

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydrofolate reductase (Escherichia coli) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli DHFR | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant human DHFR assessed as reduction in NADPH oxidation using dihydrofolate as substrate by fluorescence spectrophotometric ana... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM18069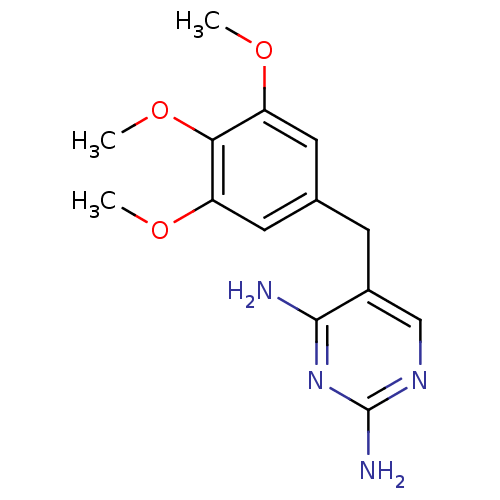 (5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-d...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dihydrof... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM210930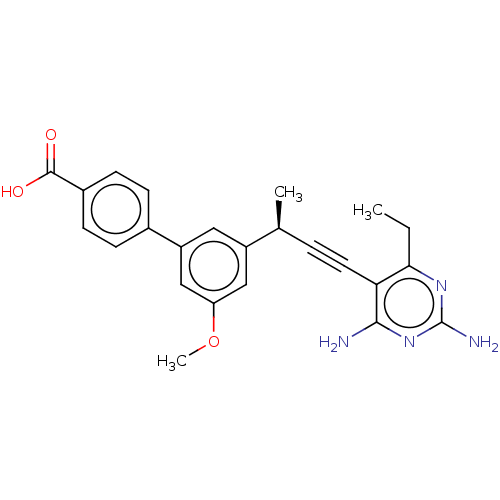 (UCP1173) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dihydrof... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50190621 (CHEMBL3827532 | US10870625, Compound 15) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dihydrof... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM210930 (UCP1173) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Staphylococcus aureus DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dih... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM210931 (UCP1175) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Staphylococcus aureus DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dih... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM210929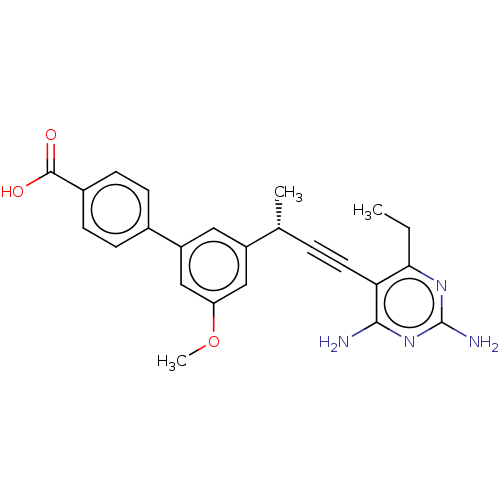 (UCP1172) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dihydrof... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50190622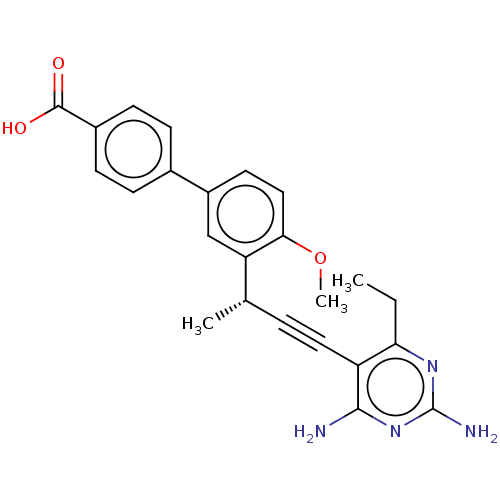 (CHEMBL3827326) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dihydrof... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50190619 (CHEMBL3827086 | US10870625, Compound 14) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dihydrof... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM210929 (UCP1172) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Staphylococcus aureus DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dih... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM210928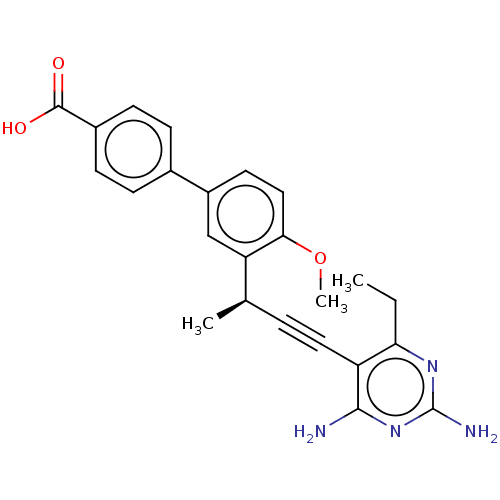 (UCP1164) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dihydrof... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM18069 (5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-d...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Staphylococcus aureus DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dih... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50190621 (CHEMBL3827532 | US10870625, Compound 15) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Staphylococcus aureus DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dih... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM210931 (UCP1175) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dihydrof... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50190622 (CHEMBL3827326) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Staphylococcus aureus DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dih... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50190620 (CHEMBL3828724) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dihydrof... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50190620 (CHEMBL3828724) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Staphylococcus aureus DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dih... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM210928 (UCP1164) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Staphylococcus aureus DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dih... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50190619 (CHEMBL3827086 | US10870625, Compound 14) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Staphylococcus aureus DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dih... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM210931 (UCP1175) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 159 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant human DHFR assessed as reduction in NADPH oxidation using dihydrofolate as substrate preincubated for 5 mins followed by su... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50190621 (CHEMBL3827532 | US10870625, Compound 15) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 201 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant human DHFR assessed as reduction in NADPH oxidation using dihydrofolate as substrate preincubated for 5 mins followed by su... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM210930 (UCP1173) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 234 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant human DHFR assessed as reduction in NADPH oxidation using dihydrofolate as substrate preincubated for 5 mins followed by su... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50190620 (CHEMBL3828724) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 346 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant human DHFR assessed as reduction in NADPH oxidation using dihydrofolate as substrate preincubated for 5 mins followed by su... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50190622 (CHEMBL3827326) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 377 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant human DHFR assessed as reduction in NADPH oxidation using dihydrofolate as substrate preincubated for 5 mins followed by su... