 Found 134 hits with Last Name = 'madison' and Initial = 'vs'
Found 134 hits with Last Name = 'madison' and Initial = 'vs' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
GTP-binding nuclear protein GSP1/CNR1
(Saccharomyces cerevisiae) | BDBM50176988
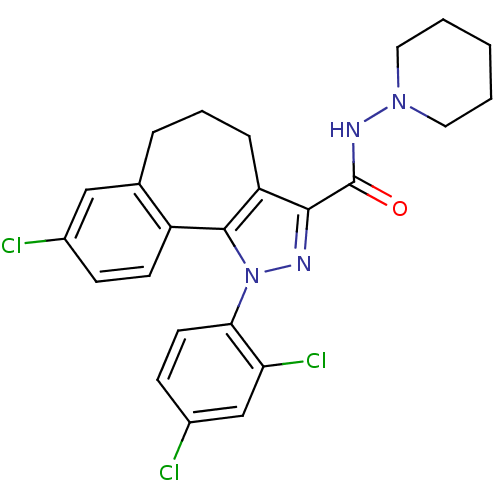
(8-Chloro-1-(2,4-dichloro-phenyl)-1,3a,4,5,6,10b-he...)Show SMILES Clc1ccc(c(Cl)c1)-n1nc(C(=O)NN2CCCCC2)c2CCCc3cc(Cl)ccc3-c12 Show InChI InChI=1S/C24H23Cl3N4O/c25-16-7-9-18-15(13-16)5-4-6-19-22(24(32)29-30-11-2-1-3-12-30)28-31(23(18)19)21-10-8-17(26)14-20(21)27/h7-10,13-14H,1-6,11-12H2,(H,29,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.000350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNS Biological Research
Curated by PDSP Ki Database
| |
J Med Chem 51: 2439-46 (2008)
Article DOI: 10.1021/jm701519h
BindingDB Entry DOI: 10.7270/Q22R3Q7S |
More data for this
Ligand-Target Pair | |
GTP-binding nuclear protein GSP1/CNR1
(Saccharomyces cerevisiae) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNS Biological Research
Curated by PDSP Ki Database
| |
J Med Chem 51: 2439-46 (2008)
Article DOI: 10.1021/jm701519h
BindingDB Entry DOI: 10.7270/Q22R3Q7S |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNS Biological Research
Curated by PDSP Ki Database
| |
J Med Chem 51: 2439-46 (2008)
Article DOI: 10.1021/jm701519h
BindingDB Entry DOI: 10.7270/Q22R3Q7S |
More data for this
Ligand-Target Pair | |
GTP-binding nuclear protein GSP1/CNR1
(Saccharomyces cerevisiae) | BDBM87019
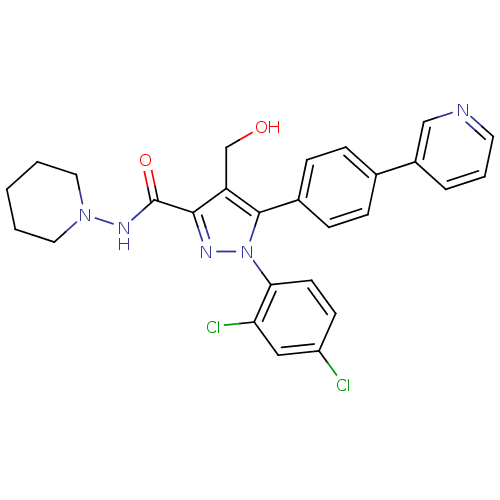
(1-(2,4-dichlorophenyl)-4-(hydroxymethyl)-N-(piperi...)Show SMILES OCc1c(nn(c1-c1ccc(cc1)-c1cccnc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C27H25Cl2N5O2/c28-21-10-11-24(23(29)15-21)34-26(19-8-6-18(7-9-19)20-5-4-12-30-16-20)22(17-35)25(31-34)27(36)32-33-13-2-1-3-14-33/h4-12,15-16,35H,1-3,13-14,17H2,(H,32,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNS Biological Research
Curated by PDSP Ki Database
| |
J Med Chem 51: 2439-46 (2008)
Article DOI: 10.1021/jm701519h
BindingDB Entry DOI: 10.7270/Q22R3Q7S |
More data for this
Ligand-Target Pair | |
GTP-binding nuclear protein GSP1/CNR1
(Saccharomyces cerevisiae) | BDBM29094
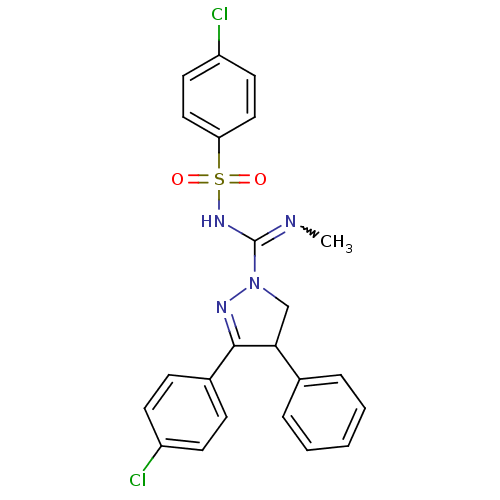
((+/-)-SLV319 | (S)-3-(4-chlorophenyl)-N-(4-chlorop...)Show SMILES CN=C(NS(=O)(=O)c1ccc(Cl)cc1)N1CC(C(=N1)c1ccc(Cl)cc1)c1ccccc1 |w:1.0,c:18| Show InChI InChI=1S/C23H20Cl2N4O2S/c1-26-23(28-32(30,31)20-13-11-19(25)12-14-20)29-15-21(16-5-3-2-4-6-16)22(27-29)17-7-9-18(24)10-8-17/h2-14,21H,15H2,1H3,(H,26,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNS Biological Research
Curated by PDSP Ki Database
| |
J Med Chem 51: 2439-46 (2008)
Article DOI: 10.1021/jm701519h
BindingDB Entry DOI: 10.7270/Q22R3Q7S |
More data for this
Ligand-Target Pair | |
GTP-binding nuclear protein GSP2/CNR2
(Saccharomyces cerevisiae) | BDBM50176988

(8-Chloro-1-(2,4-dichloro-phenyl)-1,3a,4,5,6,10b-he...)Show SMILES Clc1ccc(c(Cl)c1)-n1nc(C(=O)NN2CCCCC2)c2CCCc3cc(Cl)ccc3-c12 Show InChI InChI=1S/C24H23Cl3N4O/c25-16-7-9-18-15(13-16)5-4-6-19-22(24(32)29-30-11-2-1-3-12-30)28-31(23(18)19)21-10-8-17(26)14-20(21)27/h7-10,13-14H,1-6,11-12H2,(H,29,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNS Biological Research
Curated by PDSP Ki Database
| |
J Med Chem 51: 2439-46 (2008)
Article DOI: 10.1021/jm701519h
BindingDB Entry DOI: 10.7270/Q22R3Q7S |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM87017

(CAS_44450408 | NSC_44450408 | S-(R)-1-((2S,3R)-1-(...)Show SMILES CC(=O)SC(CCc1ccccc1)C1C(N(C1=O)c1ccc(F)cc1)c1ccc(O)cc1 Show InChI InChI=1S/C26H24FNO3S/c1-17(29)32-23(16-7-18-5-3-2-4-6-18)24-25(19-8-14-22(30)15-9-19)28(26(24)31)21-12-10-20(27)11-13-21/h2-6,8-15,23-25,30H,7,16H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 52.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNS Biological Research
Curated by PDSP Ki Database
| |
J Med Chem 51: 2439-46 (2008)
Article DOI: 10.1021/jm701519h
BindingDB Entry DOI: 10.7270/Q22R3Q7S |
More data for this
Ligand-Target Pair | |
GTP-binding nuclear protein GSP1/CNR1
(Saccharomyces cerevisiae) | BDBM50080103

