

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50483336 (CHEMBL1651153 | GRL-0476) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.00270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 54: 622-34 (2011) Article DOI: 10.1021/jm1012787 BindingDB Entry DOI: 10.7270/Q2BV7KG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM13925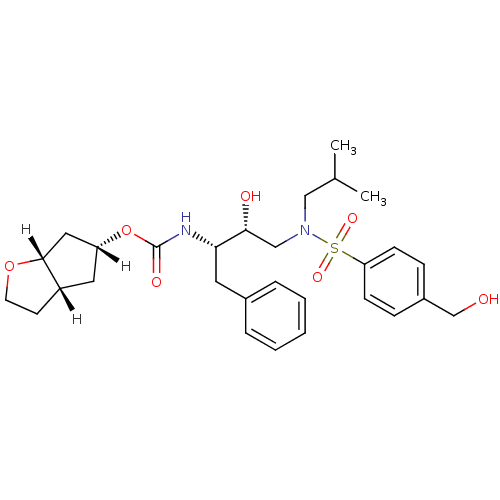 ((3aS,5R,6aR)-hexahydro-2H-cyclopenta[b]furan-5-yl ...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University Graduate School of Medical and Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 protease dimerization in MT2 cells | J Biol Chem 282: 28709-20 (2007) Article DOI: 10.1074/jbc.M703938200 BindingDB Entry DOI: 10.7270/Q2JS9T6J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM13925 ((3aS,5R,6aR)-hexahydro-2H-cyclopenta[b]furan-5-yl ...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00450 | -64.8 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 49: 5252-61 (2006) Article DOI: 10.1021/jm060561m BindingDB Entry DOI: 10.7270/Q23R0R41 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50483338 (CHEMBL1651155) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 54: 622-34 (2011) Article DOI: 10.1021/jm1012787 BindingDB Entry DOI: 10.7270/Q2BV7KG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50481584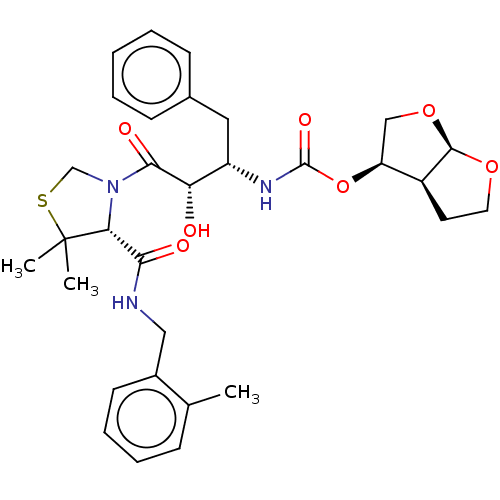 (CHEMBL589988 | GRL-0355) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem Lett 20: 1241-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.123 BindingDB Entry DOI: 10.7270/Q2CJ8HB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM4685 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University Graduate School of Medical and Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 protease dimerization in MT2 cells | J Biol Chem 282: 28709-20 (2007) Article DOI: 10.1074/jbc.M703938200 BindingDB Entry DOI: 10.7270/Q2JS9T6J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM9236 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University Graduate School of Medical and Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 protease dimerization in MT2 cells | J Biol Chem 282: 28709-20 (2007) Article DOI: 10.1074/jbc.M703938200 BindingDB Entry DOI: 