

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50527134 (CHEMBL4471306 | US20230295213, Compound a) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive reversible inhibition of human C-terminal His6-tagged CD73 expressed in HEK293 cells using AMP as substrate preincubated with substrate f... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00525 BindingDB Entry DOI: 10.7270/Q29W0K29 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium vivax (malaria parasite P. vivax)) | BDBM18788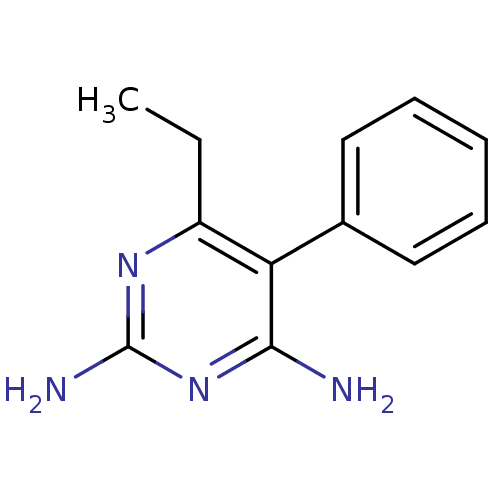 (6-ethyl-5-phenylpyrimidine-2,4-diamine | CHEMBL221...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0300 | -60.1 | 1.82E+3 | n/a | n/a | n/a | n/a | 7.0 | 25 |
National Center for Genetic Engineering and Biotechnology at Thailand | Assay Description Nineteen Pyr analogs were studied for their inhibition activity against cells expressing either WT or SP21 mutant PvDHFR-TS. The assays were conducte... | Antimicrob Agents Chemother 50: 3631-7 (2006) Article DOI: 10.1128/AAC.00448-06 BindingDB Entry DOI: 10.7270/Q2N8781P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase [1-238,S58R,S117N] (Plasmodium vivax (malaria parasite P. vivax)) | BDBM18788 (6-ethyl-5-phenylpyrimidine-2,4-diamine | CHEMBL221...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0800 | n/a | 1.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology at Thailand | Assay Description Nineteen Pyr analogs were studied for their inhibition activity against cells expressing either WT or SP21 mutant PvDHFR-TS. The assays were conducte... | Antimicrob Agents Chemother 50: 3631-7 (2006) Article DOI: 10.1128/AAC.00448-06 BindingDB Entry DOI: 10.7270/Q2N8781P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50054974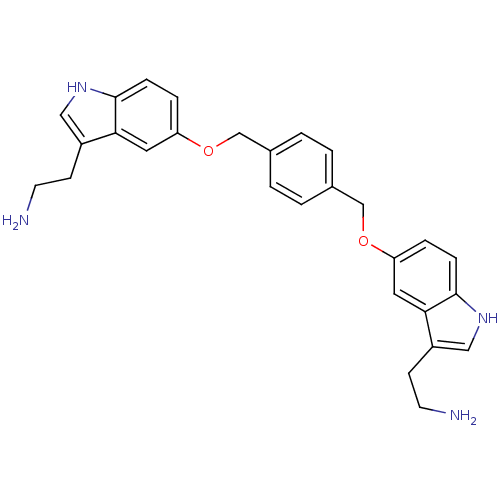 (2-(5-((4-((3-(2-aminoethyl)-1H-indol-5-yloxy)methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-hydroxytryptamine from human cloned 5HT1B receptor expressed in CHOK1 cells by liquid scintillation counting | J Med Chem 51: 3609-16 (2008) Article DOI: 10.1021/jm7011722 BindingDB Entry DOI: 10.7270/Q26T0MDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50271131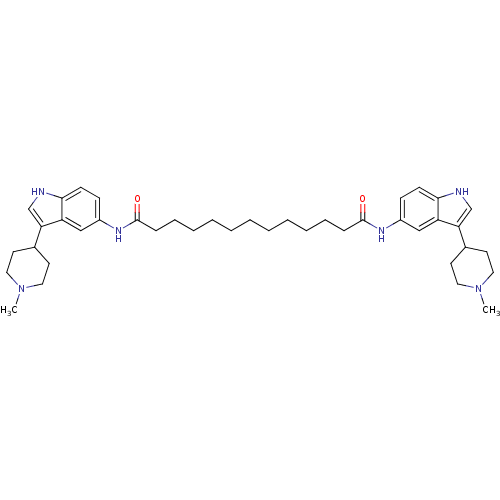 (CHEMBL374973 | N1,N13-bis(3-(1-methylpiperidin-4-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-hydroxytryptamine from human cloned 5HT1B receptor expressed in CHOK1 cells by liquid scintillation counting | J Med Chem 51: 3609-16 (2008) Article DOI: 10.1021/jm7011722 BindingDB Entry DOI: 10.7270/Q26T0MDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50200182 ((1S,3R,5Z,7E)-9,10-secocholesta-5,7,10(19)-triene-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]-1alpha25-(OH)2D3 from full length recombinant rat vitamin D receptor by scintillation counting analysis | J Med Chem 58: 6237-47 (2015) Article DOI: 10.1021/acs.jmedchem.5b00795 BindingDB Entry DOI: 10.7270/Q2H133S9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50054974 (2-(5-((4-((3-(2-aminoethyl)-1H-indol-5-yloxy)methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-hydroxytryptamine from human cloned 5HT1D receptor expressed in CHOK1 cells by liquid scintillation counting | J Med Chem 51: 3609-16 (2008) Article DOI: 10.1021/jm7011722 BindingDB Entry DOI: 10.7270/Q26T0MDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50104639 (CHEMBL3593364) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]-1alpha25-(OH)2D3 from full length recombinant rat vitamin D receptor by scintillation counting analysis | J Med Chem 58: 6237-47 (2015) Article DOI: 10.1021/acs.jmedchem.5b00795 BindingDB Entry DOI: 10.7270/Q2H133S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium vivax (malaria parasite P. vivax)) | BDBM18512 (5-(4-chlorophenyl)-6-ethylpyrimidine-2,4-diamine |...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.210 | -55.2 | 180 | n/a | n/a | n/a | n/a | 7.0 | 25 |
National Center for Genetic Engineering and Biotechnology at Thailand | Assay Description Nineteen Pyr analogs were studied for their inhibition activity against cells expressing either WT or SP21 mutant PvDHFR-TS. The assays were conducte... | Antimicrob Agents Chemother 50: 3631-7 (2006) Article DOI: 10.1128/AAC.00448-06 BindingDB Entry DOI: 10.7270/Q2N8781P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50271019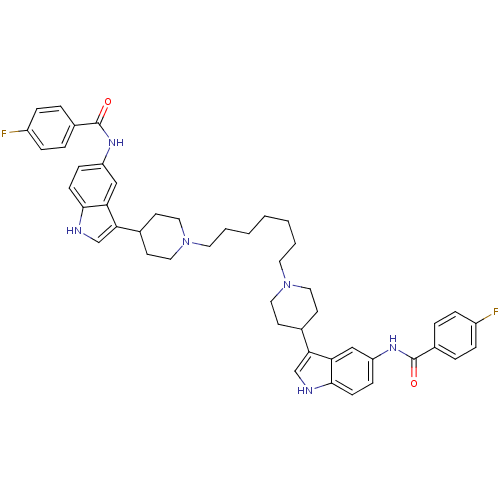 (4-fluoro-N-(3-{1-[7-(4-{5-[(4-fluorobenzene)amido]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-hydroxytryptamine from human cloned 5HT1D receptor expressed in CHOK1 cells by liquid scintillation counting | J Med Chem 51: 3609-16 (2008) Article DOI: 10.1021/jm7011722 BindingDB Entry DOI: 10.7270/Q26T0MDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50142916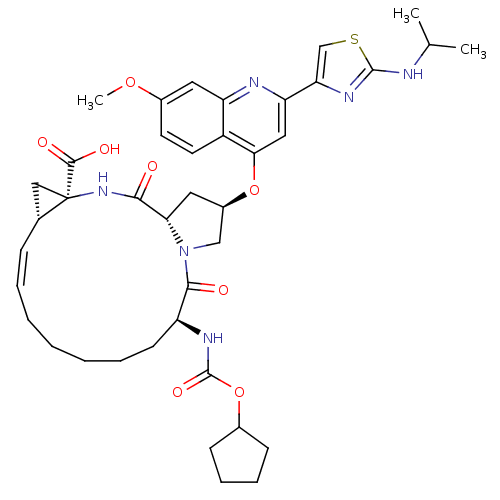 ((1S,4R,6S,14S,18R)-14-Cyclopentyloxycarbonylamino-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HCV 1a NS3 protease | Bioorg Med Chem 15: 1448-74 (2007) Article DOI: 10.1016/j.bmc.2006.11.003 BindingDB Entry DOI: 10.7270/Q22B8ZVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium vivax (malaria parasite P. vivax)) | BDBM18784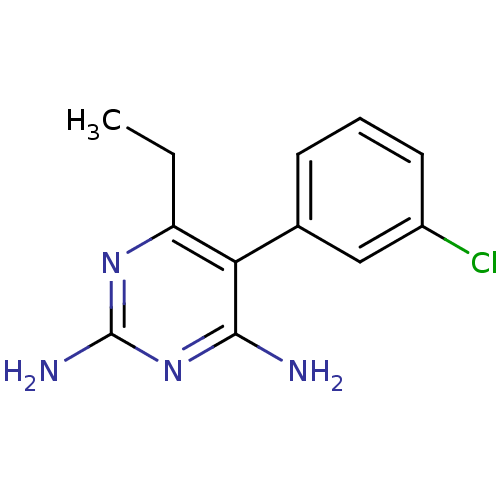 (5-(3-chlorophenyl)-6-ethylpyrimidine-2,4-diamine |...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.310 | -54.3 | 360 | n/a | n/a | n/a | n/a | 7.0 | 25 |
National Center for Genetic Engineering and Biotechnology at Thailand | Assay Description Nineteen Pyr analogs were studied for their inhibition activity against cells expressing either WT or SP21 mutant PvDHFR-TS. The assays were conducte... | Antimicrob Agents Chemother 50: 3631-7 (2006) Article DOI: 10.1128/AAC.00448-06 BindingDB Entry DOI: 10.7270/Q2N8781P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium vivax (malaria parasite P. vivax)) | BDBM18779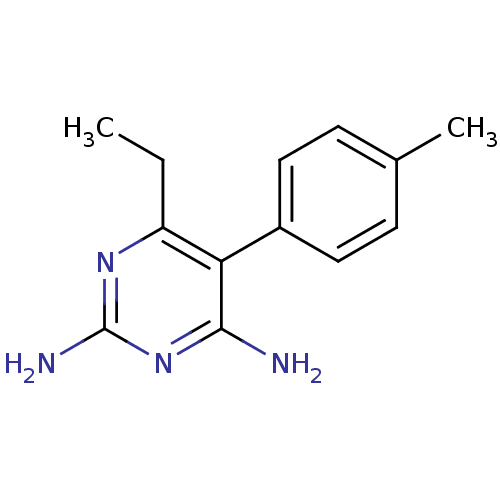 (6-ethyl-5-(4-methylphenyl)pyrimidine-2,4-diamine |...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.390 | -53.7 | 140 | n/a | n/a | n/a | n/a | 7.0 | 25 |
