 Found 1919 hits with Last Name = 'liu' and Initial = 'hm'
Found 1919 hits with Last Name = 'liu' and Initial = 'hm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50247053

(1-(3-(3-(4-chlorophenyl)propoxy)propyl)piperidine ...)Show InChI InChI=1S/C17H26ClNO/c18-17-9-7-16(8-10-17)6-4-14-20-15-5-13-19-11-2-1-3-12-19/h7-10H,1-6,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at recombinant human H3 receptor expressed in CHO-K1 cell membranes incubated for 60 mins by [35S]GTPgammaS binding assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112667
BindingDB Entry DOI: 10.7270/Q2HD80BX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM50073587

(CHEMBL3408947 | US10358436, Example A185 | US20230...)Show SMILES COc1ccc2NC(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN6C[C@H](C)O[C@H](C)C6)cc5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University
Curated by ChEMBL
| Assay Description
Competitive inhibition of PLK-4 (unknown origin) |
Eur J Med Chem 95: 35-40 (2015)
Article DOI: 10.1016/j.ejmech.2015.03.020
BindingDB Entry DOI: 10.7270/Q2NG4SBV |
More data for this
Ligand-Target Pair | |
DCN1-like protein 5
(Homo sapiens) | BDBM50525313

(CHEMBL4592844)Show InChI InChI=1S/C18H12N4S3/c1-3-9-23-17-21-15(13-6-4-12(2)5-7-13)14(11-19)16(22-17)25-18-20-8-10-24-18/h1,4-8,10H,9H2,2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University
Curated by ChEMBL
| Assay Description
Binding affinity to N-terminal GST tagged recombinant DCN5 (unknown origin) (47 to 237 residues) expressed in Escherichia coli BL21(DE3) assessed as ... |
J Med Chem 62: 5382-5403 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00003
BindingDB Entry DOI: 10.7270/Q2GT5RM9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM25117

(AG-013736 | AXITINIB | N-methyl-2-({3-[(E)-2-(pyri...)Show SMILES CNC(=O)c1ccccc1Sc1ccc2c(\C=C\c3ccccn3)n[nH]c2c1 Show InChI InChI=1S/C22H18N4OS/c1-23-22(27)18-7-2-3-8-21(18)28-16-10-11-17-19(25-26-20(17)14-16)12-9-15-6-4-5-13-24-15/h2-14H,1H3,(H,23,27)(H,25,26)/b12-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University
Curated by ChEMBL
| Assay Description
Inhibition of PLK-4 (unknown origin) |
Eur J Med Chem 95: 35-40 (2015)
Article DOI: 10.1016/j.ejmech.2015.03.020
BindingDB Entry DOI: 10.7270/Q2NG4SBV |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50271443
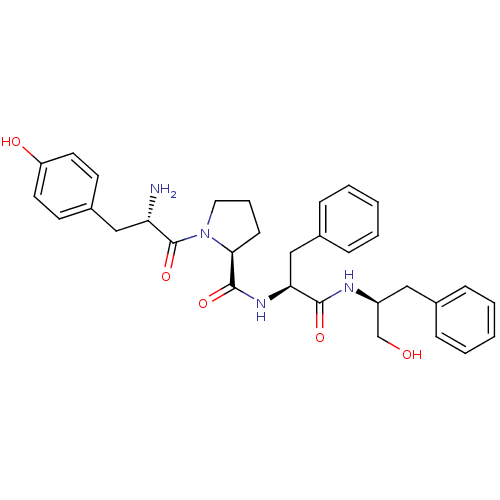
(CHEMBL522293 | Tyr-Pro-Phe-Phe-OCH2OH)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H](CO)Cc1ccccc1 |r| Show InChI InChI=1S/C32H38N4O5/c33-27(19-24-13-15-26(38)16-14-24)32(41)36-17-7-12-29(36)31(40)35-28(20-23-10-5-2-6-11-23)30(39)34-25(21-37)18-22-8-3-1-4-9-22/h1-6,8-11,13-16,25,27-29,37-38H,7,12,17-21,33H2,(H,34,39)(H,35,40)/t25-,27-,28-,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat brain membrane |
Bioorg Med Chem 16: 6415-22 (2008)
Article DOI: 10.1016/j.bmc.2008.05.001
BindingDB Entry DOI: 10.7270/Q2RR1Z1F |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50139013