(5,5-Bis-(4-bromo-phenyl)-3-(2-morpholin-4-yl-ethyl...)Show SMILES Brc1ccc(cc1)C1(NC(=O)N(CCN2CCOCC2)C1=O)c1ccc(Br)cc1 Show InChI InChI=1S/C21H21Br2N3O3/c22-17-5-1-15(2-6-17)21(16-3-7-18(23)8-4-16)19(27)26(20(28)24-21)10-9-25-11-13-29-14-12-25/h1-8H,9-14H2,(H,24,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 70.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNS Biological Research
Curated by PDSP Ki Database
| |
J Med Chem 51: 2439-46 (2008)
Article DOI: 10.1021/jm701519h
BindingDB Entry DOI: 10.7270/Q22R3Q7S |
More data for this
Ligand-Target Pair | |
GTP-binding nuclear protein GSP1/CNR1
(Saccharomyces cerevisiae) | BDBM87015

(1-(bis(4-chlorophenyl)methyl)-3-((3,5-difluorophen...)Show SMILES [#6]S(=O)(=O)[#6](=[#6]-1/[#6]-[#7](-[#6]-1)-[#6](-c1ccc(Cl)cc1)-c1ccc(Cl)cc1)\c1cc(F)cc(F)c1 Show InChI InChI=1S/C24H19Cl2F2NO2S/c1-32(30,31)24(17-10-21(27)12-22(28)11-17)18-13-29(14-18)23(15-2-6-19(25)7-3-15)16-4-8-20(26)9-5-16/h2-12,23H,13-14H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNS Biological Research
Curated by PDSP Ki Database
| |
J Med Chem 51: 2439-46 (2008)
Article DOI: 10.1021/jm701519h
BindingDB Entry DOI: 10.7270/Q22R3Q7S |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50444921

