

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Progesterone receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.0540 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Antagonist activity at progesterone receptor expressed in human T47D cells assessed as inhibition of promegestone-induced alkaline phosphatase activi... | Bioorg Med Chem Lett 17: 5754-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.064 BindingDB Entry DOI: 10.7270/Q2Z03905 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50222089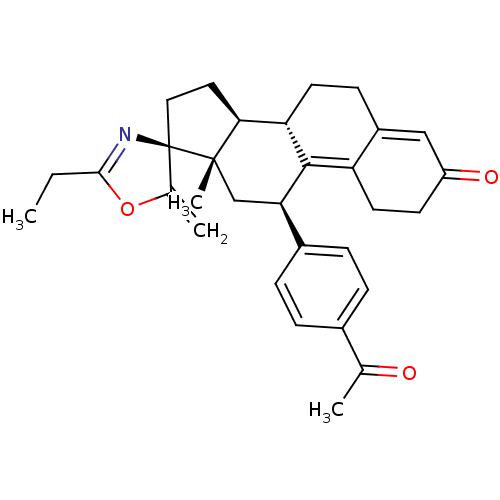 ((3S,10'S,11'S,15'S,17'R)-17'-(4-acetylphenyl)-5-et...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Antagonist activity at progesterone receptor expressed in human T47D cells assessed as inhibition of promegestone-induced alkaline phosphatase activi... | Bioorg Med Chem Lett 17: 5754-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.064 BindingDB Entry DOI: 10.7270/Q2Z03905 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50222091 ((3S,10'S,11'S,15'S,17'R)-17'-(4-acetylphenyl)-5,15...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Antagonist activity at progesterone receptor expressed in human T47D cells assessed as inhibition of promegestone-induced alkaline phosphatase activi... | Bioorg Med Chem Lett 17: 5754-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.064 BindingDB Entry DOI: 10.7270/Q2Z03905 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50222086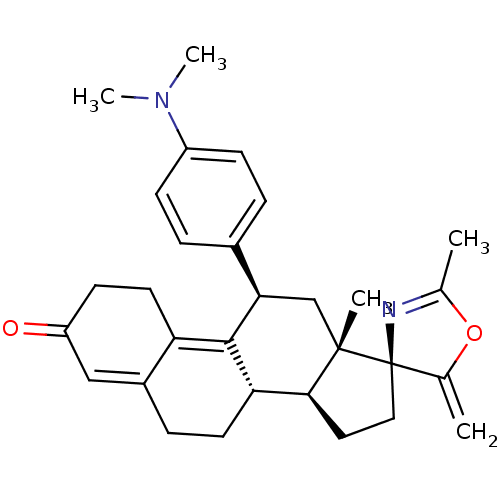 ((3S,10'S,11'S,15'S,17'R)-17'-[4-(dimethylamino)phe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Antagonist activity at progesterone receptor expressed in human T47D cells assessed as inhibition of promegestone-induced alkaline phosphatase activi... | Bioorg Med Chem Lett 17: 5754-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.064 BindingDB Entry DOI: 10.7270/Q2Z03905 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50222092 ((3S,10'S,11'S,15'S,17'R)-17'-[4-(dimethylamino)phe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Antagonist activity at progesterone receptor expressed in human T47D cells assessed as inhibition of promegestone-induced alkaline phosphatase activi... | Bioorg Med Chem Lett 17: 5754-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.064 BindingDB Entry DOI: 10.7270/Q2Z03905 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50222088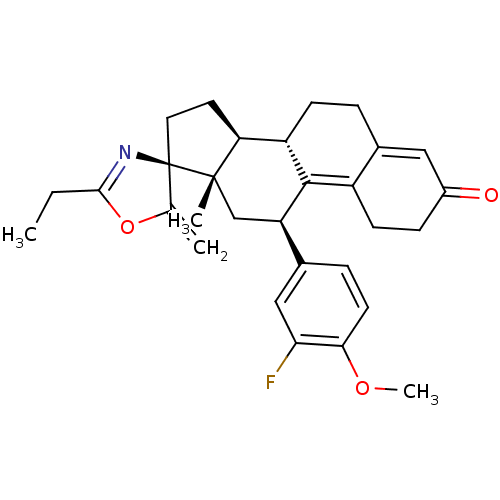 ((3S,10'S,11'S,15'S,17'R)-5-ethyl-17'-(3-fluoro-4-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Antagonist activity at progesterone receptor expressed in human T47D cells assessed as inhibition of promegestone-induced alkaline phosphatase activi... | Bioorg Med Chem Lett 17: 5754-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.064 BindingDB Entry DOI: 10.7270/Q2Z03905 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50222087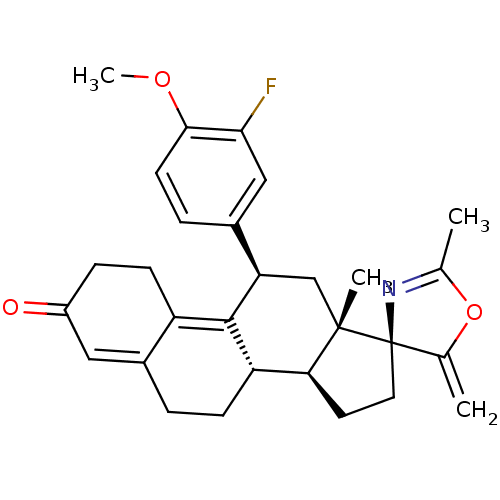 ((3S,10'S,11'S,15'S,17'R)-17'-(3-fluoro-4-methoxyph...