 Found 66 hits with Last Name = 'kondoh' and Initial = 'o'
Found 66 hits with Last Name = 'kondoh' and Initial = 'o' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
1,3-beta-glucan synthase
(Candida albicans (Yeast)) | BDBM50100335
(CHEMBL2371681 | RO-09-3655 derivative)Show SMILES [H][C@@]12C[C@@H](O)CN1C(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](C)NC(=O)[C@@]([H])(NC(=O)C[C@@H](CCCCCCCCCCCCC)OC(=O)[C@H](CCCN(CCN)C(=O)[C@H](CCCN)N(CCN)CCN)NC(=O)[C@@]([H])(NC(=O)CNC(=O)[C@@]([H])(NC(=O)[C@]1([H])[C@@H](O)CCN1C(=O)[C@@]([H])(NC(=O)[C@@]([H])(NC2=O)[C@@H](C)O)[C@@H](C)O)[C@H](O)CC(N)=O)[C@@H](C)O)[C@@H](C)O)C(C)C Show InChI InChI=1S/C82H141N19O24/c1-9-10-11-12-13-14-15-16-17-18-19-22-54-41-62(111)92-65(47(5)102)75(117)89-46(4)71(113)91-56(39-51-25-27-52(106)28-26-51)72(114)94-64(45(2)3)80(122)101-44-53(107)40-58(101)73(115)95-67(49(7)104)77(119)96-68(50(8)105)81(123)100-35-29-59(108)70(100)78(120)97-69(60(109)42-61(87)110)74(116)88-43-63(112)93-66(48(6)103)76(118)90-55(82(124)125-54)23-21-34-99(38-33-86)79(121)57(24-20-30-83)98(36-31-84)37-32-85/h25-28,45-50,53-60,64-70,102-109H,9-24,29-44,83-86H2,1-8H3,(H2,87,110)(H,88,116)(H,89,117)(H,90,118)(H,91,113)(H,92,111)(H,93,112)(H,94,114)(H,95,115)(H,96,119)(H,97,120)/t46-,47-,48-,49-,50-,53-,54-,55+,56+,57+,58+,59+,60-,64+,65+,66+,67+,68+,69+,70+/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Beta glucan synthase |
Bioorg Med Chem Lett 11: 1273-6 (2001)
BindingDB Entry DOI: 10.7270/Q2CN74FQ |
More data for this
Ligand-Target Pair | |
1,3-beta-glucan synthase
(Candida albicans (Yeast)) | BDBM50100338

(CHEMBL267407 | RO-09-3655 derivative)Show SMILES CCCCCCCCCCCCC[C@@H]1CC(=O)N[C@H]([C@@H](C)O)C(=O)N[C@H](C)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H](C(C)C)C(=O)N2C[C@H](O)C[C@H]2C(=O)N[C@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N2CC[C@H](O)[C@H]2C(=O)N[C@@H]([C@H](O)CC(N)=O)C(=O)NCC(=O)N[C@H]([C@@H](C)O)C(=O)N[C@@H](CCCN(CCCN)C(=O)[C@@H](CCCN)N(CCN)CCN)C(=O)O1 Show InChI InChI=1S/C83H143N19O24/c1-9-10-11-12-13-14-15-16-17-18-19-23-55-42-63(112)93-66(48(5)103)76(118)90-47(4)72(114)92-57(40-52-26-28-53(107)29-27-52)73(115)95-65(46(2)3)81(123)102-45-54(108)41-59(102)74(116)96-68(50(7)105)78(120)97-69(51(8)106)82(124)101-37-30-60(109)71(101)79(121)98-70(61(110)43-62(88)111)75(117)89-44-64(113)94-67(49(6)104)77(119)91-56(83(125)126-55)24-21-35-100(36-22-32-85)80(122)58(25-20-31-84)99(38-33-86)39-34-87/h26-29,46-51,54-61,65-71,103-110H,9-25,30-45,84-87H2,1-8H3,(H2,88,111)(H,89,117)(H,90,118)(H,91,119)(H,92,114)(H,93,112)(H,94,113)(H,95,115)(H,96,116)(H,97,120)(H,98,121)/t47-,48-,49-,50-,51-,54-,55-,56+,57+,58-,59+,60+,61-,65+,66-,67-,68-,69+,70+,71+/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Beta glucan synthase |
Bioorg Med Chem Lett 11: 1273-6 (2001)
BindingDB Entry DOI: 10.7270/Q2CN74FQ |
More data for this
Ligand-Target Pair | |
1,3-beta-glucan synthase
(Candida albicans (Yeast)) | BDBM50100332
(CHEMBL415843 | RO-09-3655 derivative)Show SMILES CCCCCCCCCCCCC[C@@H]1CC(=O)N[C@H]([C@@H](C)O)C(=O)N[C@H](C)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H](C(C)C)C(=O)N2C[C@H](O)C[C@H]2C(=O)N[C@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N2CC[C@H](O)[C@H]2C(=O)N[C@@H]([C@H](O)CC(N)=O)C(=O)NCC(=O)N[C@H]([C@@H](C)O)C(=O)N[C@@H](CCCN(CCCN)C(=O)[C@H](CCCN)N(CCN)CCN)C(=O)O1 Show InChI InChI=1S/C83H143N19O24/c1-9-10-11-12-13-14-15-16-17-18-19-23-55-42-63(112)93-66(48(5)103)76(118)90-47(4)72(114)92-57(40-52-26-28-53(107)29-27-52)73(115)95-65(46(2)3)81(123)102-45-54(108)41-59(102)74(116)96-68(50(7)105)78(120)97-69(51(8)106)82(124)101-37-30-60(109)71(101)79(121)98-70(61(110)43-62(88)111)75(117)89-44-64(113)94-67(49(6)104)77(119)91-56(83(125)126-55)24-21-35-100(36-22-32-85)80(122)58(25-20-31-84)99(38-33-86)39-34-87/h26-29,46-51,54-61,65-71,103-110H,9-25,30-45,84-87H2,1-8H3,(H2,88,111)(H,89,117)(H,90,118)(H,91,119)(H,92,114)(H,93,112)(H,94,113)(H,95,115)(H,96,116)(H,97,120)(H,98,121)/t47-,48-,49-,50-,51-,54-,55-,56+,57+,58+,59+,60+,61-,65+,66-,67-,68-,69+,70+,71+/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Beta glucan synthase |
Bioorg Med Chem Lett 11: 1273-6 (2001)
BindingDB Entry DOI: 10.7270/Q2CN74FQ |
More data for this
Ligand-Target Pair | |
1,3-beta-glucan synthase
(Candida albicans (Yeast)) | BDBM50100333
(CHEMBL2371765 | RO-09-3655 derivative)Show SMILES [H][C@@]12C[C@@H](O)CN1C(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](C)NC(=O)[C@@]([H])(NC(=O)C[C@@H](CCCCCCCCCCCCC)OC(=O)[C@H](CCCNC(=O)[C@@H](CCCN)N(CCN)CCN)NC(=O)[C@@]([H])(NC(=O)CNC(=O)[C@@]([H])(NC(=O)[C@]1([H])[C@@H](O)CCN1C(=O)[C@@]([H])(NC(=O)[C@@]([H])(NC2=O)[C@@H](C)O)[C@@H](C)O)[C@H](O)CC(N)=O)[C@@H](C)O)[C@@H](C)O)C(C)C Show InChI InChI=1S/C80H136N18O24/c1-9-10-11-12-13-14-15-16-17-18-19-22-52-39-60(108)90-63(45(5)99)74(115)87-44(4)69(110)89-54(37-49-25-27-50(103)28-26-49)70(111)92-62(43(2)3)78(119)98-42-51(104)38-56(98)72(113)93-65(47(7)101)76(117)94-66(48(8)102)79(120)97-34-29-57(105)68(97)77(118)95-67(58(106)40-59(84)107)73(114)86-41-61(109)91-64(46(6)100)75(116)88-53(80(121)122-52)23-21-33-85-71(112)55(24-20-30-81)96(35-31-82)36-32-83/h25-28,43-48,51-58,62-68,99-106H,9-24,29-42,81-83H2,1-8H3,(H2,84,107)(H,85,112)(H,86,114)(H,87,115)(H,88,116)(H,89,110)(H,90,108)(H,91,109)(H,92,111)(H,93,113)(H,94,117)(H,95,118)/t44-,45-,46-,47-,48-,51-,52-,53+,54+,55-,56+,57+,58-,62+,63+,64+,65+,66+,67+,68+/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Beta glucan synthase |