National Center for Genetic Engineering and Biotechnology at Thailand | Assay Description Nineteen Pyr analogs were studied for their inhibition activity against cells expressing either WT or SP21 mutant PvDHFR-TS. The assays were conducte... | Antimicrob Agents Chemother 50: 3631-7 (2006) Article DOI: 10.1128/AAC.00448-06 BindingDB Entry DOI: 10.7270/Q2N8781P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50070409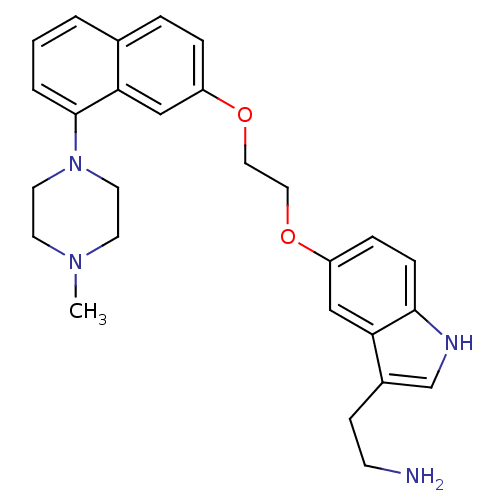 (2-(5-(2-(8-(4-methylpiperazin-1-yl)naphthalen-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-hydroxytryptamine from human cloned 5HT1D receptor expressed in CHOK1 cells by liquid scintillation counting | J Med Chem 51: 3609-16 (2008) Article DOI: 10.1021/jm7011722 BindingDB Entry DOI: 10.7270/Q26T0MDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50574836 (CHEMBL4871105) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Trypanosoma brucei rhodesain assessed as fluorescence using Cbz-Phe-Arg-AMC as substrate measured at second inhibition step by fluorome... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01002 BindingDB Entry DOI: 10.7270/Q2CC14G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50271132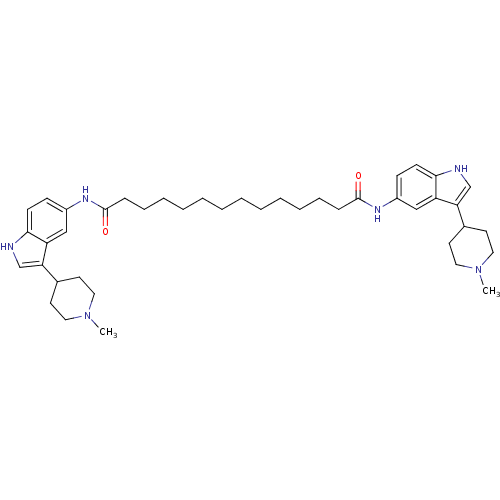 (CHEMBL502138 | N1,N14-bis(3-(1-methylpiperidin-4-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-hydroxytryptamine from human cloned 5HT1B receptor expressed in CHOK1 cells by liquid scintillation counting | J Med Chem 51: 3609-16 (2008) Article DOI: 10.1021/jm7011722 BindingDB Entry DOI: 10.7270/Q26T0MDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM10755 (14C-5-hydroxy tryptamine creatinine disulfate | 2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-hydroxytryptamine from human cloned 5HT1D receptor expressed in CHOK1 cells by liquid scintillation counting | J Med Chem 51: 3609-16 (2008) Article DOI: 10.1021/jm7011722 BindingDB Entry DOI: 10.7270/Q26T0MDK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium vivax (malaria parasite P. vivax)) | BDBM18783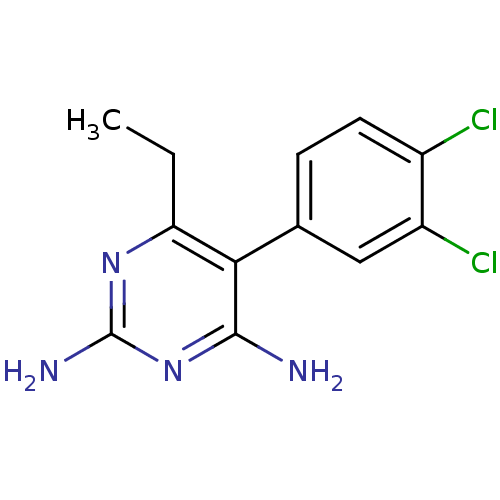 (5-(3,4-dichlorophenyl)-6-ethylpyrimidine-2,4-diami...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.520 | -53.0 | 70 | n/a | n/a | n/a | n/a | 7.0 | 25 |
National Center for Genetic Engineering and Biotechnology at Thailand | Assay Description Nineteen Pyr analogs were studied for their inhibition activity against cells expressing either WT or SP21 mutant PvDHFR-TS. The assays were conducte... | Antimicrob Agents Chemother 50: 3631-7 (2006) Article DOI: 10.1128/AAC.00448-06 BindingDB Entry DOI: 10.7270/Q2N8781P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium vivax (malaria parasite P. vivax)) | BDBM18791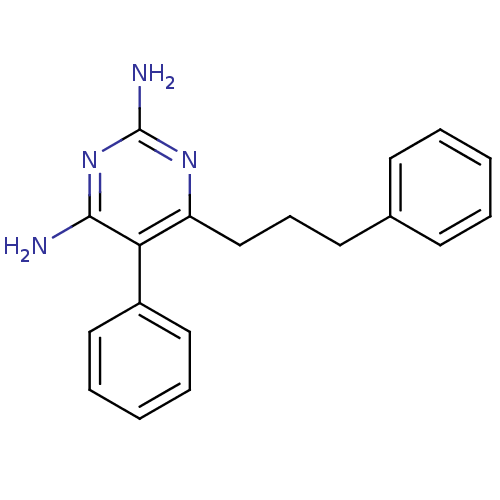 (5-phenyl-6-(3-phenylpropyl)pyrimidine-2,4-diamine ...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.530 | -52.9 | 800 | n/a | n/a | n/a | n/a | 7.0 | 25 |