((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C32H37N5O5/c33-25(18-23-13-15-24(38)16-14-23)32(42)37-17-7-12-28(37)31(41)36-27(20-22-10-5-2-6-11-22)30(40)35-26(29(34)39)19-21-8-3-1-4-9-21/h1-6,8-11,13-16,25-28,38H,7,12,17-20,33H2,(H2,34,39)(H,35,40)(H,36,41)/t25-,26-,27-,28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat brain membrane |
Bioorg Med Chem 16: 6415-22 (2008)
Article DOI: 10.1016/j.bmc.2008.05.001
BindingDB Entry DOI: 10.7270/Q2RR1Z1F |
More data for this
Ligand-Target Pair | |
DCN1-like protein 1
(Homo sapiens) | BDBM50584167
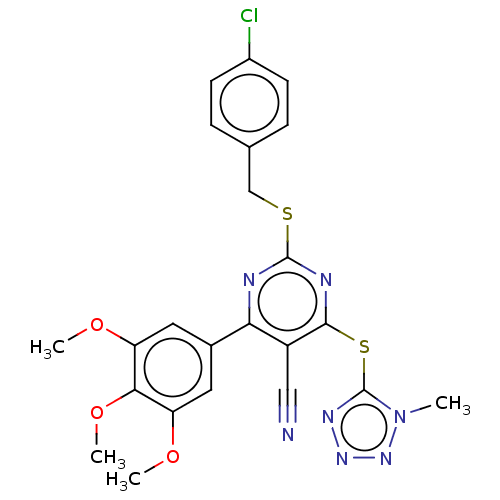
(CHEMBL5085822)Show SMILES COc1cc(cc(OC)c1OC)-c1nc(SCc2ccc(Cl)cc2)nc(Sc2nnnn2C)c1C#N | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to DCN1 (unknown origin) assessed as inhibitory constant |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01207
BindingDB Entry DOI: 10.7270/Q2JD51NJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50163909
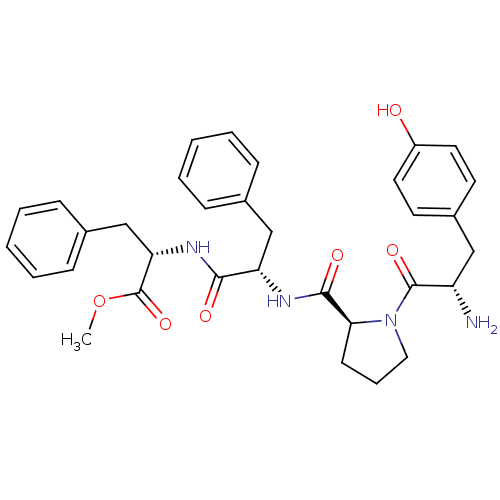
(CHEMBL361922 | Tyr-Pro-Phe-Phe-OCH3 | Tyr-Pro-Phe-...)Show SMILES COC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1ccc(O)cc1 |r| Show InChI InChI=1S/C33H38N4O6/c1-43-33(42)28(21-23-11-6-3-7-12-23)36-30(39)27(20-22-9-4-2-5-10-22)35-31(40)29-13-8-18-37(29)32(41)26(34)19-24-14-16-25(38)17-15-24/h2-7,9-12,14-17,26-29,38H,8,13,18-21,34H2,1H3,(H,35,40)(H,36,39)/t26-,27-,28-,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.07 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat brain membrane |
Bioorg Med Chem 16: 6415-22 (2008)
Article DOI: 10.1016/j.bmc.2008.05.001
BindingDB Entry DOI: 10.7270/Q2RR1Z1F |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50163913
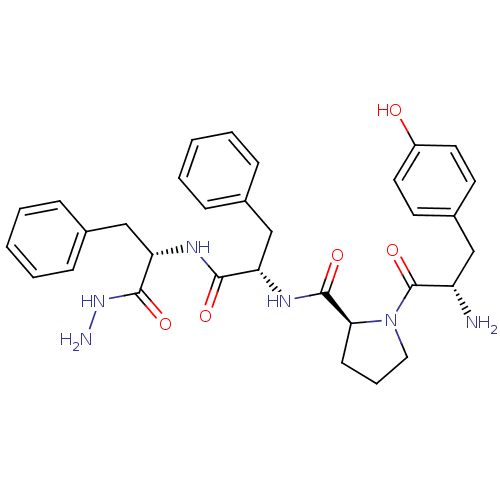
(CHEMBL180777 | Tyr-Pro-Phe-Phe-NHNH2)Show SMILES NNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1ccc(O)cc1 |r| Show InChI InChI=1S/C32H38N6O5/c33-25(18-23-13-15-24(39)16-14-23)32(43)38-17-7-12-28(38)31(42)36-26(19-21-8-3-1-4-9-21)29(40)35-27(30(41)37-34)20-22-10-5-2-6-11-22/h1-6,8-11,13-16,25-28,39H,7,12,17-20,33-34H2,(H,35,40)(H,36,42)(H,37,41)/t25-,26-,27-,28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat brain membrane |
Bioorg Med Chem 16: 6415-22 (2008)
Article DOI: 10.1016/j.bmc.2008.05.001
BindingDB Entry DOI: 10.7270/Q2RR1Z1F |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50556205

(BAY 1841788 | BAY-1841788 | BAY1841788 | Darolutam...)Show SMILES C[C@@H](Cn1ccc(n1)-c1ccc(C#N)c(Cl)c1)NC(=O)c1cc([nH]n1)C(C)O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of androgen receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112667
BindingDB Entry DOI: 10.7270/Q2HD80BX |
More data for this
Ligand-Target Pair | |
DCN1-like protein 1
(Homo sapiens) | BDBM50525354
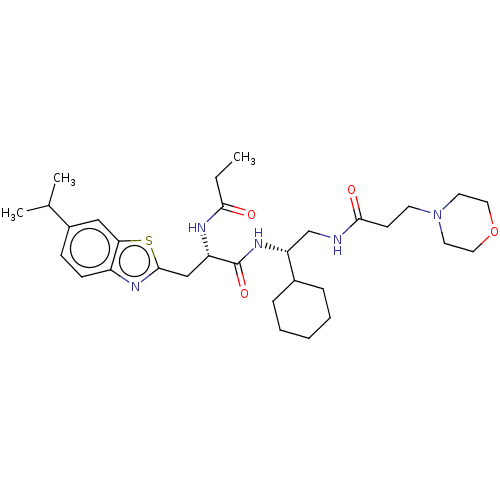
(CHEMBL4517307)Show SMILES CCC(=O)N[C@@H](Cc1nc2ccc(cc2s1)C(C)C)C(=O)N[C@H](CNC(=O)CCN1CCOCC1)C1CCCCC1 |r| Show InChI InChI=1S/C31H47N5O4S/c1-4-28(37)33-25(19-30-34-24-11-10-23(21(2)3)18-27(24)41-30)31(39)35-26(22-8-6-5-7-9-22)20-32-29(38)12-13-36-14-16-40-17-15-36/h10-11,18,21-22,25-26H,4-9,12-17,19-20H2,1-3H3,(H,32,38)(H,33,37)(H,35,39)/t25-,26+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
DCN1-like protein 1
(Homo sapiens) | BDBM50525313

(CHEMBL4592844)Show InChI InChI=1S/C18H12N4S3/c1-3-9-23-17-21-15(13-6-4-12(2)5-7-13)14(11-19)16(22-17)25-18-20-8-10-24-18/h1,4-8,10H,9H2,2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to DCN1 (unknown origin) assessed as inhibitory constant |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01207
BindingDB Entry DOI: 10.7270/Q2JD51NJ |
More data for this
Ligand-Target Pair | |
DCN1-like protein 1
(Homo sapiens) | BDBM50525313