Bioorg Med Chem Lett 11: 1273-6 (2001)
BindingDB Entry DOI: 10.7270/Q2CN74FQ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50479835
(CHEMBL510873)Show SMILES Cn1cncc1[C@](N)(c1cc2cc(cc(-c3cccc(c3)C#N)c2o1)C#N)c1ccc(cc1)C#N |r| Show InChI InChI=1S/C28H18N6O/c1-34-17-33-16-25(34)28(32,23-7-5-18(13-29)6-8-23)26-12-22-10-20(15-31)11-24(27(22)35-26)21-4-2-3-19(9-21)14-30/h2-12,16-17H,32H2,1H3/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human FTase |
Bioorg Med Chem Lett 19: 1753-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.074
BindingDB Entry DOI: 10.7270/Q28G8PGH |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50479863
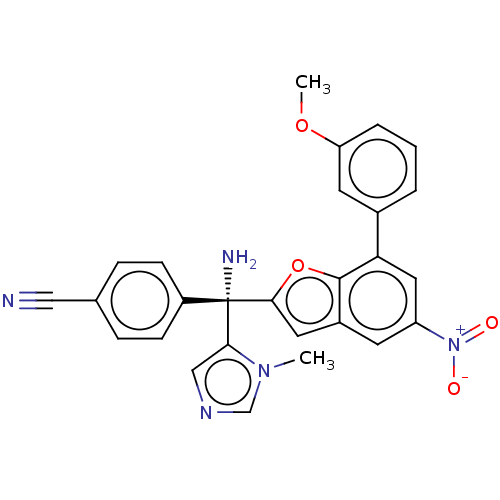
(CHEMBL521743)Show SMILES COc1cccc(c1)-c1cc(cc2cc(oc12)[C@@](N)(c1cncn1C)c1ccc(cc1)C#N)[N+]([O-])=O |r| Show InChI InChI=1S/C27H21N5O4/c1-31-16-30-15-24(31)27(29,20-8-6-17(14-28)7-9-20)25-12-19-10-21(32(33)34)13-23(26(19)36-25)18-4-3-5-22(11-18)35-2/h3-13,15-16H,29H2,1-2H3/t27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human FTase |
Bioorg Med Chem Lett 19: 1753-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.074
BindingDB Entry DOI: 10.7270/Q28G8PGH |
More data for this
Ligand-Target Pair | |
1,3-beta-glucan synthase
(Candida albicans (Yeast)) | BDBM50100331
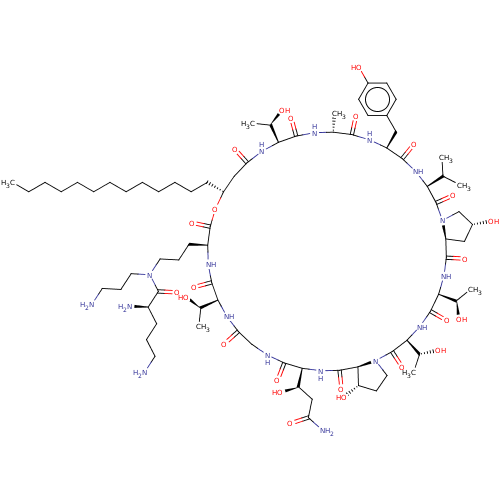
(CHEMBL2371715 | RO-09-3655 derivative)Show SMILES [H][C@@]12C[C@@H](O)CN1C(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](C)NC(=O)[C@@]([H])(NC(=O)C[C@@H](CCCCCCCCCCCCC)OC(=O)[C@H](CCCN(CCCN)C(=O)[C@H](N)CCCN)NC(=O)[C@@]([H])(NC(=O)CNC(=O)[C@@]([H])(NC(=O)[C@]1([H])[C@@H](O)CCN1C(=O)[C@@]([H])(NC(=O)[C@@]([H])(NC2=O)[C@@H](C)O)[C@@H](C)O)[C@H](O)CC(N)=O)[C@@H](C)O)[C@@H](C)O)C(C)C Show InChI InChI=1S/C79H133N17O24/c1-9-10-11-12-13-14-15-16-17-18-19-23-51-38-59(106)88-62(44(5)97)72(112)85-43(4)68(108)87-54(36-48-26-28-49(101)29-27-48)69(109)90-61(42(2)3)77(117)96-41-50(102)37-55(96)70(110)91-64(46(7)99)74(114)92-65(47(8)100)78(118)95-35-30-56(103)67(95)75(115)93-66(57(104)39-58(83)105)71(111)84-40-60(107)89-63(45(6)98)73(113)86-53(79(119)120-51)25-21-33-94(34-22-32-81)76(116)52(82)24-20-31-80/h26-29,42-47,50-57,61-67,97-104H,9-25,30-41,80-82H2,1-8H3,(H2,83,105)(H,84,111)(H,85,112)(H,86,113)(H,87,108)(H,88,106)(H,89,107)(H,90,109)(H,91,110)(H,92,114)(H,93,115)/t43-,44-,45-,46-,47-,50-,51-,52-,53+,54+,55+,56+,57-,61+,62+,63+,64+,65+,66+,67+/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Beta glucan synthase |
Bioorg Med Chem Lett 11: 1273-6 (2001)
BindingDB Entry DOI: 10.7270/Q2CN74FQ |
More data for this
Ligand-Target Pair | |
1,3-beta-glucan synthase
(Candida albicans (Yeast)) | BDBM50100329
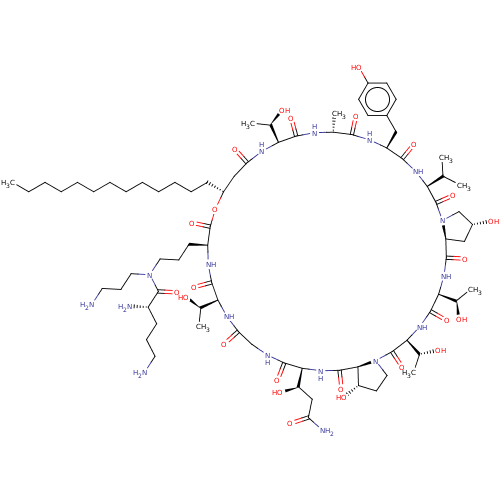
(CHEMBL2371727 | RO-09-3655 derivative)Show SMILES [H][C@@]12C[C@@H](O)CN1C(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](C)NC(=O)[C@@]([H])(NC(=O)C[C@@H](CCCCCCCCCCCCC)OC(=O)[C@H](CCCN(CCCN)C(=O)[C@@H](N)CCCN)NC(=O)[C@@]([H])(NC(=O)CNC(=O)[C@@]([H])(NC(=O)[C@]1([H])[C@@H](O)CCN1C(=O)[C@@]([H])(NC(=O)[C@@]([H])(NC2=O)[C@@H](C)O)[C@@H](C)O)[C@H](O)CC(N)=O)[C@@H](C)O)[C@@H](C)O)C(C)C Show InChI InChI=1S/C79H133N17O24/c1-9-10-11-12-13-14-15-16-17-18-19-23-51-38-59(106)88-62(44(5)97)72(112)85-43(4)68(108)87-54(36-48-26-28-49(101)29-27-48)69(109)90-61(42(2)3)77(117)96-41-50(102)37-55(96)70(110)91-64(46(7)99)74(114)92-65(47(8)100)78(118)95-35-30-56(103)67(95)75(115)93-66(57(104)39-58(83)105)71(111)84-40-60(107)89-63(45(6)98)73(113)86-53(79(119)120-51)25-21-33-94(34-22-32-81)76(116)52(82)24-20-31-80/h26-29,42-47,50-57,61-67,97-104H,9-25,30-41,80-82H2,1-8H3,(H2,83,105)(H,84,111)(H,85,112)(H,86,113)(H,87,108)(H,88,106)(H,89,107)(H,90,109)(H,91,110)(H,92,114)(H,93,115)/t43-,44-,45-,46-,47-,50-,51-,52+,53+,54+,55+,56+,57-,61+,62+,63+,64+,65+,66+,67+/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Beta glucan synthase |
Bioorg Med Chem Lett 11: 1273-6 (2001)
BindingDB Entry DOI: 10.7270/Q2CN74FQ |
More data for this
Ligand-Target Pair | |
1,3-beta-glucan synthase
(Candida albicans (Yeast)) | BDBM50100337
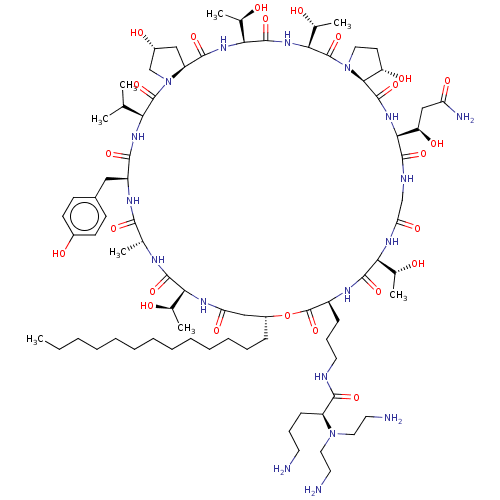
(CHEMBL2371766 | RO-09-3655 derivative)Show SMILES [H][C@@]12C[C@@H](O)CN1C(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](C)NC(=O)[C@@]([H])(NC(=O)C[C@@H](CCCCCCCCCCCCC)OC(=O)[C@H](CCCNC(=O)[C@H](CCCN)N(CCN)CCN)NC(=O)[C@@]([H])(NC(=O)CNC(=O)[C@@]([H])(NC(=O)[C@]1([H])[C@@H](O)CCN1C(=O)[C@@]([H])(NC(=O)[C@@]([H])(NC2=O)[C@@H](C)O)[C@@H](C)O)[C@H](O)CC(N)=O)[C@@H](C)O)[C@@H](C)O)C(C)C Show InChI InChI=1S/C80H136N18O24/c1-9-10-11-12-13-14-15-16-17-18-19-22-52-39-60(108)90-63(45(5)99)74(115)87-44(4)69(110)89-54(37-49-25-27-50(103)28-26-49)70(111)92-62(43(2)3)78(119)98-42-51(104)38-56(98)72(113)93-65(47(7)101)76(117)94-66(48(8)102)79(120)97-34-29-57(105)68(97)77(118)95-67(58(106)40-59(84)107)73(114)86-41-61(109)91-64(46(6)100)75(116)88-53(80(121)122-52)23-21-33-85-71(112)55(24-20-30-81)96(35-31-82)36-32-83/h25-28,43-48,51-58,62-68,99-106H,9-24,29-42,81-83H2,1-8H3,(H2,84,107)(H,85,112)(H,86,114)(H,87,115)(H,88,116)(H,89,110)(H,90,108)(H,91,109)(H,92,111)(H,93,113)(H,94,117)(H,95,118)/t44-,45-,46-,47-,48-,51-,52-,53+,54+,55+,56+,57+,58-,62+,63+,64+,65+,66+,67+,68+/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Beta glucan synthase |
Bioorg Med Chem Lett 11: 1273-6 (2001)
BindingDB Entry DOI: 10.7270/Q2CN74FQ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50479859
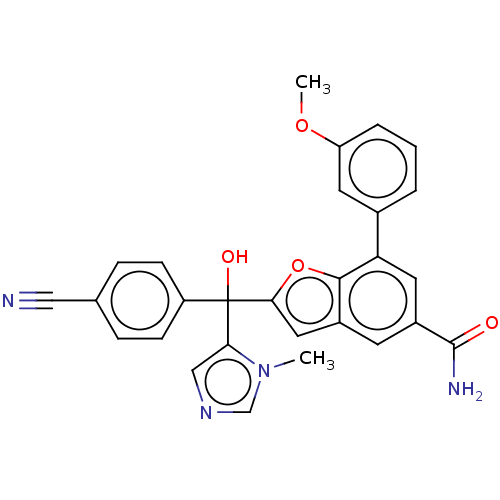