(CHEMBL3099750)Show SMILES CNC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)c1cc(C(O)=O)c2cc(\C=C\c3ccc(Cl)cc3)ccc2n1 |r| Show InChI InChI=1S/C29H24ClN3O5/c1-31-27(35)25(15-19-6-11-21(34)12-7-19)33-28(36)26-16-23(29(37)38)22-14-18(8-13-24(22)32-26)3-2-17-4-9-20(30)10-5-17/h2-14,16,25,34H,15H2,1H3,(H,31,35)(H,33,36)(H,37,38)/b3-2+/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 199-203 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.041
BindingDB Entry DOI: 10.7270/Q2SN0BF8 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50444933
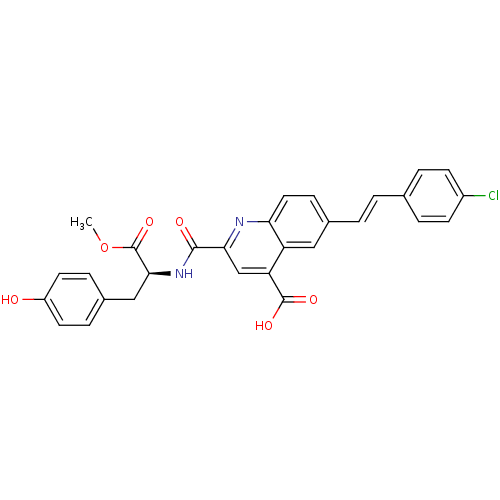
(CHEMBL3099763)Show SMILES COC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)c1cc(C(O)=O)c2cc(\C=C\c3ccc(Cl)cc3)ccc2n1 |r| Show InChI InChI=1S/C29H23ClN2O6/c1-38-29(37)26(15-19-6-11-21(33)12-7-19)32-27(34)25-16-23(28(35)36)22-14-18(8-13-24(22)31-25)3-2-17-4-9-20(30)10-5-17/h2-14,16,26,33H,15H2,1H3,(H,32,34)(H,35,36)/b3-2+/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 199-203 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.041
BindingDB Entry DOI: 10.7270/Q2SN0BF8 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50444932
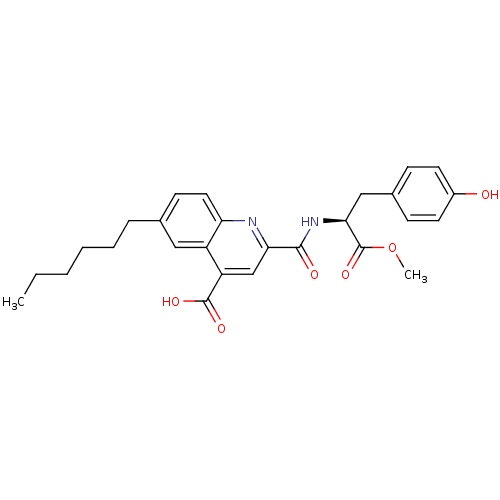
(CHEMBL3099764)Show SMILES CCCCCCc1ccc2nc(cc(C(O)=O)c2c1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)OC |r| Show InChI InChI=1S/C27H30N2O6/c1-3-4-5-6-7-17-10-13-22-20(14-17)21(26(32)33)16-23(28-22)25(31)29-24(27(34)35-2)15-18-8-11-19(30)12-9-18/h8-14,16,24,30H,3-7,15H2,1-2H3,(H,29,31)(H,32,33)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 199-203 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.041
BindingDB Entry DOI: 10.7270/Q2SN0BF8 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50444934
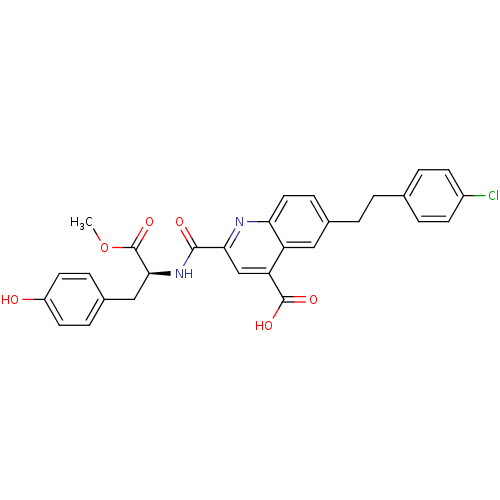
(CHEMBL3099762)Show SMILES COC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)c1cc(C(O)=O)c2cc(CCc3ccc(Cl)cc3)ccc2n1 |r| Show InChI InChI=1S/C29H25ClN2O6/c1-38-29(37)26(15-19-6-11-21(33)12-7-19)32-27(34)25-16-23(28(35)36)22-14-18(8-13-24(22)31-25)3-2-17-4-9-20(30)10-5-17/h4-14,16,26,33H,2-3,15H2,1H3,(H,32,34)(H,35,36)/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 199-203 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.041
BindingDB Entry DOI: 10.7270/Q2SN0BF8 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50444935
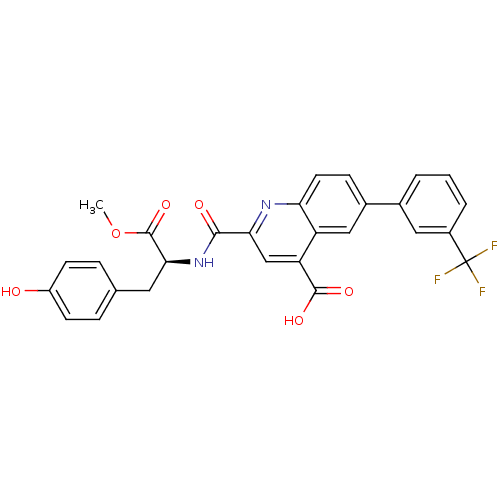
(CHEMBL3099761)Show SMILES COC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)c1cc(C(O)=O)c2cc(ccc2n1)-c1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C28H21F3N2O6/c1-39-27(38)24(11-15-5-8-19(34)9-6-15)33-25(35)23-14-21(26(36)37)20-13-17(7-10-22(20)32-23)16-3-2-4-18(12-16)28(29,30)31/h2-10,12-14,24,34H,11H2,1H3,(H,33,35)(H,36,37)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 199-203 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.041
BindingDB Entry DOI: 10.7270/Q2SN0BF8 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50444919
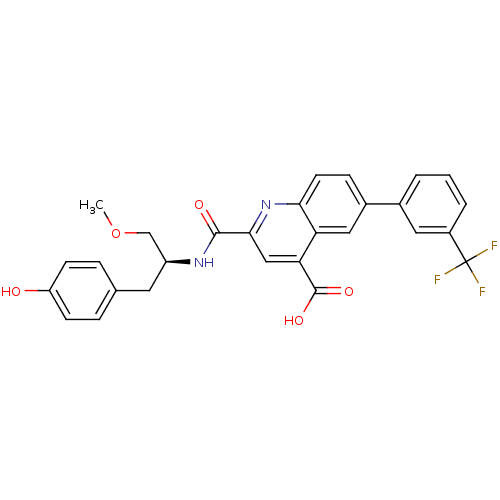
(CHEMBL3099752)Show SMILES COC[C@H](Cc1ccc(O)cc1)NC(=O)c1cc(C(O)=O)c2cc(ccc2n1)-c1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C28H23F3N2O5/c1-38-15-20(11-16-5-8-21(34)9-6-16)32-26(35)25-14-23(27(36)37)22-13-18(7-10-24(22)33-25)17-3-2-4-19(12-17)28(29,30)31/h2-10,12-14,20,34H,11,15H2,1H3,(H,32,35)(H,36,37)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 199-203 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.041
BindingDB Entry DOI: 10.7270/Q2SN0BF8 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50444918
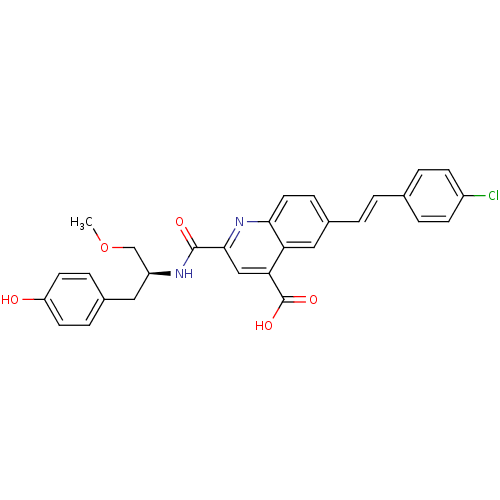
(CHEMBL3099753)Show SMILES COC[C@H](Cc1ccc(O)cc1)NC(=O)c1cc(C(O)=O)c2cc(\C=C\c3ccc(Cl)cc3)ccc2n1 |r| Show InChI InChI=1S/C29H25ClN2O5/c1-37-17-22(14-19-6-11-23(33)12-7-19)31-28(34)27-16-25(29(35)36)24-15-20(8-13-26(24)32-27)3-2-18-4-9-21(30)10-5-18/h2-13,15-16,22,33H,14,17H2,1H3,(H,31,34)(H,35,36)/b3-2+/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 199-203 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.041
BindingDB Entry DOI: 10.7270/Q2SN0BF8 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50171301
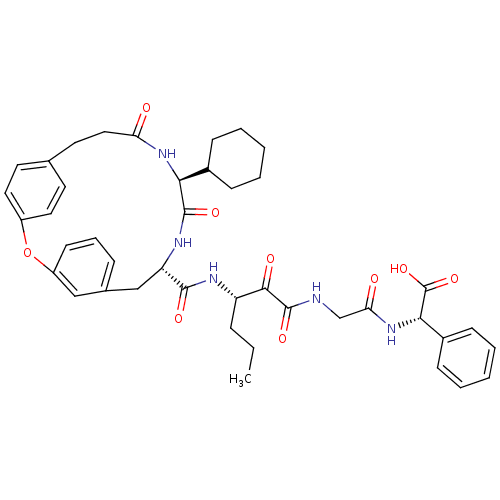
((S)-(2-{3-[((9S,12S)-12-Cyclohexyl-11,14-dioxo-2-o...)Show SMILES CCC[C@H](NC(=O)[C@@H]1Cc2cccc(Oc3ccc(CCC(=O)N[C@@H](C4CCCCC4)C(=O)N1)cc3)c2)C(=O)C(=O)NCC(=O)N[C@H](C(O)=O)c1ccccc1 Show InChI InChI=1S/C42H49N5O9/c1-2-10-32(38(50)41(53)43-25-35(49)47-37(42(54)55)29-14-7-4-8-15-29)44-39(51)33-24-27-11-9-16-31(23-27)56-30-20-17-26(18-21-30)19-22-34(48)46-36(40(52)45-33)28-12-5-3-6-13-28/h4,7-9,11,14-18,20-21,23,28,32-33,36-37H,2-3,5-6,10,12-13,19,22,24-25H2,1H3,(H,43,53)(H,44,51)(H,45,52)(H,46,48)(H,47,49)(H,54,55)/t32-,33-,36-,37-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Ability to inhibit the hydrolysis of chromogenic 4-phenylazophenyl ester from the peptide fragment Ac- DTEDVVP(Nva)-O-4-PAP in a HCV protease continu... |
J Med Chem 48: 5088-91 (2005)
Article DOI: 10.1021/jm0489556
BindingDB Entry DOI: 10.7270/Q2DN45T7 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50444941
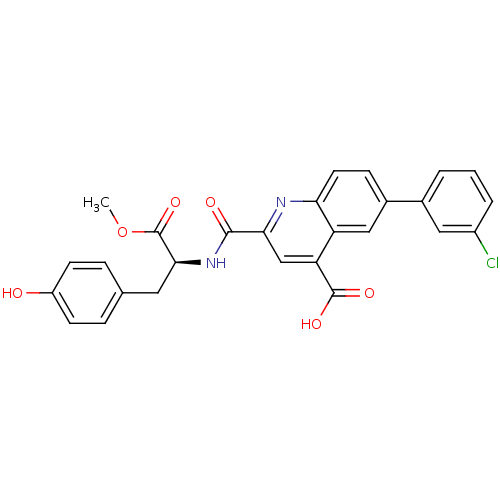
(CHEMBL3099755)Show SMILES COC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)c1cc(C(O)=O)c2cc(ccc2n1)-c1cccc(Cl)c1 |r| Show InChI InChI=1S/C27H21ClN2O6/c1-36-27(35)24(11-15-5-8-19(31)9-6-15)30-25(32)23-14-21(26(33)34)20-13-17(7-10-22(20)29-23)16-3-2-4-18(28)12-16/h2-10,12-14,24,31H,11H2,1H3,(H,30,32)(H,33,34)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 199-203 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.041
BindingDB Entry DOI: 10.7270/Q2SN0BF8 |
More data for this
Ligand-Target Pair | |
GTP-binding nuclear protein GSP1/CNR1
(Saccharomyces cerevisiae) | BDBM87014
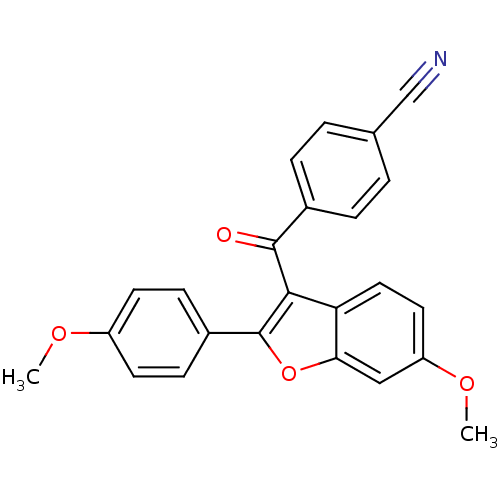
(4-(6-methoxy-2-(4-methoxyphenyl)benzofuran-3-carbo...)Show SMILES COc1ccc(cc1)-c1oc2cc(OC)ccc2c1C(=O)c1ccc(cc1)C#N Show InChI InChI=1S/C24H17NO4/c1-27-18-9-7-17(8-10-18)24-22(20-12-11-19(28-2)13-21(20)29-24)23(26)16-5-3-15(14-25)4-6-16/h3-13H,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 141 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNS Biological Research
Curated by PDSP Ki Database
| |
J Med Chem 51: 2439-46 (2008)
Article DOI: 10.1021/jm701519h
BindingDB Entry DOI: 10.7270/Q22R3Q7S |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50444920
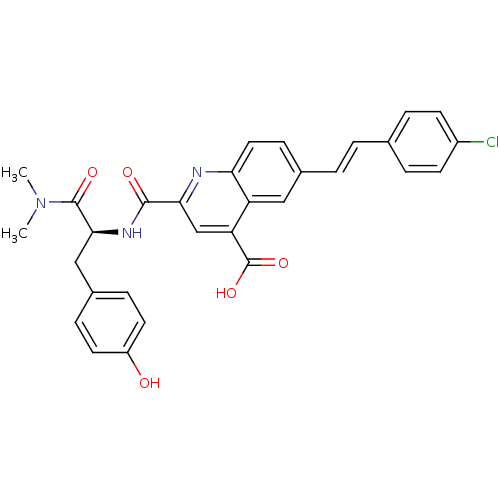
(CHEMBL3099751)Show SMILES CN(C)C(=O)[C@H](Cc1ccc(O)cc1)NC(=O)c1cc(C(O)=O)c2cc(\C=C\c3ccc(Cl)cc3)ccc2n1 |r| Show InChI InChI=1S/C30H26ClN3O5/c1-34(2)29(37)27(16-20-7-12-22(35)13-8-20)33-28(36)26-17-24(30(38)39)23-15-19(9-14-25(23)32-26)4-3-18-5-10-21(31)11-6-18/h3-15,17,27,35H,16H2,1-2H3,(H,33,36)(H,38,39)/b4-3+/t27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 199-203 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.041
BindingDB Entry DOI: 10.7270/Q2SN0BF8 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50171302
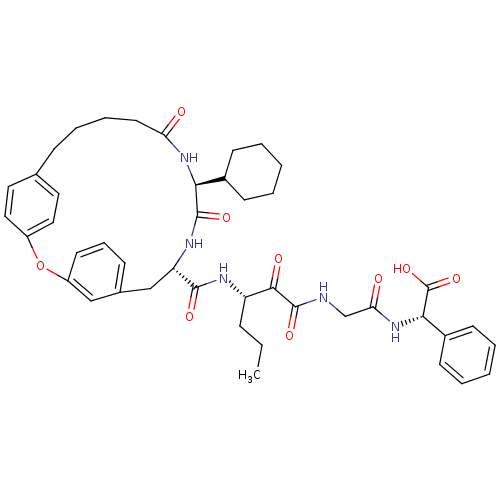
((S)-(2-{3-[((9S,12S)-12-Cyclohexyl-11,14-dioxo-2-o...)Show SMILES CCC[C@H](NC(=O)[C@@H]1Cc2cccc(Oc3ccc(CCCCC(=O)N[C@@H](C4CCCCC4)C(=O)N1)cc3)c2)C(=O)C(=O)NCC(=O)N[C@H](C(O)=O)c1ccccc1 Show InChI InChI=1S/C44H53N5O9/c1-2-12-34(40(52)43(55)45-27-37(51)49-39(44(56)57)31-17-7-4-8-18-31)46-41(53)35-26-29-14-11-19-33(25-29)58-32-23-21-28(22-24-32)13-9-10-20-36(50)48-38(42(54)47-35)30-15-5-3-6-16-30/h4,7-8,11,14,17-19,21-25,30,34-35,38-39H,2-3,5-6,9-10,12-13,15-16,20,26-27H2,1H3,(H,45,55)(H,46,53)(H,47,54)(H,48,50)(H,49,51)(H,56,57)/t34-,35-,38-,39-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Ability to inhibit the hydrolysis of chromogenic 4-phenylazophenyl ester from the peptide fragment Ac- DTEDVVP(Nva)-O-4-PAP in a HCV protease continu... |
J Med Chem 48: 5088-91 (2005)
Article DOI: 10.1021/jm0489556
BindingDB Entry DOI: 10.7270/Q2DN45T7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM87013

