

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50292544 (3,4,6,7-tetrahydroxyxanthone | CHEMBL477740) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of ACE by Lineweaver-Burk plot | J Nat Prod 55: 691-695 (1992) Article DOI: 10.1021/np50083a025 BindingDB Entry DOI: 10.7270/Q21C1WXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50292547 (1,3,5,6-tetrahydroxyxanthone | 1,3,5,6-tetrahydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 3.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of ACE by Lineweaver-Burk plot | J Nat Prod 55: 691-695 (1992) Article DOI: 10.1021/np50083a025 BindingDB Entry DOI: 10.7270/Q21C1WXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50292546 (3,4,5,6-tetrahydroxyxanthone | CHEMBL477921 | US91...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.26E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of ACE by Lineweaver-Burk plot | J Nat Prod 55: 691-695 (1992) Article DOI: 10.1021/np50083a025 BindingDB Entry DOI: 10.7270/Q21C1WXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50155447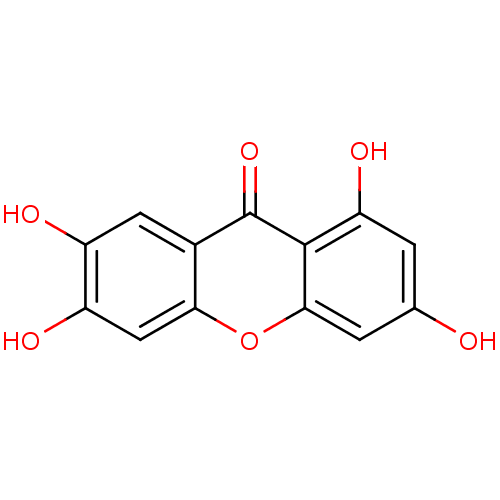 (1,3,6,7-Tetrahydroxy-xanthen-9-one | 1,3,6,7-tetra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of ACE by Lineweaver-Burk plot | J Nat Prod 55: 691-695 (1992) Article DOI: 10.1021/np50083a025 BindingDB Entry DOI: 10.7270/Q21C1WXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM35440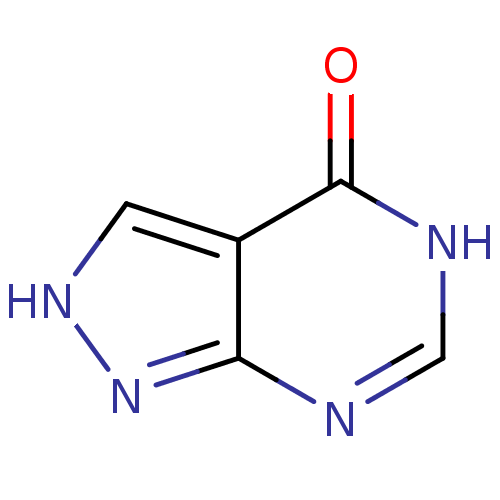 (ALLOPURINOL | MLS000069453 | SMR000059083 | cid_20...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibition of xanthine oxidase activity assessed as uric acid formation pretreated for 15 mins before substrate addition measured after 5 mins | Eur J Med Chem 46: 1222-31 (2011) Article DOI: 10.1016/j.ejmech.2011.01.043 BindingDB Entry DOI: 10.7270/Q2RB74X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM35440 (ALLOPURINOL | MLS000069453 | SMR000059083 | cid_20...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibition of xanthine oxidase (unknown origin) | J Nat Prod 71: 1027-31 (2008) Article DOI: 10.1021/np8001145 BindingDB Entry DOI: 10.7270/Q25T3K7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM35440 (ALLOPURINOL | MLS000069453 | SMR000059083 | cid_20...