

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50456028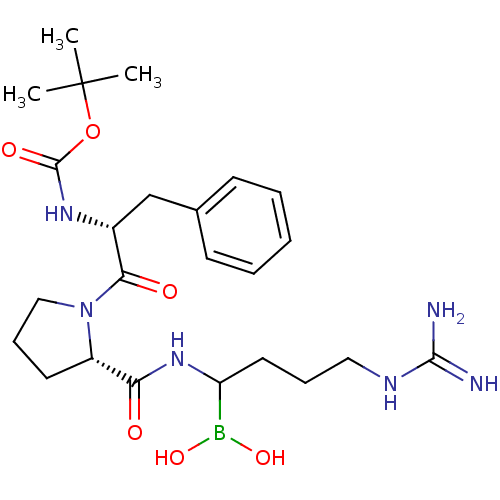 (CHEMBL2028993) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Du Pont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition constant for binding with thrombin was determined | J Med Chem 36: 1831-8 (1993) BindingDB Entry DOI: 10.7270/Q21G0KB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50008834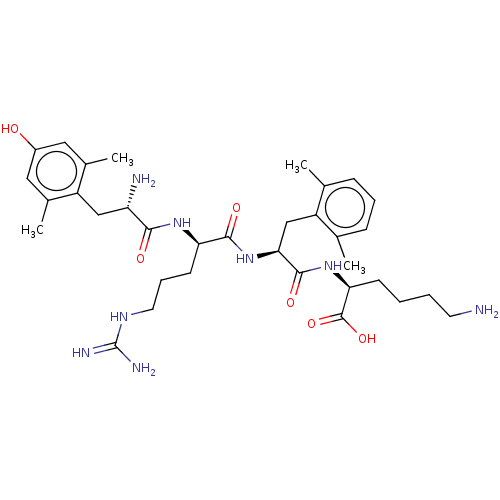 (CHEMBL3236671) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.00935 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain membranes after 2 hrs | Bioorg Med Chem 22: 2333-8 (2014) Article DOI: 10.1016/j.bmc.2014.02.011 BindingDB Entry DOI: 10.7270/Q20P11JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI (Staphylococcus aureus) | BDBM16297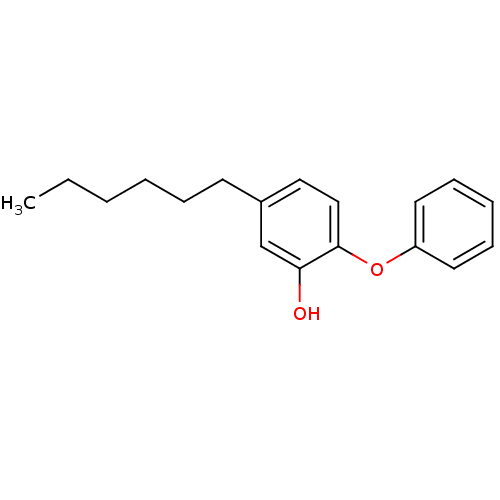 (5-Hexyl-2-phenoxy-phenol | 5-hexyl-2-phenoxylpheno...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Stony Brook University | Assay Description ThermoFluor experiments were carried out in 96-well plates (Concord) using the CFX96 RealTime PCR Detection system and C1000 Thermal Cycler (BioRad). | Biochemistry 52: 4217-28 (2013) Article DOI: 10.1021/bi400413c BindingDB Entry DOI: 10.7270/Q2610XZJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI (Staphylococcus aureus) | BDBM97445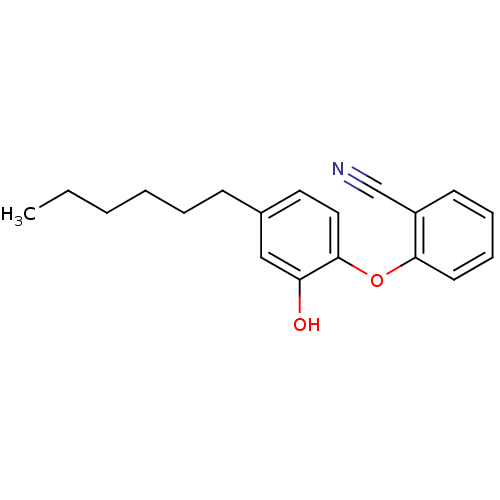 (PT119) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Stony Brook University | Assay Description ThermoFluor experiments were carried out in 96-well plates (Concord) using the CFX96 RealTime PCR Detection system and C1000 Thermal Cycler (BioRad). | Biochemistry 52: 4217-28 (2013) Article DOI: 10.1021/bi400413c BindingDB Entry DOI: 10.7270/Q2610XZJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50184069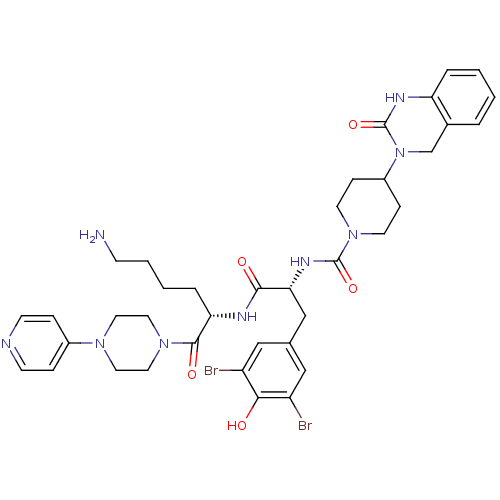 (CHEMBL207197 | N-((R)-1-((S)-6-amino-1-oxo-1-(4-(p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Displacement of [125I]CGRP from