((3R,4S)-3-(benzofuran-2-ylmethyl)-4-(4-methoxyphen...)Show SMILES COc1ccc(cc1)C1C(Cc2cc3ccccc3o2)C(=O)N1c1ccccc1 Show InChI InChI=1S/C25H21NO3/c1-28-20-13-11-17(12-14-20)24-22(25(27)26(24)19-8-3-2-4-9-19)16-21-15-18-7-5-6-10-23(18)29-21/h2-15,22,24H,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 194 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNS Biological Research
Curated by PDSP Ki Database
| |
J Med Chem 51: 2439-46 (2008)
Article DOI: 10.1021/jm701519h
BindingDB Entry DOI: 10.7270/Q22R3Q7S |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50444938
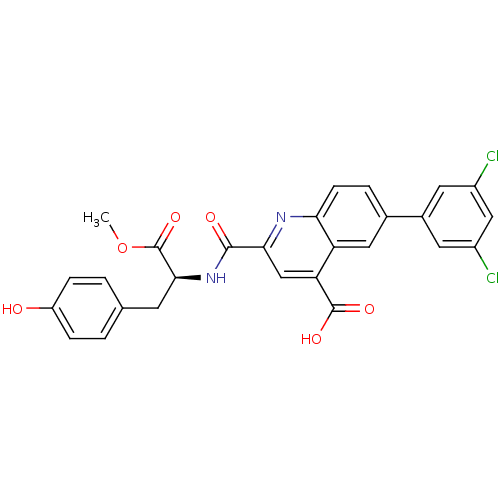
(CHEMBL3099758)Show SMILES COC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)c1cc(C(O)=O)c2cc(ccc2n1)-c1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C27H20Cl2N2O6/c1-37-27(36)24(8-14-2-5-19(32)6-3-14)31-25(33)23-13-21(26(34)35)20-11-15(4-7-22(20)30-23)16-9-17(28)12-18(29)10-16/h2-7,9-13,24,32H,8H2,1H3,(H,31,33)(H,34,35)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 199-203 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.041
BindingDB Entry DOI: 10.7270/Q2SN0BF8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM87020

((3R,4S)-4-(4-methoxyphenyl)-1-phenyl-3-(3-(pyridin...)Show SMILES COc1ccc(cc1)C1C(CCCc2cccnc2)C(=O)N1c1ccccc1 Show InChI InChI=1S/C24H24N2O2/c1-28-21-14-12-19(13-15-21)23-22(11-5-7-18-8-6-16-25-17-18)24(27)26(23)20-9-3-2-4-10-20/h2-4,6,8-10,12-17,22-23H,5,7,11H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 244 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNS Biological Research
Curated by PDSP Ki Database
| |
J Med Chem 51: 2439-46 (2008)
Article DOI: 10.1021/jm701519h
BindingDB Entry DOI: 10.7270/Q22R3Q7S |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM87017