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibition of xanthine oxidase (unknown origin) | Bioorg Med Chem 16: 7270-6 (2008) Article DOI: 10.1016/j.bmc.2008.06.031 BindingDB Entry DOI: 10.7270/Q2S182BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-glucuronidase (Rattus norvegicus) | BDBM7458 (5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibitory effect of compound on the release of Beta-glucuronidase in rat neutrophils stimulated with fMLP/CB | Bioorg Med Chem Lett 14: 1011-4 (2004) Article DOI: 10.1016/j.bmcl.2003.11.074 BindingDB Entry DOI: 10.7270/Q2C829VG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-glucuronidase (Rattus norvegicus) | BDBM50142192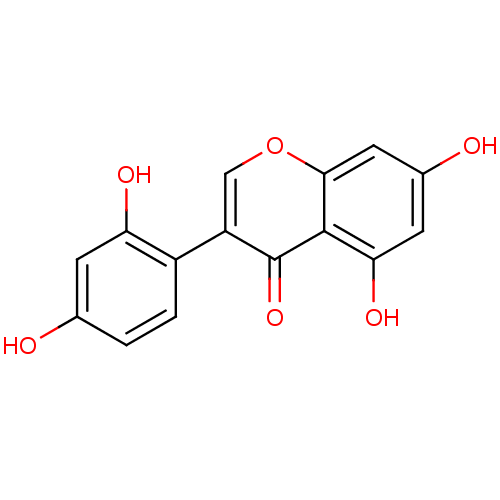 (3-(2,4-Dihydroxy-phenyl)-5,7-dihydroxy-chromen-4-o...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibitory effect of compound on the release of Beta-glucuronidase in rat neutrophils stimulated with fMLP/CB | Bioorg Med Chem Lett 14: 1011-4 (2004) Article DOI: 10.1016/j.bmcl.2003.11.074 BindingDB Entry DOI: 10.7270/Q2C829VG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-glucuronidase (Rattus norvegicus) | BDBM79181 (10-[3-(4-methyl-1-piperazinyl)propyl]-2-(trifluoro...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibitory effect of compound on the release of Beta-glucuronidase in rat neutrophils stimulated with fMLP/CB | Bioorg Med Chem Lett 14: 1011-4 (2004) Article DOI: 10.1016/j.bmcl.2003.11.074 BindingDB Entry DOI: 10.7270/Q2C829VG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysozyme C-1 (Rattus norvegicus) | BDBM79181 (10-[3-(4-methyl-1-piperazinyl)propyl]-2-(trifluoro...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibitory effect of compound on the release of lysozyme in rat neutrophils stimulated with fMLP/CB | Bioorg Med Chem Lett 14: 1011-4 (2004) Article DOI: 10.1016/j.bmcl.2003.11.074 BindingDB Entry DOI: 10.7270/Q2C829VG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysozyme C-1 (Rattus norvegicus) | BDBM50142192 (3-(2,4-Dihydroxy-phenyl)-5,7-dihydroxy-chromen-4-o...