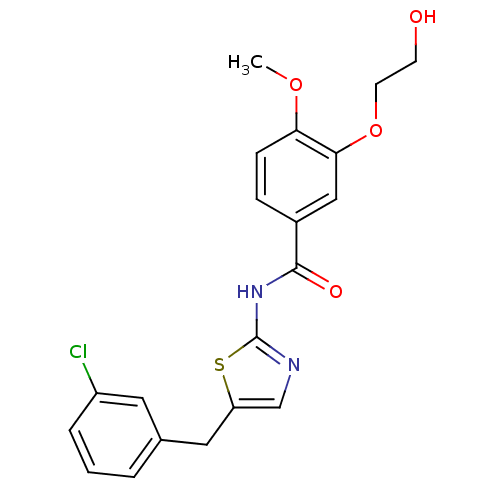 (CHEMBL561588 | N-(5-(3-chlorobenzyl)thiazol-2-yl)-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of SCD1 in mouse microsomes assessed as conversion of [14C]stearate to [14C]oleate after 60 mins | Bioorg Med Chem Lett 19: 4151-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.119 BindingDB Entry DOI: 10.7270/Q21836H1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fructose-1,6-bisphosphatase 1 (Homo sapiens (Human)) | BDBM50310094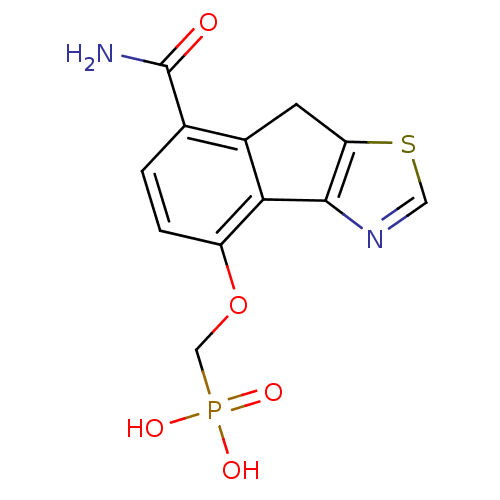 ((7-carbamoyl-8H-indeno[1,2-d]thiazol-4-yloxy)methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co, Ltd Curated by ChEMBL | Assay Description Inhibition of human liver recombinant FBPase | Bioorg Med Chem Lett 20: 1004-7 (2010) Article DOI: 10.1016/j.bmcl.2009.12.056 BindingDB Entry DOI: 10.7270/Q2JW8F1D | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acyl-CoA desaturase 1 (Mus musculus) | BDBM50296529 (CHEMBL552270 | N-(5-(3-chloro-4-fluorobenzyl)thiaz...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of SCD1 in mouse microsomes assessed as conversion of [14C]stearate to [14C]oleate after 60 mins | Bioorg Med Chem Lett 19: 4151-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.119 BindingDB Entry DOI: 10.7270/Q21836H1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM50559633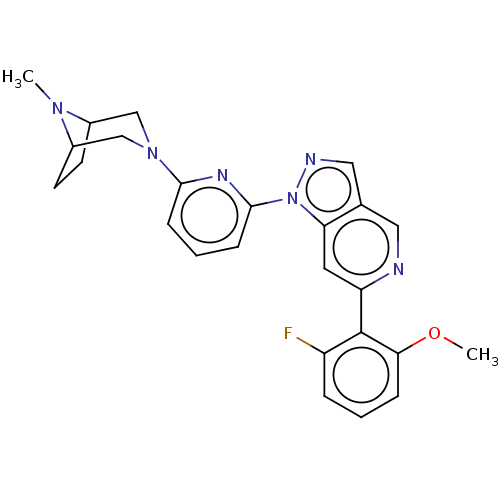 (CHEMBL4797075) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of Tracer 222 from N-terminal GST-tagged human recombinant HPK1 (1 to 346 residues ) expressed in baculovirus-infected Sf9 cells incubat... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00672 BindingDB Entry DOI: 10.7270/Q28G8QD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM50559634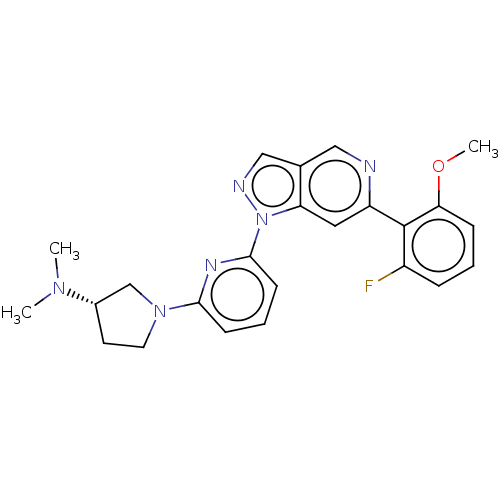 (CHEMBL4763655) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of Tracer 222 from N-terminal GST-tagged human recombinant HPK1 (1 to 346 residues ) expressed in baculovirus-infected Sf9 cells incubat... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00672 BindingDB Entry DOI: 10.7270/Q28G8QD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM50559649 (CHEMBL4778477) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of Tracer 222 from N-terminal GST-tagged human recombinant HPK1 (1 to 346 residues ) expressed in baculovirus-infected Sf9 cells incubat... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00672 BindingDB Entry DOI: 10.7270/Q28G8QD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM50559635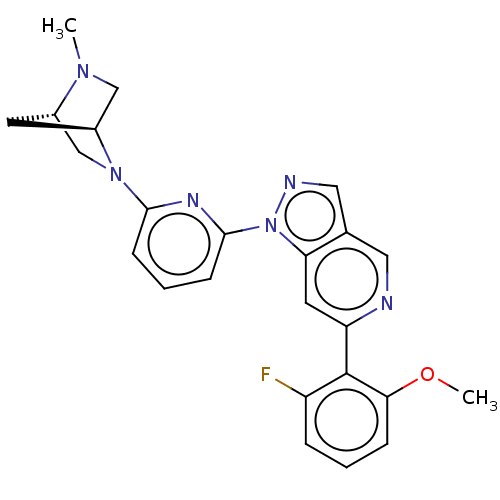 (CHEMBL4794486) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of Tracer 222 from N-terminal GST-tagged human recombinant HPK1 (1 to 346 residues ) expressed in baculovirus-infected Sf9 cells incubat... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00672 BindingDB Entry DOI: 10.7270/Q28G8QD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Mus musculus) | BDBM50296294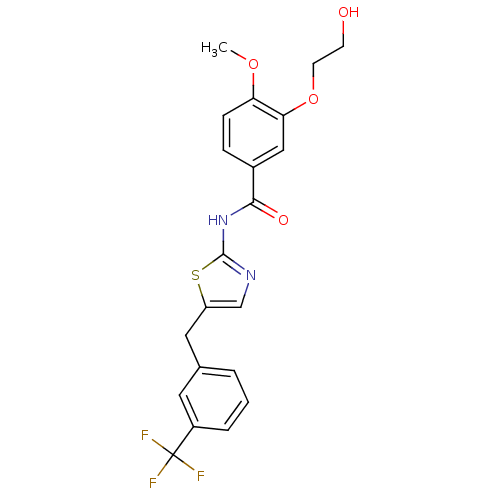 (3-(2-hydroxyethoxy)-4-methoxy-N-(5-(3-(trifluorome...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of SCD1 in mouse microsomes assessed as conversion of [14C]stearate to [14C]oleate after 60 mins | Bioorg Med Chem Lett 19: 4151-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.119 BindingDB Entry DOI: 10.7270/Q21836H1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Mus musculus) | BDBM50296533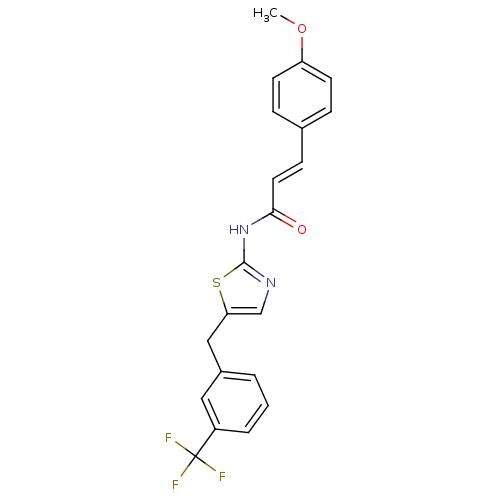 (3-(4-methoxyphenyl)-N-(5-(3-(trifluoromethyl)benzy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of SCD1 in mouse microsomes assessed as conversion of [14C]stearate to [14C]oleate after 60 mins | Bioorg Med Chem Lett 19: 4151-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.119 BindingDB Entry DOI: 10.7270/Q21836H1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 378 total ) | Next | Last >> |