(CAS_44450408 | NSC_44450408 | S-(R)-1-((2S,3R)-1-(...)Show SMILES CC(=O)SC(CCc1ccccc1)C1C(N(C1=O)c1ccc(F)cc1)c1ccc(O)cc1 Show InChI InChI=1S/C26H24FNO3S/c1-17(29)32-23(16-7-18-5-3-2-4-6-18)24-25(19-8-14-22(30)15-9-19)28(26(24)31)21-12-10-20(27)11-13-21/h2-6,8-15,23-25,30H,7,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 282 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNS Biological Research
Curated by PDSP Ki Database
| |
J Med Chem 51: 2439-46 (2008)
Article DOI: 10.1021/jm701519h
BindingDB Entry DOI: 10.7270/Q22R3Q7S |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50171305

((S)-(2-{3-[((9S,12S)-12-Cyclohexyl-11,14-dioxo-2-o...)Show SMILES CCC[C@H](NC(=O)[C@@H]1Cc2cccc(Oc3ccc(CCCC(=O)N[C@@H](C4CCCCC4)C(=O)N1)cc3)c2)C(=O)C(=O)NCC(=O)N[C@H](C(O)=O)c1ccccc1 Show InChI InChI=1S/C43H51N5O9/c1-2-11-33(39(51)42(54)44-26-36(50)48-38(43(55)56)30-16-7-4-8-17-30)45-40(52)34-25-28-13-9-18-32(24-28)57-31-22-20-27(21-23-31)12-10-19-35(49)47-37(41(53)46-34)29-14-5-3-6-15-29/h4,7-9,13,16-18,20-24,29,33-34,37-38H,2-3,5-6,10-12,14-15,19,25-26H2,1H3,(H,44,54)(H,45,52)(H,46,53)(H,47,49)(H,48,50)(H,55,56)/t33-,34-,37-,38-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Ability to inhibit the hydrolysis of chromogenic 4-phenylazophenyl ester from the peptide fragment Ac- DTEDVVP(Nva)-O-4-PAP in a HCV protease continu... |
J Med Chem 48: 5088-91 (2005)
Article DOI: 10.1021/jm0489556
BindingDB Entry DOI: 10.7270/Q2DN45T7 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50444922
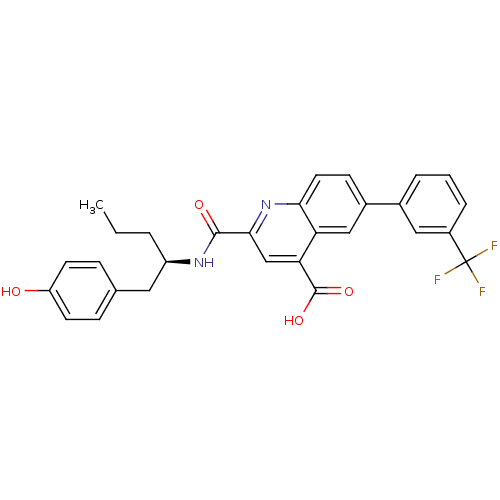
(CHEMBL3099749)Show SMILES CCC[C@H](Cc1ccc(O)cc1)NC(=O)c1cc(C(O)=O)c2cc(ccc2n1)-c1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C29H25F3N2O4/c1-2-4-21(13-17-7-10-22(35)11-8-17)33-27(36)26-16-24(28(37)38)23-15-19(9-12-25(23)34-26)18-5-3-6-20(14-18)29(30,31)32/h3,5-12,14-16,21,35H,2,4,13H2,1H3,(H,33,36)(H,37,38)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 199-203 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.041
BindingDB Entry DOI: 10.7270/Q2SN0BF8 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50171302

((S)-(2-{3-[((9S,12S)-12-Cyclohexyl-11,14-dioxo-2-o...)Show SMILES CCC[C@H](NC(=O)[C@@H]1Cc2cccc(Oc3ccc(CCCCC(=O)N[C@@H](C4CCCCC4)C(=O)N1)cc3)c2)C(=O)C(=O)NCC(=O)N[C@H](C(O)=O)c1ccccc1 Show InChI InChI=1S/C44H53N5O9/c1-2-12-34(40(52)43(55)45-27-37(51)49-39(44(56)57)31-17-7-4-8-18-31)46-41(53)35-26-29-14-11-19-33(25-29)58-32-23-21-28(22-24-32)13-9-10-20-36(50)48-38(42(54)47-35)30-15-5-3-6-16-30/h4,7-8,11,14,17-19,21-25,30,34-35,38-39H,2-3,5-6,9-10,12-13,15-16,20,26-27H2,1H3,(H,45,55)(H,46,53)(H,47,54)(H,48,50)(H,49,51)(H,56,57)/t34-,35-,38-,39-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Ability to inhibit the hydrolysis of chromogenic 4-chlorophenylbutyric acid ester from the peptide fragment Ac- DTEDVVP(Nva)-O-4-PAP in a HCV proteas... |
J Med Chem 48: 5088-91 (2005)
Article DOI: 10.1021/jm0489556
BindingDB Entry DOI: 10.7270/Q2DN45T7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM87018
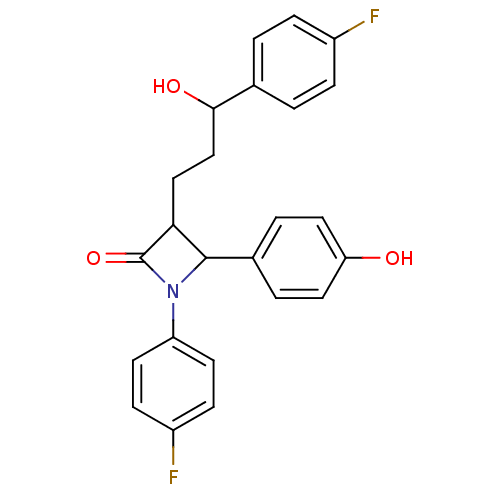
((3S,4R)-1-(4-fluorophenyl)-3-(3-(4-fluorophenyl)-3...)Show SMILES OC(CCC1C(N(C1=O)c1ccc(F)cc1)c1ccc(O)cc1)c1ccc(F)cc1 Show InChI InChI=1S/C24H21F2NO3/c25-17-5-1-15(2-6-17)22(29)14-13-21-23(16-3-11-20(28)12-4-16)27(24(21)30)19-9-7-18(26)8-10-19/h1-12,21-23,28-29H,13-14H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 301 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNS Biological Research
Curated by PDSP Ki Database
| |
J Med Chem 51: 2439-46 (2008)
Article DOI: 10.1021/jm701519h
BindingDB Entry DOI: 10.7270/Q22R3Q7S |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM87016