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibitory effect of compound on the release of lysozyme in rat neutrophils stimulated with fMLP/CB | Bioorg Med Chem Lett 14: 1011-4 (2004) Article DOI: 10.1016/j.bmcl.2003.11.074 BindingDB Entry DOI: 10.7270/Q2C829VG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-glucuronidase (Rattus norvegicus) | BDBM50077323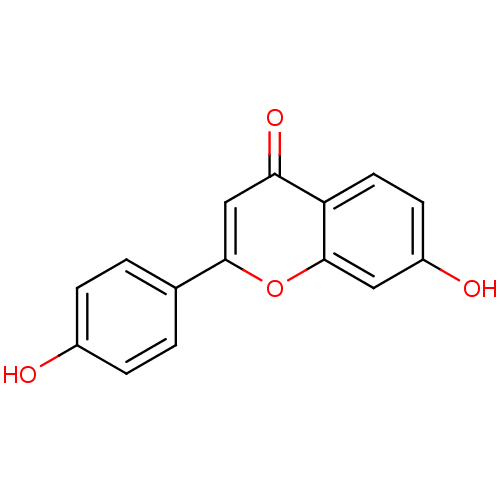 (7,4'-Dihydroxyflavone | 7-hydroxy-2-(4-hydroxyphen...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibitory effect of compound on the release of Beta-glucuronidase in rat neutrophils stimulated with fMLP/CB | Bioorg Med Chem Lett 14: 1011-4 (2004) Article DOI: 10.1016/j.bmcl.2003.11.074 BindingDB Entry DOI: 10.7270/Q2C829VG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysozyme C-1 (Rattus norvegicus) | BDBM7458 (5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibitory effect of compound on the release of lysozyme in rat neutrophils stimulated with fMLP/CB | Bioorg Med Chem Lett 14: 1011-4 (2004) Article DOI: 10.1016/j.bmcl.2003.11.074 BindingDB Entry DOI: 10.7270/Q2C829VG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM50263178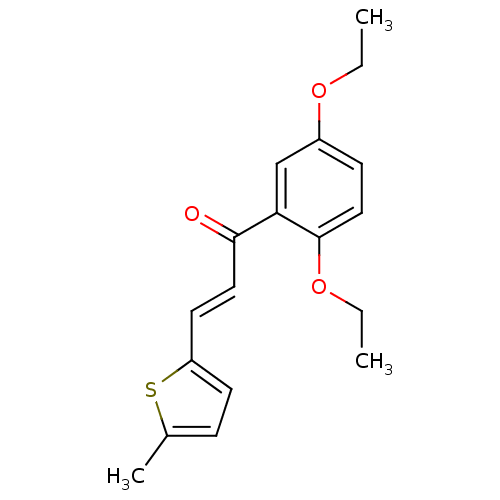 (2',5'-Diethoxy-2-(5-methylthienyl)chalcone | CHEMB...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibition of xanthine oxidase (unknown origin) | Bioorg Med Chem 16: 7270-6 (2008) Article DOI: 10.1016/j.bmc.2008.06.031 BindingDB Entry DOI: 10.7270/Q2S182BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysozyme C-1 (Rattus norvegicus) | BDBM50142193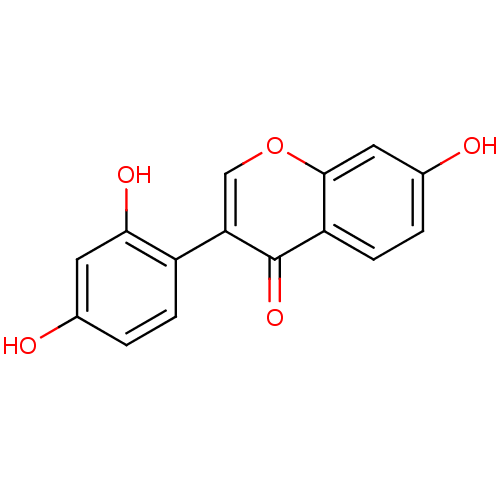 (2'-hydroxydaidzein | 3-(2,4-dihydroxyphenyl)-7-hyd...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibitory effect of compound on the release of lysozyme in rat neutrophils stimulated with fMLP/CB | Bioorg Med Chem Lett 14: 1011-4 (2004) Article DOI: 10.1016/j.bmcl.2003.11.074 BindingDB Entry DOI: 10.7270/Q2C829VG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysozyme C-1 (Rattus norvegicus) | BDBM50142191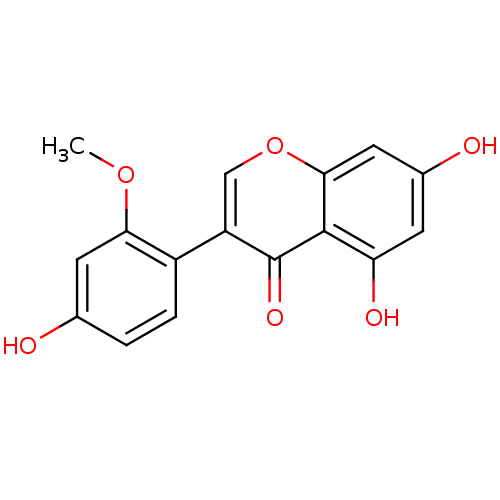 (5,7-Dihydroxy-3-(4-hydroxy-2-methoxy-phenyl)-chrom...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibitory effect of compound on the release of lysozyme in rat neutrophils stimulated with fMLP/CB | Bioorg Med Chem Lett 14: 1011-4 (2004) Article DOI: 10.1016/j.bmcl.2003.11.074 BindingDB Entry DOI: 10.7270/Q2C829VG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysozyme C-1 (Rattus norvegicus) | BDBM50077323 (7,4'-Dihydroxyflavone | 7-hydroxy-2-(4-hydroxyphen...