(CHEMBL4592844)Show InChI InChI=1S/C18H12N4S3/c1-3-9-23-17-21-15(13-6-4-12(2)5-7-13)14(11-19)16(22-17)25-18-20-8-10-24-18/h1,4-8,10H,9H2,2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University
Curated by ChEMBL
| Assay Description
Binding affinity to N-terminal GST tagged recombinant human DCN1 (58 to 259 residues) expressed in Escherichia coli BL21(DE3) assessed as reduction i... |
J Med Chem 62: 5382-5403 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00003
BindingDB Entry DOI: 10.7270/Q2GT5RM9 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50271441
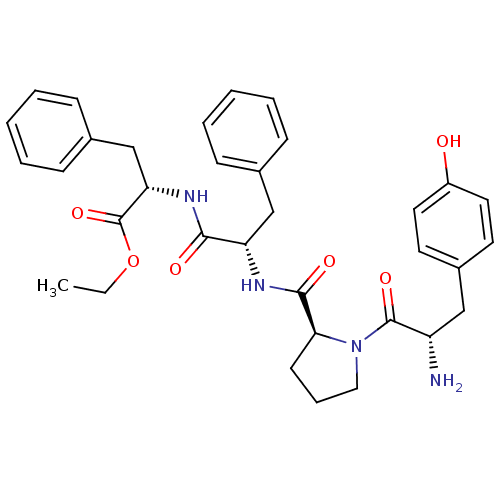
(CHEMBL505502 | Tyr-Pro-Phe-Phe-OCH2CH3)Show SMILES CCOC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1ccc(O)cc1 |r| Show InChI InChI=1S/C34H40N4O6/c1-2-44-34(43)29(22-24-12-7-4-8-13-24)37-31(40)28(21-23-10-5-3-6-11-23)36-32(41)30-14-9-19-38(30)33(42)27(35)20-25-15-17-26(39)18-16-25/h3-8,10-13,15-18,27-30,39H,2,9,14,19-22,35H2,1H3,(H,36,41)(H,37,40)/t27-,28-,29-,30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15.0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat brain membrane |
Bioorg Med Chem 16: 6415-22 (2008)
Article DOI: 10.1016/j.bmc.2008.05.001
BindingDB Entry DOI: 10.7270/Q2RR1Z1F |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50407800
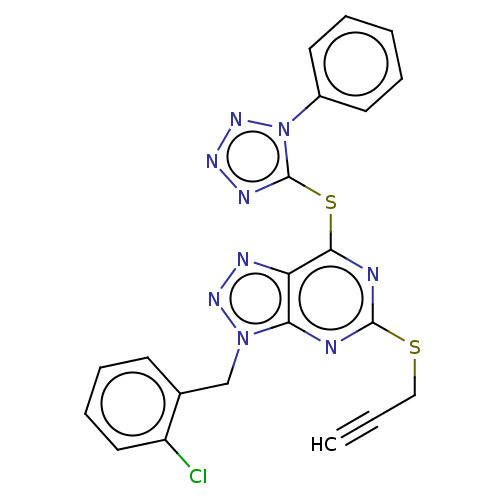
(CHEMBL5291138)Show SMILES NC(=N)NCCC[C@@H]1NC(=O)[C@@H]2CC3CCCCC3N2C(=O)[C@H]2Cc3ccccc3CN2C(=O)[C@H](Cc2cccs2)NC(=O)[C@H](CCCNC(N)=N)NC1=O |r| Show InChI InChI=1S/C38H53N11O5S/c39-37(40)43-15-5-12-26-33(51)47-28(20-25-11-7-17-55-25)35(53)48-21-24-10-2-1-8-22(24)18-31(48)36(54)49-29-14-4-3-9-23(29)19-30(49)34(52)46-27(32(50)45-26)13-6-16-44-38(41)42/h1-2,7-8,10-11,17,23,26-31H,3-6,9,12-16,18-21H2,(H,45,50)(H,46,52)(H,47,51)(H4,39,40,43)(H4,41,42,44)/t23?,26-,27-,28-,29?,30-,31+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mean functional activity against human H3 receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | |
DCN1-like protein 1
(Homo sapiens) | BDBM50525313

(CHEMBL4592844)Show InChI InChI=1S/C18H12N4S3/c1-3-9-23-17-21-15(13-6-4-12(2)5-7-13)14(11-19)16(22-17)25-18-20-8-10-24-18/h1,4-8,10H,9H2,2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University
Curated by ChEMBL
| Assay Description
Binding affinity to N-terminal His6-tagged recombinant human DCN1 expressed in Escherichia coli BL21-AI assessed as reduction in DCN1-FAM-labelled N-... |
J Med Chem 62: 5382-5403 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00003
BindingDB Entry DOI: 10.7270/Q2GT5RM9 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50553786