CGRP receptor in human SK-N-MC cell membranes preincubated for 30 mins followed by radioligand addition measured afte... | Bioorg Med Chem Lett 24: 845-9 (2014) Article DOI: 10.1016/j.bmcl.2013.12.089 BindingDB Entry DOI: 10.7270/Q2FJ2J84 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029704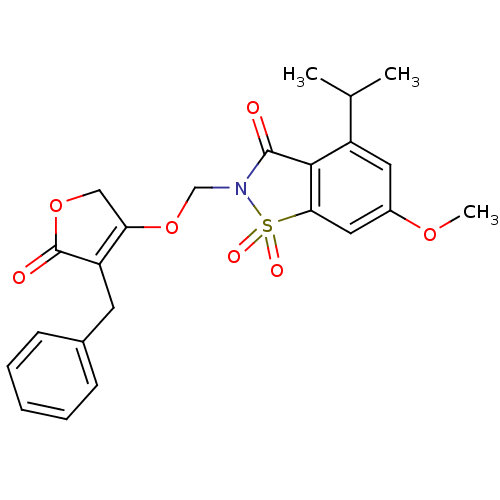 (2-(4-Benzyl-5-oxo-2,5-dihydro-furan-3-yloxymethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029709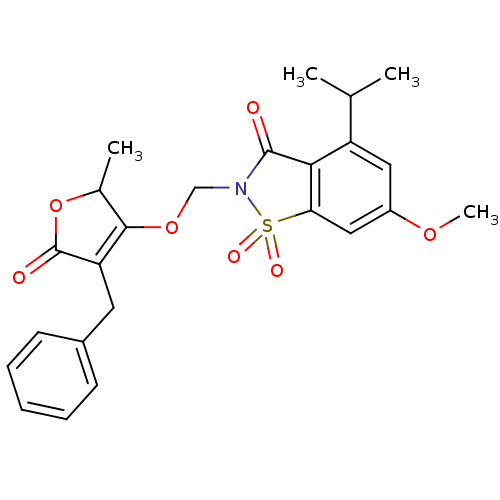 (2-(4-Benzyl-2-methyl-5-oxo-2,5-dihydro-furan-3-ylo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50447327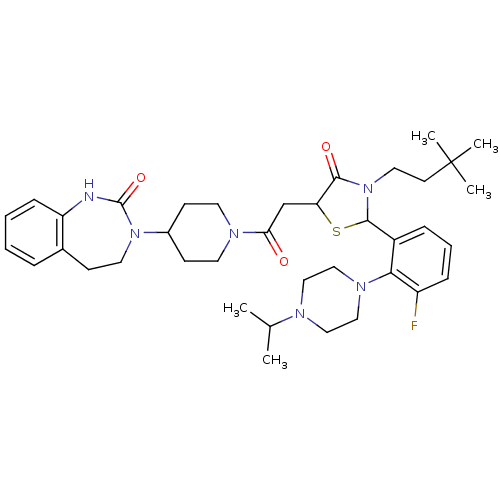 (CHEMBL3114495) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Displacement of [125I]CGRP from CGRP receptor in human SK-N-MC cell membranes preincubated for 30 mins followed by radioligand addition measured afte... | Bioorg Med Chem Lett 24: 845-9 (2014) Article DOI: 10.1016/j.bmcl.2013.12.089 BindingDB Entry DOI: 10.7270/Q2FJ2J84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029710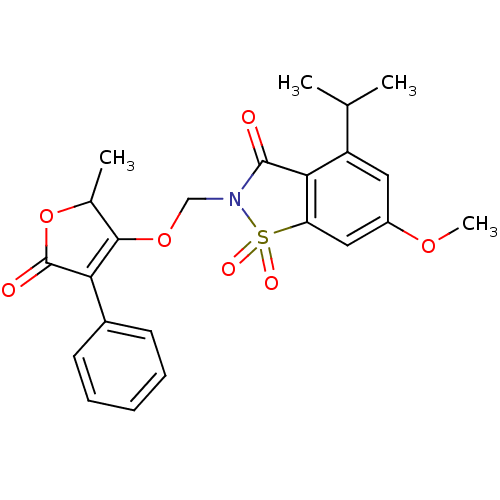 (4-Isopropyl-6-methoxy-2-(2-methyl-5-oxo-4-phenyl-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50285289 (2,6-Dichloro-3-(2-morpholin-4-yl-ethoxy)-benzoic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Human Leukocyte Elastase (HLE) as apparent binding constant (kreact/kinact) | Bioorg Med Chem Lett 5: 105-109 (1995) Article DOI: 10.1016/0960-894X(94)00466-S BindingDB Entry DOI: 10.7270/Q2V69JJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029699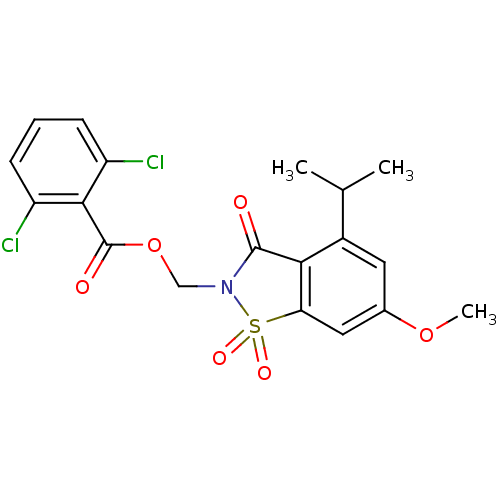 (2,6-Dichloro-benzoic acid 4-isopropyl-6-methoxy-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029699 (2,6-Dichloro-benzoic acid 4-isopropyl-6-methoxy-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Human leukocyte elastase (HLE) | Bioorg Med Chem Lett 5: 331-336 (1995) Article DOI: 10.1016/0960-894X(95)00030-W BindingDB Entry DOI: 