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibitory effect of compound on the release of lysozyme in rat neutrophils stimulated with fMLP/CB | Bioorg Med Chem Lett 14: 1011-4 (2004) Article DOI: 10.1016/j.bmcl.2003.11.074 BindingDB Entry DOI: 10.7270/Q2C829VG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysozyme C-1 (Rattus norvegicus) | BDBM50366927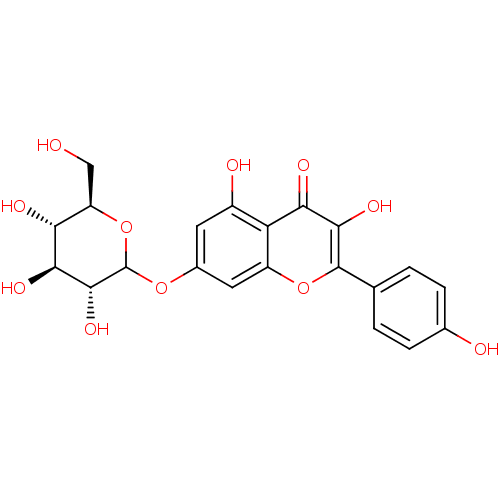 (CHEMBL1159471) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibitory effect of compound on the release of lysozyme in rat neutrophils stimulated with fMLP/CB | Bioorg Med Chem Lett 14: 1011-4 (2004) Article DOI: 10.1016/j.bmcl.2003.11.074 BindingDB Entry DOI: 10.7270/Q2C829VG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-glucuronidase (Rattus norvegicus) | BDBM50142193 (2'-hydroxydaidzein | 3-(2,4-dihydroxyphenyl)-7-hyd...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibitory effect of compound on the release of Beta-glucuronidase in rat neutrophils stimulated with fMLP/CB | Bioorg Med Chem Lett 14: 1011-4 (2004) Article DOI: 10.1016/j.bmcl.2003.11.074 BindingDB Entry DOI: 10.7270/Q2C829VG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-glucuronidase (Rattus norvegicus) | BDBM50366927 (CHEMBL1159471) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibitory effect of compound on the release of Beta-glucuronidase in rat neutrophils stimulated with fMLP/CB | Bioorg Med Chem Lett 14: 1011-4 (2004) Article DOI: 10.1016/j.bmcl.2003.11.074 BindingDB Entry DOI: 10.7270/Q2C829VG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysozyme C-1 (Rattus norvegicus) | BDBM50142190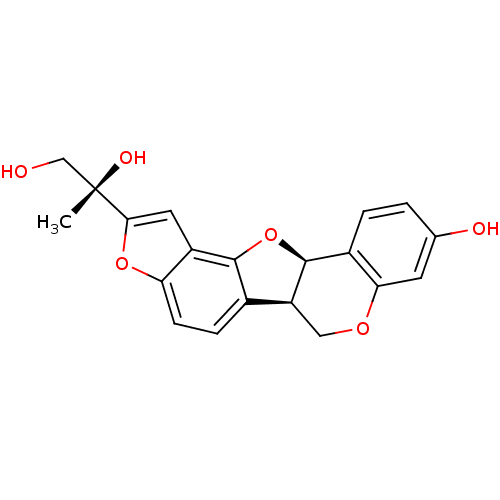 (2-((11bR,12R)-9-Hydroxy-5b,11b-dihydro-6H-3,7,12-t...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibitory effect of compound on the release of lysozyme in rat neutrophils stimulated with fMLP/CB | Bioorg Med Chem Lett 14: 1011-4 (2004) Article DOI: 10.1016/j.bmcl.2003.11.074 BindingDB Entry DOI: 10.7270/Q2C829VG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-glucuronidase (Rattus norvegicus) | BDBM50142191 (5,7-Dihydroxy-3-(4-hydroxy-2-methoxy-phenyl)-chrom...