(CHEMBL4789106)Show SMILES CC1(CN)CCN(CC1)c1cnc(Sc2cccc(Cl)c2Cl)c(N)n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114106
BindingDB Entry DOI: 10.7270/Q26977NN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50142238
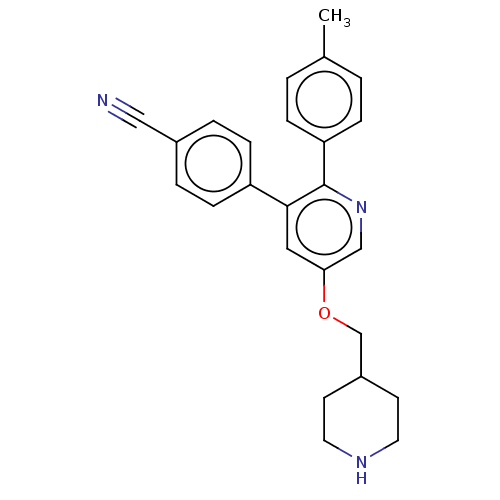
(CHEMBL3759201)Show SMILES Cc1ccc(cc1)-c1ncc(OCC2CCNCC2)cc1-c1ccc(cc1)C#N Show InChI InChI=1S/C25H25N3O/c1-18-2-6-22(7-3-18)25-24(21-8-4-19(15-26)5-9-21)14-23(16-28-25)29-17-20-10-12-27-13-11-20/h2-9,14,16,20,27H,10-13,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University
Curated by ChEMBL
| Assay Description
Dissociation constant against galectin-3 using competitive fluorescence polarization |
Eur J Med Chem 125: 940-951 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.021
BindingDB Entry DOI: 10.7270/Q2ZC8597 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50271439
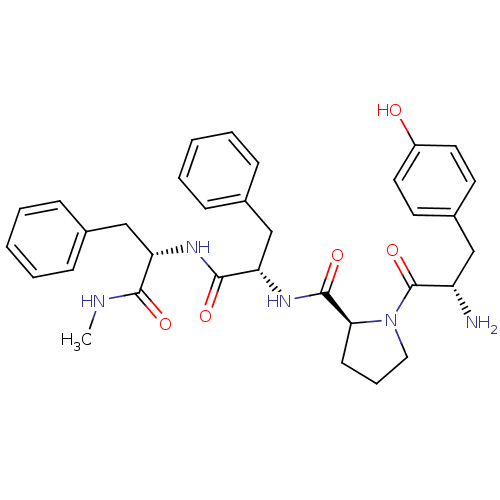
(CHEMBL453689 | Tyr-Pro-Phe-Phe-NHCH3)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1ccc(O)cc1 |r| Show InChI InChI=1S/C33H39N5O5/c1-35-30(40)27(20-22-9-4-2-5-10-22)36-31(41)28(21-23-11-6-3-7-12-23)37-32(42)29-13-8-18-38(29)33(43)26(34)19-24-14-16-25(39)17-15-24/h2-7,9-12,14-17,26-29,39H,8,13,18-21,34H2,1H3,(H,35,40)(H,36,41)(H,37,42)/t26-,27-,28-,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat brain membrane |
Bioorg Med Chem 16: 6415-22 (2008)
Article DOI: 10.1016/j.bmc.2008.05.001
BindingDB Entry DOI: 10.7270/Q2RR1Z1F |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50346870
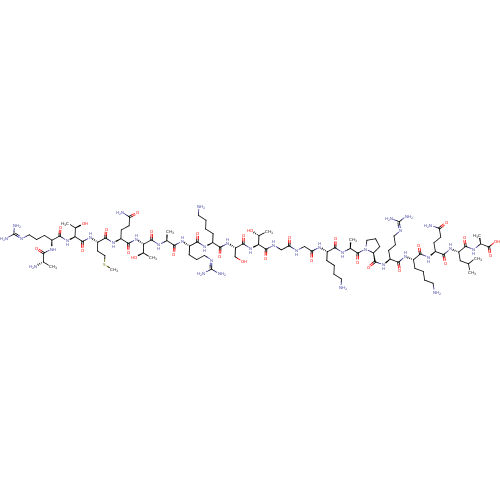
(CHEMBL1797647)Show SMILES [#6]-[#16]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7])-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](-[#8])=O |r| Show InChI InChI=1S/C93H169N35O28S/c1-45(2)41-62(83(148)113-49(6)90(155)156)123-79(144)59(28-30-65(98)133)119-75(140)54(22-12-15-34-95)117-77(142)57(25-18-37-107-92(102)103)121-85(150)64-27-20-39-128(64)89(154)48(5)112-74(139)53(21-11-14-33-94)114-68(136)43-109-67(135)42-110-86(151)69(50(7)130)125-84(149)63(44-129)124-78(143)55(23-13-16-35-96)118-76(141)56(24-17-36-106-91(100)101)116-73(138)47(4)111-87(152)70(51(8)131)126-82(147)60(29-31-66(99)134)120-80(145)61(32-40-157-10)122-88(153)71(52(9)132)127-81(146)58(115-72(137)46(3)97)26-19-38-108-93(104)105/h45-64,69-71,129-132H,11-44,94-97H2,1-10H3,(H2,98,133)(H2,99,134)(H,109,135)(H,110,151)(H,111,152)(H,112,139)(H,113,148)(H,114,136)(H,115,137)(H,116,138)(H,117,142)(H,118,141)(H,119,140)(H,120,145)(H,121,150)(H,122,153)(H,123,144)(H,124,143)(H,125,149)(H,126,147)(H,127,146)(H,155,156)(H4,100,101,106)(H4,102,103,107)(H4,104,105,108)/t46-,47-,48-,49-,50+,51+,52+,53-,54-,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-,69-,70-,71-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Blocking activity was assessed by antagonism of (-)-noradrenaline induced contraction of rat prostatic vas deferens (alpha1A adrenoceptor) |
Citation and Details
|
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50346870

(CHEMBL1797647)Show SMILES [#6]-[#16]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7])-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](-[#8])=O |r| Show InChI InChI=1S/C93H169N35O28S/c1-45(2)41-62(83(148)113-49(6)90(155)156)123-79(144)59(28-30-65(98)133)119-75(140)54(22-12-15-34-95)117-77(142)57(25-18-37-107-92(102)103)121-85(150)64-27-20-39-128(64)89(154)48(5)112-74(139)53(21-11-14-33-94)114-68(136)43-109-67(135)42-110-86(151)69(50(7)130)125-84(149)63(44-129)124-78(143)55(23-13-16-35-96)118-76(141)56(24-17-36-106-91(100)101)116-73(138)47(4)111-87(152)70(51(8)131)126-82(147)60(29-31-66(99)134)120-80(145)61(32-40-157-10)122-88(153)71(52(9)132)127-81(146)58(115-72(137)46(3)97)26-19-38-108-93(104)105/h45-64,69-71,129-132H,11-44,94-97H2,1-10H3,(H2,98,133)(H2,99,134)(H,109,135)(H,110,151)(H,111,152)(H,112,139)(H,113,148)(H,114,136)(H,115,137)(H,116,138)(H,117,142)(H,118,141)(H,119,140)(H,120,145)(H,121,150)(H,122,153)(H,123,144)(H,124,143)(H,125,149)(H,126,147)(H,127,146)(H,155,156)(H4,100,101,106)(H4,102,103,107)(H4,104,105,108)/t46-,47-,48-,49-,50+,51+,52+,53-,54-,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-,69-,70-,71-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mean functional activity against human H3 receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50271442
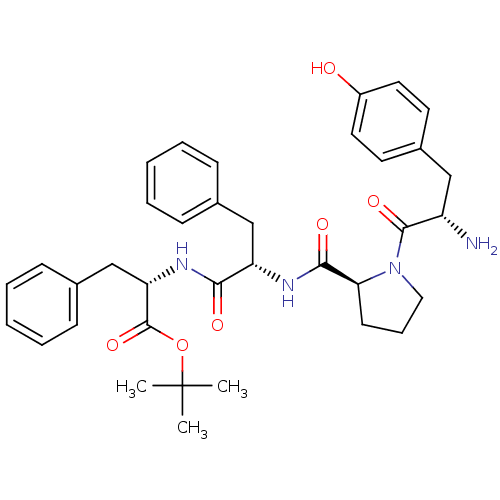
(CHEMBL500195 | Tyr-Pro-Phe-Phe-OC(CH3)3)Show SMILES CC(C)(C)OC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1ccc(O)cc1 |r| Show InChI InChI=1S/C36H44N4O6/c1-36(2,3)46-35(45)30(23-25-13-8-5-9-14-25)39-32(42)29(22-24-11-6-4-7-12-24)38-33(43)31-15-10-20-40(31)34(44)28(37)21-26-16-18-27(41)19-17-26/h4-9,11-14,16-19,28-31,41H,10,15,20-23,37H2,1-3H3,(H,38,43)(H,39,42)/t28-,29-,30-,31-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat brain membrane |
Bioorg Med Chem 16: 6415-22 (2008)
Article DOI: 10.1016/j.bmc.2008.05.001
BindingDB Entry DOI: 10.7270/Q2RR1Z1F |
More data for this
Ligand-Target Pair | |
DCN1-like protein 2
(Homo sapiens) | BDBM50525313