National Center for Genetic Engineering and Biotechnology at Thailand | Assay Description Nineteen Pyr analogs were studied for their inhibition activity against cells expressing either WT or SP21 mutant PvDHFR-TS. The assays were conducte... | Antimicrob Agents Chemother 50: 3631-7 (2006) Article DOI: 10.1128/AAC.00448-06 BindingDB Entry DOI: 10.7270/Q2N8781P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase [1-238,S58R,S117N] (Plasmodium vivax (malaria parasite P. vivax)) | BDBM18784 (5-(3-chlorophenyl)-6-ethylpyrimidine-2,4-diamine |...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.540 | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology at Thailand | Assay Description Nineteen Pyr analogs were studied for their inhibition activity against cells expressing either WT or SP21 mutant PvDHFR-TS. The assays were conducte... | Antimicrob Agents Chemother 50: 3631-7 (2006) Article DOI: 10.1128/AAC.00448-06 BindingDB Entry DOI: 10.7270/Q2N8781P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50151114 (CHEMBL3770682) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX from human TRPV1 expressed in CHO cells after 60 mins by scintillation counting analysis | Bioorg Med Chem 24: 1231-40 (2016) Article DOI: 10.1016/j.bmc.2016.01.051 BindingDB Entry DOI: 10.7270/Q25D8TP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50271133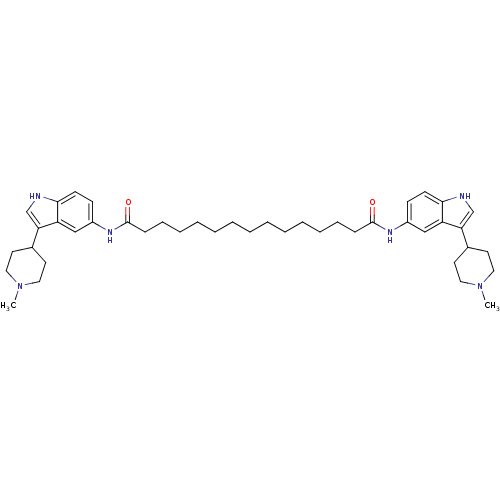 (CHEMBL500284 | N1,N15-bis(3-(1-methylpiperidin-4-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-hydroxytryptamine from human cloned 5HT1B receptor expressed in CHOK1 cells by liquid scintillation counting | J Med Chem 51: 3609-16 (2008) Article DOI: 10.1021/jm7011722 BindingDB Entry DOI: 10.7270/Q26T0MDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50271018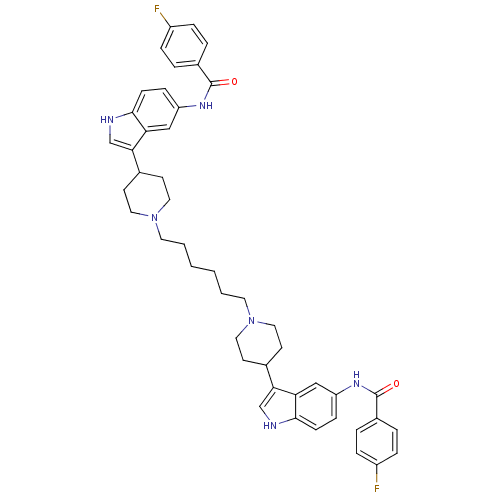 (4-fluoro-N-(3-{1-[6-(4-{5-[(4-fluorobenzene)amido]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-hydroxytryptamine from human cloned 5HT1D receptor expressed in CHOK1 cells by liquid scintillation counting | J Med Chem 51: 3609-16 (2008) Article DOI: 10.1021/jm7011722 BindingDB Entry DOI: 10.7270/Q26T0MDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium vivax (malaria parasite P. vivax)) | BDBM18787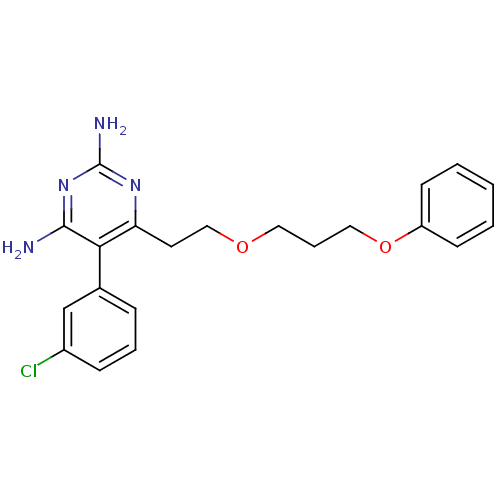 (5-(3-chlorophenyl)-6-[2-(3-phenoxypropoxy)ethyl]py...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | 0.630 | -52.5 | 2.27E+3 | n/a | n/a | n/a | n/a | 7.0 | 25 |
National Center for Genetic Engineering and Biotechnology at Thailand | Assay Description Nineteen Pyr analogs were studied for their inhibition activity against cells expressing either WT or SP21 mutant PvDHFR-TS. The assays were conducte... | Antimicrob Agents Chemother 50: 3631-7 (2006) Article DOI: 10.1128/AAC.00448-06 BindingDB Entry DOI: 10.7270/Q2N8781P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50069315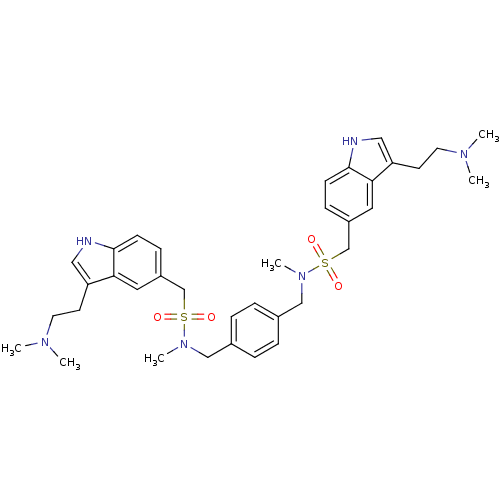 (C-[3-(2-Dimethylamino-ethyl)-1H-indol-5-yl]-N-[4-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-hydroxytryptamine from human cloned 5HT1B receptor expressed in CHOK1 cells by liquid scintillation counting | J Med Chem 51: 3609-16 (2008) Article DOI: 10.1021/jm7011722 BindingDB Entry DOI: 10.7270/Q26T0MDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium vivax (malaria parasite P. vivax)) | BDBM18778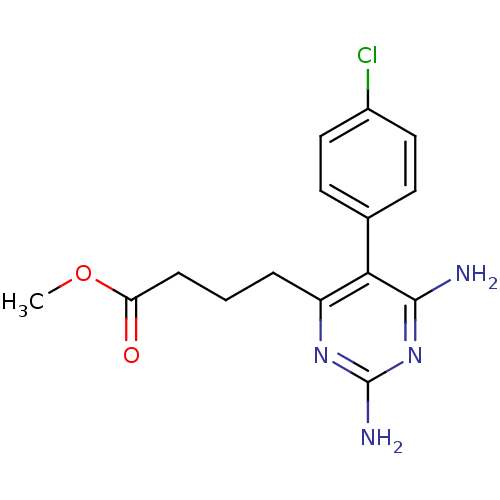 (CHEMBL22405 | P16 | methyl 4-[2,6-diamino-5-(4-chl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.690 | -52.3 | 200 | n/a | n/a | n/a | n/a | 7.0 | 25 |
National Center for Genetic Engineering and Biotechnology at Thailand | Assay Description Nineteen Pyr analogs were studied for their inhibition activity against cells expressing either WT or SP21 mutant PvDHFR-TS. The assays were conducte... | Antimicrob Agents Chemother 50: 3631-7 (2006) Article DOI: 10.1128/AAC.00448-06 BindingDB Entry DOI: 10.7270/Q2N8781P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium vivax (malaria parasite P. vivax)) | BDBM18785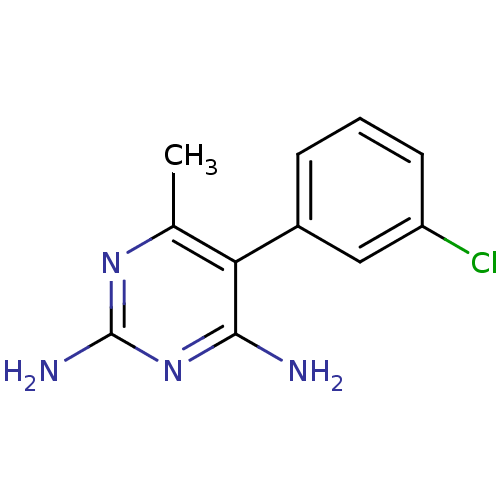 (5-(3-chlorophenyl)-6-methylpyrimidine-2,4-diamine ...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.690 | -52.3 | 2.12E+3 | n/a | n/a | n/a | n/a | 7.0 | 25 |
National Center for Genetic Engineering and Biotechnology at Thailand | Assay Description Nineteen Pyr analogs were studied for their inhibition activity against cells expressing either WT or SP21 mutant PvDHFR-TS. The assays were conducte... | Antimicrob Agents Chemother 50: 3631-7 (2006) Article DOI: 10.1128/AAC.00448-06 BindingDB Entry DOI: 10.7270/Q2N8781P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50080960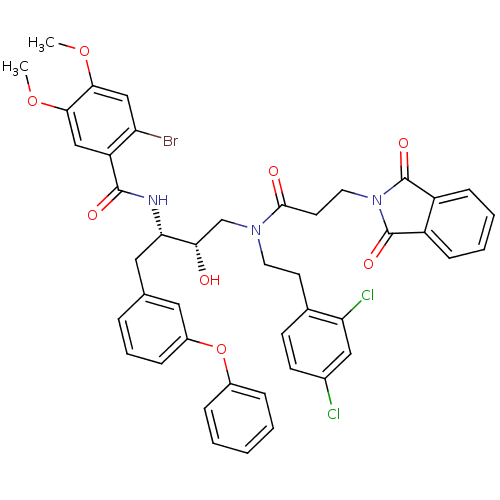 (2-Bromo-N-[(1S,2S)-3-{[2-(2,4-dichloro-phenyl)-eth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Inhibition of cathepsin D (unknown origin) | Bioorg Med Chem Lett 24: 4141-50 (2014) Article DOI: 10.1016/j.bmcl.2014.07.054 BindingDB Entry DOI: 10.7270/Q2W95BV1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50602541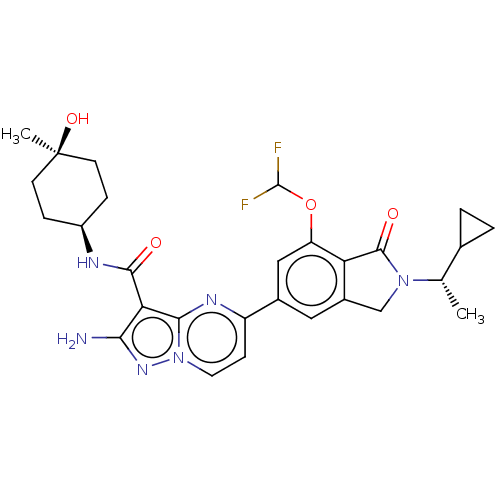 (CHEMBL5209268) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01153 BindingDB Entry DOI: 10.7270/Q2KH0SCH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50150646 (CHEMBL3770091) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX from human TRPV1 expressed in CHO cells after 60 mins by scintillation counting analysis | Bioorg Med Chem 24: 1231-40 (2016) Article DOI: 10.1016/j.bmc.2016.01.051 BindingDB Entry DOI: 10.7270/Q25D8TP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50271127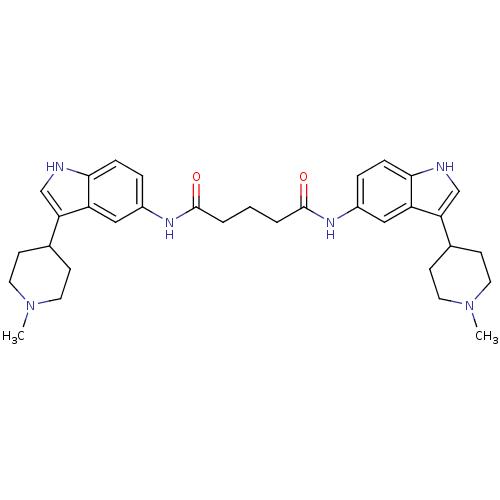 (CHEMBL444404 | N1,N5-bis(3-(1-methylpiperidin-4-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-hydroxytryptamine from human cloned 5HT1B receptor expressed in CHOK1 cells by liquid scintillation counting | J Med Chem 51: 3609-16 (2008) Article DOI: 10.1021/jm7011722 BindingDB Entry DOI: 