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibitory effect of compound on the release of Beta-glucuronidase in rat neutrophils stimulated with fMLP/CB | Bioorg Med Chem Lett 14: 1011-4 (2004) Article DOI: 10.1016/j.bmcl.2003.11.074 BindingDB Entry DOI: 10.7270/Q2C829VG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-glucuronidase (Rattus norvegicus) | BDBM50142190 (2-((11bR,12R)-9-Hydroxy-5b,11b-dihydro-6H-3,7,12-t...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibitory effect of compound on the release of Beta-glucuronidase in rat neutrophils stimulated with fMLP/CB | Bioorg Med Chem Lett 14: 1011-4 (2004) Article DOI: 10.1016/j.bmcl.2003.11.074 BindingDB Entry DOI: 10.7270/Q2C829VG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50292544 (3,4,6,7-tetrahydroxyxanthone | CHEMBL477740) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of ACE | J Nat Prod 55: 691-695 (1992) Article DOI: 10.1021/np50083a025 BindingDB Entry DOI: 10.7270/Q21C1WXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM50340832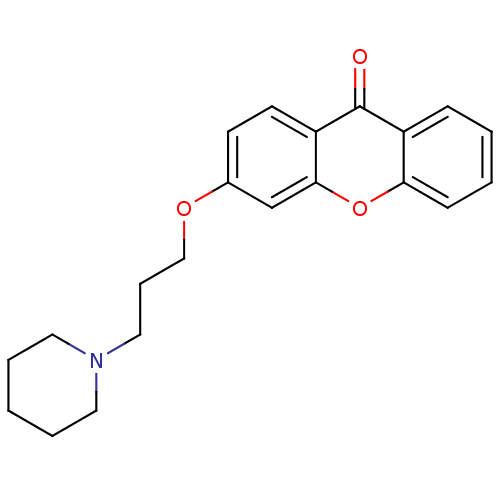 (3-[3-(Piperidin-1-yl)-propoxy]xanthone | CHEMBL176...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibition of xanthine oxidase activity assessed as uric acid formation pretreated for 15 mins before substrate addition measured after 5 mins | Eur J Med Chem 46: 1222-31 (2011) Article DOI: 10.1016/j.ejmech.2011.01.043 BindingDB Entry DOI: 10.7270/Q2RB74X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM50244273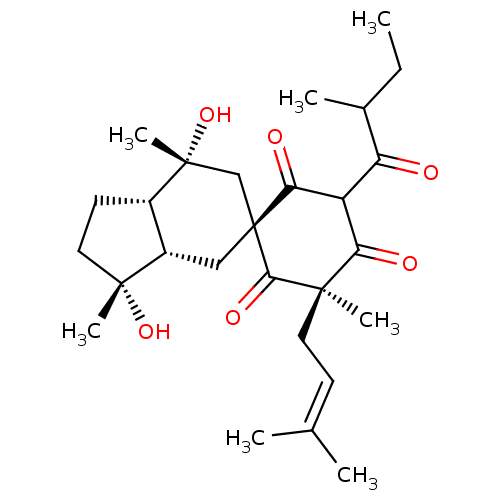 (CHEMBL470950 | CHEMBL511791 | Hyperielliptone HB) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibition of xanthine oxidase (unknown origin) | J Nat Prod 71: 1027-31 (2008) Article DOI: 10.1021/np8001145 BindingDB Entry DOI: 10.7270/Q25T3K7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM50244273 (CHEMBL470950 | CHEMBL511791 | Hyperielliptone HB) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibition of xanthine oxidase (unknown origin) | J Nat Prod 71: 1027-31 (2008) Article DOI: 10.1021/np8001145 BindingDB Entry DOI: 10.7270/Q25T3K7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM50340843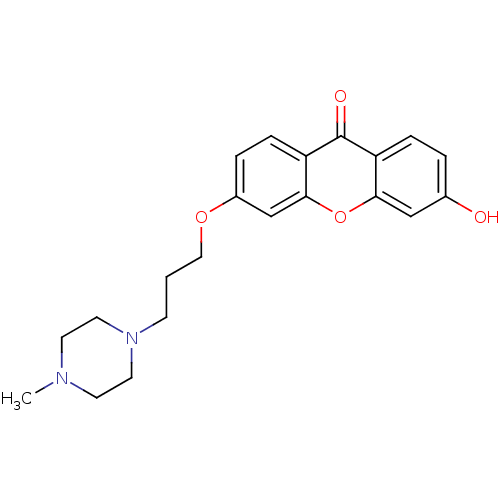 (3-Hydroxy-6-[3-(methylpiperazylamino)-propoxy]xant...