(CHEMBL4592844)Show InChI InChI=1S/C18H12N4S3/c1-3-9-23-17-21-15(13-6-4-12(2)5-7-13)14(11-19)16(22-17)25-18-20-8-10-24-18/h1,4-8,10H,9H2,2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University
Curated by ChEMBL
| Assay Description
Binding affinity to N-terminal GST tagged recombinant DCN2 (unknown origin) (62 to 259 residues) expressed in Escherichia coli BL21(DE3) assessed as ... |
J Med Chem 62: 5382-5403 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00003
BindingDB Entry DOI: 10.7270/Q2GT5RM9 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM38019

(US10093646, Compound 1 | US10301278, Example 00003...)Show InChI InChI=1S/C24H34N4O2/c1-18-8-7-9-19-16-20(23(29)26-22(18)19)17-28(15-14-27-12-5-6-13-27)24(30)25-21-10-3-2-4-11-21/h7-9,16,21H,2-6,10-15,17H2,1H3,(H,25,30)(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114106
BindingDB Entry DOI: 10.7270/Q26977NN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM50425732
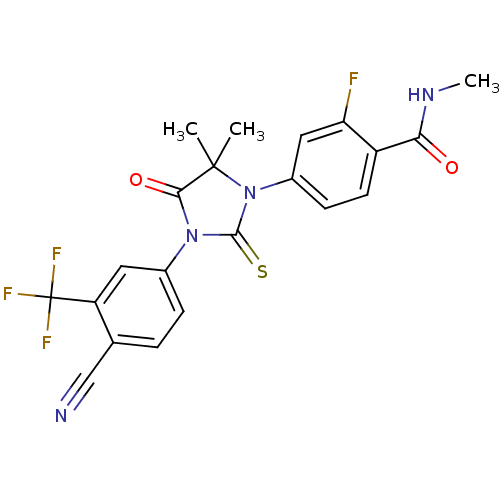
(ENZALUTAMIDE | US10053433, FC 4.129 | US10806720, ...)Show SMILES CNC(=O)c1ccc(cc1F)N1C(=S)N(C(=O)C1(C)C)c1ccc(C#N)c(c1)C(F)(F)F Show InChI InChI=1S/C21H16F4N4O2S/c1-20(2)18(31)28(12-5-4-11(10-26)15(8-12)21(23,24)25)19(32)29(20)13-6-7-14(16(22)9-13)17(30)27-3/h4-9H,1-3H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of androgen receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112667
BindingDB Entry DOI: 10.7270/Q2HD80BX |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50094975

(956104-40-8 | ARN-509 | JNJ-56021927 | US10053433,...)Show SMILES CNC(=O)c1ccc(cc1F)N1C(=S)N(C(=O)C11CCC1)c1cnc(C#N)c(c1)C(F)(F)F Show InChI InChI=1S/C21H15F4N5O2S/c1-27-17(31)13-4-3-11(8-15(13)22)30-19(33)29(18(32)20(30)5-2-6-20)12-7-14(21(23,24)25)16(9-26)28-10-12/h3-4,7-8,10H,2,5-6H2,1H3,(H,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of androgen receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112667
BindingDB Entry DOI: 10.7270/Q2HD80BX |
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50586363
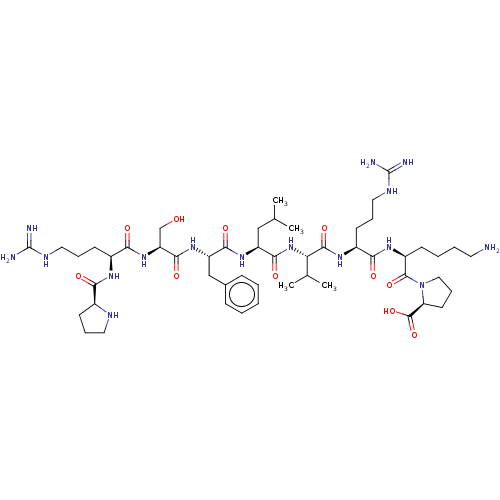
(CHEMBL5079374)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(O)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Blocking activity was assessed by antagonism of (-)-noradrenaline induced contraction of rat prostatic vas deferens (alpha1A adrenoceptor) |
Citation and Details
|
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50407795