10.7270/Q2SX6D60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50012477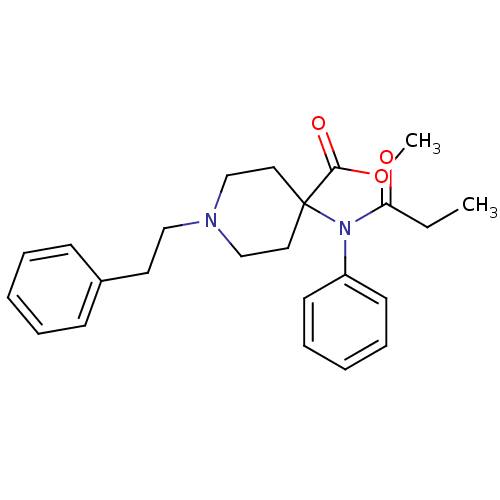 (1-Phenethyl-4-(phenyl-propionyl-amino)-piperidine-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain membranes | Bioorg Med Chem 22: 4581-6 (2014) Article DOI: 10.1016/j.bmc.2014.07.033 BindingDB Entry DOI: 10.7270/Q2P270WT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029717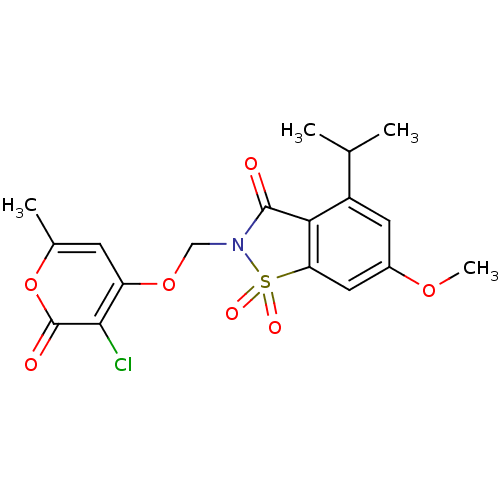 (2-(3-Chloro-6-methyl-2-oxo-2H-pyran-4-yloxymethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029698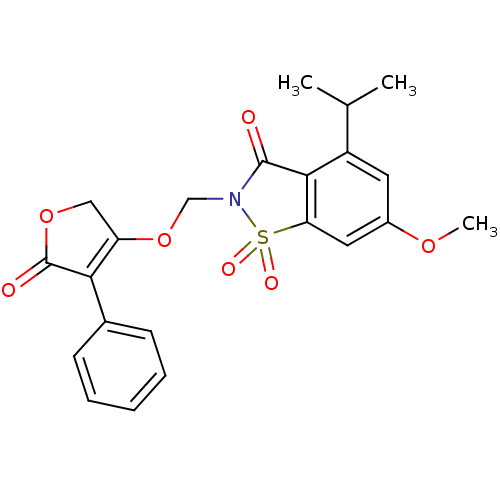 (4-Isopropyl-6-methoxy-1,1-dioxo-2-(5-oxo-4-phenyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029696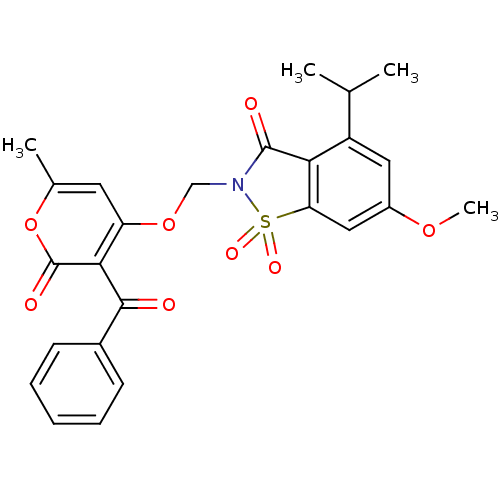 (2-(3-Benzoyl-6-methyl-2-oxo-2H-pyran-4-yloxymethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12693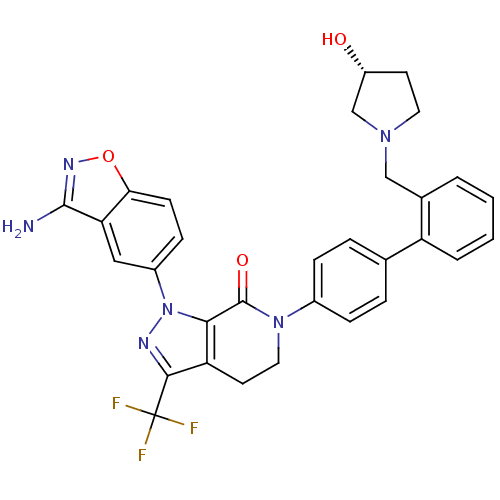 (1-(3-amino-1,2-benzoxazol-5-yl)-6-[4-(2-{[(3R)-3-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0300 | -59.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029712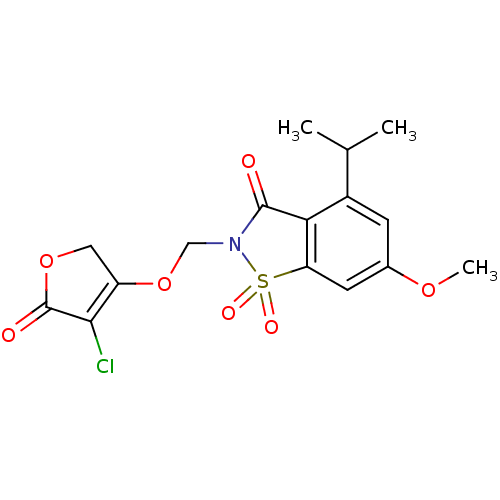 (2-(4-Chloro-5-oxo-2,5-dihydro-furan-3-yloxymethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50039631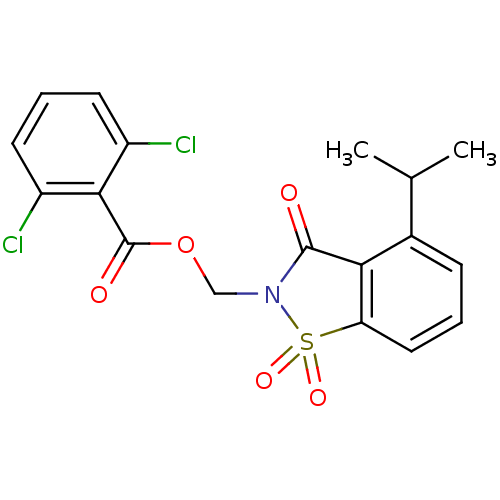 (2,6-Dichloro-benzoic acid 4-isopropyl-1,1,3-trioxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Human leukocyte elastase (HLE) | Bioorg Med Chem Lett 5: 331-336 (1995) Article DOI: 10.1016/0960-894X(95)00030-W BindingDB Entry DOI: 10.7270/Q2SX6D60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50447325 (CHEMBL3114676) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Displacement of [125I]CGRP from CGRP receptor in human SK-N-MC cell membranes preincubated for 30 mins followed by radioligand addition measured afte... | Bioorg Med Chem Lett 24: 845-9 (2014) Article DOI: 10.1016/j.bmcl.2013.12.089 BindingDB Entry DOI: 10.7270/Q2FJ2J84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50039631 (2,6-Dichloro-benzoic acid 