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibition of xanthine oxidase activity assessed as uric acid formation pretreated for 15 mins before substrate addition measured after 5 mins | Eur J Med Chem 46: 1222-31 (2011) Article DOI: 10.1016/j.ejmech.2011.01.043 BindingDB Entry DOI: 10.7270/Q2RB74X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM50340834 (3-[3-(4-Methylpiperazino)-propoxy]xanthone | CHEMB...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibition of xanthine oxidase activity assessed as uric acid formation pretreated for 15 mins before substrate addition measured after 5 mins | Eur J Med Chem 46: 1222-31 (2011) Article DOI: 10.1016/j.ejmech.2011.01.043 BindingDB Entry DOI: 10.7270/Q2RB74X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM50340841 (3-[3-(Diethylamino)-propoxy]-6-hydroxyxanthone | C...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibition of xanthine oxidase activity assessed as uric acid formation pretreated for 15 mins before substrate addition measured after 5 mins | Eur J Med Chem 46: 1222-31 (2011) Article DOI: 10.1016/j.ejmech.2011.01.043 BindingDB Entry DOI: 10.7270/Q2RB74X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM50340836 (3-[3-(Diethylamino)-propoxy]xanthone | CHEMBL17612...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibition of xanthine oxidase activity assessed as uric acid formation pretreated for 15 mins before substrate addition measured after 5 mins | Eur J Med Chem 46: 1222-31 (2011) Article DOI: 10.1016/j.ejmech.2011.01.043 BindingDB Entry DOI: 10.7270/Q2RB74X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM50340839 (3-[3-(Cyclopropylamino)-propoxy]-6-hydroxyxanthone...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibition of xanthine oxidase activity assessed as uric acid formation pretreated for 15 mins before substrate addition measured after 5 mins | Eur J Med Chem 46: 1222-31 (2011) Article DOI: 10.1016/j.ejmech.2011.01.043 BindingDB Entry DOI: 10.7270/Q2RB74X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM50340833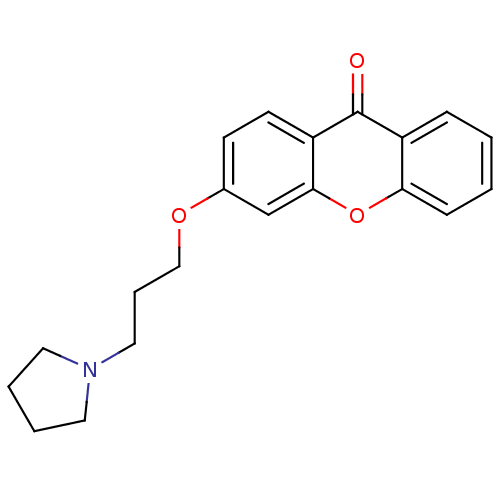 (3-[3-(Pyrrolidin-1-yl)-propoxy]xanthone | CHEMBL17...