10.7270/Q26T0MDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50151119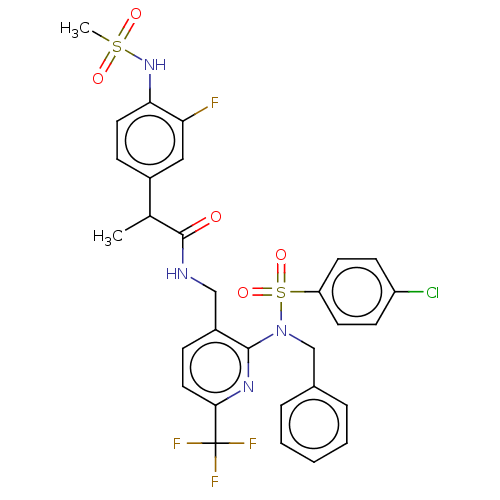 (CHEMBL3769821) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX from human TRPV1 expressed in CHO cells after 60 mins by scintillation counting analysis | Bioorg Med Chem 24: 1231-40 (2016) Article DOI: 10.1016/j.bmc.2016.01.051 BindingDB Entry DOI: 10.7270/Q25D8TP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50070409 (2-(5-(2-(8-(4-methylpiperazin-1-yl)naphthalen-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-hydroxytryptamine from human cloned 5HT1B receptor expressed in CHOK1 cells by liquid scintillation counting | J Med Chem 51: 3609-16 (2008) Article DOI: 10.1021/jm7011722 BindingDB Entry DOI: 10.7270/Q26T0MDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium vivax (malaria parasite P. vivax)) | BDBM18786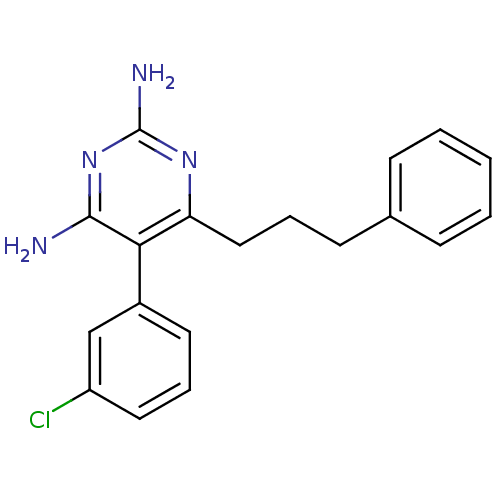 (5-(3-chlorophenyl)-6-(3-phenylpropyl)pyrimidine-2,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.770 | -52.0 | 2.38E+3 | n/a | n/a | n/a | n/a | 7.0 | 25 |
National Center for Genetic Engineering and Biotechnology at Thailand | Assay Description Nineteen Pyr analogs were studied for their inhibition activity against cells expressing either WT or SP21 mutant PvDHFR-TS. The assays were conducte... | Antimicrob Agents Chemother 50: 3631-7 (2006) Article DOI: 10.1128/AAC.00448-06 BindingDB Entry DOI: 10.7270/Q2N8781P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium vivax (malaria parasite P. vivax)) | BDBM18781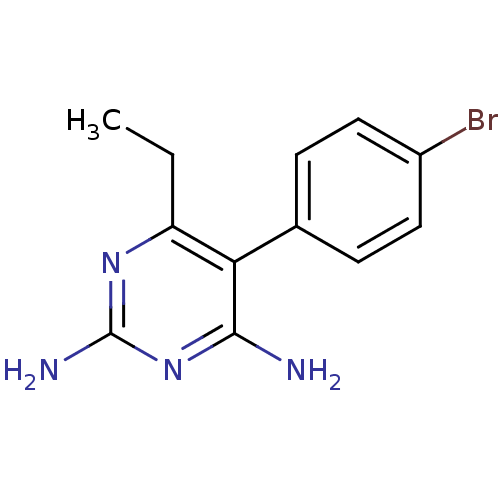 (5-(4-bromophenyl)-6-ethylpyrimidine-2,4-diamine | ...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.780 | -52.0 | 90 | n/a | n/a | n/a | n/a | 7.0 | 25 |
National Center for Genetic Engineering and Biotechnology at Thailand | Assay Description Nineteen Pyr analogs were studied for their inhibition activity against cells expressing either WT or SP21 mutant PvDHFR-TS. The assays were conducte... | Antimicrob Agents Chemother 50: 3631-7 (2006) Article DOI: 10.1128/AAC.00448-06 BindingDB Entry DOI: 10.7270/Q2N8781P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium vivax (malaria parasite P. vivax)) | BDBM18775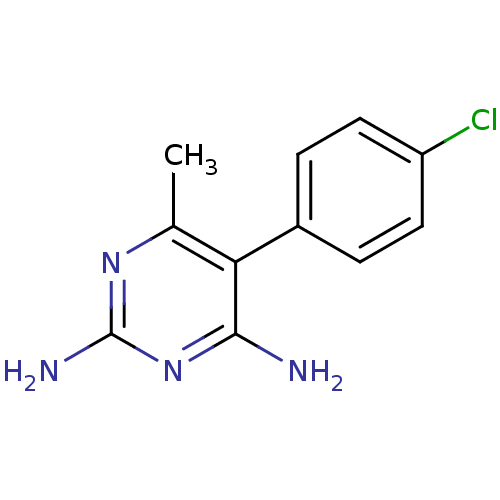 (5-(4-chlorophenyl)-6-methylpyrimidine-2,4-diamine ...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.780 | -52.0 | 1.95E+3 | n/a | n/a | n/a | n/a | 7.0 | 25 |
National Center for Genetic Engineering and Biotechnology at Thailand | Assay Description Nineteen Pyr analogs were studied for their inhibition activity against cells expressing either WT or SP21 mutant PvDHFR-TS. The assays were conducte... | Antimicrob Agents Chemother 50: 3631-7 (2006) Article DOI: 10.1128/AAC.00448-06 BindingDB Entry DOI: 10.7270/Q2N8781P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50271129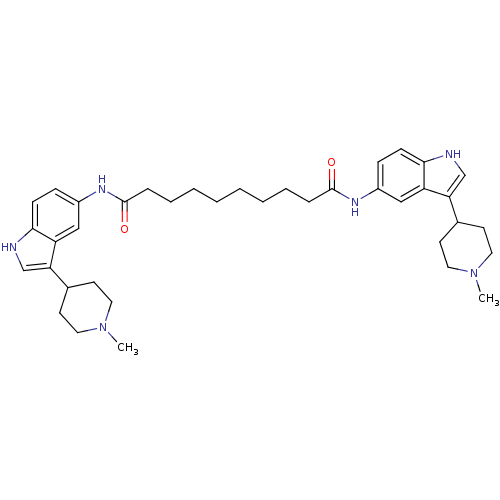 (CHEMBL452387 | N1,N10-bis(3-(1-methylpiperidin-4-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-hydroxytryptamine