

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50444550 (CHEMBL3099704) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP11B1 using 11-deoxycortisol as substrate by cell-based assay | ACS Med Chem Lett 4: 1203-7 (2013) Article DOI: 10.1021/ml400324c BindingDB Entry DOI: 10.7270/Q2NZ8938 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Rattus norvegicus) | BDBM50444550 (CHEMBL3099704) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of rat recombinant CYP11B1 using 11-deoxycortisol as substrate by cell-based assay | ACS Med Chem Lett 4: 1203-7 (2013) Article DOI: 10.1021/ml400324c BindingDB Entry DOI: 10.7270/Q2NZ8938 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50323359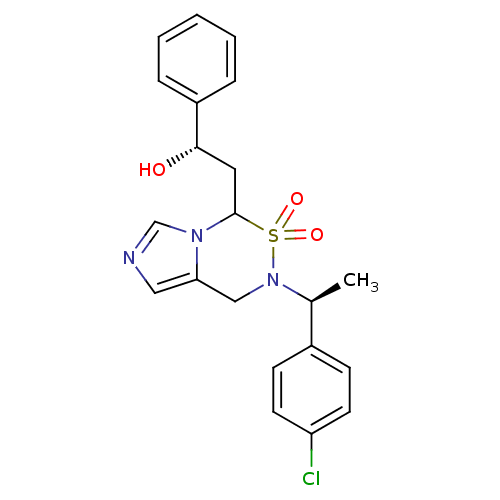 ((S)-2-{6-[(S)-1-(4-Chloro-phenyl)-ethyl]-5,5-dioxo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP11B2 by cell-based assay | Bioorg Med Chem Lett 20: 4324-7 (2010) Article DOI: 10.1016/j.bmcl.2010.06.086 BindingDB Entry DOI: 10.7270/Q2QJ7HGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50444550 (CHEMBL3099704) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP11B2 using 11-deoxycorticosterone as substrate by cell-based assay | ACS Med Chem Lett 4: 1203-7 (2013) Article DOI: 10.1021/ml400324c BindingDB Entry DOI: 10.7270/Q2NZ8938 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50444549 (CHEMBL3099695) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP11B2 using 11-deoxycorticosterone as substrate after 2 hrs by scintillation proximity assay | J Med Chem 58: 9382-94 (2015) Article DOI: 10.1021/acs.jmedchem.5b01545 BindingDB Entry DOI: 10.7270/Q2CC13QV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50444549 (CHEMBL3099695) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP11B2 using 11-deoxycorticosterone as substrate by cell-based assay | ACS Med Chem Lett 4: 1203-7 (2013) Article DOI: 10.1021/ml400324c BindingDB Entry DOI: 10.7270/Q2NZ8938 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50323356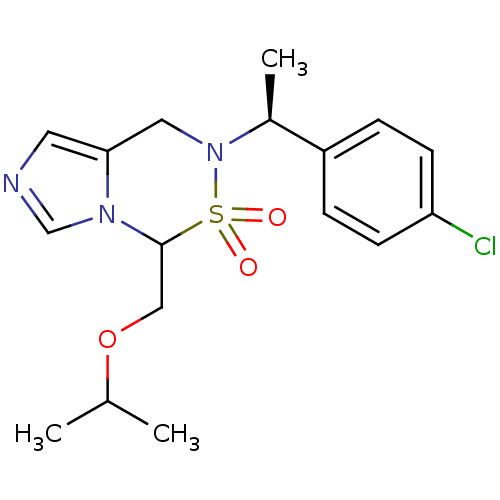 (6-[(S)-1-(4-Chloro-phenyl)-ethyl]-4-isopropoxymeth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP11B2 by cell-based assay | Bioorg Med Chem Lett 20: 4324-7 (2010) Article DOI: 10.1016/j.bmcl.2010.06.086 BindingDB Entry DOI: 10.7270/Q2QJ7HGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50444548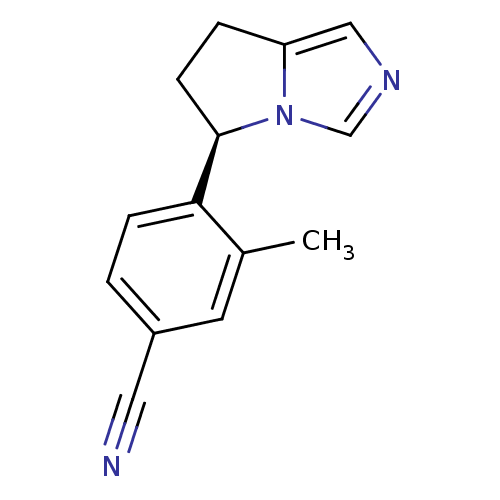 (CHEMBL3099696) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP11B2 using 11-deoxycorticosterone as substrate by cell-based assay | ACS Med Chem Lett 4: 1203-7 (2013) Article DOI: 