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM210929 (UCP1172) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 389 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant human DHFR assessed as reduction in NADPH oxidation using dihydrofolate as substrate preincubated for 5 mins followed by su... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM210928 (UCP1164) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 451 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant human DHFR assessed as reduction in NADPH oxidation using dihydrofolate as substrate preincubated for 5 mins followed by su... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50190619 (CHEMBL3827086 | US10870625, Compound 14) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant human DHFR assessed as reduction in NADPH oxidation using dihydrofolate as substrate preincubated for 5 mins followed by su... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM18069 (5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 4.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant human DHFR assessed as reduction in NADPH oxidation using dihydrofolate as substrate preincubated for 5 mins followed by su... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Metallo-beta-lactamase type 2 (Klebsiella pneumoniae) | BDBM262675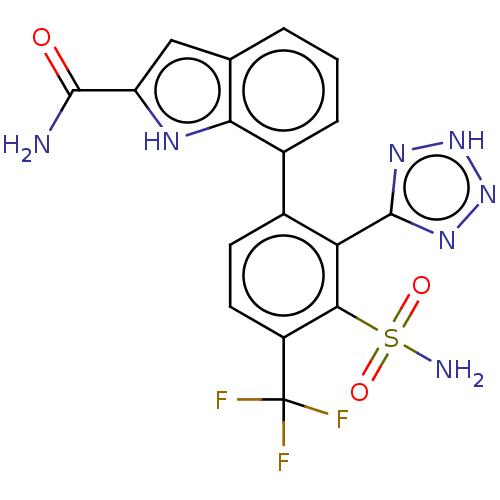 (7-(3-Sulfamoyl-2-(2H-tetrazol-5-yl)- 4-(trifluorom...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0250 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US9708336 (2017) BindingDB Entry DOI: 10.7270/Q2FN186K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal Pro-X carboxypeptidase (Mus musculus) | BDBM50364407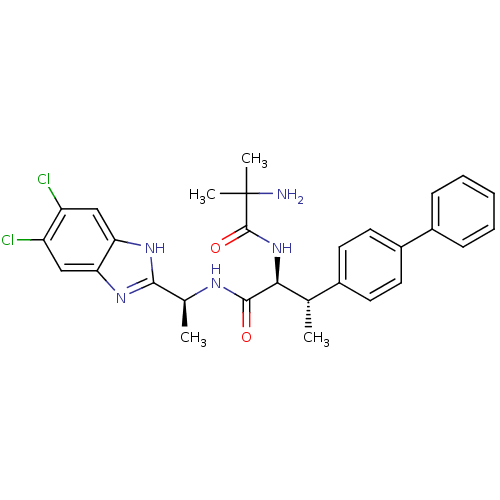 (CHEMBL1950444) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of mouse recombinant PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate for 30 mins by continuous fluorimetric assay | Bioorg Med Chem Lett 22: 1774-8 (2012) Article DOI: 10.1016/j.bmcl.2011.12.064 BindingDB Entry DOI: 10.7270/Q27H1K28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Klebsiella pneumoniae) | BDBM262700 (3-(2-amino-1,3-benzothiazol- 7-yl)-2-(1H-tetrazol-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US9708336 (2017) BindingDB Entry DOI: 10.7270/Q2FN186K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| VIM-1 metallo-beta-lactamase (Klebsiella pneumoniae) | BDBM262700 (3-(2-amino-1,3-benzothiazol- 7-yl)-2-(1H-tetrazol-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0620 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US9708336 (2017) BindingDB Entry DOI: 10.7270/Q2FN186K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| VIM-1 metallo-beta-lactamase (Klebsiella pneumoniae) | BDBM262696 (3-(2-amino-1,3-benzothiazol- 6-yl)-2-(1H-tetrazol-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0630 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US9708336 (2017) BindingDB Entry DOI: 10.7270/Q2FN186K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| VIM-1 metallo-beta-lactamase (Klebsiella pneumoniae) | BDBM262972 (4-[4-[3-sulfamoyl-2-(1H-tetrazol-5- yl)-4- (triflu...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0810 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US9708336 (2017) BindingDB Entry DOI: 10.7270/Q2FN186K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| VIM-1 metallo-beta-lactamase (Klebsiella pneumoniae) | BDBM262672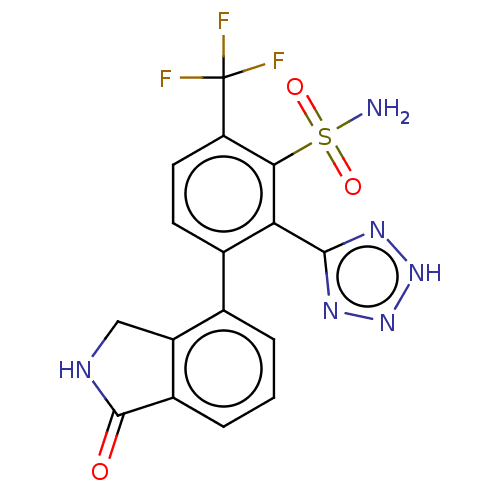 (3-(1-Oxoisoindolin-4-yl)-2-(2H- tetrazol-5-yl)-6- ...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US9708336 (2017) BindingDB Entry DOI: 10.7270/Q2FN186K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Klebsiella pneumoniae) | BDBM262715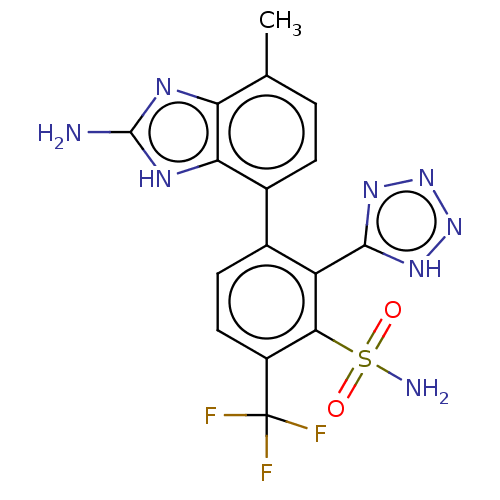 (3-(2-amino-4-methyl-1H-benzo[d]imidazol-7-yl)-2-(1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0980 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US9708336 (2017) BindingDB Entry DOI: 10.7270/Q2FN186K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM476445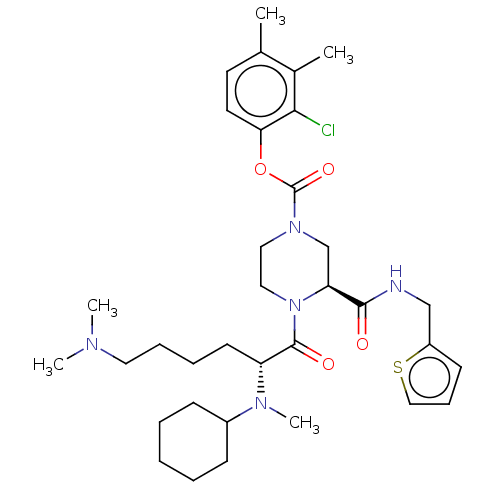 (2-chloro-3,4-dimethylphenyl (3S)-4- (N2-cyclohexyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIIa can be determined using a relevant purified serin... | US Patent US10875851 (2020) BindingDB Entry DOI: 10.7270/Q2JD50W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50582290 (CHEMBL5082614 | US20230286958, Example 198) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2DB8609 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| VIM-1 metallo-beta-lactamase (Klebsiella pneumoniae) | BDBM262898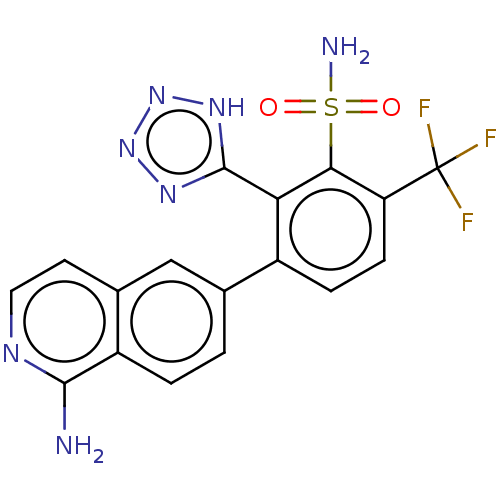 (3-(1-aminoisoquinolin-6-yl)-2- (1H-tetrazol-5-yl)-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.108 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US9708336 (2017) BindingDB Entry DOI: 10.7270/Q2FN186K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| VIM-1 metallo-beta-lactamase (Klebsiella pneumoniae) | BDBM262926 (3-[4-(4-piperidyl)-1-piperidyl]- 2-(1H-tetrazol-5-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US9708336 (2017) BindingDB Entry DOI: 10.7270/Q2FN186K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Klebsiella pneumoniae) | BDBM262701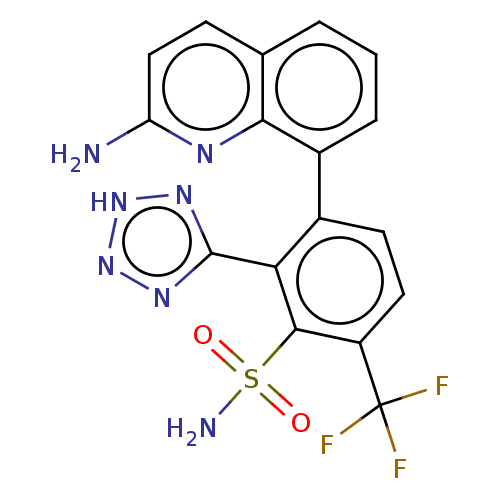 (3-(2-amino-8-quinolyl)-2-(2H- tetrazol-5-yl)-6- (t...