10.7270/Q2JS9T6J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50483334 (CHEMBL1651160) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 54: 622-34 (2011) Article DOI: 10.1021/jm1012787 BindingDB Entry DOI: 10.7270/Q2BV7KG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM35968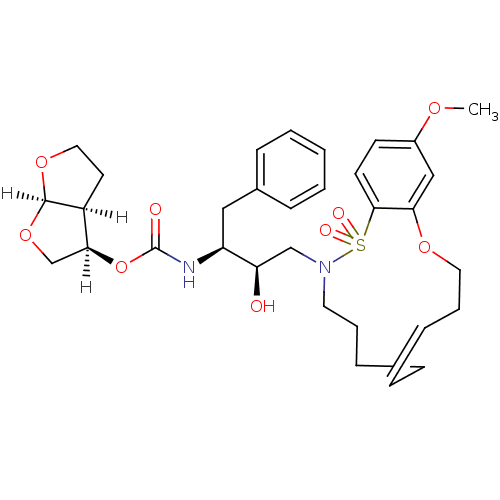 (cyclic compound, 14c | cyclic compound, 14c-Z) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0120 | -62.3 | n/a | n/a | 4.60 | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 52: 7689-705 (2009) Article DOI: 10.1021/jm900695w BindingDB Entry DOI: 10.7270/Q2G44NN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50476647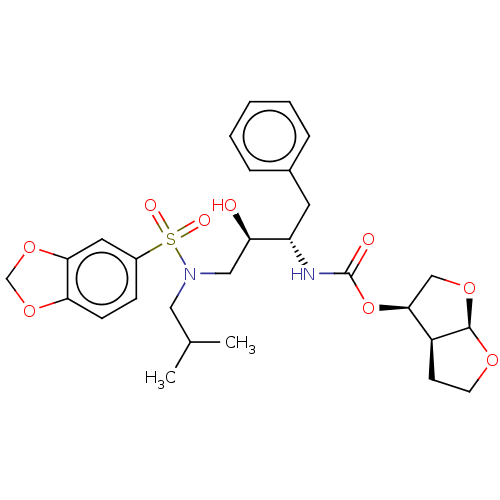 (CHEMBL178593 | GRL-98065) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University Graduate School of Medical and Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 protease dimerization in MT2 cells | J Biol Chem 282: 28709-20 (2007) Article DOI: 10.1074/jbc.M703938200 BindingDB Entry DOI: 10.7270/Q2JS9T6J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0140 | -62.0 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 49: 5252-61 (2006) Article DOI: 10.1021/jm060561m BindingDB Entry DOI: 10.7270/Q23R0R41 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9236 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0140 | -62.0 | n/a | n/a | 1.20 | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 52: 7689-705 (2009) Article DOI: 10.1021/jm900695w BindingDB Entry DOI: 10.7270/Q2G44NN7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University Graduate School of Medical and Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 protease dimerization in MT2 cells | J Biol Chem 282: 28709-20 (2007) Article DOI: 10.1074/jbc.M703938200 BindingDB Entry DOI: 10.7270/Q2JS9T6J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0160 | -61.6 | n/a | n/a | 1.60 | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 52: 7689-705 (2009) Article DOI: 10.1021/jm900695w BindingDB Entry DOI: 10.7270/Q2G44NN7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem Lett 20: 1241-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.123 BindingDB Entry DOI: 10.7270/Q2CJ8HB8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM35984 (cyclic compound, 15d) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0170 | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 52: 7689-705 (2009) Article DOI: 10.1021/jm900695w BindingDB Entry DOI: 10.7270/Q2G44NN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM25367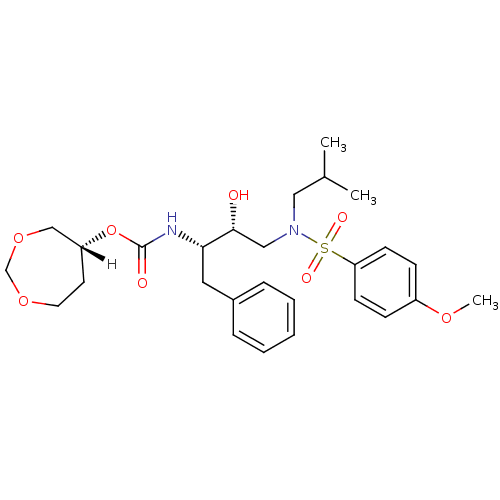 ((5R)-1,3-dioxepan-5-yl N-[(2S,3R)-3-hydroxy-4-[(4-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB Article PubMed | 0.0260 | -60.4 | 4.90 | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 51: 6021-33 (2008) Article DOI: 10.1021/jm8004543 BindingDB Entry DOI: 10.7270/Q2G15Z51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50478337 (CHEMBL403306 | GRL-0036A) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University Graduate School of Medical and Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 protease dimerization in MT2 cells | J Biol Chem 282: 28709-20 (2007) Article DOI: 10.1074/jbc.M703938200 BindingDB Entry DOI: 10.7270/Q2JS9T6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM31822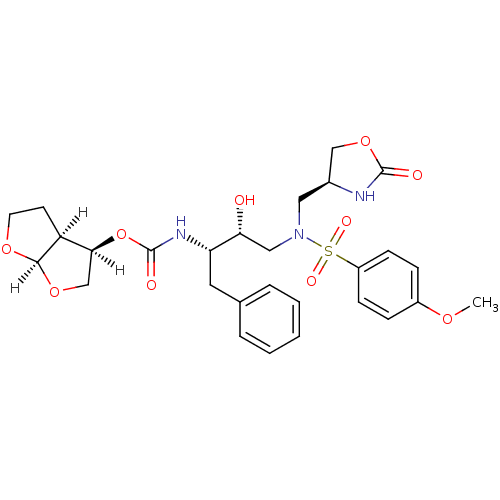 (oxazolidinone, 31) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0350 | -59.7 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 52: 3902-14 (2009) Article DOI: 10.1021/jm900303m BindingDB Entry DOI: 10.7270/Q20G3HHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM25371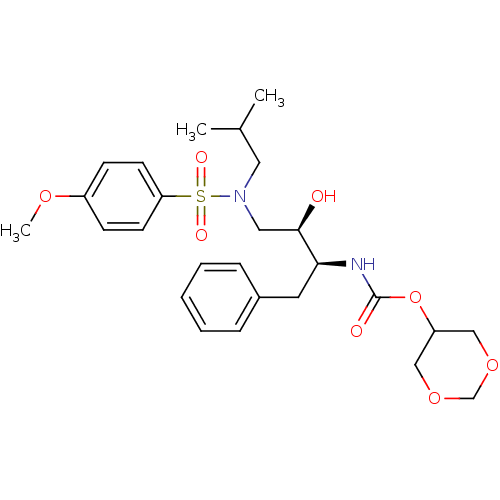 ((R)-(hydroxyethylamino)sulfonamide isostere, 3h | ...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0410 | -59.3 | 3.40 | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 51: 6021-33 (2008) Article DOI: 10.1021/jm8004543 BindingDB Entry DOI: 10.7270/Q2G15Z51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM35968 (cyclic compound, 14c | cyclic compound, 14c-Z) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0450 | -59.1 | n/a | n/a | 2 | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 52: 7689-705 (2009) Article DOI: 10.1021/jm900695w BindingDB Entry DOI: 10.7270/Q2G44NN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM35974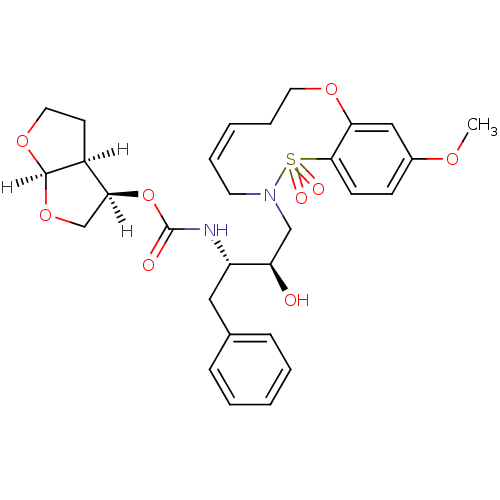 (cyclic compound, 14g) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0510 | -58.7 | n/a | n/a | 15 | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 52: 7689-705 (2009) Article DOI: 10.1021/jm900695w BindingDB Entry DOI: 10.7270/Q2G44NN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM35970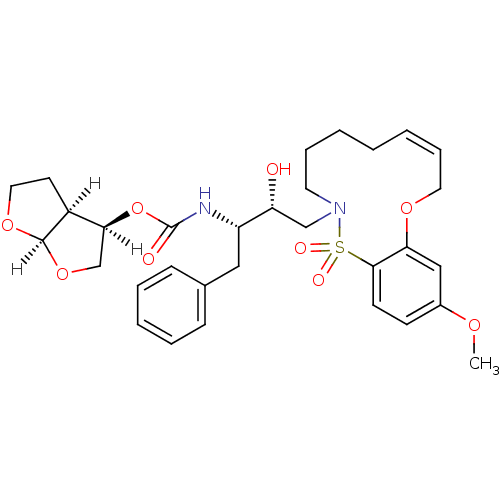 (cyclic compound, 14d) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0580 | -58.4 | n/a | n/a | 14 | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 52: 7689-705 (2009) Article DOI: 10.1021/jm900695w BindingDB Entry DOI: 10.7270/Q2G44NN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM35982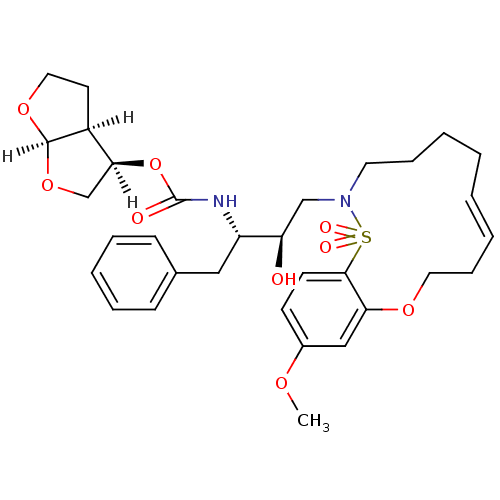 (cyclic compound, 14c-E) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 0.0600 | -58.3 | n/a | n/a | 7 | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 52: 7689-705 (2009) Article DOI: 10.1021/jm900695w BindingDB Entry DOI: 10.7270/Q2G44NN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50483337 (CHEMBL1651154) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 54: 622-34 (2011) Article DOI: 10.1021/jm1012787 BindingDB Entry DOI: 10.7270/Q2BV7KG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM35996 (cyclic compound, 22c) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | 170 | n/a | n/a | n/a | n/a |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 52: 7689-705 (2009) Article DOI: 10.1021/jm900695w BindingDB Entry DOI: 10.7270/Q2G44NN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM35972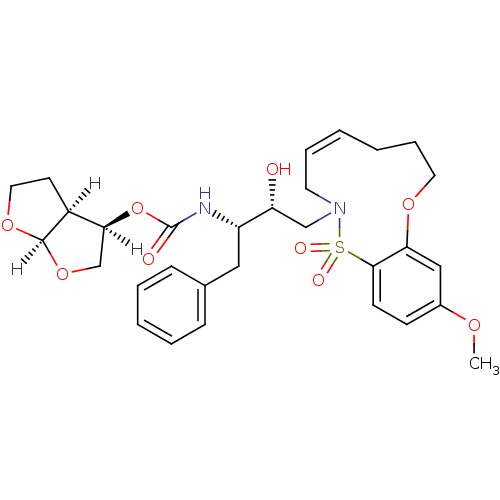 (cyclic compound, 14f) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0770 | -57.7 | n/a | n/a | 20 | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 52: 7689-705 (2009) Article DOI: 10.1021/jm900695w BindingDB Entry DOI: 10.7270/Q2G44NN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM35971 (cyclic compound, 14e) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | -57.6 | n/a | n/a | 23 | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 52: 7689-705 (2009) Article DOI: 10.1021/jm900695w BindingDB Entry DOI: 10.7270/Q2G44NN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM35966 (E/Z ratio 1:4 | cyclic compound, 14b) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0820 | -57.6 | n/a | n/a | 4 | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 52: 7689-705 (2009) Article DOI: 10.1021/jm900695w BindingDB Entry DOI: 10.7270/Q2G44NN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50483335 (CHEMBL1651161) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 54: 622-34 (2011) Article DOI: 10.1021/jm1012787 BindingDB Entry DOI: 10.7270/Q2BV7KG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM35975 (cyclic compound, 15g) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | -57.3 | n/a | n/a | 5.5 | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 52: 7689-705 (2009) Article DOI: 10.1021/jm900695w BindingDB Entry DOI: 10.7270/Q2G44NN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM31817 (GRL-02031 | methyl-2-pyrrolidinone, 19b) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0990 | -57.1 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 52: 3902-14 (2009) Article DOI: 10.1021/jm900303m BindingDB Entry DOI: 10.7270/Q20G3HHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM35963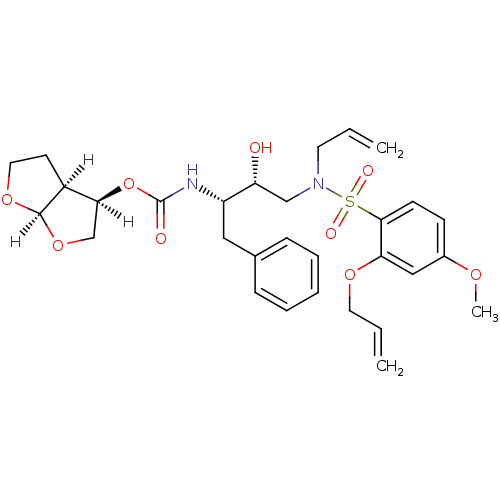 (acyclic compound, 13h) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.100 | -57.1 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 52: 7689-705 (2009) Article DOI: 10.1021/jm900695w BindingDB Entry DOI: 10.7270/Q2G44NN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127171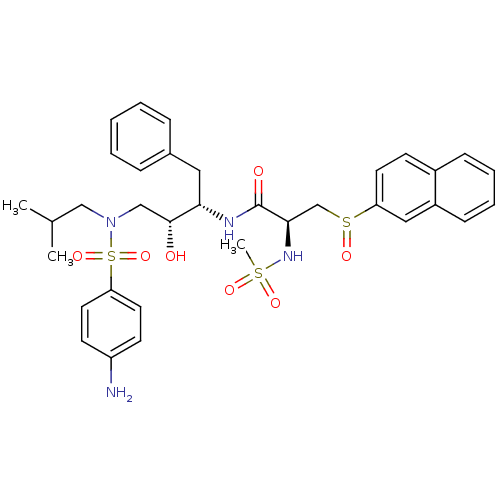 (CHEMBL299578 | N-{3-[(4-Amino-benzenesulfonyl)-iso...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV-1 protease | J Med Chem 46: 1764-8 (2003) Article DOI: 10.1021/jm020537i BindingDB Entry DOI: 10.7270/Q2805218 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50483341 (CHEMBL1651159) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 54: 622-34 (2011) Article DOI: 10.1021/jm1012787 BindingDB Entry DOI: 10.7270/Q2BV7KG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM810 ((3S,4aS,8aS)-N-tert-butyl-2-[(2R,3S)-2-hydroxy-3-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV-1 protease | J Med Chem 46: 1764-8 (2003) Article DOI: 10.1021/jm020537i BindingDB Entry DOI: 10.7270/Q2805218 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM31816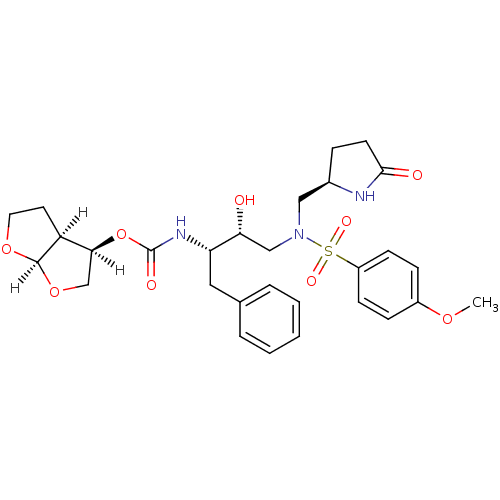 (methyl-2-pyrrolidinone, 19a) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.120 | -56.6 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 52: 3902-14 (2009) Article DOI: 10.1021/jm900303m BindingDB Entry DOI: 10.7270/Q20G3HHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM13924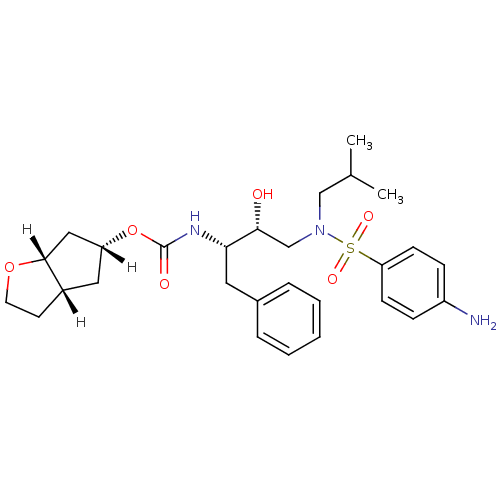 ((3aS,5R,6aR)-hexahydro-2H-cyclopenta[b]furan-5-yl ...