10.1021/ml400324c BindingDB Entry DOI: 10.7270/Q2NZ8938 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50323355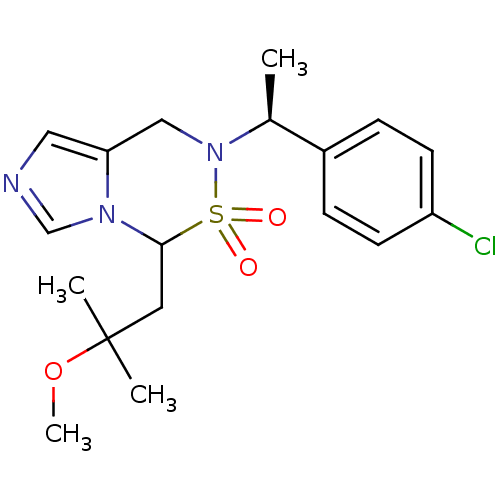 (6-[(S)-1-(4-Chloro-phenyl)-ethyl]-4-(2-methoxy-2-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP11B2 by cell-based assay | Bioorg Med Chem Lett 20: 4324-7 (2010) Article DOI: 10.1016/j.bmcl.2010.06.086 BindingDB Entry DOI: 10.7270/Q2QJ7HGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50444550 (CHEMBL3099704) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of CYP11B2 in human NCI-H295R cells assessed as inhibition of angiotensin-2-induced aldosterone production after 24 hrs by RIA | ACS Med Chem Lett 4: 1203-7 (2013) Article DOI: 10.1021/ml400324c BindingDB Entry DOI: 10.7270/Q2NZ8938 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Rattus norvegicus) | BDBM50444550 (CHEMBL3099704) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of rat recombinant CYP11B2 using 11-deoxycorticosterone as substrate by cell-based assay | ACS Med Chem Lett 4: 1203-7 (2013) Article DOI: 10.1021/ml400324c BindingDB Entry DOI: 10.7270/Q2NZ8938 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase WNK1 [166-489] (Homo sapiens (Human)) | BDBM203827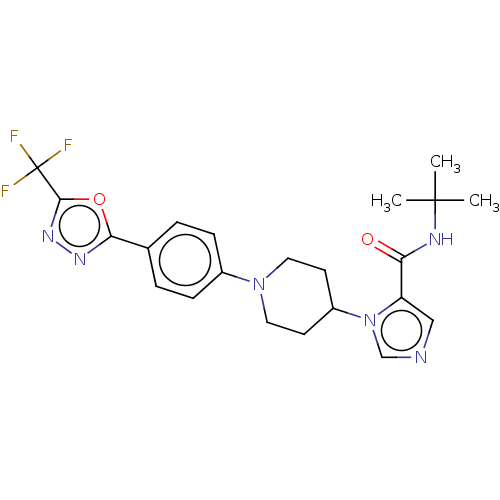 (N-(tert-butyl)-1-(1-(5-(5-(trifluoromethyl)-1,3,4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.3 | 25 |
Novartis Institutes | Assay Description Each well of the MBP-coated ScintiPlates held 100 ul of a solution containing 20 mM HEPES pH 7.3, 5 mM MnCl2 (WNK1 and WNK4) or 3 mM MnCl2 (WNK2 and ... | Nat Chem Biol 12: 896-898 (2016) Article DOI: 10.1038/nchembio.2168 BindingDB Entry DOI: 10.7270/Q2ZK5FH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50500190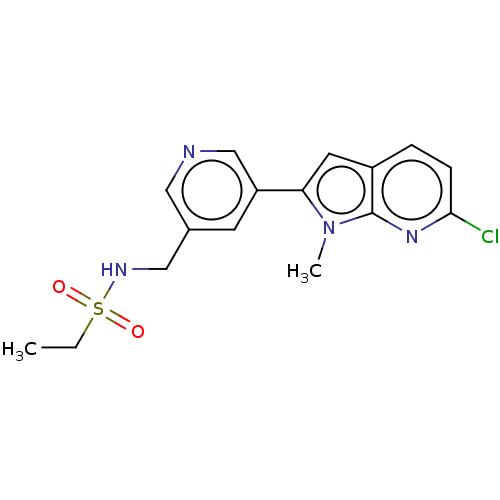 (CHEMBL3746080) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP11B2 using 11-deoxycorticosterone as substrate after 2 hrs by scintillation proximity assay | J Med Chem 58: 9382-94 (2015) Article DOI: 10.1021/acs.jmedchem.5b01545 BindingDB Entry DOI: 10.7270/Q2CC13QV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50323357 (6-[(S)-1-(4-Chloro-phenyl)-ethyl]-4-cyclopropylmet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP11B2 by cell-based assay | Bioorg Med Chem Lett 20: 4324-7 (2010) Article DOI: 10.1016/j.bmcl.2010.06.086 BindingDB Entry DOI: 10.7270/Q2QJ7HGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50500191 (CHEMBL3747584) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP11B2 using 11-deoxycorticosterone as substrate after 2 hrs by scintillation