from human cloned 5HT1B receptor expressed in CHOK1 cells by liquid scintillation counting | J Med Chem 51: 3609-16 (2008) Article DOI: 10.1021/jm7011722 BindingDB Entry DOI: 10.7270/Q26T0MDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50503902 (CHEMBL4588688) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma US Inc. Curated by ChEMBL | Assay Description Displacement of Europium-labeled angiotensin-2 from human AT1 receptor expressed in CHOK1 cell membranes after 120 mins by DELFIA | ACS Med Chem Lett 10: 86-91 (2019) Article DOI: 10.1021/acsmedchemlett.8b00462 BindingDB Entry DOI: 10.7270/Q2D50R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase [1-238,S58R,S117N] (Plasmodium vivax (malaria parasite P. vivax)) | BDBM18791 (5-phenyl-6-(3-phenylpropyl)pyrimidine-2,4-diamine ...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.810 | n/a | 2.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology at Thailand | Assay Description Nineteen Pyr analogs were studied for their inhibition activity against cells expressing either WT or SP21 mutant PvDHFR-TS. The assays were conducte... | Antimicrob Agents Chemother 50: 3631-7 (2006) Article DOI: 10.1128/AAC.00448-06 BindingDB Entry DOI: 10.7270/Q2N8781P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50271134 (CHEMBL500488 | N1,N16-bis(3-(1-methylpiperidin-4-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-hydroxytryptamine from human cloned 5HT1B receptor expressed in CHOK1 cells by liquid scintillation counting | J Med Chem 51: 3609-16 (2008) Article DOI: 10.1021/jm7011722 BindingDB Entry DOI: 10.7270/Q26T0MDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18512 (5-(4-chlorophenyl)-6-ethylpyrimidine-2,4-diamine |...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Science and Technology Development Agency Curated by ChEMBL | Assay Description Inhibition of pyrimethamine-resistant form-2 Plasmodium falciparum N51I, C59R, S108N, I164L, K96N mutant expressed in Escherichia coli BL21(DE3) by M... | Antimicrob Agents Chemother 51: 4356-60 (2007) Article DOI: 10.1128/aac.00577-07 BindingDB Entry DOI: 10.7270/Q2JS9T70 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50069315 (C-[3-(2-Dimethylamino-ethyl)-1H-indol-5-yl]-N-[4-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-hydroxytryptamine from human cloned 5HT1D receptor expressed in CHOK1 cells by liquid scintillation counting | J Med Chem 51: 3609-16 (2008) Article DOI: 10.1021/jm7011722 BindingDB Entry DOI: 10.7270/Q26T0MDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase [1-238,S58R,S117N] (Plasmodium vivax (malaria parasite P. vivax)) | BDBM18790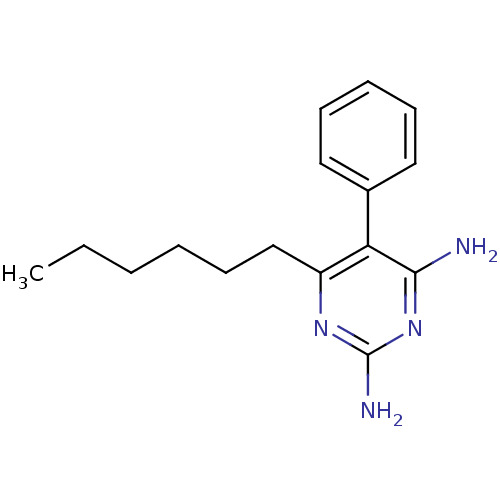 (6-hexyl-5-phenylpyrimidine-2,4-diamine | CHEMBL416...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 0.900 | n/a | 3.