(CHEMBL508937)Show SMILES COc1cccc(c1)-c1cc(cc2cc(oc12)C(O)(c1cncn1C)c1ccc(cc1)C#N)C(N)=O Show InChI InChI=1S/C28H22N4O4/c1-32-16-31-15-24(32)28(34,21-8-6-17(14-29)7-9-21)25-13-19-10-20(27(30)33)12-23(26(19)36-25)18-4-3-5-22(11-18)35-2/h3-13,15-16,34H,1-2H3,(H2,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human FTase |
Bioorg Med Chem Lett 19: 1753-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.074
BindingDB Entry DOI: 10.7270/Q28G8PGH |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50479847
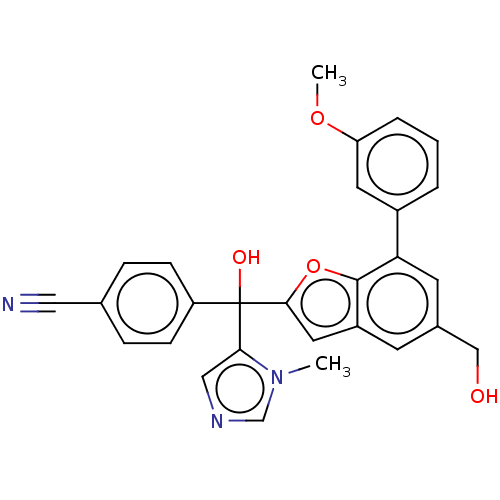
(CHEMBL514300)Show SMILES COc1cccc(c1)-c1cc(CO)cc2cc(oc12)C(O)(c1cncn1C)c1ccc(cc1)C#N Show InChI InChI=1S/C28H23N3O4/c1-31-17-30-15-25(31)28(33,22-8-6-18(14-29)7-9-22)26-13-21-10-19(16-32)11-24(27(21)35-26)20-4-3-5-23(12-20)34-2/h3-13,15,17,32-33H,16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human FTase |
Bioorg Med Chem Lett 19: 1753-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.074
BindingDB Entry DOI: 10.7270/Q28G8PGH |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50479856
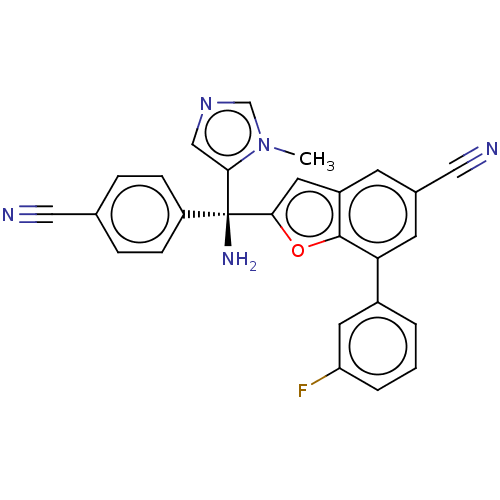
(CHEMBL454405)Show SMILES Cn1cncc1[C@](N)(c1cc2cc(cc(-c3cccc(F)c3)c2o1)C#N)c1ccc(cc1)C#N |r| Show InChI InChI=1S/C27H18FN5O/c1-33-16-32-15-24(33)27(31,21-7-5-17(13-29)6-8-21)25-12-20-9-18(14-30)10-23(26(20)34-25)19-3-2-4-22(28)11-19/h2-12,15-16H,31H2,1H3/t27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human FTase |
Bioorg Med Chem Lett 19: 1753-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.074
BindingDB Entry DOI: 10.7270/Q28G8PGH |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50479848
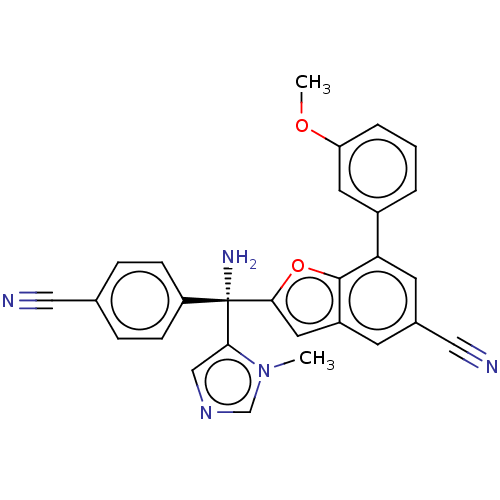
(CHEMBL489318)Show SMILES COc1cccc(c1)-c1cc(cc2cc(oc12)[C@@](N)(c1cncn1C)c1ccc(cc1)C#N)C#N |r| Show InChI InChI=1S/C28H21N5O2/c1-33-17-32-16-25(33)28(31,22-8-6-18(14-29)7-9-22)26-13-21-10-19(15-30)11-24(27(21)35-26)20-4-3-5-23(12-20)34-2/h3-13,16-17H,31H2,1-2H3/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human FTase |
Bioorg Med Chem Lett 19: 1753-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.074
BindingDB Entry DOI: 10.7270/Q28G8PGH |
More data for this
Ligand-Target Pair | |
1,3-beta-glucan synthase
(Candida albicans (Yeast)) | BDBM50366679
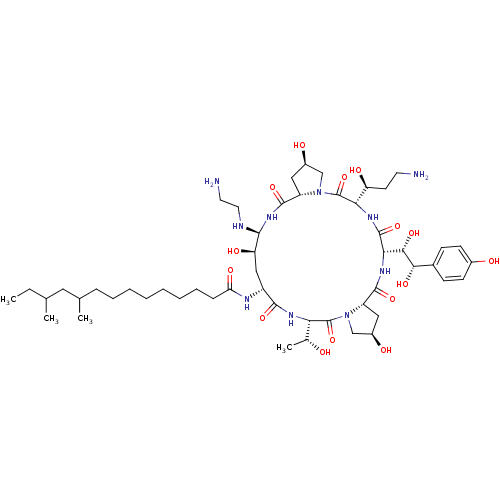
(CHEMBL1793852 | MK-991)Show SMILES CCC(C)CC(C)CCCCCCCCC(=O)N[C@@H]1C[C@@H](O)[C@@H](NCCN)NC(=O)[C@@H]2C[C@@H](O)CN2C(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@@H]2C[C@@H](O)CN2C(=O)[C@@H](NC1=O)[C@@H](C)O)[C@H](O)[C@@H](O)c1ccc(O)cc1)[C@@H](O)CCN Show InChI InChI=1S/C52H88N10O15/c1-5-28(2)22-29(3)12-10-8-6-7-9-11-13-40(69)56-35-25-39(68)46(55-21-20-54)60-49(74)37-24-34(66)27-62(37)52(77)42(38(67)18-19-53)58-50(75)43(45(71)44(70)31-14-16-32(64)17-15-31)59-48(73)36-23-33(65)26-61(36)51(76)41(30(4)63)57-47(35)72/h14-17,28-30,33-39,41-46,55,63-68,70-71H,5-13,18-27,53-54H2,1-4H3,(H,56,69)(H,57,72)(H,58,75)(H,59,73)(H,60,74)/t28?,29?,30-,33-,34-,35-,36+,37+,38+,39-,41+,42+,43+,44+,45+,46+/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Beta glucan synthase |
Bioorg Med Chem Lett 11: 1273-6 (2001)
BindingDB Entry DOI: 10.7270/Q2CN74FQ |
More data for this
Ligand-Target Pair | |
1,3-beta-glucan synthase
(Candida albicans (Yeast)) | BDBM50100336
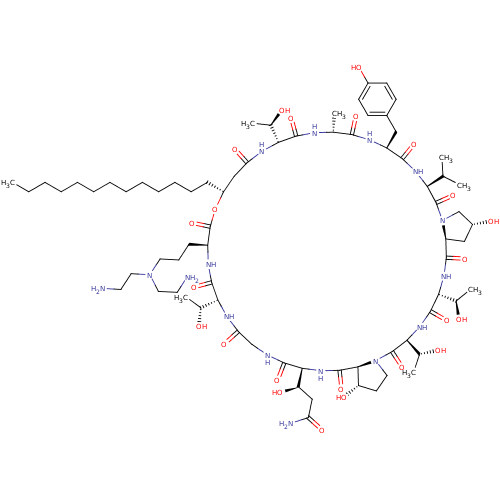
(CHEMBL405487 | RO-09-3655 derivative)Show SMILES CCCCCCCCCCCCC[C@@H]1CC(=O)N[C@H]([C@@H](C)O)C(=O)N[C@H](C)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H](C(C)C)C(=O)N2C[C@H](O)C[C@H]2C(=O)N[C@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N2CC[C@H](O)[C@H]2C(=O)N[C@@H]([C@H](O)CC(N)=O)C(=O)NCC(=O)N[C@H]([C@@H](C)O)C(=O)N[C@@H](CCCN(CCN)CCN)C(=O)O1 Show InChI InChI=1S/C75H126N16O23/c1-9-10-11-12-13-14-15-16-17-18-19-21-49-36-56(101)83-59(42(5)92)69(107)80-41(4)65(103)82-51(34-46-23-25-47(96)26-24-46)66(104)85-58(40(2)3)73(111)91-39-48(97)35-52(91)67(105)86-61(44(7)94)71(109)87-62(45(8)95)74(112)90-31-27-53(98)64(90)72(110)88-63(54(99)37-55(78)100)68(106)79-38-57(102)84-60(43(6)93)70(108)81-50(75(113)114-49)22-20-30-89(32-28-76)33-29-77/h23-26,40-45,48-54,58-64,92-99H,9-22,27-39,76-77H2,1-8H3,(H2,78,100)(H,79,106)(H,80,107)(H,81,108)(H,82,103)(H,83,101)(H,84,102)(H,85,104)(H,86,105)(H,87,109)(H,88,110)/t41-,42-,43-,44-,45-,48-,49-,50+,51+,52+,53+,54-,58+,59-,60-,61-,62+,63+,64+/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Beta glucan synthase |
Bioorg Med Chem Lett 11: 1273-6 (2001)
BindingDB Entry DOI: 10.7270/Q2CN74FQ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50479849
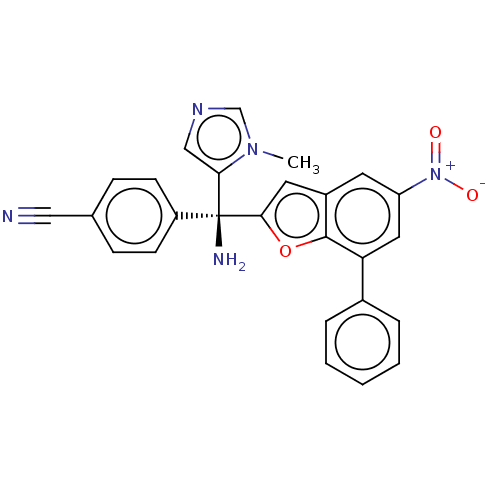
(CHEMBL489121)Show SMILES Cn1cncc1[C@](N)(c1cc2cc(cc(-c3ccccc3)c2o1)[N+]([O-])=O)c1ccc(cc1)C#N |r| Show InChI InChI=1S/C26H19N5O3/c1-30-16-29-15-23(30)26(28,20-9-7-17(14-27)8-10-20)24-12-19-11-21(31(32)33)13-22(25(19)34-24)18-5-3-2-4-6-18/h2-13,15-16H,28H2,1H3/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human FTase |
Bioorg Med Chem Lett 19: 1753-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.074
BindingDB Entry DOI: 10.7270/Q28G8PGH |
More data for this
Ligand-Target Pair | |
1,3-beta-glucan synthase
(Candida albicans (Yeast)) | BDBM50096797
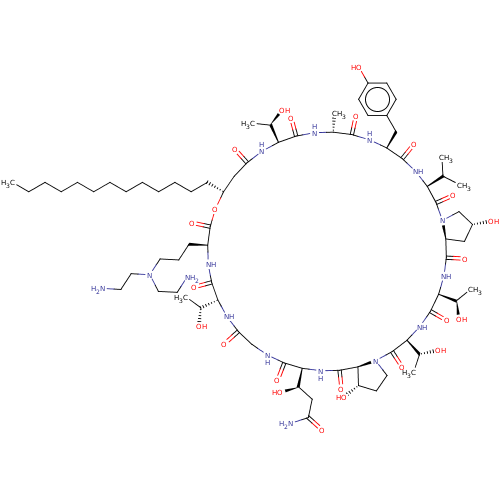