proximity assay | J Med Chem 58: 9382-94 (2015) Article DOI: 10.1021/acs.jmedchem.5b01545 BindingDB Entry DOI: 10.7270/Q2CC13QV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50444552 (CHEMBL3099689) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of CYP11B2 in human NCI-H295R cells assessed as inhibition of angiotensin-2-induced aldosterone production after 24 hrs by RIA | ACS Med Chem Lett 4: 1203-7 (2013) Article DOI: 10.1021/ml400324c BindingDB Entry DOI: 10.7270/Q2NZ8938 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50444548 (CHEMBL3099696) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP11B1 using 11-deoxycortisol as substrate by cell-based assay | ACS Med Chem Lett 4: 1203-7 (2013) Article DOI: 10.1021/ml400324c BindingDB Entry DOI: 10.7270/Q2NZ8938 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50047262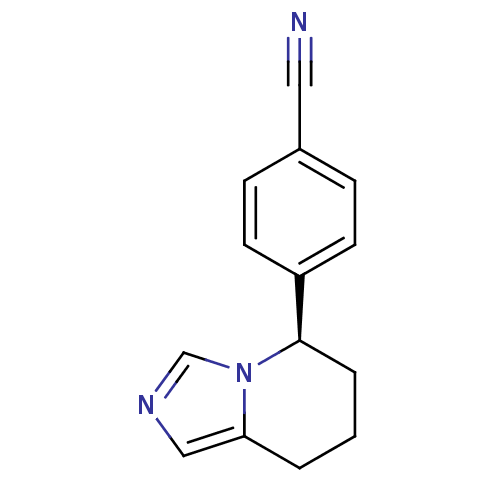 ((R)-4-(5,6,7,8-tetrahydroimidazo[1,5-a]pyridin-5-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP11B2 by cell-based assay | Bioorg Med Chem Lett 20: 4324-7 (2010) Article DOI: 10.1016/j.bmcl.2010.06.086 BindingDB Entry DOI: 10.7270/Q2QJ7HGS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50047262 ((R)-4-(5,6,7,8-tetrahydroimidazo[1,5-a]pyridin-5-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP11B2 using 11-deoxycorticosterone as substrate by cell-based assay | ACS Med Chem Lett 4: 1203-7 (2013) Article DOI: 10.1021/ml400324c BindingDB Entry DOI: 10.7270/Q2NZ8938 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50500189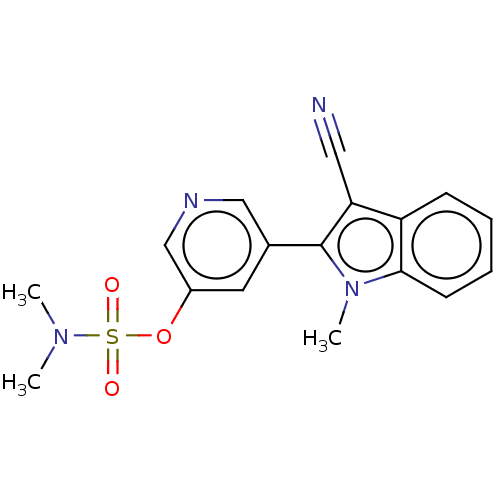 (CHEMBL3746175) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP11B2 using 11-deoxycorticosterone as substrate after 2 hrs by scintillation proximity assay | J Med Chem 58: 9382-94 (2015) Article DOI: 10.1021/acs.jmedchem.5b01545 BindingDB Entry DOI: 10.7270/Q2CC13QV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50323360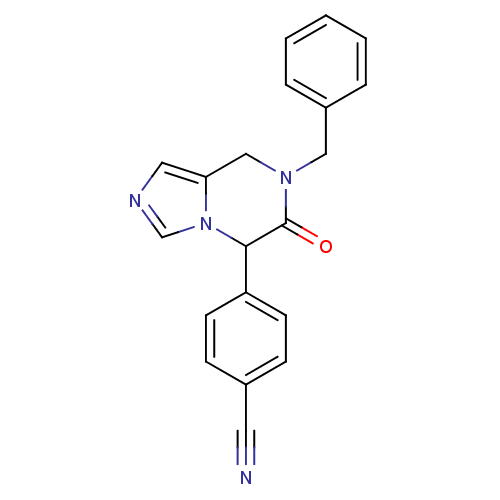 (4-(7-benzyl-6-oxo-5,6,7,8-tetrahydroimidazo[1,5-a]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP11B1 by cell-based assay | Bioorg Med Chem Lett 20: 4324-7 (2010) Article DOI: 10.1016/j.bmcl.2010.06.086 BindingDB Entry DOI: 10.7270/Q2QJ7HGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50444564 (CHEMBL3099682) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of CYP11B2 in human NCI-H295R cells assessed as inhibition of angiotensin-2-induced aldosterone production after 24 hrs by RIA | ACS Med Chem Lett 4: 1203-7 (2013) Article DOI: 10.1021/ml400324c BindingDB Entry DOI: 10.7270/Q2NZ8938 