4-isopropyl-1,1,3-trioxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ratio of Kreact to that of Kinact was determined on human leukocyte elastase(HLE) | Bioorg Med Chem Lett 5: 325-330 (1995) Article DOI: 10.1016/0960-894X(95)00029-S BindingDB Entry DOI: 10.7270/Q2XP74WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase (Homo sapiens (Human)) | BDBM50117772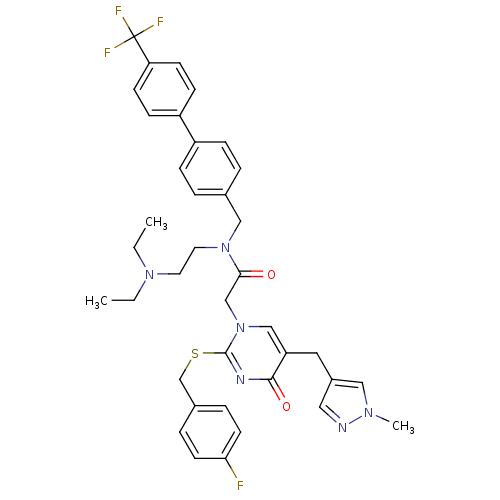 (CHEMBL10921 | N-(2-Diethylamino-ethyl)-2-[2-(4-flu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Steady state and transient kinetics to a freely reversible, non-covalently bound, human recombinant Phospholipase A2 (rhLp-PLA2) was determined | Bioorg Med Chem Lett 12: 2603-6 (2002) BindingDB Entry DOI: 10.7270/Q2G44PNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (OK) | BDBM81811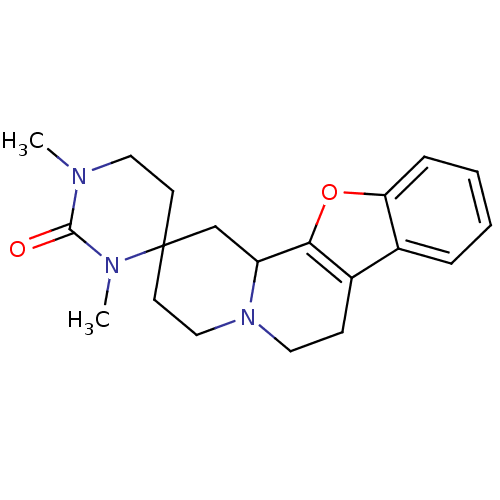 (CAS_123679 | L-657,743 | MK-912 | NSC_123679) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Curated by PDSP Ki Database | J Pharmacol Exp Ther 259: 323-9 (1991) BindingDB Entry DOI: 10.7270/Q2Z899WV | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50286326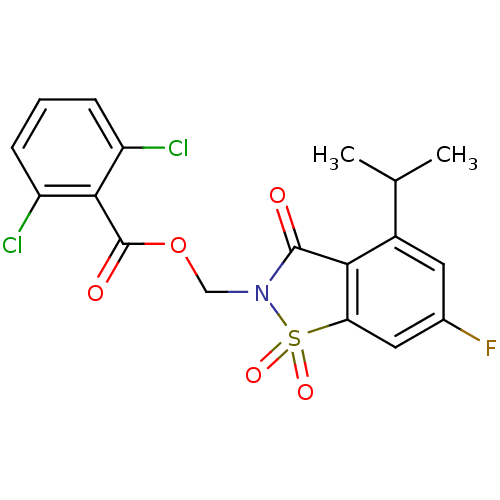 (2,6-Dichloro-benzoic acid 6-fluoro-4-isopropyl-1,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Human leukocyte elastase (HLE) | Bioorg Med Chem Lett 5: 331-336 (1995) Article DOI: 10.1016/0960-894X(95)00030-W BindingDB Entry DOI: 10.7270/Q2SX6D60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50039631 (2,6-Dichloro-benzoic acid 4-isopropyl-1,1,3-trioxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0302 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Observed binding affinity against Human leukocyte elastase (HLE) | Bioorg Med Chem Lett 5: 325-330 (1995) Article DOI: 10.1016/0960-894X(95)00029-S BindingDB Entry DOI: 10.7270/Q2XP74WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50198754 (CHEMBL3924888) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0323 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from