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.115 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US9708336 (2017) BindingDB Entry DOI: 10.7270/Q2FN186K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| VIM-1 metallo-beta-lactamase (Klebsiella pneumoniae) | BDBM262703 (3-(2-aminoquinazolin-5-yl)-2- (2H-tetrazol-5-yl)-6...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.122 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US9708336 (2017) BindingDB Entry DOI: 10.7270/Q2FN186K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Klebsiella pneumoniae) | BDBM262703 (3-(2-aminoquinazolin-5-yl)-2- (2H-tetrazol-5-yl)-6...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.125 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US9708336 (2017) BindingDB Entry DOI: 10.7270/Q2FN186K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| VIM-1 metallo-beta-lactamase (Klebsiella pneumoniae) | BDBM262641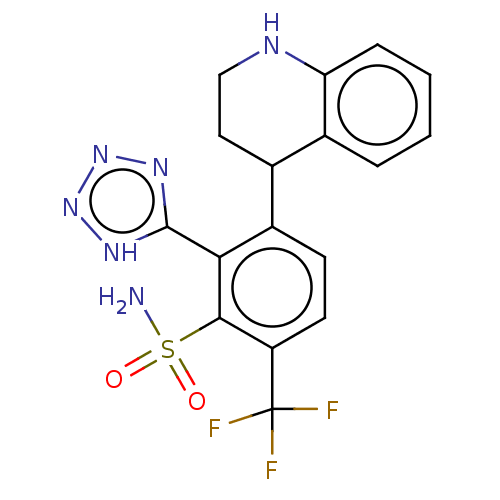 (3-(1,2,3,4-Tetrahydroquinolin-4-yl)-2-(1H-tetrazol...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.133 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US9708336 (2017) BindingDB Entry DOI: 10.7270/Q2FN186K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Klebsiella pneumoniae) | BDBM262688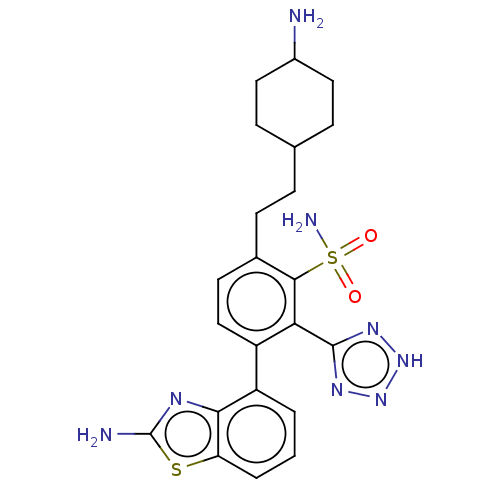 (3-(2-Aminobenzo[d]thiazol-4-yl)-6- (2-(4-aminocycl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.133 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US9708336 (2017) BindingDB Entry DOI: 10.7270/Q2FN186K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| VIM-1 metallo-beta-lactamase (Klebsiella pneumoniae) | BDBM262634 (3-(Quinolin-5-yl)-2-(2H-tetrazol-5-yl)-6-(trifluor...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.134 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US9708336 (2017) BindingDB Entry DOI: 10.7270/Q2FN186K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| VIM-1 metallo-beta-lactamase (Klebsiella pneumoniae) | BDBM262661 (5-(3-Sulfamoyl-2-(2H-tetrazol-5-yl)- 4-(trifluorom...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.138 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US9708336 (2017) BindingDB Entry DOI: 10.7270/Q2FN186K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Klebsiella pneumoniae) | BDBM262698 (3-(2-amino-3H-benzimidazol- 4-yl)-2-(1H-tetrazol-5...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.142 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US9708336 (2017) BindingDB Entry DOI: 10.7270/Q2FN186K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3936 total ) | Next | Last >> |