(CHEMBL5281080)Show SMILES C[C@@H]1NC(=O)[C@H](Cc2cccs2)NC(=O)CCCNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]2CC3CCCCC3N2C(=O)[C@H]2Cc3ccccc3CN2C1=O |r| Show InChI InChI=1S/C39H53N9O6S/c1-23-37(53)47-22-26-11-3-2-9-24(26)19-32(47)38(54)48-30-14-5-4-10-25(30)20-31(48)36(52)46-28(13-6-17-43-39(40)41)34(50)42-16-7-15-33(49)45-29(35(51)44-23)21-27-12-8-18-55-27/h2-3,8-9,11-12,18,23,25,28-32H,4-7,10,13-17,19-22H2,1H3,(H,42,50)(H,44,51)(H,45,49)(H,46,52)(H4,40,41,43)/t23-,25?,28-,29-,30?,31-,32+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 157 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mean functional activity against human H3 receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50407796
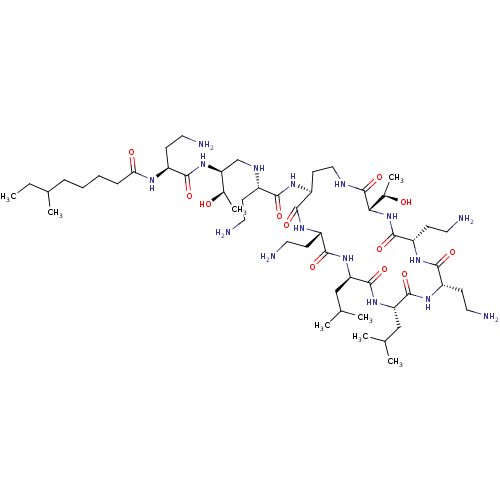
(CHEMBL1255711)Show SMILES [#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-c2ccccc2-[#6]-[#6@H]-1-[#6](=O)-[#7]-1-[#6]-2-[#6]-[#6]-[#6]-[#6]-[#6]-2-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](-[#8])=O |r| Show InChI InChI=1S/C36H48N8O6/c37-20-31(45)41-27(17-22-9-2-1-3-10-22)33(47)43-21-25-13-5-4-11-23(25)18-30(43)34(48)44-28-15-7-6-12-24(28)19-29(44)32(46)42-26(35(49)50)14-8-16-40-36(38)39/h1-5,9-11,13,24,26-30H,6-8,12,14-21,37H2,(H,41,45)(H,42,46)(H,49,50)(H4,38,39,40)/t24?,26-,27-,28?,29-,30-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 193 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mean functional activity against human H3 receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50407722

(CHEMBL5287743)Show InChI InChI=1S/C13H11N3OS2/c1-8-6-12(19-16-8)15-13(17)14-10-2-3-11-9(7-10)4-5-18-11/h2-7H,1H3,(H2,14,15,17) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Blocking activity was assessed by antagonism of (-)-phenylephrine induced contraction of rat spleen (alpha1B adrenoceptor) |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50271442

(CHEMBL500195 | Tyr-Pro-Phe-Phe-OC(CH3)3)Show SMILES CC(C)(C)OC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1ccc(O)cc1 |r| Show InChI InChI=1S/C36H44N4O6/c1-36(2,3)46-35(45)30(23-25-13-8-5-9-14-25)39-32(42)29(22-24-11-6-4-7-12-24)38-33(43)31-15-10-20-40(31)34(44)28(37)21-26-16-18-27(41)19-17-26/h4-9,11-14,16-19,28-31,41H,10,15,20-23,37H2,1-3H3,(H,38,43)(H,39,42)/t28-,29-,30-,31-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 247 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in rat brain membrane |
Bioorg Med Chem 16: 6415-22 (2008)
Article DOI: 10.1016/j.bmc.2008.05.001
BindingDB Entry DOI: 10.7270/Q2RR1Z1F |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50271440

(CHEMBL505975 | Tyr-Pro-Phe-Phe-N(CH3)2)Show SMILES CN(C)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1ccc(O)cc1 |r| Show InChI InChI=1S/C34H41N5O5/c1-38(2)34(44)29(22-24-12-7-4-8-13-24)37-31(41)28(21-23-10-5-3-6-11-23)36-32(42)30-14-9-19-39(30)33(43)27(35)20-25-15-17-26(40)18-16-25/h3-8,10-13,15-18,27-30,40H,9,14,19-22,35H2,1-2H3,(H,36,42)(H,37,41)/t27-,28-,29-,30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 273 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat brain membrane |
Bioorg Med Chem 16: 6415-22 (2008)
Article DOI: 10.1016/j.bmc.2008.05.001
BindingDB Entry DOI: 10.7270/Q2RR1Z1F |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50568522
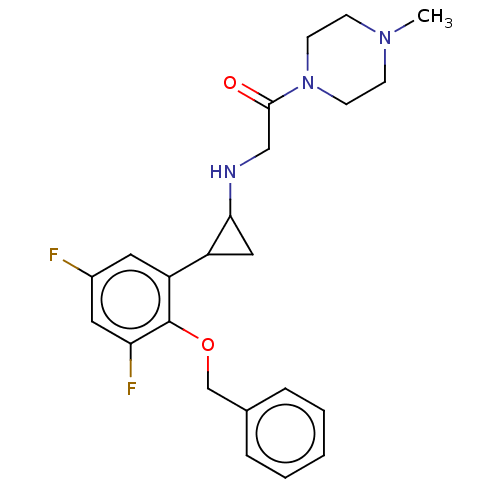
(CHEMBL4864352)Show SMILES CN1CCN(CC1)C(=O)CNC1CC1c1cc(F)cc(F)c1OCc1ccccc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human LSD1 (172 to 833 residues) using Lys4-dimethylated H3 (1-20) peptide as substrate incubated for 30 mins by peroxidase-coupled ass... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00919
BindingDB Entry DOI: 10.7270/Q28K7DTB |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50346586
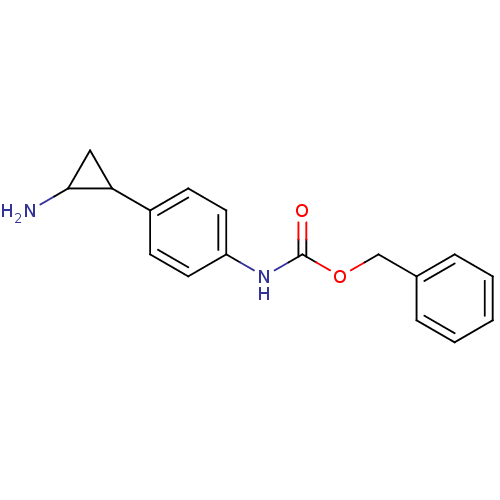
(CHEMBL1795981 | US8765820, 5a)Show InChI InChI=1S/C17H18N2O2/c18-16-10-15(16)13-6-8-14(9-7-13)19-17(20)21-11-12-4-2-1-3-5-12/h1-9,15-16H,10-11,18H2,(H,19,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human MAO-A expressed in Pichia pastoris using kynuramine as substrate by peroxidase-coupled method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00919
BindingDB Entry DOI: 10.7270/Q28K7DTB |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50568521
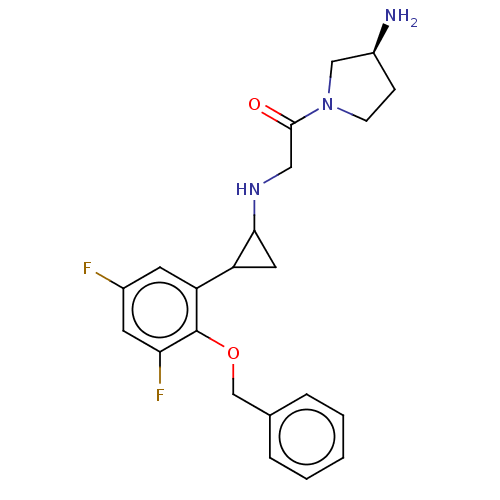
(CHEMBL4878787)Show SMILES N[C@H]1CCN(C1)C(=O)CNC1CC1c1cc(F)cc(F)c1OCc1ccccc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human LSD1 (172 to 833 residues) using Lys4-dimethylated H3 (1-20) peptide as substrate incubated for 30 mins by peroxidase-coupled ass... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00919
BindingDB Entry DOI: 10.7270/Q28K7DTB |
More data for this
Ligand-Target Pair | |
DCN1-like protein 3
(Homo sapiens) | BDBM50525313