(4-(4-methoxyphenyl)-1-phenyl-3-(2-(phenylsulfonyl)...)Show SMILES COc1ccc(cc1)C1C(CCS(=O)(=O)c2ccccc2)C(=O)N1c1ccccc1 Show InChI InChI=1S/C24H23NO4S/c1-29-20-14-12-18(13-15-20)23-22(24(26)25(23)19-8-4-2-5-9-19)16-17-30(27,28)21-10-6-3-7-11-21/h2-15,22-23H,16-17H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 452 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNS Biological Research
Curated by PDSP Ki Database
| |
J Med Chem 51: 2439-46 (2008)
Article DOI: 10.1021/jm701519h
BindingDB Entry DOI: 10.7270/Q22R3Q7S |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNS Biological Research
Curated by PDSP Ki Database
| |
J Med Chem 51: 2439-46 (2008)
Article DOI: 10.1021/jm701519h
BindingDB Entry DOI: 10.7270/Q22R3Q7S |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50444940
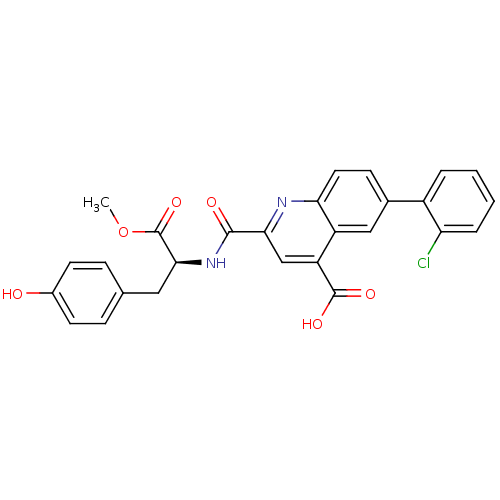
(CHEMBL3099756)Show SMILES COC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)c1cc(C(O)=O)c2cc(ccc2n1)-c1ccccc1Cl |r| Show InChI InChI=1S/C27H21ClN2O6/c1-36-27(35)24(12-15-6-9-17(31)10-7-15)30-25(32)23-14-20(26(33)34)19-13-16(8-11-22(19)29-23)18-4-2-3-5-21(18)28/h2-11,13-14,24,31H,12H2,1H3,(H,30,32)(H,33,34)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 199-203 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.041
BindingDB Entry DOI: 10.7270/Q2SN0BF8 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50370596
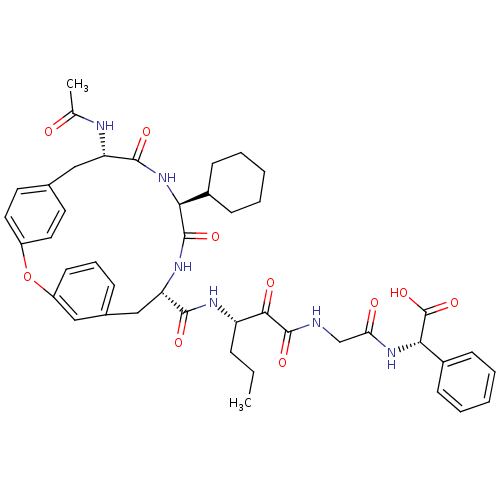
(CHEMBL1169404)Show SMILES CCC[C@H](NC(=O)[C@@H]1Cc2cccc(Oc3ccc(C[C@H](NC(C)=O)C(=O)N[C@@H](C4CCCCC4)C(=O)N1)cc3)c2)C(=O)C(=O)NCC(=O)N[C@H](C(O)=O)c1ccccc1 |r| Show InChI InChI=1S/C44H52N6O10/c1-3-11-33(39(53)43(57)45-25-36(52)49-38(44(58)59)30-15-8-5-9-16-30)47-40(54)35-24-28-12-10-17-32(22-28)60-31-20-18-27(19-21-31)23-34(46-26(2)51)41(55)50-37(42(56)48-35)29-13-6-4-7-14-29/h5,8-10,12,15-22,29,33-35,37-38H,3-4,6-7,11,13-14,23-25H2,1-2H3,(H,45,57)(H,46,51)(H,47,54)(H,48,56)(H,49,52)(H,50,55)(H,58,59)/t33-,34-,35-,37-,38-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Ability to inhibit the hydrolysis of chromogenic 4-phenylazophenyl ester from the peptide fragment Ac- DTEDVVP(Nva)-O-4-PAP in a HCV protease continu... |
J Med Chem 48: 5088-91 (2005)
Article DOI: 10.1021/jm0489556
BindingDB Entry DOI: 10.7270/Q2DN45T7 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50444942
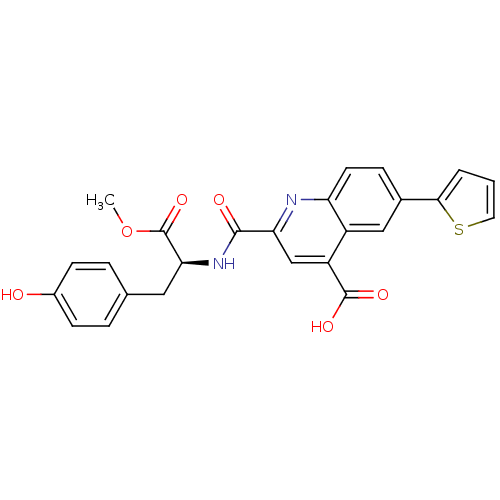
(CHEMBL3099754)Show SMILES COC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)c1cc(C(O)=O)c2cc(ccc2n1)-c1cccs1 |r| Show InChI InChI=1S/C25H20N2O6S/c1-33-25(32)21(11-14-4-7-16(28)8-5-14)27-23(29)20-13-18(24(30)31)17-12-15(6-9-19(17)26-20)22-3-2-10-34-22/h2-10,12-13,21,28H,11H2,1H3,(H,27,29)(H,30,31)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 199-203 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.041
BindingDB Entry DOI: 10.7270/Q2SN0BF8 |
More data for this
Ligand-Target Pair | |
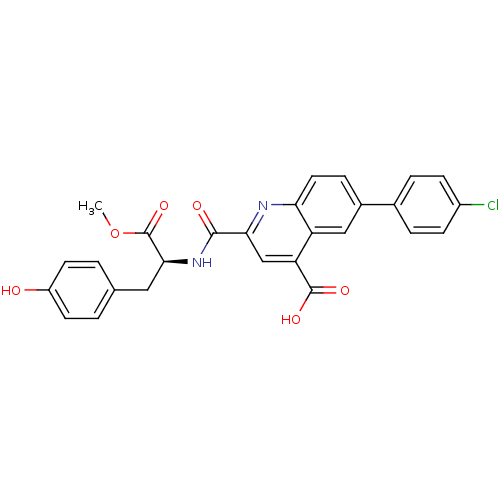
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50444939
(CHEMBL3099757)Show SMILES COC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)c1cc(C(O)=O)c2cc(ccc2n1)-c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C27H21ClN2O6/c1-36-27(35)24(12-15-2-9-19(31)10-3-15)30-25(32)23-14-21(26(33)34)20-13-17(6-11-22(20)29-23)16-4-7-18(28)8-5-16/h2-11,13-14,24,31H,12H2,1H3,(H,30,32)(H,33,34)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 199-203 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.041
BindingDB Entry DOI: 10.7270/Q2SN0BF8 |
More data for this
Ligand-Target Pair | |
GTP-binding nuclear protein GSP2/CNR2
(Saccharomyces cerevisiae) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNS Biological Research
Curated by PDSP Ki Database
| |
J Med Chem 51: 2439-46 (2008)
Article DOI: 10.1021/jm701519h
BindingDB Entry DOI: 10.7270/Q22R3Q7S |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM87020

((3R,4S)-4-(4-methoxyphenyl)-1-phenyl-3-(3-(pyridin...)Show SMILES COc1ccc(cc1)C1C(CCCc2cccnc2)C(=O)N1c1ccccc1 Show InChI InChI=1S/C24H24N2O2/c1-28-21-14-12-19(13-15-21)23-22(11-5-7-18-8-6-16-25-17-18)24(27)26(23)20-9-3-2-4-10-20/h2-4,6,8-10,12-17,22-23H,5,7,11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNS Biological Research
Curated by PDSP Ki Database
| |
J Med Chem 51: 2439-46 (2008)
Article DOI: 10.1021/jm701519h
BindingDB Entry DOI: 10.7270/Q22R3Q7S |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM87016

(4-(4-methoxyphenyl)-1-phenyl-3-(2-(phenylsulfonyl)...)Show SMILES COc1ccc(cc1)C1C(CCS(=O)(=O)c2ccccc2)C(=O)N1c1ccccc1 Show InChI InChI=1S/C24H23NO4S/c1-29-20-14-12-18(13-15-20)23-22(24(26)25(23)19-8-4-2-5-9-19)16-17-30(27,28)21-10-6-3-7-11-21/h2-15,22-23H,16-17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNS Biological Research
Curated by PDSP Ki Database
| |
J Med Chem 51: 2439-46 (2008)
Article DOI: 10.1021/jm701519h
BindingDB Entry DOI: 10.7270/Q22R3Q7S |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM87018