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibition of xanthine oxidase activity assessed as uric acid formation pretreated for 15 mins before substrate addition measured after 5 mins | Eur J Med Chem 46: 1222-31 (2011) Article DOI: 10.1016/j.ejmech.2011.01.043 BindingDB Entry DOI: 10.7270/Q2RB74X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM50340838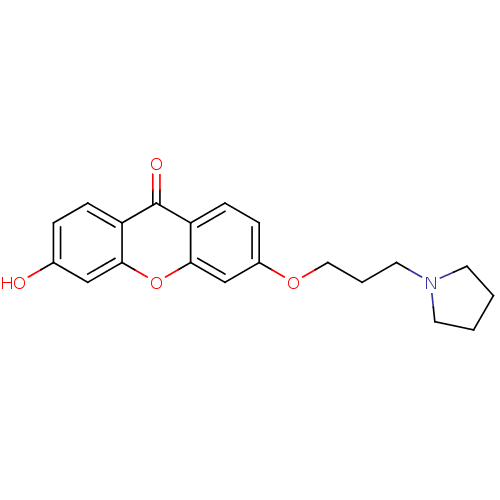 (3-Hydroxy-6-[3-(pyrrolidin-1-yl)-propoxy]xanthone ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibition of xanthine oxidase activity assessed as uric acid formation pretreated for 15 mins before substrate addition measured after 5 mins | Eur J Med Chem 46: 1222-31 (2011) Article DOI: 10.1016/j.ejmech.2011.01.043 BindingDB Entry DOI: 10.7270/Q2RB74X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50292547 (1,3,5,6-tetrahydroxyxanthone | 1,3,5,6-tetrahydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of ACE | J Nat Prod 55: 691-695 (1992) Article DOI: 10.1021/np50083a025 BindingDB Entry DOI: 10.7270/Q21C1WXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM50340840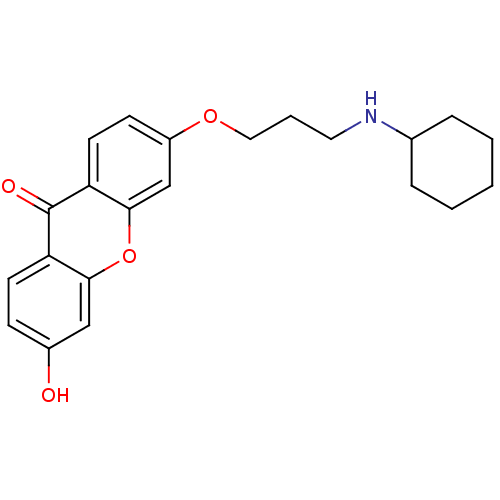 (3-[3-(Cyclohexylamino)-propoxy]-6-hydroxyxanthone ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibition of xanthine oxidase activity assessed as uric acid formation pretreated for 15 mins before substrate addition measured after 5 mins | Eur J Med Chem 46: 1222-31 (2011) Article DOI: 10.1016/j.ejmech.2011.01.043 BindingDB Entry DOI: 10.7270/Q2RB74X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM50340837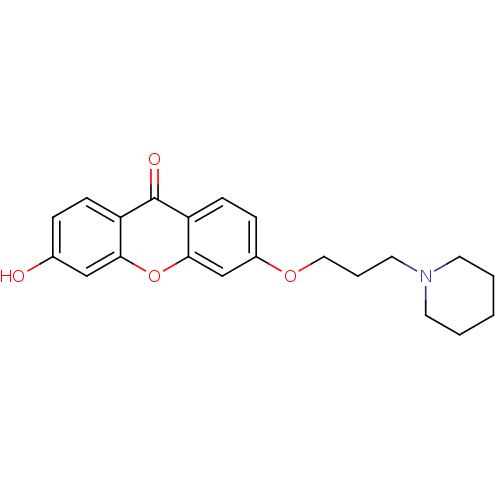 (3-Hydroxy-6-[3-(piperidin-1-yl)-propoxy]xanthone |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibition of xanthine oxidase activity assessed as uric acid formation pretreated for 15 mins before substrate addition measured after 5 mins | Eur J Med Chem 46: 1222-31 (2011) Article DOI: 10.1016/j.ejmech.2011.01.043 BindingDB Entry DOI: 10.7270/Q2RB74X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM50340842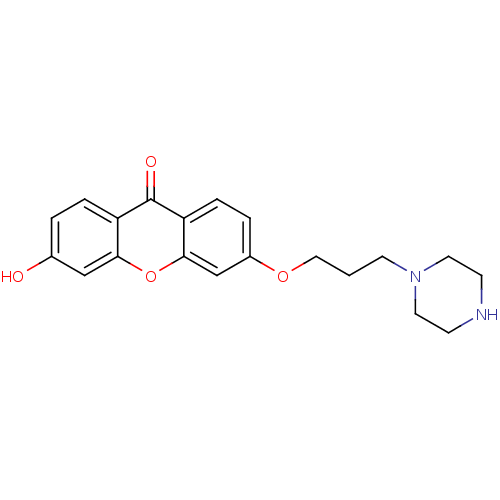 (3-Hydroxy-6-[3-(piperazino)-propoxy]xanthone | CHE...