MOR in rat brain membrane measured after 2 hrs | J Med Chem 59: 9243-9254 (2016) Article DOI: 10.1021/acs.jmedchem.6b01200 BindingDB Entry DOI: 10.7270/Q23B623F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029713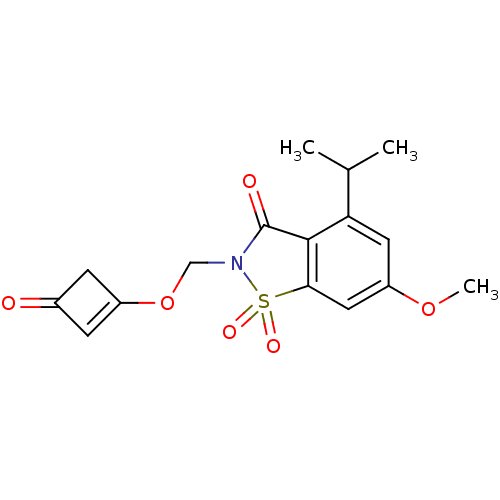 (4-Isopropyl-6-methoxy-1,1-dioxo-2-(3-oxo-cyclobut-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50156455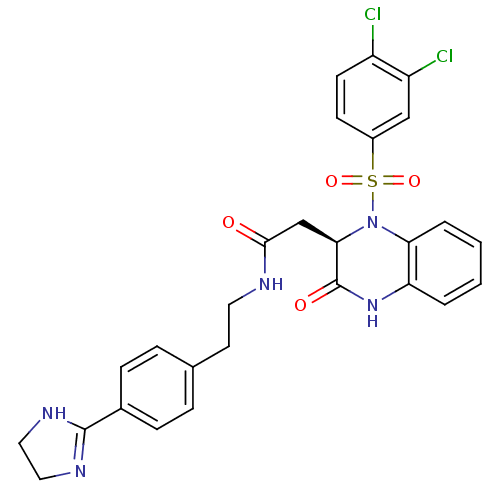 ((R)-N-(4-(4,5-dihydro-1H-imidazol-2-yl)phenethyl)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHO-D-/aequorin cells by rapid filtration technique | Bioorg Med Chem Lett 18: 4477-81 (2008) Article DOI: 10.1016/j.bmcl.2008.07.055 BindingDB Entry DOI: 10.7270/Q2F76CCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029691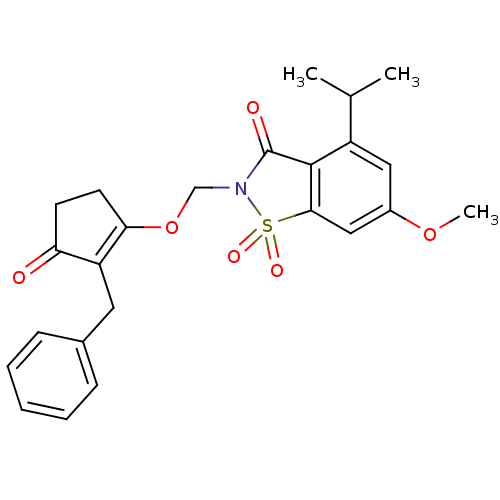 (2-(2-Benzyl-3-oxo-cyclopent-1-enyloxymethyl)-4-iso...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50034671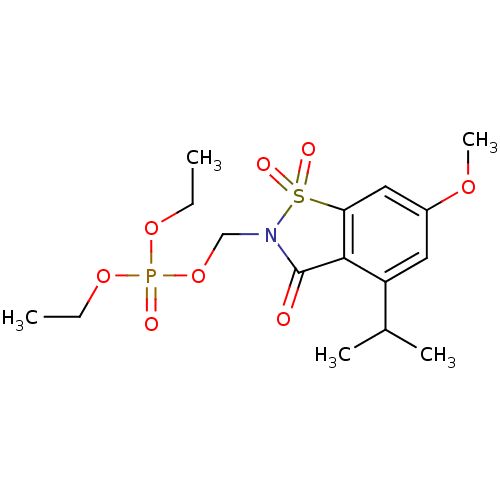 (CHEMBL41327 | Phosphoric acid diethyl ester 4-isop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description In vitro inhibitory activity against Human leukocyte elastase | J Med Chem 38: 1571-4 (1995) BindingDB Entry DOI: 10.7270/Q2M32TS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50288406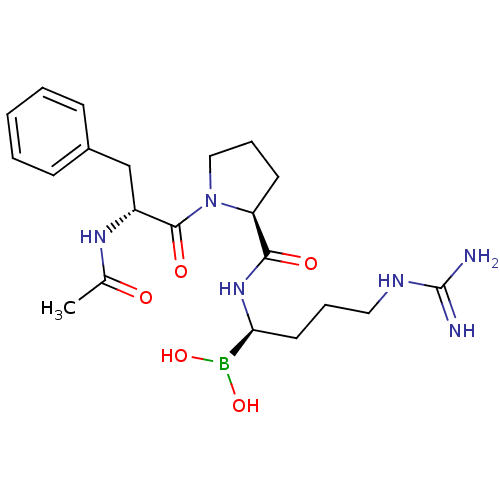 (1-(2-Acetylamino-3-phenyl-propionyl)-pyrrolidine-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 7: 1595-1600 (1997) Article DOI: 10.1016/S0960-894X(97)00254-0 BindingDB Entry DOI: 10.7270/Q2VQ32PJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50069922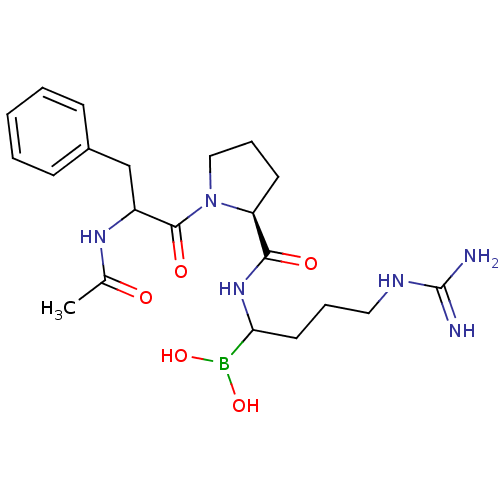 (Boropeptide analogue | CHEMBL102069) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for the binding affinity against thrombin | Bioorg Med Chem Lett 7: 79-84 (1997) Checked by Author Article DOI: 10.1016/S0960-894X(96)00584-7 BindingDB Entry DOI: 10.7270/Q2FN16P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI (Staphylococcus aureus) | BDBM16296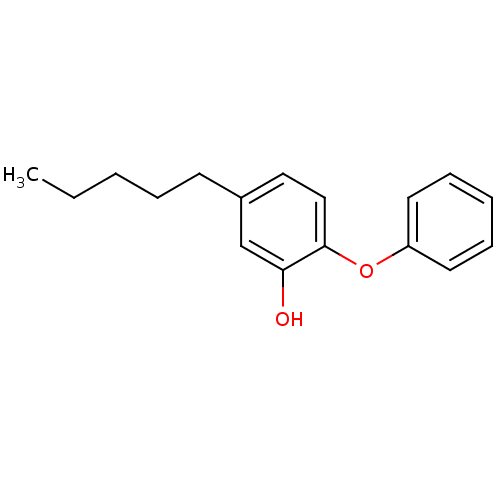 (5-Pentyl-2-phenoxy-phenol | 5-pentyl-2-phenoxylphe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Stony Brook University | Assay Description ThermoFluor experiments were carried out in 96-well plates (Concord) using the CFX96 RealTime PCR Detection system and C1000 Thermal Cycler (BioRad). | Biochemistry 52: 4217-28 (2013) Article DOI: 10.1021/bi400413c BindingDB Entry DOI: 10.7270/Q2610XZJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50288632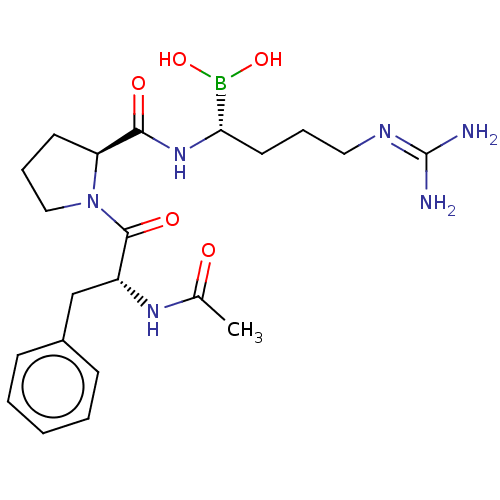 (Boropeptide | CHEMBL607008) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Binding affinity towards thrombin was tested. | Bioorg Med Chem Lett 8: 301-6 (1999) BindingDB Entry DOI: 10.7270/Q2542MRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (OK) | BDBM50013515 ((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Curated by PDSP Ki Database | J Pharmacol Exp Ther 259: 323-9 (1991) BindingDB Entry DOI: 10.7270/Q2Z899WV | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12681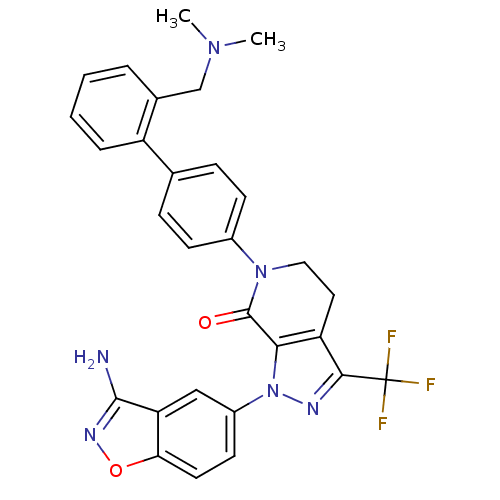 (1-(3-amino-1,2-benzoxazol-5-yl)-6-(4-{2-[(dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0400 | -58.8 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50514220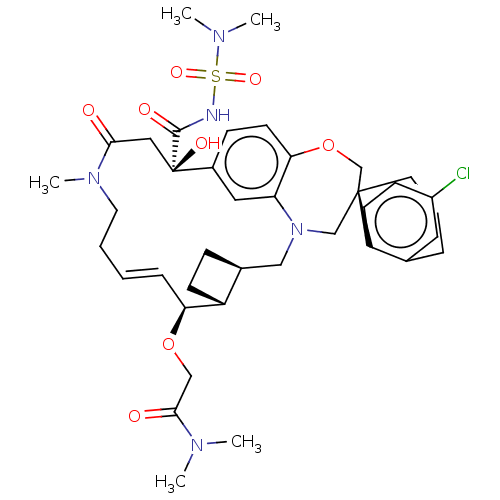 (CHEMBL4535151 | US11274105, Example 188) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... | J Med Chem 62: 10258-10271 (2019) Article DOI: 10.1021/acs.jmedchem.9b01310 BindingDB Entry DOI: 10.7270/Q2TQ64WW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50288632 (Boropeptide | CHEMBL607008) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity to human thrombin. | Bioorg Med Chem Lett 6: 301-306 (1996) Article DOI: 10.1016/0960-894X(96)00016-9 BindingDB Entry DOI: 10.7270/Q2959HJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50288406 (1-(2-Acetylamino-3-phenyl-propionyl)-pyrrolidine-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article | 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro for inhibition of thrombin. | Bioorg Med Chem Lett 6: 2913-2918 (1996) Article DOI: 10.1016/S0960-894X(96)00525-2 BindingDB Entry DOI: 10.7270/Q2V40V6S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50451005 (CHEMBL290376 | DuP-714) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB PubMed | 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Du Pont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition constant for binding with thrombin was determined | J Med Chem 36: 1831-8 (1993) BindingDB Entry DOI: 10.7270/Q21G0KB8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50069922 (Boropeptide analogue | CHEMBL102069) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for selectivity against trypsin | Bioorg Med Chem Lett 7: 79-84 (1997) Checked by Author Article DOI: 10.1016/S0960-894X(96)00584-7 BindingDB Entry DOI: 10.7270/Q2FN16P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50288406 (1-(2-Acetylamino-3-phenyl-propionyl)-pyrrolidine-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of trypsin | Bioorg Med Chem Lett 7: 1595-1600 (1997) Article DOI: 10.1016/S0960-894X(97)00254-0 BindingDB Entry DOI: 10.7270/Q2VQ32PJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50288406 (1-(2-Acetylamino-3-phenyl-propionyl)-pyrrolidine-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested in vitro for inhibition of trypsin | Bioorg Med Chem Lett 6: 2913-2918 (1996) Article DOI: 10.1016/S0960-894X(96)00525-2 BindingDB Entry DOI: 10.7270/Q2V40V6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50285280 (2,6-Dichloro-benzoic acid 4-ethoxy-6-methoxy-1,1,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Human Leukocyte Elastase (HLE) as apparent binding constant (kreact/kinact) | Bioorg Med Chem Lett 5: 105-109 (1995) Article DOI: 10.1016/0960-894X(94)00466-S BindingDB Entry DOI: 10.7270/Q2V69JJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM81806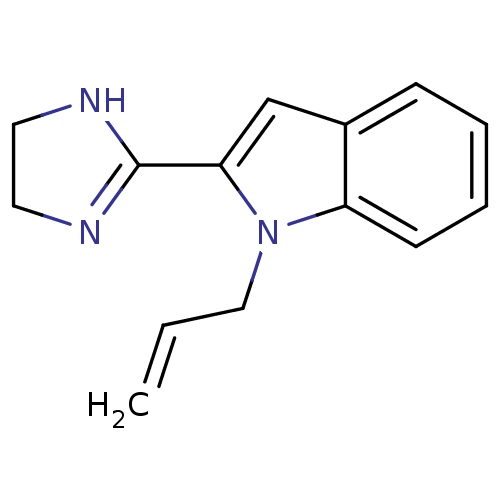 (2-(4,5-dihydro-1h-imidazol-2-yl)-2,3-dihydro-1-(2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Curated by PDSP Ki Database | J Pharmacol Exp Ther 259: 323-9 (1991) BindingDB Entry DOI: 10.7270/Q2Z899WV | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029692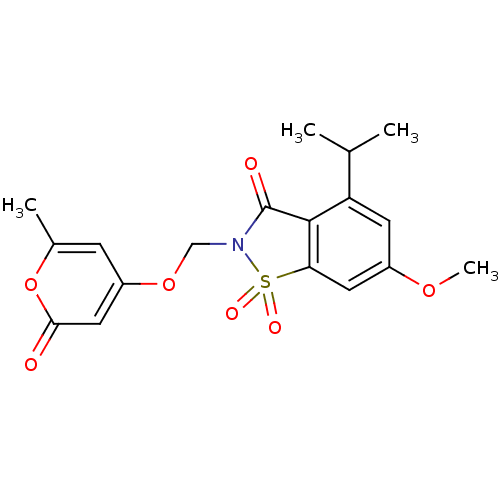 (4-Isopropyl-6-methoxy-2-(6-methyl-2-oxo-2H-pyran-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50514222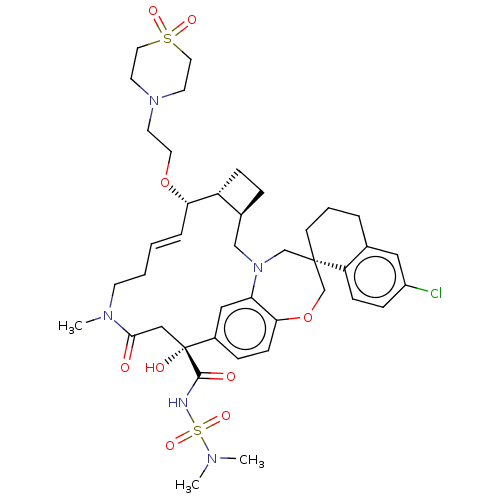 (CHEMBL4580244 | US11274105, Example 193) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... | J Med Chem 62: 10258-10271 (2019) Article DOI: 10.1021/acs.jmedchem.9b01310 BindingDB Entry DOI: 10.7270/Q2TQ64WW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50514203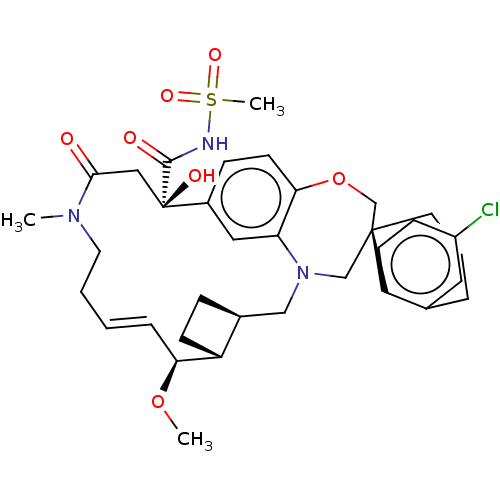 (CHEMBL4593361 | US11274105, Example 6) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... | J Med Chem 62: 10258-10271 (2019) Article DOI: 10.1021/acs.jmedchem.9b01310 BindingDB Entry DOI: 10.7270/Q2TQ64WW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50319772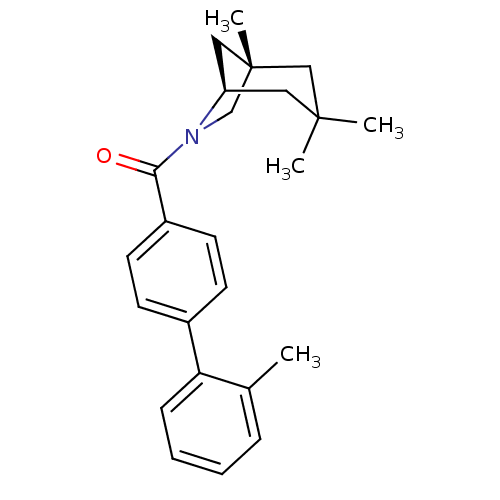 ((2'-methylbiphenyl-4-yl)((1S,5R)-1,3,3-trimethyl-6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement [3H]Arg human recombinant Vasopressin V1a receptor | Bioorg Med Chem Lett 20: 3742-5 (2010) Article DOI: 10.1016/j.bmcl.2010.04.068 BindingDB Entry DOI: 10.7270/Q24F1QW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI (Staphylococcus aureus) | BDBM8726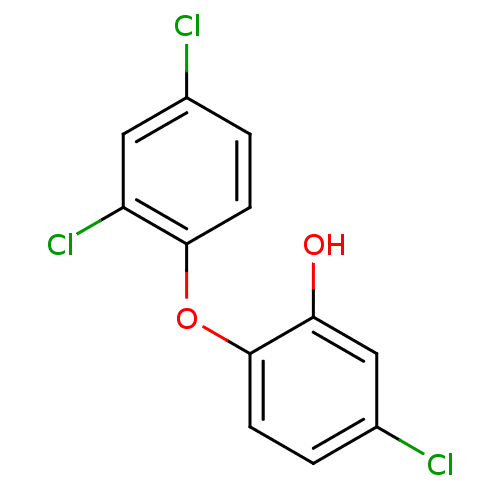 (5-chloro-2-(2,4-dichlorophenoxy)phenol | CHEMBL849...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Stony Brook University | Assay Description ThermoFluor experiments were carried out in 96-well plates (Concord) using the CFX96 RealTime PCR Detection system and C1000 Thermal Cycler (BioRad). | Biochemistry 52: 4217-28 (2013) Article DOI: 10.1021/bi400413c BindingDB Entry DOI: 10.7270/Q2610XZJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 41591 total ) | Next | Last >> |