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.140 | -56.2 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 49: 5252-61 (2006) Article DOI: 10.1021/jm060561m BindingDB Entry DOI: 10.7270/Q23R0R41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM35973 (cyclic compound, 15f) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.150 | -56.1 | n/a | n/a | 95 | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 52: 7689-705 (2009) Article DOI: 10.1021/jm900695w BindingDB Entry DOI: 10.7270/Q2G44NN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM25364 ((5S)-1,3-dioxocan-5-yl N-[(2S,3R)-3-hydroxy-4-[(4-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.150 | -56.1 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 51: 6021-33 (2008) Article DOI: 10.1021/jm8004543 BindingDB Entry DOI: 10.7270/Q2G15Z51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM35995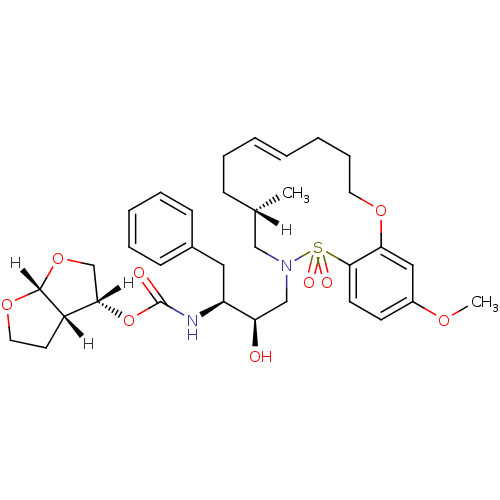 (cyclic compound, 21c) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.150 | n/a | n/a | n/a | 52 | n/a | n/a | n/a | n/a |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 52: 7689-705 (2009) Article DOI: 10.1021/jm900695w BindingDB Entry DOI: 10.7270/Q2G44NN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM25365 ((5S)-1,3-dioxepan-5-yl N-[(2S,3R)-3-hydroxy-4-[(4-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.160 | -55.9 | 30 | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 51: 6021-33 (2008) Article DOI: 10.1021/jm8004543 BindingDB Entry DOI: 10.7270/Q2G15Z51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM35964 (E/Z ratio 3:1 | cyclic compound, 14a | cyclic comp...