(CHEMBL2370665 | macrocyclic lipopeptidolactone der...)Show SMILES [H][C@@]12C[C@@H](O)CN1C(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](C)NC(=O)[C@@]([H])(NC(=O)C[C@@H](CCCCCCCCCCCCC)OC(=O)[C@H](CCCN(CCN)CCN)NC(=O)[C@]([H])(NC(=O)CNC(=O)[C@@]([H])(NC(=O)[C@]1([H])[C@@H](O)CCN1C(=O)[C@@]([H])(NC(=O)[C@@]([H])(NC2=O)[C@@H](C)O)[C@@H](C)O)[C@H](O)CC(N)=O)[C@@H](C)O)[C@@H](C)O)C(C)C Show InChI InChI=1S/C75H126N16O23/c1-9-10-11-12-13-14-15-16-17-18-19-21-49-36-56(101)83-59(42(5)92)69(107)80-41(4)65(103)82-51(34-46-23-25-47(96)26-24-46)66(104)85-58(40(2)3)73(111)91-39-48(97)35-52(91)67(105)86-61(44(7)94)71(109)87-62(45(8)95)74(112)90-31-27-53(98)64(90)72(110)88-63(54(99)37-55(78)100)68(106)79-38-57(102)84-60(43(6)93)70(108)81-50(75(113)114-49)22-20-30-89(32-28-76)33-29-77/h23-26,40-45,48-54,58-64,92-99H,9-22,27-39,76-77H2,1-8H3,(H2,78,100)(H,79,106)(H,80,107)(H,81,108)(H,82,103)(H,83,101)(H,84,102)(H,85,104)(H,86,105)(H,87,109)(H,88,110)/t41-,42-,43-,44-,45-,48-,49-,50+,51+,52+,53+,54-,58+,59+,60-,61+,62+,63+,64+/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of 1,3-beta-D-glucan synthase (GS) from candida albicans |
Bioorg Med Chem Lett 11: 395-8 (2001)
BindingDB Entry DOI: 10.7270/Q28051W2 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50479853
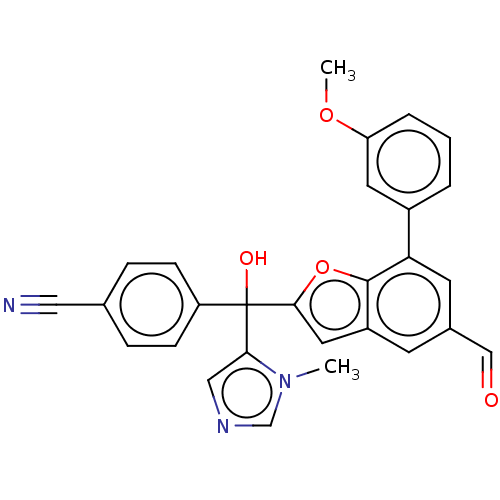
(CHEMBL511113)Show SMILES COc1cccc(c1)-c1cc(C=O)cc2cc(oc12)C(O)(c1cncn1C)c1ccc(cc1)C#N Show InChI InChI=1S/C28H21N3O4/c1-31-17-30-15-25(31)28(33,22-8-6-18(14-29)7-9-22)26-13-21-10-19(16-32)11-24(27(21)35-26)20-4-3-5-23(12-20)34-2/h3-13,15-17,33H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human FTase |
Bioorg Med Chem Lett 19: 1753-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.074
BindingDB Entry DOI: 10.7270/Q28G8PGH |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50479850
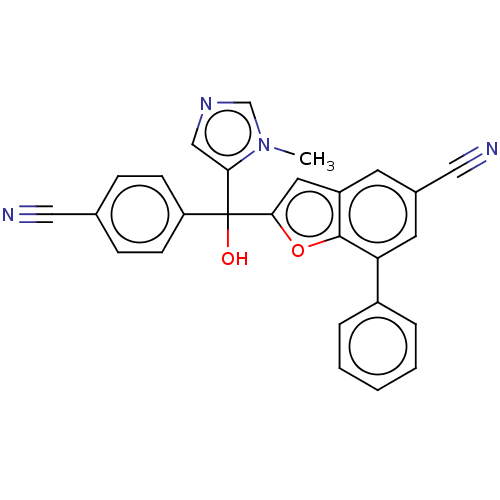
(CHEMBL475149)Show SMILES Cn1cncc1C(O)(c1cc2cc(cc(-c3ccccc3)c2o1)C#N)c1ccc(cc1)C#N Show InChI InChI=1S/C27H18N4O2/c1-31-17-30-16-24(31)27(32,22-9-7-18(14-28)8-10-22)25-13-21-11-19(15-29)12-23(26(21)33-25)20-5-3-2-4-6-20/h2-13,16-17,32H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human FTase |
Bioorg Med Chem Lett 19: 1753-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.074
BindingDB Entry DOI: 10.7270/Q28G8PGH |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50479864

(CHEMBL510021)Show SMILES COc1cccc(c1)-c1cc(cc2cc(oc12)C(O)(c1cncn1C)c1ccc(cc1)C#N)C(=O)NN1CCOCC1 Show InChI InChI=1S/C32H29N5O5/c1-36-20-34-19-28(36)32(39,25-8-6-21(18-33)7-9-25)29-17-23-14-24(31(38)35-37-10-12-41-13-11-37)16-27(30(23)42-29)22-4-3-5-26(15-22)40-2/h3-9,14-17,19-20,39H,10-13H2,1-2H3,(H,35,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human FTase |
Bioorg Med Chem Lett 19: 1753-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.074
BindingDB Entry DOI: 10.7270/Q28G8PGH |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50479860
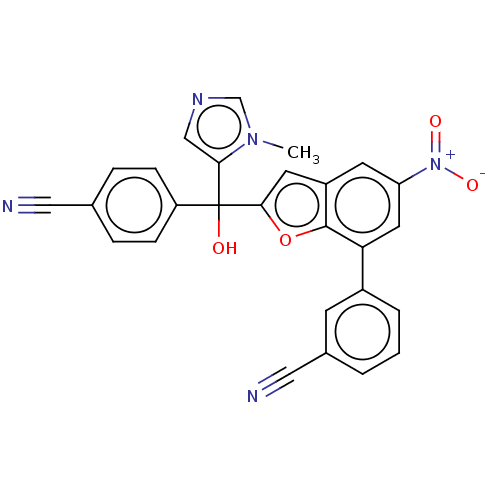
(CHEMBL473148)Show SMILES Cn1cncc1C(O)(c1cc2cc(cc(-c3cccc(c3)C#N)c2o1)[N+]([O-])=O)c1ccc(cc1)C#N Show InChI InChI=1S/C27H17N5O4/c1-31-16-30-15-24(31)27(33,21-7-5-17(13-28)6-8-21)25-11-20-10-22(32(34)35)12-23(26(20)36-25)19-4-2-3-18(9-19)14-29/h2-12,15-16,33H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human FTase |
Bioorg Med Chem Lett 19: 1753-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.074
BindingDB Entry DOI: 10.7270/Q28G8PGH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50479851
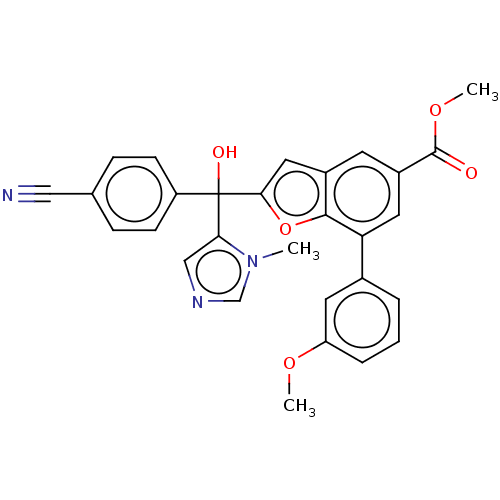
(CHEMBL462021)Show SMILES COC(=O)c1cc(-c2cccc(OC)c2)c2oc(cc2c1)C(O)(c1cncn1C)c1ccc(cc1)C#N Show InChI InChI=1S/C29H23N3O5/c1-32-17-31-16-25(32)29(34,22-9-7-18(15-30)8-10-22)26-14-20-11-21(28(33)36-3)13-24(27(20)37-26)19-5-4-6-23(12-19)35-2/h4-14,16-17,34H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human FTase |
Bioorg Med Chem Lett 19: 1753-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.074
BindingDB Entry DOI: 10.7270/Q28G8PGH |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50479857
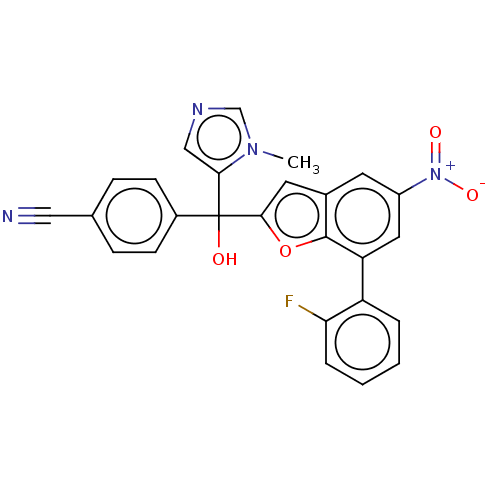
(CHEMBL472956)Show SMILES Cn1cncc1C(O)(c1cc2cc(cc(-c3ccccc3F)c2o1)[N+]([O-])=O)c1ccc(cc1)C#N Show InChI InChI=1S/C26H17FN4O4/c1-30-15-29-14-23(30)26(32,18-8-6-16(13-28)7-9-18)24-11-17-10-19(31(33)34)12-21(25(17)35-24)20-4-2-3-5-22(20)27/h2-12,14-15,32H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human FTase |
Bioorg Med Chem Lett 19: 1753-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.074
BindingDB Entry DOI: 10.7270/Q28G8PGH |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50479858
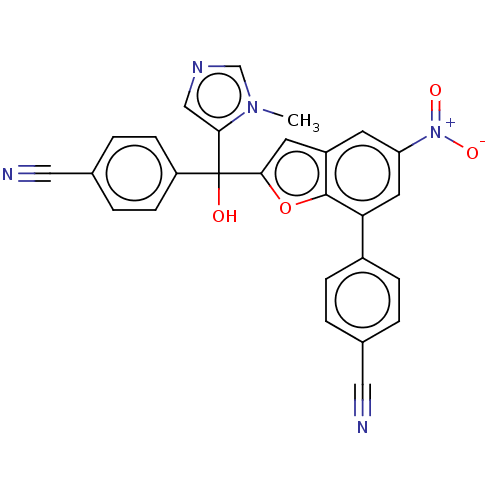
(CHEMBL453395)Show SMILES Cn1cncc1C(O)(c1cc2cc(cc(-c3ccc(cc3)C#N)c2o1)[N+]([O-])=O)c1ccc(cc1)C#N Show InChI InChI=1S/C27H17N5O4/c1-31-16-30-15-24(31)27(33,21-8-4-18(14-29)5-9-21)25-11-20-10-22(32(34)35)12-23(26(20)36-25)19-6-2-17(13-28)3-7-19/h2-12,15-16,33H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human FTase |
Bioorg Med Chem Lett 19: 1753-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.074
BindingDB Entry DOI: 10.7270/Q28G8PGH |
More data for this
Ligand-Target Pair | |
1,3-beta-glucan synthase
(Candida albicans (Yeast)) | BDBM50100334