(CHEMBL4592844)Show InChI InChI=1S/C18H12N4S3/c1-3-9-23-17-21-15(13-6-4-12(2)5-7-13)14(11-19)16(22-17)25-18-20-8-10-24-18/h1,4-8,10H,9H2,2H3 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 592 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University
Curated by ChEMBL
| Assay Description
Binding affinity to N-terminal GST tagged recombinant DCN3 (unknown origin) (86 to 304 residues) expressed in Escherichia coli BL21(DE3) assessed as ... |
J Med Chem 62: 5382-5403 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00003
BindingDB Entry DOI: 10.7270/Q2GT5RM9 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50346585
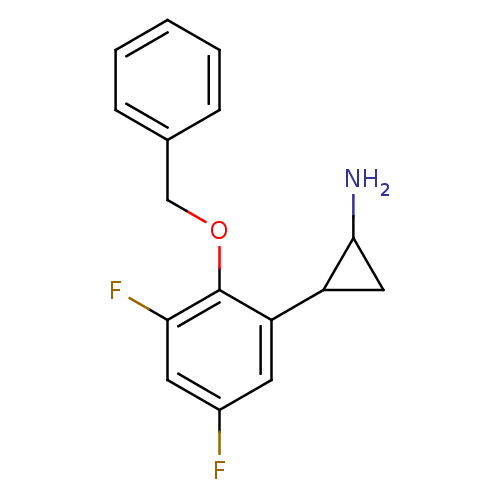
(CHEMBL1795980 | S2101)Show InChI InChI=1S/C16H15F2NO/c17-11-6-13(12-8-15(12)19)16(14(18)7-11)20-9-10-4-2-1-3-5-10/h1-7,12,15H,8-9,19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of hexahistidine-tagged human LSD1 (172 to 833 residues) expressed in Escherichia coli Rosetta (DE3) cells using H3K4me2 peptide as substr... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00919
BindingDB Entry DOI: 10.7270/Q28K7DTB |
More data for this
Ligand-Target Pair | |
DCN1-like protein 4
(Homo sapiens) | BDBM50525313

(CHEMBL4592844)Show InChI InChI=1S/C18H12N4S3/c1-3-9-23-17-21-15(13-6-4-12(2)5-7-13)14(11-19)16(22-17)25-18-20-8-10-24-18/h1,4-8,10H,9H2,2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 807 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University
Curated by ChEMBL
| Assay Description
Binding affinity to N-terminal GST tagged recombinant DCN4 (unknown origin) (102 to 292 residues) expressed in Escherichia coli BL21(DE3) assessed as... |
J Med Chem 62: 5382-5403 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00003
BindingDB Entry DOI: 10.7270/Q2GT5RM9 |
More data for this
Ligand-Target Pair | |
DCN1-like protein 4
(Homo sapiens) | BDBM50525313

(CHEMBL4592844)Show InChI InChI=1S/C18H12N4S3/c1-3-9-23-17-21-15(13-6-4-12(2)5-7-13)14(11-19)16(22-17)25-18-20-8-10-24-18/h1,4-8,10H,9H2,2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to DCN4 (unknown origin) assessed as inhibitory constant |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01207
BindingDB Entry DOI: 10.7270/Q2JD51NJ |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50346585

(CHEMBL1795980 | S2101)Show InChI InChI=1S/C16H15F2NO/c17-11-6-13(12-8-15(12)19)16(14(18)7-11)20-9-10-4-2-1-3-5-10/h1-7,12,15H,8-9,19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human LSD1 (172 to 833 residues) using Lys4-dimethylated H3 (1-20) peptide as substrate incubated for 30 mins by peroxidase-coupled ass... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00919
BindingDB Entry DOI: 10.7270/Q28K7DTB |
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50346863
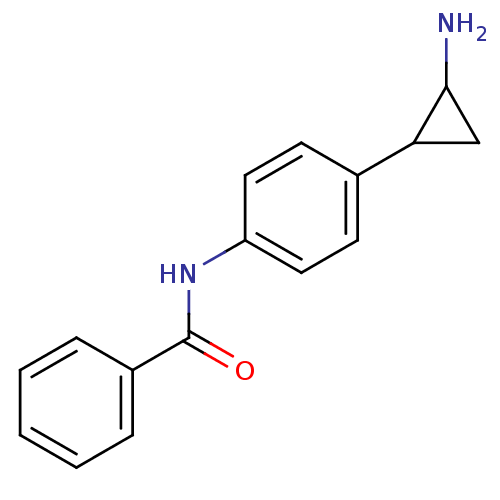
(CHEMBL1797640 | US8765820, 5b)Show InChI InChI=1S/C16H16N2O/c17-15-10-14(15)11-6-8-13(9-7-11)18-16(19)12-4-2-1-3-5-12/h1-9,14-15H,10,17H2,(H,18,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human LSD1/CoREST expressed in Escherichia coli using histone H3 peptide monomethylated at Lys4 as substrate by peroxidase-coupled assa... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00919
BindingDB Entry DOI: 10.7270/Q28K7DTB |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50346864
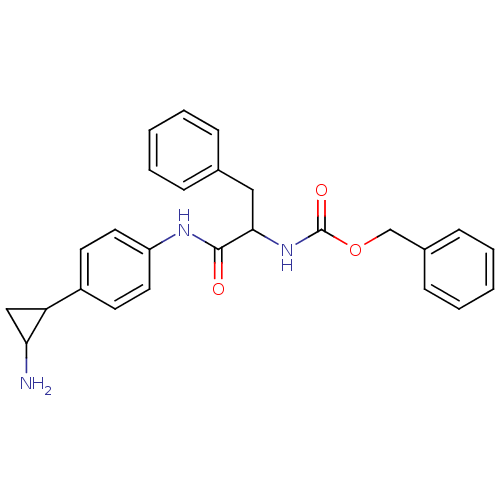
(CHEMBL1797641 | CHEMBL3104337 | US8765820, 8)Show SMILES NC1CC1c1ccc(NC(=O)C(Cc2ccccc2)NC(=O)OCc2ccccc2)cc1 Show InChI InChI=1S/C26H27N3O3/c27-23-16-22(23)20-11-13-21(14-12-20)28-25(30)24(15-18-7-3-1-4-8-18)29-26(31)32-17-19-9-5-2-6-10-19/h1-14,22-24H,15-17,27H2,(H,28,30)(H,29,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human MAO-A expressed in Pichia pastoris using kynuramine as substrate by peroxidase-coupled method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00919
BindingDB Entry DOI: 10.7270/Q28K7DTB |
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50346864