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.160 | -55.9 | n/a | n/a | >1.00E+3 | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 52: 7689-705 (2009) Article DOI: 10.1021/jm900695w BindingDB Entry DOI: 10.7270/Q2G44NN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM25366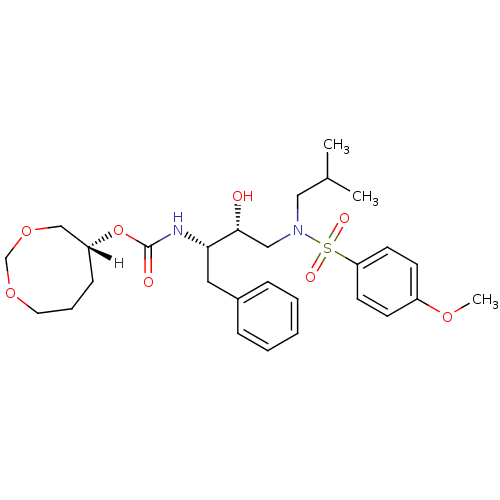 ((5R)-1,3-dioxocan-5-yl N-[(2S,3R)-3-hydroxy-4-[(4-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.160 | -55.9 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 51: 6021-33 (2008) Article DOI: 10.1021/jm8004543 BindingDB Entry DOI: 10.7270/Q2G15Z51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM50060964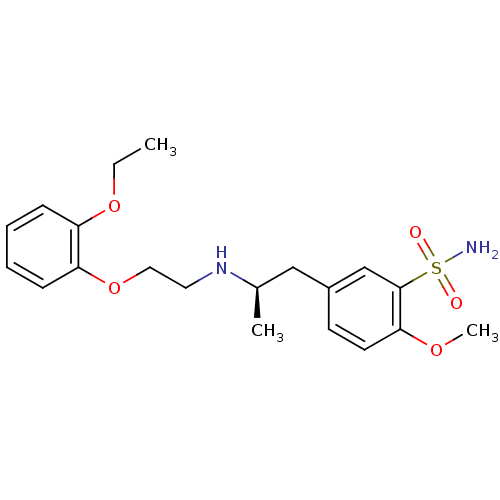 ((R)-5-(2-((2-(2-ethoxyphenoxy)ethyl)amino)propyl)-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I] HEAT from human alpha1D adrenergic receptor expressed in Chlorocebus aethiops COS1 cell membranes incubated for 60 mins by liq... | J Med Chem 59: 2989-3002 (2016) Article DOI: 10.1021/acs.jmedchem.5b01528 BindingDB Entry DOI: 10.7270/Q20G3N2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM35980 (cyclic compound, 14b-E) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.180 | -55.6 | n/a | n/a | 6.60 | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 52: 7689-705 (2009) Article DOI: 10.1021/jm900695w BindingDB Entry DOI: 10.7270/Q2G44NN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM519 ((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV-1 protease | J Med Chem 46: 1764-8 (2003) Article DOI: 10.1021/jm020537i BindingDB Entry DOI: 10.7270/Q2805218 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50481586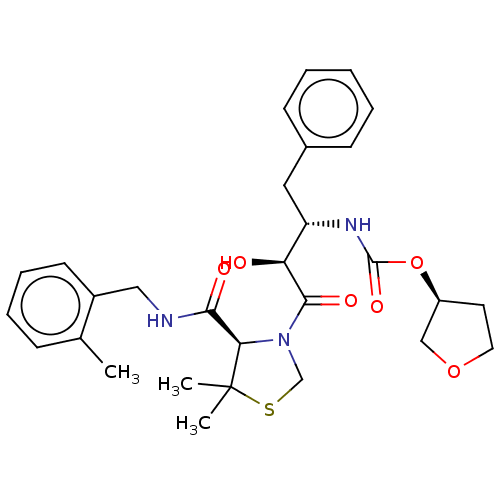 (CHEMBL604931) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem Lett 20: 1241-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.123 BindingDB Entry DOI: 10.7270/Q2CJ8HB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM35978 (cyclic compound, 14a-E) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.240 | -54.9 | n/a | n/a | 360 | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 52: 7689-705 (2009) Article DOI: 10.1021/jm900695w BindingDB Entry DOI: 10.7270/Q2G44NN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM31823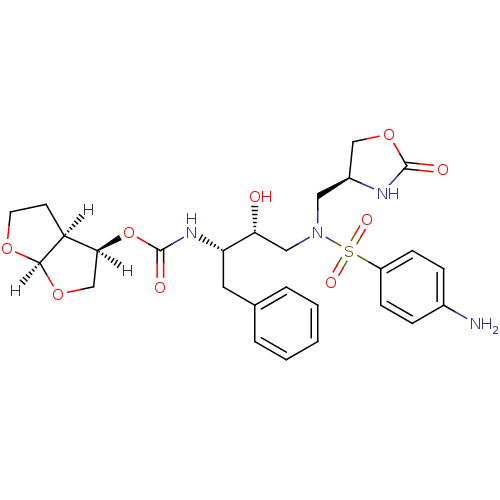 (oxazolidinone, 32) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.240 | -54.9 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 52: 3902-14 (2009) Article DOI: 10.1021/jm900303m BindingDB Entry DOI: 10.7270/Q20G3HHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1759 total ) | Next | Last >> |