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50444565 (CHEMBL3099604) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of CYP11B2 in human NCI-H295R cells assessed as inhibition of angiotensin-2-induced aldosterone production after 24 hrs by RIA | ACS Med Chem Lett 4: 1203-7 (2013) Article DOI: 10.1021/ml400324c BindingDB Entry DOI: 10.7270/Q2NZ8938 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50323358 ((R)-2-{6-[(S)-1-(4-Chloro-phenyl)-ethyl]-5,5-dioxo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP11B2 by cell-based assay | Bioorg Med Chem Lett 20: 4324-7 (2010) Article DOI: 10.1016/j.bmcl.2010.06.086 BindingDB Entry DOI: 10.7270/Q2QJ7HGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50500187 (CHEMBL3747269) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP11B2 using 11-deoxycorticosterone as substrate after 2 hrs by scintillation proximity assay | J Med Chem 58: 9382-94 (2015) Article DOI: 10.1021/acs.jmedchem.5b01545 BindingDB Entry DOI: 10.7270/Q2CC13QV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50323342 (6-(4-Fluoro-benzyl)-4-isobutyl-6,7-dihydro-5-thia-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP11B2 by cell-based assay | Bioorg Med Chem Lett 20: 4324-7 (2010) Article DOI: 10.1016/j.bmcl.2010.06.086 BindingDB Entry DOI: 10.7270/Q2QJ7HGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50444549 (CHEMBL3099695) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP11B1 using 11-deoxycortisol as substrate by cell-based assay | ACS Med Chem Lett 4: 1203-7 (2013) Article DOI: 10.1021/ml400324c BindingDB Entry DOI: 10.7270/Q2NZ8938 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50444549 (CHEMBL3099695) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition human recombinant CYP11B1 using 11-deoxycorticosterone as substrate after 2 hrs by scintillation proximity assay | J Med Chem 58: 9382-94 (2015) Article DOI: 10.1021/acs.jmedchem.5b01545 BindingDB Entry DOI: 10.7270/Q2CC13QV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50444561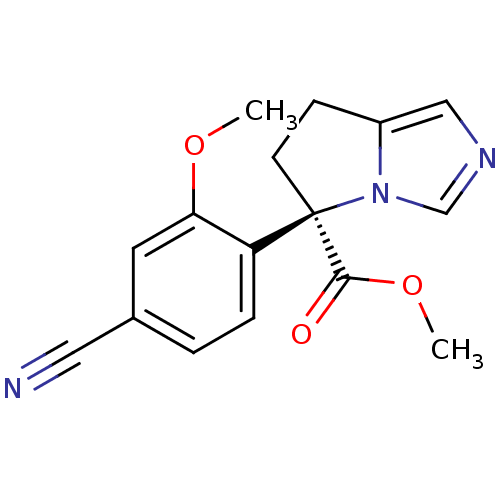 (CHEMBL3099687) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of CYP11B2 in human NCI-H295R cells assessed as inhibition of angiotensin-2-induced aldosterone production after 24 hrs by RIA | ACS Med Chem Lett 4: 1203-7 (2013) Article DOI: 10.1021/ml400324c BindingDB Entry DOI: 10.7270/Q2NZ8938 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50500185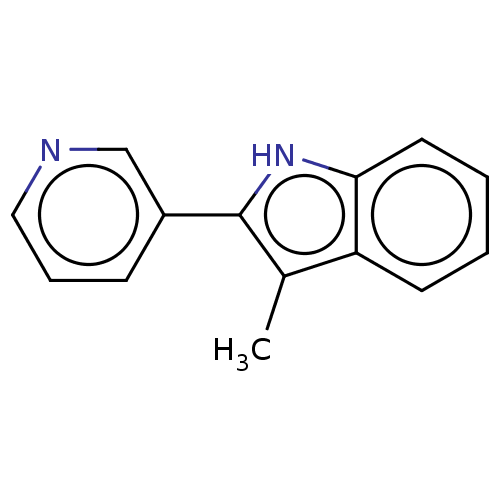 (CHEMBL3746125) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP11B2 using 11-deoxycorticosterone