(CHEMBL1797641 | CHEMBL3104337 | US8765820, 8)Show SMILES NC1CC1c1ccc(NC(=O)C(Cc2ccccc2)NC(=O)OCc2ccccc2)cc1 Show InChI InChI=1S/C26H27N3O3/c27-23-16-22(23)20-11-13-21(14-12-20)28-25(30)24(15-18-7-3-1-4-8-18)29-26(31)32-17-19-9-5-2-6-10-19/h1-14,22-24H,15-17,27H2,(H,28,30)(H,29,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human LSD1/CoREST expressed in Escherichia coli using histone H3 peptide monomethylated at Lys4 as substrate by peroxidase-coupled assa... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00919
BindingDB Entry DOI: 10.7270/Q28K7DTB |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50158869
(CHEMBL3786182 | US10836743, Compound GSK-2879552 |...)Show SMILES OC(=O)c1ccc(CN2CCC(CN[C@@H]3C[C@H]3c3ccccc3)CC2)cc1 |r| Show InChI InChI=1S/C23H28N2O2/c26-23(27)20-8-6-18(7-9-20)16-25-12-10-17(11-13-25)15-24-22-14-21(22)19-4-2-1-3-5-19/h1-9,17,21-22,24H,10-16H2,(H,26,27)/t21-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of LSD1 (unknown origin) using H3K4(diMe) peptide as substrate measured after 60 mins by amplex red dye based HRP-coupled assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00919
BindingDB Entry DOI: 10.7270/Q28K7DTB |
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50346586

(CHEMBL1795981 | US8765820, 5a)Show InChI InChI=1S/C17H18N2O2/c18-16-10-15(16)13-6-8-14(9-7-13)19-17(20)21-11-12-4-2-1-3-5-12/h1-9,15-16H,10-11,18H2,(H,19,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human LSD1/CoREST expressed in Escherichia coli using histone H3 peptide monomethylated at Lys4 as substrate by peroxidase-coupled assa... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00919
BindingDB Entry DOI: 10.7270/Q28K7DTB |
More data for this
Ligand-Target Pair | |
DCN1-like protein 5
(Homo sapiens) | BDBM50525313

(CHEMBL4592844)Show InChI InChI=1S/C18H12N4S3/c1-3-9-23-17-21-15(13-6-4-12(2)5-7-13)14(11-19)16(22-17)25-18-20-8-10-24-18/h1,4-8,10H,9H2,2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to DCN5 (unknown origin) assessed as inhibitory constant |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01207
BindingDB Entry DOI: 10.7270/Q2JD51NJ |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50346863

(CHEMBL1797640 | US8765820, 5b)Show InChI InChI=1S/C16H16N2O/c17-15-10-14(15)11-6-8-13(9-7-11)18-16(19)12-4-2-1-3-5-12/h1-9,14-15H,10,17H2,(H,18,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human MAO-A expressed in Pichia pastoris using kynuramine as substrate by peroxidase-coupled method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00919
BindingDB Entry DOI: 10.7270/Q28K7DTB |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50407723
(CHEMBL5274037)Show InChI InChI=1S/C13H11N3O2S/c1-8-6-12(18-16-8)15-13(17)14-10-2-3-11-9(7-10)4-5-19-11/h2-7H,1H3,(H2,14,15,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mean functional activity against human H3 receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50346866
(CHEMBL1797643 | S1201)Show InChI InChI=1S/C16H16FNO/c17-14-8-4-7-12(13-9-15(13)18)16(14)19-10-11-5-2-1-3-6-11/h1-8,13,15H,9-10,18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of hexahistidine-tagged human LSD1 (172 to 833 residues) expressed in Escherichia coli Rosetta (DE3) cells using H3K4me2 peptide as substr... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00919
BindingDB Entry DOI: 10.7270/Q28K7DTB |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50271441

(CHEMBL505502 | Tyr-Pro-Phe-Phe-OCH2CH3)Show SMILES CCOC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1ccc(O)cc1 |r| Show InChI InChI=1S/C34H40N4O6/c1-2-44-34(43)29(22-24-12-7-4-8-13-24)37-31(40)28(21-23-10-5-3-6-11-23)36-32(41)30-14-9-19-38(30)33(42)27(35)20-25-15-17-26(39)18-16-25/h3-8,10-13,15-18,27-30,39H,2,9,14,19-22,35H2,1H3,(H,36,41)(H,37,40)/t27-,28-,29-,30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in rat brain membrane |
Bioorg Med Chem 16: 6415-22 (2008)
Article DOI: 10.1016/j.bmc.2008.05.001
BindingDB Entry DOI: 10.7270/Q2RR1Z1F |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data