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology at Thailand | Assay Description Nineteen Pyr analogs were studied for their inhibition activity against cells expressing either WT or SP21 mutant PvDHFR-TS. The assays were conducte... | Antimicrob Agents Chemother 50: 3631-7 (2006) Article DOI: 10.1128/AAC.00448-06 BindingDB Entry DOI: 10.7270/Q2N8781P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50271130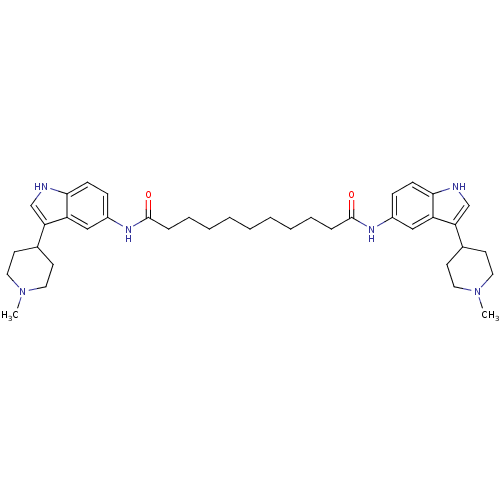 (CHEMBL446745 | N1,N11-bis(3-(1-methylpiperidin-4-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-hydroxytryptamine from human cloned 5HT1D receptor expressed in CHOK1 cells by liquid scintillation counting | J Med Chem 51: 3609-16 (2008) Article DOI: 10.1021/jm7011722 BindingDB Entry DOI: 10.7270/Q26T0MDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50150058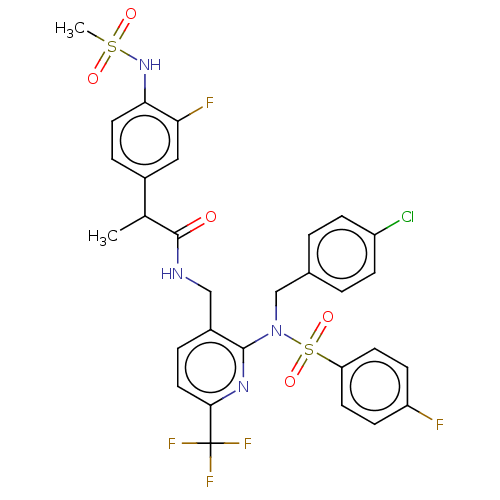 (CHEMBL3770670) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX from human TRPV1 expressed in CHO cells after 60 mins by scintillation counting analysis | Bioorg Med Chem 24: 1231-40 (2016) Article DOI: 10.1016/j.bmc.2016.01.051 BindingDB Entry DOI: 10.7270/Q25D8TP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50271128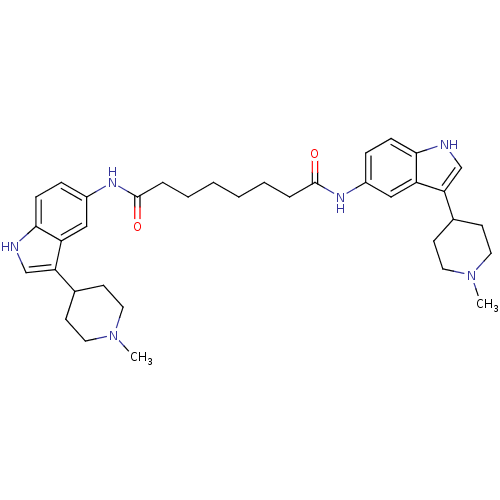 (CHEMBL448902 | N1,N8-bis(3-(1-methylpiperidin-4-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-hydroxytryptamine from human cloned 5HT1D receptor expressed in CHOK1 cells by liquid scintillation counting | J Med Chem 51: 3609-16 (2008) Article DOI: 10.1021/jm7011722 BindingDB Entry DOI: 10.7270/Q26T0MDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50064786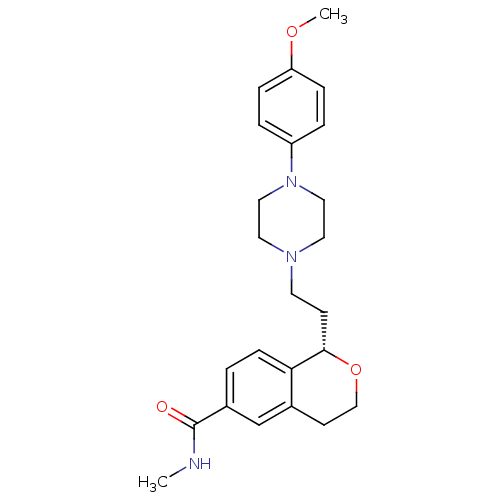 ((S)-1-(2-(4-(4-methoxyphenyl)piperazin-1-yl)ethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-hydroxytryptamine from human cloned 5HT1D receptor expressed in CHOK1 cells by liquid scintillation counting | J Med Chem 51: 3609-16 (2008) Article DOI: 10.1021/jm7011722 BindingDB Entry DOI: 10.7270/Q26T0MDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50271129 (CHEMBL452387 | N1,N10-bis(3-(1-methylpiperidin-4-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-hydroxytryptamine from human cloned 5HT1D receptor expressed in CHOK1 cells by liquid scintillation counting | J Med Chem 51: 3609-16 (2008) Article DOI: 10.1021/jm7011722 BindingDB Entry DOI: 10.7270/Q26T0MDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50271127 (CHEMBL444404 | N1,N5-bis(3-(1-methylpiperidin-4-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-hydroxytryptamine from human cloned 5HT1D receptor expressed in CHOK1 cells by liquid scintillation counting | J Med Chem 51: 3609-16 (2008) Article DOI: 10.1021/jm7011722 BindingDB Entry DOI: 10.7270/Q26T0MDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50070409 (2-(5-(2-(8-(4-methylpiperazin-1-yl)naphthalen-2-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Binding affinity to 5HT1A receptor | J Med Chem 51: 3609-16 (2008) Article DOI: 10.1021/jm7011722 BindingDB Entry DOI: 10.7270/Q26T0MDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 5282 total ) | Next | Last >> |