as substrate after 2 hrs by scintillation proximity assay | J Med Chem 58: 9382-94 (2015) Article DOI: 10.1021/acs.jmedchem.5b01545 BindingDB Entry DOI: 10.7270/Q2CC13QV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50444551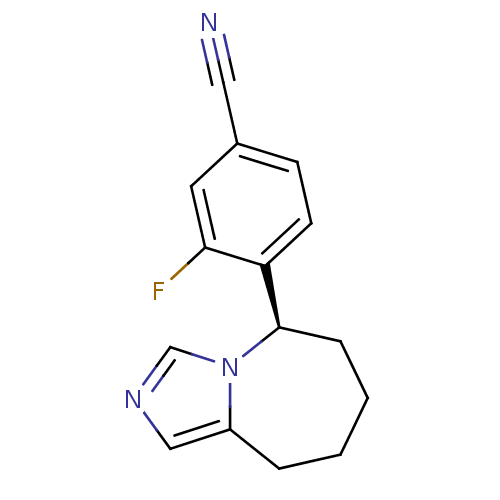 (CHEMBL3099683) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP11B2 using 11-deoxycorticosterone as substrate by cell-based assay | ACS Med Chem Lett 4: 1203-7 (2013) Article DOI: 10.1021/ml400324c BindingDB Entry DOI: 10.7270/Q2NZ8938 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50500188 (CHEMBL3747562) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP11B2 using 11-deoxycorticosterone as substrate after 2 hrs by scintillation proximity assay | J Med Chem 58: 9382-94 (2015) Article DOI: 10.1021/acs.jmedchem.5b01545 BindingDB Entry DOI: 10.7270/Q2CC13QV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50444552 (CHEMBL3099689) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP11B2 using 11-deoxycorticosterone as substrate by cell-based assay | ACS Med Chem Lett 4: 1203-7 (2013) Article DOI: 10.1021/ml400324c BindingDB Entry DOI: 10.7270/Q2NZ8938 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50500187 (CHEMBL3747269) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of CYP11B2 in human H285R cells assessed as decrease in [125I] angiotensin-2-induced aldosterone secretion after 24 hrs by RIA | J Med Chem 58: 9382-94 (2015) Article DOI: 10.1021/acs.jmedchem.5b01545 BindingDB Entry DOI: 10.7270/Q2CC13QV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase WNK1 [1-491] (Homo sapiens (Human)) | BDBM203827 (N-(tert-butyl)-1-(1-(5-(5-(trifluoromethyl)-1,3,4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.3 | 25 |
Novartis Institutes | Assay Description Each well of the MBP-coated ScintiPlates held 100 ul of a solution containing 20 mM HEPES pH 7.3, 5 mM MnCl2 (WNK1 and WNK4) or 3 mM MnCl2 (WNK2 and ... | Nat Chem Biol 12: 896-898 (2016) Article DOI: 10.1038/nchembio.2168 BindingDB Entry DOI: 10.7270/Q2ZK5FH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50444559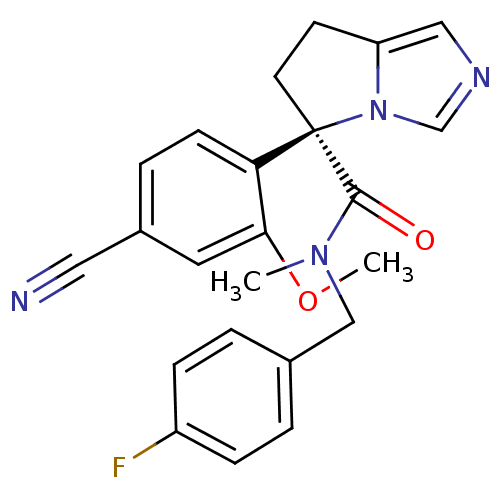 (CHEMBL3099690) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of CYP11B2 in human NCI-H295R cells assessed as inhibition of angiotensin-2-induced aldosterone production after 24 hrs by RIA | ACS Med Chem Lett 4: 1203-7 (2013) Article DOI: 10.1021/ml400324c BindingDB Entry DOI: 10.7270/Q2NZ8938 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50444555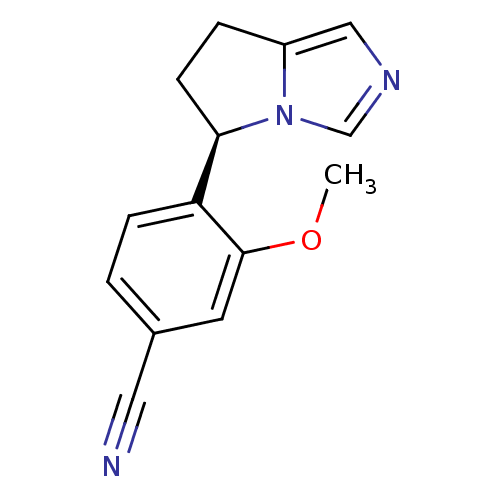 (CHEMBL3099694) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of CYP11B2 in human NCI-H295R cells assessed as inhibition of angiotensin-2-induced aldosterone production after 24 hrs by RIA | ACS Med Chem Lett 4: 1203-7 (2013) Article DOI: 10.1021/ml400324c BindingDB Entry DOI: 10.7270/Q2NZ8938 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50323343 (6-(4-Chloro-benzyl)-4-isobutyl-6,7-dihydro-5-thia-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP11B2 by cell-based assay | Bioorg Med Chem Lett 20: 4324-7 (2010) Article DOI: 10.1016/j.bmcl.2010.06.086 BindingDB Entry DOI: 10.7270/Q2QJ7HGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50500188 (CHEMBL3747562) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of CYP11B2 in human H285R cells assessed as decrease in [125I] angiotensin-2-induced aldosterone secretion after 24 hrs by RIA | J Med Chem 58: 9382-94 (2015) Article DOI: 10.1021/acs.jmedchem.5b01545 BindingDB Entry DOI: 10.7270/Q2CC13QV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase WNK1 [1-434] (Homo sapiens (Human)) | BDBM203827 (N-(tert-butyl)-1-(1-(5-(5-(trifluoromethyl)-1,3,4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.3 | 25 |
Novartis Institutes | Assay Description Each well of the MBP-coated ScintiPlates held 100 ul of a solution containing 20 mM HEPES pH 7.3, 5 mM MnCl2 (WNK1 and WNK4) or 3 mM MnCl2 (WNK2 and ... | Nat Chem Biol 12: 896-898 (2016) Article DOI: 10.1038/nchembio.2168 BindingDB Entry DOI: 10.7270/Q2ZK5FH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50500190 (CHEMBL3746080) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of CYP11B2 in human H285R cells assessed as decrease in [125I] angiotensin-2-induced aldosterone secretion after 24 hrs by RIA | J Med Chem 58: 9382-94 (2015) Article DOI: 10.1021/acs.jmedchem.5b01545 BindingDB Entry DOI: 10.7270/Q2CC13QV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50444551 (CHEMBL3099683) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP11B1 using 11-deoxycortisol as substrate by cell-based assay | ACS Med Chem Lett 4: 1203-7 (2013) Article DOI: 10.1021/ml400324c BindingDB Entry DOI: 10.7270/Q2NZ8938 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50323354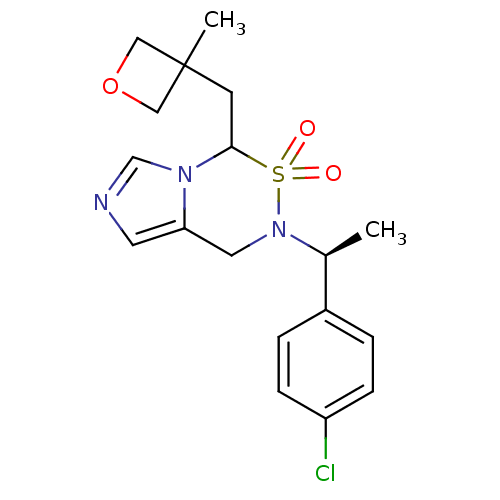 (6-[(S)-1-(4-Chloro-phenyl)-ethyl]-4-(3-methyl-oxet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP11B2 by cell-based assay | Bioorg Med Chem Lett 20: 4324-7 (2010) Article DOI: 10.1016/j.bmcl.2010.06.086 BindingDB Entry DOI: 10.7270/Q2QJ7HGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50444548 (CHEMBL3099696) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of CYP11B2 in human NCI-H295R cells assessed as inhibition of angiotensin-2-induced aldosterone production after 24 hrs by RIA | ACS Med Chem Lett 4: 1203-7 (2013) Article DOI: 10.1021/ml400324c BindingDB Entry DOI: 10.7270/Q2NZ8938 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50444549 (CHEMBL3099695) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of CYP11B2 in human NCI-H295R cells assessed as inhibition of angiotensin-2-induced aldosterone production after 24 hrs by RIA | ACS Med Chem Lett 4: 1203-7 (2013) Article DOI: 10.1021/ml400324c BindingDB Entry DOI: 10.7270/Q2NZ8938 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase WNK1 [1-444] (Homo sapiens (Human)) | BDBM203827 (N-(tert-butyl)-1-(1-(5-(5-(trifluoromethyl)-1,3,4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.3 | 25 |
Novartis Institutes | Assay Description Each well of the MBP-coated ScintiPlates held 100 ul of a solution containing 20 mM HEPES pH 7.3, 5 mM MnCl2 (WNK1 and WNK4) or 3 mM MnCl2 (WNK2 and ... | Nat Chem Biol 12: 896-898 (2016) Article DOI: 10.1038/nchembio.2168 BindingDB Entry DOI: 10.7270/Q2ZK5FH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50500191 (CHEMBL3747584) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of CYP11B2 in human H285R cells assessed as decrease in [125I] angiotensin-2-induced aldosterone secretion after 24 hrs by RIA | J Med Chem 58: 9382-94 (2015) Article DOI: 10.1021/acs.jmedchem.5b01545 BindingDB Entry DOI: 10.7270/Q2CC13QV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50323351 (6-[(S)-1-(4-Chloro-phenyl)-ethyl]-4-isobutyl-6,7-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP11B2 by cell-based assay | Bioorg Med Chem Lett 20: 4324-7 (2010) Article DOI: 10.1016/j.bmcl.2010.06.086 BindingDB Entry DOI: 10.7270/Q2QJ7HGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50047262 ((R)-4-(5,6,7,8-tetrahydroimidazo[1,5-a]pyridin-5-y...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP11B1 using 11-deoxycortisol as substrate by cell-based assay | ACS Med Chem Lett 4: 1203-7 (2013) Article DOI: 10.1021/ml400324c BindingDB Entry DOI: 10.7270/Q2NZ8938 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50047262 ((R)-4-(5,6,7,8-tetrahydroimidazo[1,5-a]pyridin-5-y...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP11B1 by cell-based assay | Bioorg Med Chem Lett 20: 4324-7 (2010) Article DOI: 10.1016/j.bmcl.2010.06.086 BindingDB Entry DOI: 10.7270/Q2QJ7HGS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 175 total ) | Next | Last >> |