((3S,4R)-1-(4-fluorophenyl)-3-(3-(4-fluorophenyl)-3...)Show SMILES OC(CCC1C(N(C1=O)c1ccc(F)cc1)c1ccc(O)cc1)c1ccc(F)cc1 Show InChI InChI=1S/C24H21F2NO3/c25-17-5-1-15(2-6-17)22(29)14-13-21-23(16-3-11-20(28)12-4-16)27(24(21)30)19-9-7-18(26)8-10-19/h1-12,21-23,28-29H,13-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNS Biological Research
Curated by PDSP Ki Database
| |
J Med Chem 51: 2439-46 (2008)
Article DOI: 10.1021/jm701519h
BindingDB Entry DOI: 10.7270/Q22R3Q7S |
More data for this
Ligand-Target Pair | |
GTP-binding nuclear protein GSP2/CNR2
(Saccharomyces cerevisiae) | BDBM87019

(1-(2,4-dichlorophenyl)-4-(hydroxymethyl)-N-(piperi...)Show SMILES OCc1c(nn(c1-c1ccc(cc1)-c1cccnc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C27H25Cl2N5O2/c28-21-10-11-24(23(29)15-21)34-26(19-8-6-18(7-9-19)20-5-4-12-30-16-20)22(17-35)25(31-34)27(36)32-33-13-2-1-3-14-33/h4-12,15-16,35H,1-3,13-14,17H2,(H,32,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNS Biological Research
Curated by PDSP Ki Database
| |
J Med Chem 51: 2439-46 (2008)
Article DOI: 10.1021/jm701519h
BindingDB Entry DOI: 10.7270/Q22R3Q7S |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50444928
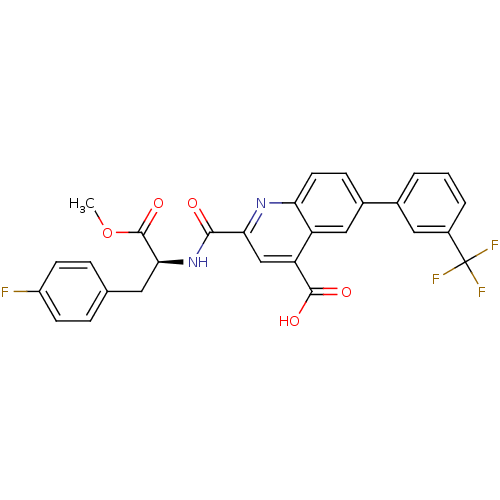
(CHEMBL3099743)Show SMILES COC(=O)[C@H](Cc1ccc(F)cc1)NC(=O)c1cc(C(O)=O)c2cc(ccc2n1)-c1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C28H20F4N2O5/c1-39-27(38)24(11-15-5-8-19(29)9-6-15)34-25(35)23-14-21(26(36)37)20-13-17(7-10-22(20)33-23)16-3-2-4-18(12-16)28(30,31)32/h2-10,12-14,24H,11H2,1H3,(H,34,35)(H,36,37)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 199-203 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.041
BindingDB Entry DOI: 10.7270/Q2SN0BF8 |
More data for this
Ligand-Target Pair | |
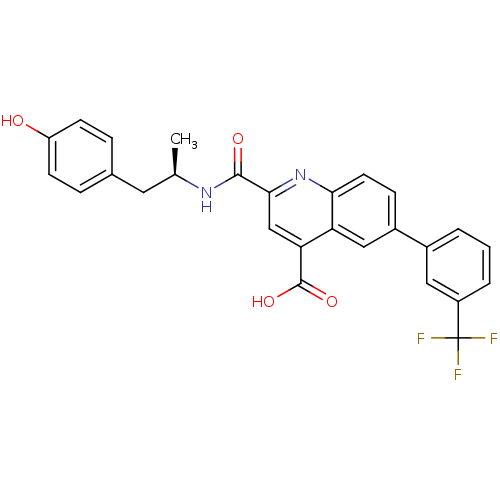
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50444923
(CHEMBL3099748)Show SMILES C[C@H](Cc1ccc(O)cc1)NC(=O)c1cc(C(O)=O)c2cc(ccc2n1)-c1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C27H21F3N2O4/c1-15(11-16-5-8-20(33)9-6-16)31-25(34)24-14-22(26(35)36)21-13-18(7-10-23(21)32-24)17-3-2-4-19(12-17)27(28,29)30/h2-10,12-15,33H,11H2,1H3,(H,31,34)(H,35,36)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 199-203 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.041
BindingDB Entry DOI: 10.7270/Q2SN0BF8 |
More data for this
Ligand-Target Pair | |
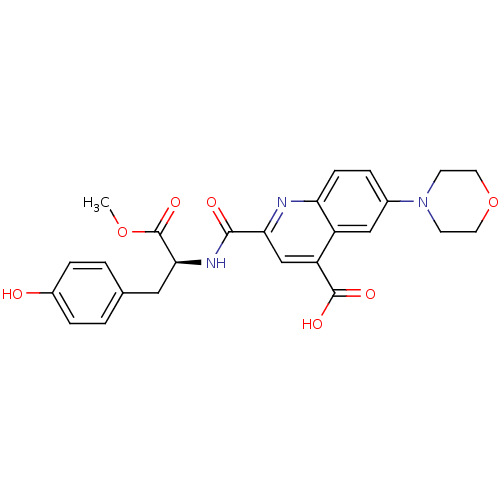
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50444936
(CHEMBL3099760)Show SMILES COC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)c1cc(C(O)=O)c2cc(ccc2n1)N1CCOCC1 |r| Show InChI InChI=1S/C25H25N3O7/c1-34-25(33)22(12-15-2-5-17(29)6-3-15)27-23(30)21-14-19(24(31)32)18-13-16(4-7-20(18)26-21)28-8-10-35-11-9-28/h2-7,13-14,22,29H,8-12H2,1H3,(H,27,30)(H,31,32)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 199-203 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.041
BindingDB Entry DOI: 10.7270/Q2SN0BF8 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50171301