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibition of xanthine oxidase activity assessed as uric acid formation pretreated for 15 mins before substrate addition measured after 5 mins | Eur J Med Chem 46: 1222-31 (2011) Article DOI: 10.1016/j.ejmech.2011.01.043 BindingDB Entry DOI: 10.7270/Q2RB74X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM50340835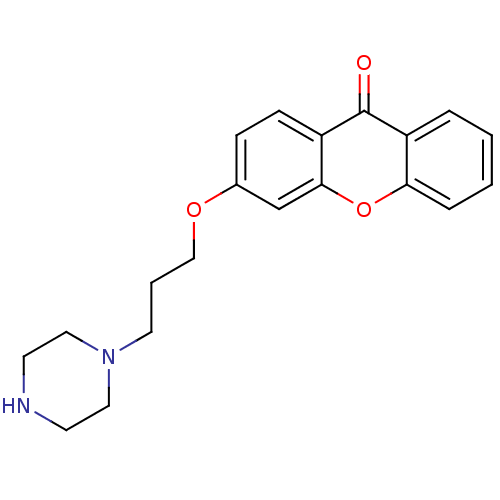 (3-[3-(Piperazino)-propoxy]xanthone | CHEMBL1761211) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.09E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibition of xanthine oxidase activity assessed as uric acid formation pretreated for 15 mins before substrate addition measured after 5 mins | Eur J Med Chem 46: 1222-31 (2011) Article DOI: 10.1016/j.ejmech.2011.01.043 BindingDB Entry DOI: 10.7270/Q2RB74X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM50263222 (2'-Ethoxy-5'-methoxy-2-(5-methylthienyl)chalcone |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.31E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibition of xanthine oxidase (unknown origin) | Bioorg Med Chem 16: 7270-6 (2008) Article DOI: 10.1016/j.bmc.2008.06.031 BindingDB Entry DOI: 10.7270/Q2S182BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50292546 (3,4,5,6-tetrahydroxyxanthone | CHEMBL477921 | US91...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.39E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of ACE | J Nat Prod 55: 691-695 (1992) Article DOI: 10.1021/np50083a025 BindingDB Entry DOI: 10.7270/Q21C1WXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50155447 (1,3,6,7-Tetrahydroxy-xanthen-9-one | 1,3,6,7-tetra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.31E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of ACE | J Nat Prod 55: 691-695 (1992) Article DOI: 10.1021/np50083a025 BindingDB Entry DOI: 10.7270/Q21C1WXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50292545 (2,3,6,7-Tethrahydroxyxanthone | CHEMBL12700) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.69E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of ACE | J Nat Prod 55: 691-695 (1992) Article DOI: 10.1021/np50083a025 BindingDB Entry DOI: 10.7270/Q21C1WXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||