(CHEMBL407924 | RO-09-3655 derivative)Show SMILES CCCCCCCCCCCCC[C@@H]1CC(=O)N[C@H]([C@@H](C)O)C(=O)N[C@H](C)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H](C(C)C)C(=O)N2C[C@H](O)C[C@H]2C(=O)N[C@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N2CC[C@H](O)[C@H]2C(=O)N[C@@H]([C@H](O)CC(N)=O)C(=O)NCC(=O)N[C@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(=O)[C@H](N)CCCN)C(=O)O1 Show InChI InChI=1S/C76H126N16O24/c1-9-10-11-12-13-14-15-16-17-18-19-22-48-35-56(102)85-59(41(5)93)70(109)82-40(4)65(104)84-51(33-45-25-27-46(97)28-26-45)67(106)87-58(39(2)3)74(113)92-38-47(98)34-52(92)68(107)88-61(43(7)95)72(111)89-62(44(8)96)75(114)91-32-29-53(99)64(91)73(112)90-63(54(100)36-55(79)101)69(108)81-37-57(103)86-60(42(6)94)71(110)83-50(76(115)116-48)24-21-31-80-66(105)49(78)23-20-30-77/h25-28,39-44,47-54,58-64,93-100H,9-24,29-38,77-78H2,1-8H3,(H2,79,101)(H,80,105)(H,81,108)(H,82,109)(H,83,110)(H,84,104)(H,85,102)(H,86,103)(H,87,106)(H,88,107)(H,89,111)(H,90,112)/t40-,41-,42-,43-,44-,47-,48-,49-,50+,51+,52+,53+,54-,58+,59-,60-,61-,62+,63+,64+/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Beta glucan synthase |
Bioorg Med Chem Lett 11: 1273-6 (2001)
BindingDB Entry DOI: 10.7270/Q2CN74FQ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50479836
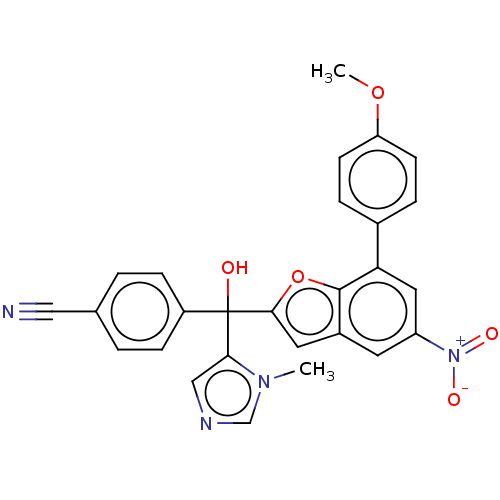
(CHEMBL511072)Show SMILES COc1ccc(cc1)-c1cc(cc2cc(oc12)C(O)(c1cncn1C)c1ccc(cc1)C#N)[N+]([O-])=O Show InChI InChI=1S/C27H20N4O5/c1-30-16-29-15-24(30)27(32,20-7-3-17(14-28)4-8-20)25-12-19-11-21(31(33)34)13-23(26(19)36-25)18-5-9-22(35-2)10-6-18/h3-13,15-16,32H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human FTase |
Bioorg Med Chem Lett 19: 1753-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.074
BindingDB Entry DOI: 10.7270/Q28G8PGH |
More data for this
Ligand-Target Pair | |
1,3-beta-glucan synthase
(Candida albicans (Yeast)) | BDBM50096795
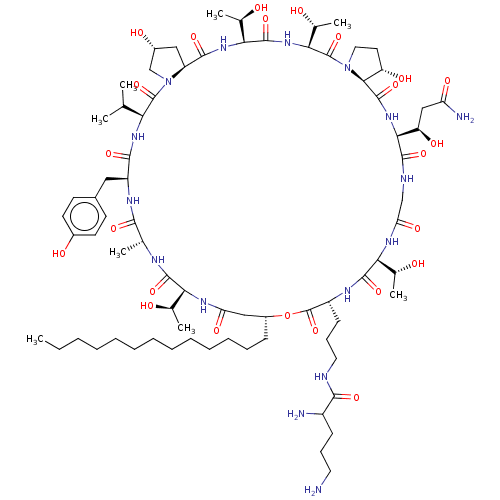
(CHEMBL2370654 | Macrocyclic lipopeptidolactone der...)Show SMILES [H][C@@]12C[C@@H](O)CN1C(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](C)NC(=O)[C@@]([H])(NC(=O)C[C@@H](CCCCCCCCCCCCC)OC(=O)[C@@H](CCCNC(=O)C(N)CCCN)NC(=O)[C@@]([H])(NC(=O)CNC(=O)[C@@]([H])(NC(=O)[C@]1([H])[C@@H](O)CCN1C(=O)[C@@]([H])(NC(=O)[C@@]([H])(NC2=O)[C@@H](C)O)[C@@H](C)O)[C@H](O)CC(N)=O)[C@@H](C)O)[C@@H](C)O)C(C)C Show InChI InChI=1S/C76H126N16O24/c1-9-10-11-12-13-14-15-16-17-18-19-22-48-35-56(102)85-59(41(5)93)70(109)82-40(4)65(104)84-51(33-45-25-27-46(97)28-26-45)67(106)87-58(39(2)3)74(113)92-38-47(98)34-52(92)68(107)88-61(43(7)95)72(111)89-62(44(8)96)75(114)91-32-29-53(99)64(91)73(112)90-63(54(100)36-55(79)101)69(108)81-37-57(103)86-60(42(6)94)71(110)83-50(76(115)116-48)24-21-31-80-66(105)49(78)23-20-30-77/h25-28,39-44,47-54,58-64,93-100H,9-24,29-38,77-78H2,1-8H3,(H2,79,101)(H,80,105)(H,81,108)(H,82,109)(H,83,110)(H,84,104)(H,85,102)(H,86,103)(H,87,106)(H,88,107)(H,89,111)(H,90,112)/t40-,41-,42-,43-,44-,47-,48-,49?,50-,51+,52+,53+,54-,58+,59+,60+,61+,62+,63+,64+/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of 1,3-beta-D-glucan synthase (GS) from candida albicans |
Bioorg Med Chem Lett 11: 395-8 (2001)
BindingDB Entry DOI: 10.7270/Q28051W2 |
More data for this
Ligand-Target Pair | |
1,3-beta-glucan synthase
(Candida albicans (Yeast)) | BDBM50096793
(CHEMBL2370666 | macrocyclic lipopeptidolactone der...)Show SMILES [H][C@@]12C[C@@H](O)CN1C(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](C)NC(=O)[C@@]([H])(NC(=O)C[C@@H](CCCCCCCCCCCCC)OC(=O)[C@H](CCN)NC(=O)[C@@]([H])(NC(=O)CNC(=O)[C@@]([H])(NC(=O)[C@]1([H])[C@@H](O)CCN1C(=O)[C@@]([H])(NC(=O)[C@@]([H])(NC2=O)[C@@H](C)O)[C@@H](C)O)[C@H](O)CCN)[C@@H](C)O)[C@@H](C)O)C(C)C Show InChI InChI=1S/C70H116N14O22/c1-9-10-11-12-13-14-15-16-17-18-19-20-45-33-51(93)77-54(38(5)85)64(99)74-37(4)60(95)76-47(31-42-21-23-43(89)24-22-42)61(96)79-53(36(2)3)68(103)84-35-44(90)32-48(84)62(97)80-56(40(7)87)66(101)81-57(41(8)88)69(104)83-30-27-50(92)59(83)67(102)82-58(49(91)26-29-72)63(98)73-34-52(94)78-55(39(6)86)65(100)75-46(25-28-71)70(105)106-45/h21-24,36-41,44-50,53-59,85-92H,9-20,25-35,71-72H2,1-8H3,(H,73,98)(H,74,99)(H,75,100)(H,76,95)(H,77,93)(H,78,94)(H,79,96)(H,80,97)(H,81,101)(H,82,102)/t37-,38-,39-,40-,41-,44-,45-,46+,47+,48+,49-,50+,53+,54+,55+,56+,57+,58+,59+/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of 1,3-beta-D-glucan synthase (GS) from candida albicans |
Bioorg Med Chem Lett 11: 395-8 (2001)
BindingDB Entry DOI: 10.7270/Q28051W2 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50479854
(CHEMBL453394)Show SMILES Cn1cncc1C(O)(c1cc2cc(cc(-c3cccc(F)c3)c2o1)[N+]([O-])=O)c1ccc(cc1)C#N Show InChI InChI=1S/C26H17FN4O4/c1-30-15-29-14-23(30)26(32,19-7-5-16(13-28)6-8-19)24-11-18-10-21(31(33)34)12-22(25(18)35-24)17-3-2-4-20(27)9-17/h2-12,14-15,32H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human FTase |
Bioorg Med Chem Lett 19: 1753-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.074
BindingDB Entry DOI: 10.7270/Q28G8PGH |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50479837
(CHEMBL516034)Show SMILES Cn1cncc1C(O)(c1cc2cc(cc(-c3ccccc3)c2o1)[N+]([O-])=O)c1ccc(cc1)C#N Show InChI InChI=1S/C26H18N4O4/c1-29-16-28-15-23(29)26(31,20-9-7-17(14-27)8-10-20)24-12-19-11-21(30(32)33)13-22(25(19)34-24)18-5-3-2-4-6-18/h2-13,15-16,31H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human FTase |
Bioorg Med Chem Lett 19: 1753-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.074
BindingDB Entry DOI: 10.7270/Q28G8PGH |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50479838
(CHEMBL513211)Show SMILES Cn1cncc1C(O)(c1cc2cc(cc(-c3ccccc3)c2o1)C(=O)NN1CCOCC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C30H27ClN4O4/c1-34-19-32-18-26(34)30(37,23-7-9-24(31)10-8-23)27-17-21-15-22(29(36)33-35-11-13-38-14-12-35)16-25(28(21)39-27)20-5-3-2-4-6-20/h2-10,15-19,37H,11-14H2,1H3,(H,33,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human FTase |
Bioorg Med Chem Lett 19: 1753-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.074