((S)-(2-{3-[((9S,12S)-12-Cyclohexyl-11,14-dioxo-2-o...)Show SMILES CCC[C@H](NC(=O)[C@@H]1Cc2cccc(Oc3ccc(CCC(=O)N[C@@H](C4CCCCC4)C(=O)N1)cc3)c2)C(=O)C(=O)NCC(=O)N[C@H](C(O)=O)c1ccccc1 Show InChI InChI=1S/C42H49N5O9/c1-2-10-32(38(50)41(53)43-25-35(49)47-37(42(54)55)29-14-7-4-8-15-29)44-39(51)33-24-27-11-9-16-31(23-27)56-30-20-17-26(18-21-30)19-22-34(48)46-36(40(52)45-33)28-12-5-3-6-13-28/h4,7-9,11,14-18,20-21,23,28,32-33,36-37H,2-3,5-6,10,12-13,19,22,24-25H2,1H3,(H,43,53)(H,44,51)(H,45,52)(H,46,48)(H,47,49)(H,54,55)/t32-,33-,36-,37-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding constant towards Human neutrophil elastase HNE protease |
J Med Chem 48: 5088-91 (2005)
Article DOI: 10.1021/jm0489556
BindingDB Entry DOI: 10.7270/Q2DN45T7 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50171304
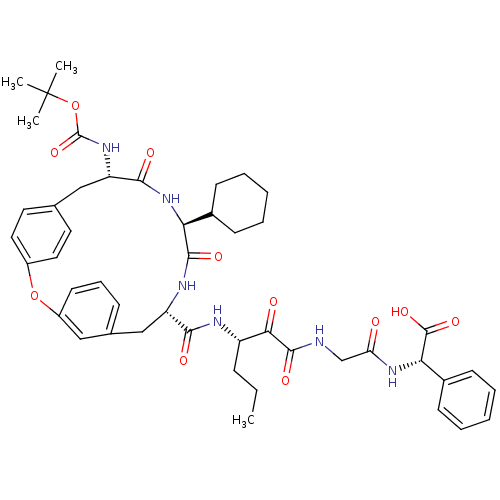
((S)-(2-{(S)-3-[((9S,12S,15S)-15-tert-Butoxycarbony...)Show SMILES CCC[C@H](NC(=O)[C@@H]1Cc2cccc(Oc3ccc(C[C@H](NC(=O)OC(C)(C)C)C(=O)N[C@@H](C4CCCCC4)C(=O)N1)cc3)c2)C(=O)C(=O)NCC(=O)N[C@H](C(O)=O)c1ccccc1 Show InChI InChI=1S/C47H58N6O11/c1-5-13-34(40(55)44(59)48-27-37(54)52-39(45(60)61)31-17-10-7-11-18-31)49-41(56)35-26-29-14-12-19-33(24-29)63-32-22-20-28(21-23-32)25-36(51-46(62)64-47(2,3)4)42(57)53-38(43(58)50-35)30-15-8-6-9-16-30/h7,10-12,14,17-24,30,34-36,38-39H,5-6,8-9,13,15-16,25-27H2,1-4H3,(H,48,59)(H,49,56)(H,50,58)(H,51,62)(H,52,54)(H,53,57)(H,60,61)/t34-,35-,36-,38-,39-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Ability to inhibit the hydrolysis of chromogenic 4-phenylazophenyl ester from the peptide fragment Ac- DTEDVVP(Nva)-O-4-PAP in a HCV protease continu... |
J Med Chem 48: 5088-91 (2005)
Article DOI: 10.1021/jm0489556
BindingDB Entry DOI: 10.7270/Q2DN45T7 |
More data for this
Ligand-Target Pair | |
GTP-binding nuclear protein GSP2/CNR2
(Saccharomyces cerevisiae) | BDBM29094

((+/-)-SLV319 | (S)-3-(4-chlorophenyl)-N-(4-chlorop...)Show SMILES CN=C(NS(=O)(=O)c1ccc(Cl)cc1)N1CC(C(=N1)c1ccc(Cl)cc1)c1ccccc1 |w:1.0,c:18| Show InChI InChI=1S/C23H20Cl2N4O2S/c1-26-23(28-32(30,31)20-13-11-19(25)12-14-20)29-15-21(16-5-3-2-4-6-16)22(27-29)17-7-9-18(24)10-8-17/h2-14,21H,15H2,1H3,(H,26,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNS Biological Research
Curated by PDSP Ki Database
| |
J Med Chem 51: 2439-46 (2008)
Article DOI: 10.1021/jm701519h
BindingDB Entry DOI: 10.7270/Q22R3Q7S |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50444937
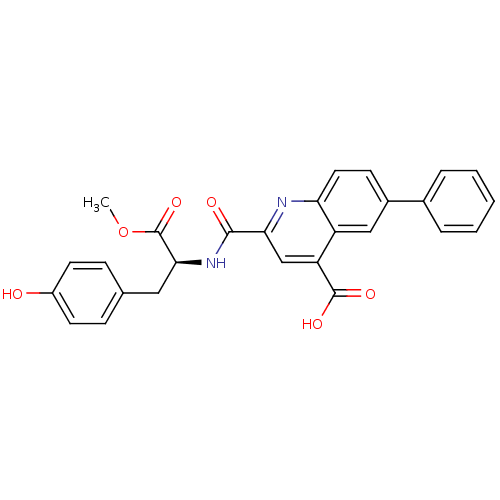
(CHEMBL3099759)Show SMILES COC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)c1cc(C(O)=O)c2cc(ccc2n1)-c1ccccc1 |r| Show InChI InChI=1S/C27H22N2O6/c1-35-27(34)24(13-16-7-10-19(30)11-8-16)29-25(31)23-15-21(26(32)33)20-14-18(9-12-22(20)28-23)17-5-3-2-4-6-17/h2-12,14-15,24,30H,13H2,1H3,(H,29,31)(H,32,33)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 199-203 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.041
BindingDB Entry DOI: 10.7270/Q2SN0BF8 |
More data for this
Ligand-Target Pair | |
GTP-binding nuclear protein GSP2/CNR2
(Saccharomyces cerevisiae) | BDBM87014

(4-(6-methoxy-2-(4-methoxyphenyl)benzofuran-3-carbo...)Show SMILES COc1ccc(cc1)-c1oc2cc(OC)ccc2c1C(=O)c1ccc(cc1)C#N Show InChI InChI=1S/C24H17NO4/c1-27-18-9-7-17(8-10-18)24-22(20-12-11-19(28-2)13-21(20)29-24)23(26)16-5-3-15(14-25)4-6-16/h3-13H,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNS Biological Research
Curated by PDSP Ki Database
| |
J Med Chem 51: 2439-46 (2008)
Article DOI: 10.1021/jm701519h
BindingDB Entry DOI: 10.7270/Q22R3Q7S |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50171303

(CHEMBL370096 | {3-[((9S,12S,15S)-15-tert-Butoxycar...)Show SMILES CCC[C@H](NC(=O)[C@@H]1Cc2cccc(Oc3ccc(C[C@H](NC(=O)OC(C)(C)C)C(=O)N[C@@H](C4CCCCC4)C(=O)N1)cc3)c2)C(=O)C(=O)NCC(O)=O Show InChI InChI=1S/C39H51N5O10/c1-5-10-28(33(47)37(51)40-22-31(45)46)41-34(48)29-21-24-11-9-14-27(19-24)53-26-17-15-23(16-18-26)20-30(43-38(52)54-39(2,3)4)35(49)44-32(36(50)42-29)25-12-7-6-8-13-25/h9,11,14-19,25,28-30,32H,5-8,10,12-13,20-22H2,1-4H3,(H,40,51)(H,41,48)(H,42,50)(H,43,52)(H,44,49)(H,45,46)/t28-,29-,30-,32-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Ability to inhibit the hydrolysis of chromogenic 4-phenylazophenyl ester from the peptide fragment Ac- DTEDVVP(Nva)-O-4-PAP in a HCV protease continu... |
J Med Chem 48: 5088-91 (2005)
Article DOI: 10.1021/jm0489556
BindingDB Entry DOI: 10.7270/Q2DN45T7 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50444924
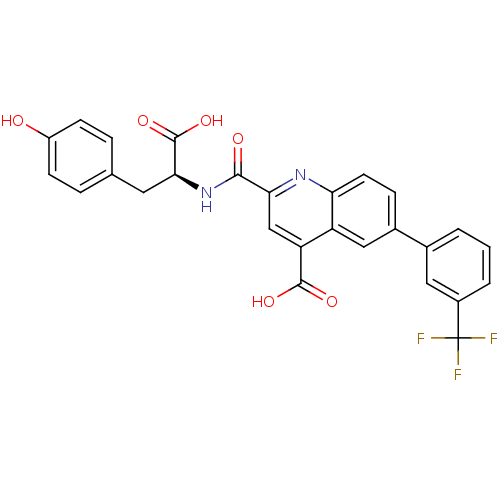
(CHEMBL3099747)Show SMILES OC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)c1cc(C(O)=O)c2cc(ccc2n1)-c1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C27H19F3N2O6/c28-27(29,30)17-3-1-2-15(11-17)16-6-9-21-19(12-16)20(25(35)36)13-22(31-21)24(34)32-23(26(37)38)10-14-4-7-18(33)8-5-14/h1-9,11-13,23,33H,10H2,(H,32,34)(H,35,36)(H,37,38)/t23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 199-203 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.041
BindingDB Entry DOI: 10.7270/Q2SN0BF8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data