BindingDB Entry DOI: 10.7270/Q28G8PGH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50479839
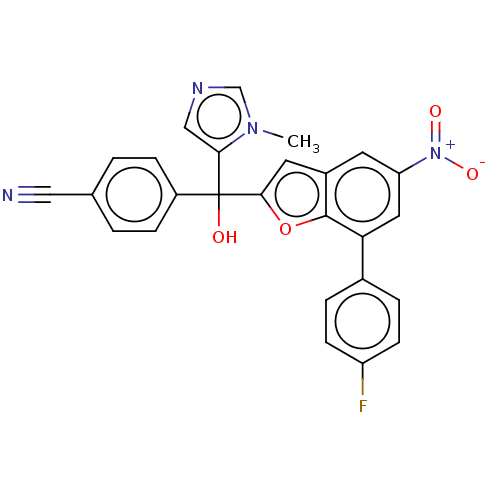
(CHEMBL517280)Show SMILES Cn1cncc1C(O)(c1cc2cc(cc(-c3ccc(F)cc3)c2o1)[N+]([O-])=O)c1ccc(cc1)C#N Show InChI InChI=1S/C26H17FN4O4/c1-30-15-29-14-23(30)26(32,19-6-2-16(13-28)3-7-19)24-11-18-10-21(31(33)34)12-22(25(18)35-24)17-4-8-20(27)9-5-17/h2-12,14-15,32H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human FTase |
Bioorg Med Chem Lett 19: 1753-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.074
BindingDB Entry DOI: 10.7270/Q28G8PGH |
More data for this
Ligand-Target Pair | |
1,3-beta-glucan synthase
(Candida albicans (Yeast)) | BDBM50096799
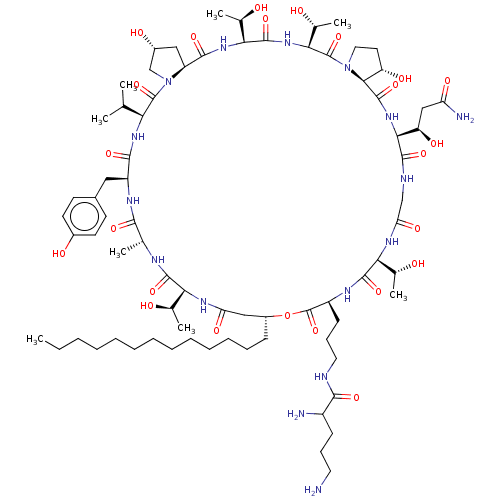
(CHEMBL2369134 | macrocyclic lipopeptidolactone der...)Show SMILES [H][C@@]12C[C@@H](O)CN1C(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](C)NC(=O)[C@@]([H])(NC(=O)C[C@@H](CCCCCCCCCCCCC)OC(=O)[C@H](CCCNC(=O)C(N)CCCN)NC(=O)[C@@]([H])(NC(=O)CNC(=O)[C@@]([H])(NC(=O)[C@]1([H])[C@@H](O)CCN1C(=O)[C@@]([H])(NC(=O)[C@@]([H])(NC2=O)[C@@H](C)O)[C@@H](C)O)[C@H](O)CC(N)=O)[C@@H](C)O)[C@@H](C)O)C(C)C Show InChI InChI=1S/C76H126N16O24/c1-9-10-11-12-13-14-15-16-17-18-19-22-48-35-56(102)85-59(41(5)93)70(109)82-40(4)65(104)84-51(33-45-25-27-46(97)28-26-45)67(106)87-58(39(2)3)74(113)92-38-47(98)34-52(92)68(107)88-61(43(7)95)72(111)89-62(44(8)96)75(114)91-32-29-53(99)64(91)73(112)90-63(54(100)36-55(79)101)69(108)81-37-57(103)86-60(42(6)94)71(110)83-50(76(115)116-48)24-21-31-80-66(105)49(78)23-20-30-77/h25-28,39-44,47-54,58-64,93-100H,9-24,29-38,77-78H2,1-8H3,(H2,79,101)(H,80,105)(H,81,108)(H,82,109)(H,83,110)(H,84,104)(H,85,102)(H,86,103)(H,87,106)(H,88,107)(H,89,111)(H,90,112)/t40-,41-,42-,43-,44-,47-,48-,49?,50+,51+,52+,53+,54-,58+,59+,60+,61+,62+,63+,64+/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of 1,3-beta-D-glucan synthase (GS) from candida albicans |
Bioorg Med Chem Lett 11: 395-8 (2001)
BindingDB Entry DOI: 10.7270/Q28051W2 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50479861
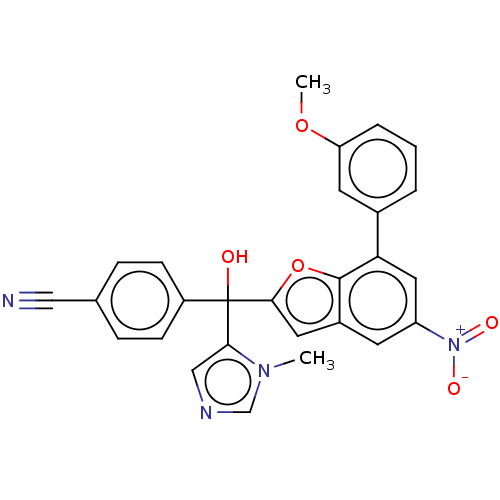
(CHEMBL473151)Show SMILES COc1cccc(c1)-c1cc(cc2cc(oc12)C(O)(c1cncn1C)c1ccc(cc1)C#N)[N+]([O-])=O Show InChI InChI=1S/C27H20N4O5/c1-30-16-29-15-24(30)27(32,20-8-6-17(14-28)7-9-20)25-12-19-10-21(31(33)34)13-23(26(19)36-25)18-4-3-5-22(11-18)35-2/h3-13,15-16,32H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human FTase |
Bioorg Med Chem Lett 19: 1753-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.074
BindingDB Entry DOI: 10.7270/Q28G8PGH |
More data for this
Ligand-Target Pair | |
1,3-beta-glucan synthase
(Candida albicans (Yeast)) | BDBM50100339
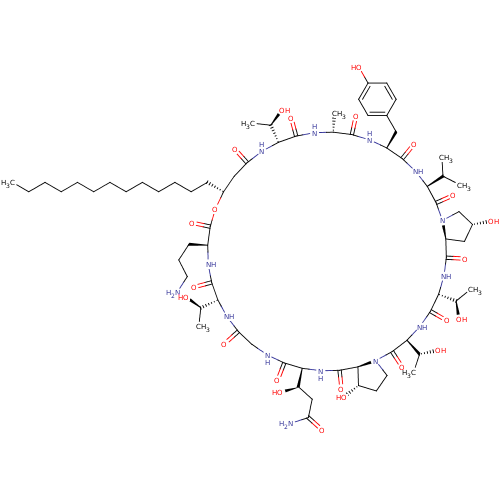
(CHEMBL438198 | FR-901469 | FR901469 | Lipopeptidol...)Show SMILES CCCCCCCCCCCCC[C@@H]1CC(=O)N[C@H]([C@@H](C)O)C(=O)N[C@H](C)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H](C(C)C)C(=O)N2C[C@H](O)C[C@H]2C(=O)N[C@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N2CC[C@H](O)[C@H]2C(=O)N[C@@H]([C@H](O)CC(N)=O)C(=O)NCC(=O)N[C@H]([C@@H](C)O)C(=O)N[C@@H](CCCN)C(=O)O1 Show InChI InChI=1S/C71H116N14O23/c1-9-10-11-12-13-14-15-16-17-18-19-21-45-32-52(95)78-55(38(5)86)65(101)75-37(4)61(97)77-47(30-42-23-25-43(90)26-24-42)62(98)80-54(36(2)3)69(105)85-35-44(91)31-48(85)63(99)81-57(40(7)88)67(103)82-58(41(8)89)70(106)84-29-27-49(92)60(84)68(104)83-59(50(93)33-51(73)94)64(100)74-34-53(96)79-56(39(6)87)66(102)76-46(22-20-28-72)71(107)108-45/h23-26,36-41,44-50,54-60,86-93H,9-22,27-35,72H2,1-8H3,(H2,73,94)(H,74,100)(H,75,101)(H,76,102)(H,77,97)(H,78,95)(H,79,96)(H,80,98)(H,81,99)(H,82,103)(H,83,104)/t37-,38-,39-,40-,41-,44-,45-,46+,47+,48+,49+,50-,54+,55-,56-,57-,58+,59+,60+/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Beta glucan synthase |
Bioorg Med Chem Lett 11: 1273-6 (2001)
BindingDB Entry DOI: 10.7270/Q2CN74FQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50338198

(3-(2-morpholino-7-(pyridin-4-yl)-6,7-dihydro-5H-py...)Show SMILES Oc1cccc(c1)-c1nc(nc2N(CCc12)c1ccncc1)N1CCOCC1 Show InChI InChI=1S/C21H21N5O2/c27-17-3-1-2-15(14-17)19-18-6-9-26(16-4-7-22-8-5-16)20(18)24-21(23-19)25-10-12-28-13-11-25/h1-5,7-8,14,27H,6,9-13H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 21: 1767-72 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.065
BindingDB Entry DOI: 10.7270/Q23B60F3 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50479852
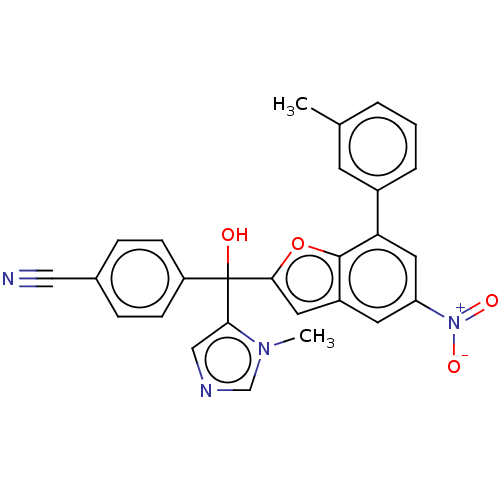
(CHEMBL473149)Show SMILES Cc1cccc(c1)-c1cc(cc2cc(oc12)C(O)(c1cncn1C)c1ccc(cc1)C#N)[N+]([O-])=O Show InChI InChI=1S/C27H20N4O4/c1-17-4-3-5-19(10-17)23-13-22(31(33)34)11-20-12-25(35-26(20)23)27(32,24-15-29-16-30(24)2)21-8-6-18(14-28)7-9-21/h3-13,15-16,32H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human FTase |
Bioorg Med Chem Lett 19: 1753-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.074
BindingDB Entry DOI: 10.7270/Q28G8PGH |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50479840
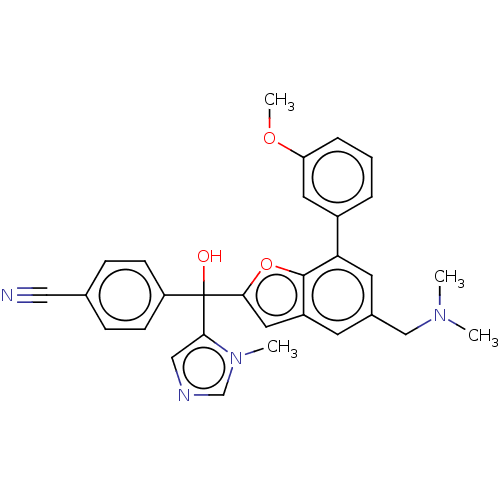
(CHEMBL475148)Show SMILES COc1cccc(c1)-c1cc(CN(C)C)cc2cc(oc12)C(O)(c1cncn1C)c1ccc(cc1)C#N Show InChI InChI=1S/C30H28N4O3/c1-33(2)18-21-12-23-15-28(37-29(23)26(13-21)22-6-5-7-25(14-22)36-4)30(35,27-17-32-19-34(27)3)24-10-8-20(16-31)9-11-24/h5-15,17,19,35H,18H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human FTase |
Bioorg Med Chem Lett 19: 1753-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.074
BindingDB Entry DOI: 10.7270/Q28G8PGH |
More data for this
Ligand-Target Pair | |
1,3-beta-glucan synthase
(Candida albicans (Yeast)) | BDBM50096798
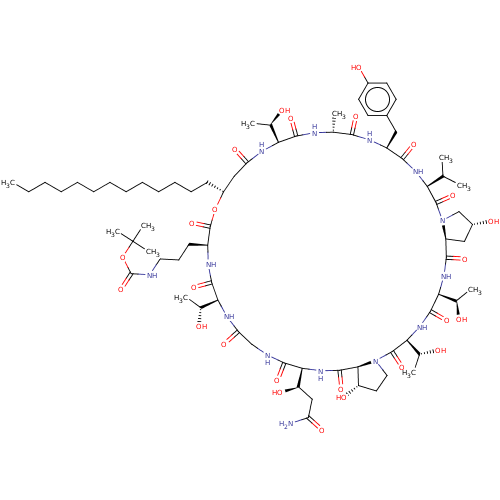
(CHEMBL2370655 | macrocyclic lipopeptidolactone der...)Show SMILES [H][C@@]12C[C@@H](O)CN1C(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](C)NC(=O)[C@@]([H])(NC(=O)C[C@@H](CCCCCCCCCCCCC)OC(=O)[C@H](CCCNC(=O)OC(C)(C)C)NC(=O)[C@@]([H])(NC(=O)CNC(=O)[C@@]([H])(NC(=O)[C@]1([H])[C@@H](O)CCN1C(=O)[C@@]([H])(NC(=O)[C@@]([H])(NC2=O)[C@@H](C)O)[C@@H](C)O)[C@H](O)CC(N)=O)[C@@H](C)O)[C@@H](C)O)C(C)C Show InChI InChI=1S/C76H124N14O25/c1-12-13-14-15-16-17-18-19-20-21-22-24-48-35-55(100)83-58(41(5)91)68(106)80-40(4)64(102)82-50(33-45-26-28-46(95)29-27-45)65(103)85-57(39(2)3)72(110)90-38-47(96)34-51(90)66(104)86-60(43(7)93)70(108)87-61(44(8)94)73(111)89-32-30-52(97)63(89)71(109)88-62(53(98)36-54(77)99)67(105)79-37-56(101)84-59(42(6)92)69(107)81-49(74(112)114-48)25-23-31-78-75(113)115-76(9,10)11/h26-29,39-44,47-53,57-63,91-98H,12-25,30-38H2,1-11H3,(H2,77,99)(H,78,113)(H,79,105)(H,80,106)(H,81,107)(H,82,102)(H,83,100)(H,84,101)(H,85,103)(H,86,104)(H,87,108)(H,88,109)/t40-,41-,42-,43-,44-,47-,48-,49+,50+,51+,52+,53-,57+,58+,59+,60+,61+,62+,63+/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of 1,3-beta-D-glucan synthase (GS) from candida albicans |
Bioorg Med Chem Lett 11: 395-8 (2001)
BindingDB Entry DOI: 10.7270/Q28051W2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50338197
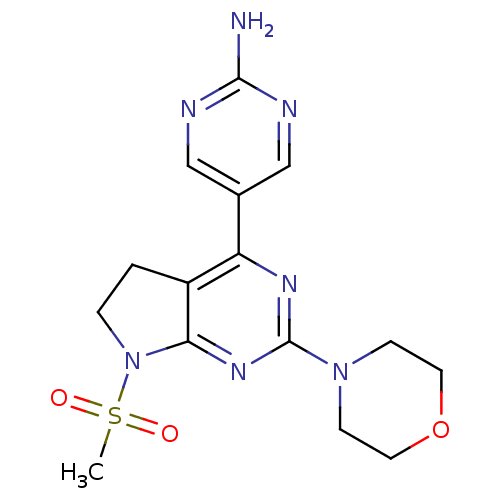
(5-(7-(methylsulfonyl)-2-morpholino-6,7-dihydro-5H-...)Show SMILES CS(=O)(=O)N1CCc2c1nc(nc2-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C15H19N7O3S/c1-26(23,24)22-3-2-11-12(10-8-17-14(16)18-9-10)19-15(20-13(11)22)21-4-6-25-7-5-21/h8-9H,2-7H2,1H3,(H2,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 21: 1767-72 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.065
BindingDB Entry DOI: 10.7270/Q23B60F3 |
More data for this
Ligand-Target Pair | |
1,3-beta-glucan synthase
(Candida albicans (Yeast)) | BDBM50100330
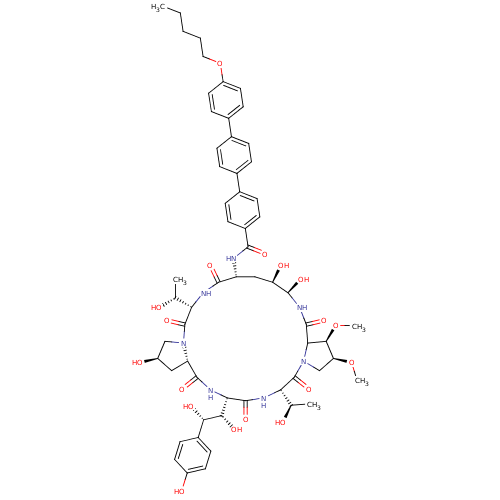
(CHEMBL413199 | LY-303366 | LY-303366 derivative)Show SMILES CCCCCOc1ccc(cc1)-c1ccc(cc1)-c1ccc(cc1)C(=O)N[C@@H]1C[C@@H](O)[C@@H](O)NC(=O)C2[C@@H](OC)[C@H](CN2C(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@@H]2C[C@@H](O)CN2C(=O)[C@@H](NC1=O)[C@@H](C)O)[C@H](O)[C@@H](O)c1ccc(O)cc1)[C@@H](C)O)OC Show InChI InChI=1S/C59H75N7O18/c1-6-7-8-25-84-40-23-19-35(20-24-40)33-11-9-32(10-12-33)34-13-15-37(16-14-34)52(74)60-41-27-43(71)55(77)64-57(79)48-51(83-5)44(82-4)29-66(48)59(81)46(31(3)68)62-56(78)47(50(73)49(72)36-17-21-38(69)22-18-36)63-54(76)42-26-39(70)28-65(42)58(80)45(30(2)67)61-53(41)75/h9-24,30-31,39,41-51,55,67-73,77H,6-8,25-29H2,1-5H3,(H,60,74)(H,61,75)(H,62,78)(H,63,76)(H,64,79)/t30-,31-,39-,41-,42+,43-,44+,45+,46+,47+,48?,49+,50+,51+,55-/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Beta glucan synthase |
Bioorg Med Chem Lett 11: 1273-6 (2001)
BindingDB Entry DOI: 10.7270/Q2CN74FQ |
More data for this
Ligand-Target Pair | |
1,3-beta-glucan synthase
(Candida albicans (Yeast)) | BDBM50451308
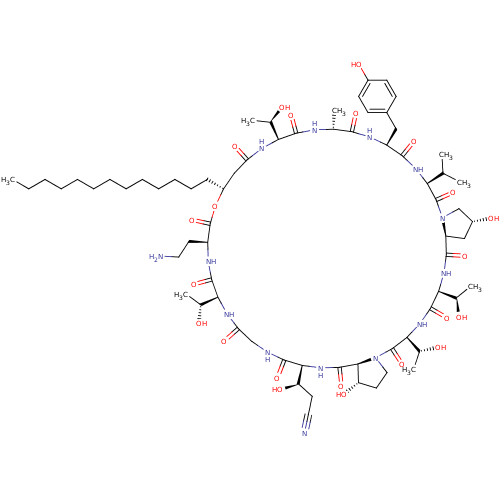
(CHEMBL2370657)Show SMILES [H][C@@]12C[C@@H](O)CN1C(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](C)NC(=O)[C@@]([H])(NC(=O)C[C@@H](CCCCCCCCCCCCC)OC(=O)[C@H](CCN)NC(=O)[C@@]([H])(NC(=O)CNC(=O)[C@@]([H])(NC(=O)[C@]1([H])[C@@H](O)CCN1C(=O)[C@@]([H])(NC(=O)[C@@]([H])(NC2=O)[C@@H](C)O)[C@@H](C)O)[C@H](O)CC#N)[C@@H](C)O)[C@@H](C)O)C(C)C Show InChI InChI=1S/C70H112N14O22/c1-9-10-11-12-13-14-15-16-17-18-19-20-45-33-51(93)77-54(38(5)85)64(99)74-37(4)60(95)76-47(31-42-21-23-43(89)24-22-42)61(96)79-53(36(2)3)68(103)84-35-44(90)32-48(84)62(97)80-56(40(7)87)66(101)81-57(41(8)88)69(104)83-30-27-50(92)59(83)67(102)82-58(49(91)26-29-72)63(98)73-34-52(94)78-55(39(6)86)65(100)75-46(25-28-71)70(105)106-45/h21-24,36-41,44-50,53-59,85-92H,9-20,25-28,30-35,71H2,1-8H3,(H,73,98)(H,74,99)(H,75,100)(H,76,95)(H,77,93)(H,78,94)(H,79,96)(H,80,97)(H,81,101)(H,82,102)/t37-,38-,39-,40-,41-,44-,45-,46+,47+,48+,49-,50+,53+,54+,55+,56+,57+,58+,59+/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of 1,3-beta-D-glucan synthase (GS) from candida albicans |
Bioorg Med Chem Lett 11: 395-8 (2001)
BindingDB Entry DOI: 10.7270/Q28051W2 |
More data for this
Ligand-Target Pair | |
1,3-beta-glucan synthase
(Candida albicans (Yeast)) | BDBM50096800
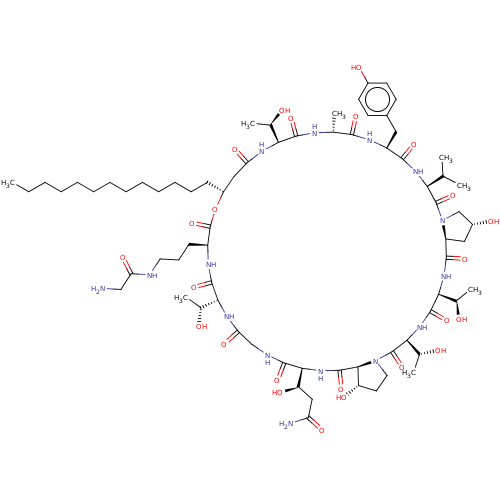
(CHEMBL2370652 | macrocyclic lipopeptidolactone der...)Show SMILES [H][C@@]12C[C@@H](O)CN1C(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](C)NC(=O)[C@@]([H])(NC(=O)C[C@@H](CCCCCCCCCCCCC)OC(=O)[C@H](CCCNC(=O)CN)NC(=O)[C@]([H])(NC(=O)CNC(=O)[C@@]([H])(NC(=O)[C@]1([H])[C@@H](O)CCN1C(=O)[C@@]([H])(NC(=O)[C@@]([H])(NC2=O)[C@@H](C)O)[C@@H](C)O)[C@H](O)CC(N)=O)[C@@H](C)O)[C@@H](C)O)C(C)C Show InChI InChI=1S/C73H119N15O24/c1-9-10-11-12-13-14-15-16-17-18-19-21-46-32-53(98)81-57(39(5)89)67(105)78-38(4)63(101)80-48(30-43-23-25-44(93)26-24-43)64(102)83-56(37(2)3)71(109)88-36-45(94)31-49(88)65(103)84-59(41(7)91)69(107)85-60(42(8)92)72(110)87-29-27-50(95)62(87)70(108)86-61(51(96)33-52(75)97)66(104)77-35-55(100)82-58(40(6)90)68(106)79-47(73(111)112-46)22-20-28-76-54(99)34-74/h23-26,37-42,45-51,56-62,89-96H,9-22,27-36,74H2,1-8H3,(H2,75,97)(H,76,99)(H,77,104)(H,78,105)(H,79,106)(H,80,101)(H,81,98)(H,82,100)(H,83,102)(H,84,103)(H,85,107)(H,86,108)/t38-,39-,40-,41-,42-,45-,46-,47+,48+,49+,50+,51-,56+,57+,58-,59+,60+,61+,62+/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of 1,3-beta-D-glucan synthase (GS) from candida albicans |
Bioorg Med Chem Lett 11: 395-8 (2001)
BindingDB Entry DOI: 10.7270/Q28051W2 |
More data for this
Ligand-Target Pair | |
1,3-beta-glucan synthase
(Candida albicans (Yeast)) | BDBM50096794

(CHEMBL2370668 | macrocyclic lipopeptidolactone der...)Show SMILES [H][C@@]12C[C@@H](O)CN1C(=O)[C@@H](NC(=O)[C@H](Cc1ccc(OC)cc1)NC(=O)[C@@H](C)NC(=O)[C@@]([H])(NC(=O)C[C@@H](CCCCCCCCCCCCC)OC(=O)[C@H](CCCN)NC(=O)[C@@]([H])(NC(=O)CNC(=O)[C@@]([H])(NC(=O)[C@]1([H])[C@@H](O)CCN1C(=O)[C@@]([H])(NC(=O)[C@@]([H])(NC2=O)[C@@H](C)O)[C@@H](C)O)[C@H](O)CC(N)=O)[C@@H](C)O)[C@@H](C)O)C(C)C Show InChI InChI=1S/C72H118N14O23/c1-10-11-12-13-14-15-16-17-18-19-20-22-46-33-53(95)79-56(39(5)87)66(101)76-38(4)62(97)78-48(31-43-24-26-45(108-9)27-25-43)63(98)81-55(37(2)3)70(105)86-36-44(91)32-49(86)64(99)82-58(41(7)89)68(103)83-59(42(8)90)71(106)85-30-28-50(92)61(85)69(104)84-60(51(93)34-52(74)94)65(100)75-35-54(96)80-57(40(6)88)67(102)77-47(23-21-29-73)72(107)109-46/h24-27,37-42,44,46-51,55-61,87-93H,10-23,28-36,73H2,1-9H3,(H2,74,94)(H,75,100)(H,76,101)(H,77,102)(H,78,97)(H,79,95)(H,80,96)(H,81,98)(H,82,99)(H,83,103)(H,84,104)/t38-,39-,40-,41-,42-,44-,46-,47+,48+,49+,50+,51-,55+,56+,57+,58+,59+,60+,61+/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of 1,3-beta-D-glucan synthase (GS) from candida albicans |
Bioorg Med Chem Lett 11: 395-8 (2001)
BindingDB Entry DOI: 10.7270/Q28051W2 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50479841
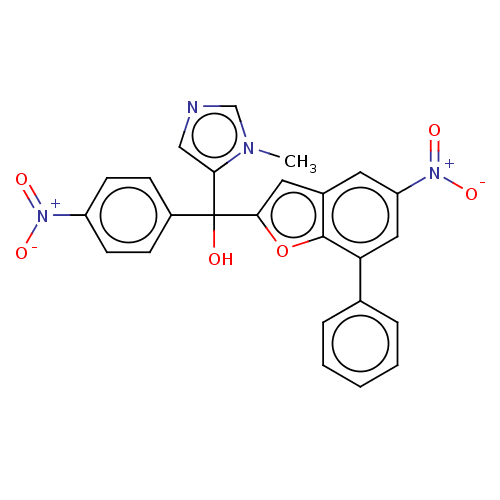
(CHEMBL516185)Show SMILES Cn1cncc1C(O)(c1cc2cc(cc(-c3ccccc3)c2o1)[N+]([O-])=O)c1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C25H18N4O6/c1-27-15-26-14-22(27)25(30,18-7-9-19(10-8-18)28(31)32)23-12-17-11-20(29(33)34)13-21(24(17)35-23)16-5-3-2-4-6-16/h2-15,30H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human FTase |
Bioorg Med Chem Lett 19: 1753-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.074
BindingDB Entry DOI: 10.7270/Q28G8PGH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50338199

(5-(2-Morpholin-4-yl-7-pyridin-3-yl-6,7-dihydro-5H-...)Show SMILES Nc1ncc(cn1)-c1nc(nc2N(CCc12)c1cccnc1)N1CCOCC1 Show InChI InChI=1S/C19H20N8O/c20-18-22-10-13(11-23-18)16-15-3-5-27(14-2-1-4-21-12-14)17(15)25-19(24-16)26-6-8-28-9-7-26/h1-2,4,10-12H,3,5-9H2,(H2,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 21: 1767-72 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.065
BindingDB Entry DOI: 10.7270/Q23B60F3 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50338197

(5-(7-(methylsulfonyl)-2-morpholino-6,7-dihydro-5H-...)Show SMILES CS(=O)(=O)N1CCc2c1nc(nc2-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C15H19N7O3S/c1-26(23,24)22-3-2-11-12(10-8-17-14(16)18-9-10)19-15(20-13(11)22)21-4-6-25-7-5-21/h8-9H,2-7H2,1H3,(H2,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma |
Bioorg Med Chem Lett 21: 1767-72 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.065
BindingDB Entry DOI: 10.7270/Q23B60F3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50338200

(3-(2-morpholino-7-(pyridin-3-yl)-6,7-dihydro-5H-py...)Show SMILES Oc1cccc(c1)-c1nc(nc2N(CCc12)c1cccnc1)N1CCOCC1 Show InChI InChI=1S/C21H21N5O2/c27-17-5-1-3-15(13-17)19-18-6-8-26(16-4-2-7-22-14-16)20(18)24-21(23-19)25-9-11-28-12-10-25/h1-5,7,13-14,27H,6,8-12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 21: 1767-72 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.065
BindingDB Entry DOI: 10.7270/Q23B60F3 |
More data for this
Ligand-Target Pair | |
1,3-beta-glucan synthase
(Candida albicans (Yeast)) | BDBM50096803
(CHEMBL2370664 | macrocyclic lipopeptidolactone der...)Show SMILES [H][C@@]12C[C@@H](O)CN1C(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](C)NC(=O)[C@@]([H])(NC(=O)C[C@@H](CCCCCCCCCCCCC)OC(=O)[C@H](CCCN(C)C)NC(=O)[C@@]([H])(NC(=O)CNC(=O)[C@@]([H])(NC(=O)[C@]1([H])[C@@H](O)CCN1C(=O)[C@@]([H])(NC(=O)[C@@]([H])(NC2=O)[C@@H](C)O)[C@@H](C)O)[C@H](O)CC(N)=O)[C@@H](C)O)[C@@H](C)O)C(C)C Show InChI InChI=1S/C73H120N14O23/c1-11-12-13-14-15-16-17-18-19-20-21-23-47-34-54(97)79-57(40(5)88)67(103)76-39(4)63(99)78-49(32-44-25-27-45(92)28-26-44)64(100)81-56(38(2)3)71(107)87-37-46(93)33-50(87)65(101)82-59(42(7)90)69(105)83-60(43(8)91)72(108)86-31-29-51(94)62(86)70(106)84-61(52(95)35-53(74)96)66(102)75-36-55(98)80-58(41(6)89)68(104)77-48(73(109)110-47)24-22-30-85(9)10/h25-28,38-43,46-52,56-62,88-95H,11-24,29-37H2,1-10H3,(H2,74,96)(H,75,102)(H,76,103)(H,77,104)(H,78,99)(H,79,97)(H,80,98)(H,81,100)(H,82,101)(H,83,105)(H,84,106)/t39-,40-,41-,42-,43-,46-,47-,48+,49+,50+,51+,52-,56+,57+,58+,59+,60+,61+,62+/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of 1,3-beta-D-glucan synthase (GS) from candida albicans |
Bioorg Med Chem Lett 11: 395-8 (2001)
BindingDB Entry DOI: 10.7270/Q28051W2 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50479862
(CHEMBL489122)Show SMILES Cn1cncc1[C@@](N)(c1cc2cc(cc(-c3ccccc3)c2o1)[N+]([O-])=O)c1ccc(cc1)C#N |r| Show InChI InChI=1S/C26H19N5O3/c1-30-16-29-15-23(30)26(28,20-9-7-17(14-27)8-10-20)24-12-19-11-21(31(32)33)13-22(25(19)34-24)18-5-3-2-4-6-18/h2-13,15-16H,28H2,1H3/t26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human FTase |
Bioorg Med Chem Lett 19: 1753-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.074
BindingDB Entry DOI: 10.7270/Q28G8PGH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data