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Antagonist activity at progesterone receptor expressed in human T47D cells assessed as inhibition of promegestone-induced alkaline phosphatase activi... | Bioorg Med Chem Lett 17: 5754-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.064 BindingDB Entry DOI: 10.7270/Q2Z03905 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor expressed in A549 cells assessed as inhibition of corticoid-induced gene transcription by luciferase r... | Bioorg Med Chem Lett 17: 5754-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.064 BindingDB Entry DOI: 10.7270/Q2Z03905 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50222085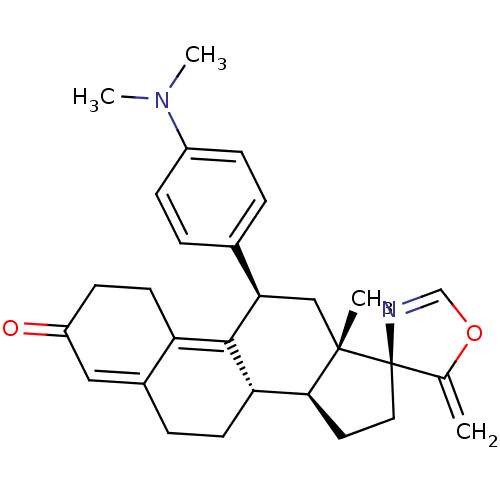 ((3S,10'S,11'S,15'S,17'R)-17'-[4-(dimethylamino)phe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Antagonist activity at progesterone receptor expressed in human T47D cells assessed as inhibition of promegestone-induced alkaline phosphatase activi... | Bioorg Med Chem Lett 17: 5754-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.064 BindingDB Entry DOI: 10.7270/Q2Z03905 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50222092 ((3S,10'S,11'S,15'S,17'R)-17'-[4-(dimethylamino)phe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor expressed in A549 cells assessed as inhibition of corticoid-induced gene transcription by luciferase r... | Bioorg Med Chem Lett 17: 5754-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.064 BindingDB Entry DOI: 10.7270/Q2Z03905 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50036133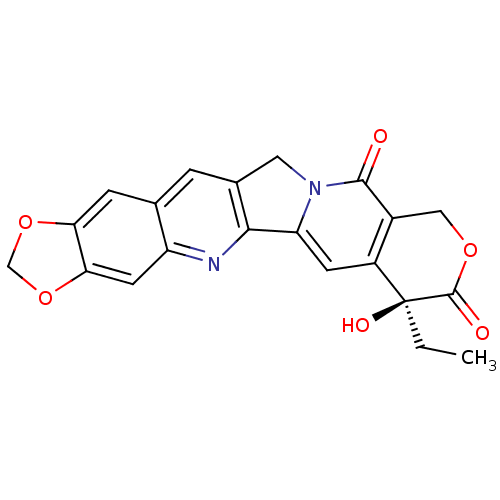 (7-ethyl-7-hydroxy-(7S)-7,8,11,13-tetrahydro-10H-[1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of Topoisomerase I by cleavage complex formation in human HL-60 cells | J Med Chem 36: 2689-700 (1993) BindingDB Entry DOI: 10.7270/Q2KP82RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50045377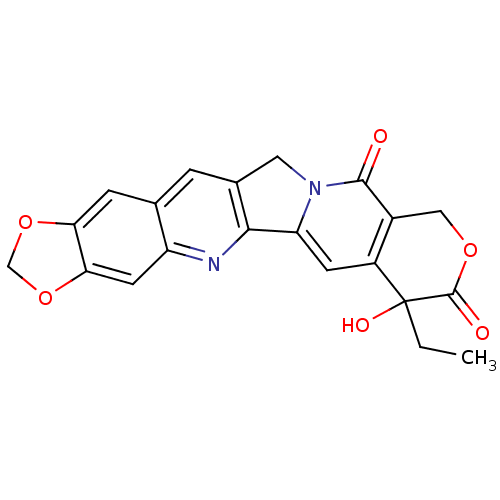 ((20R)-7-ethyl-7-hydroxy-7,8,11,13-tetrahydro-10H-[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of Topoisomerase I by cleavage complex formation in intact human HL-60 cells | J Med Chem 36: 2689-700 (1993) BindingDB Entry DOI: 10.7270/Q2KP82RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50422003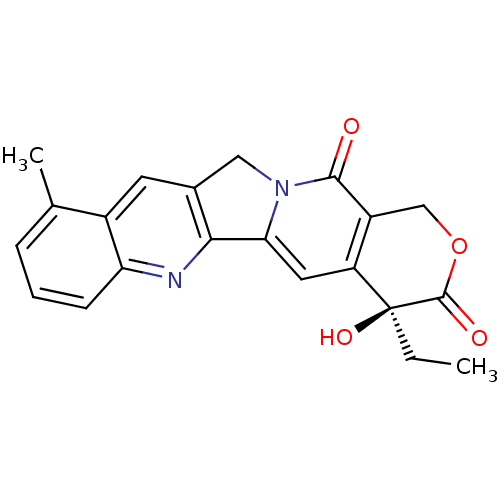 (CHEMBL10741) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of Topoisomerase I by cleavage complex formation in human HL-60 cells | J Med Chem 36: 2689-700 (1993) BindingDB Entry DOI: 10.7270/Q2KP82RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50406999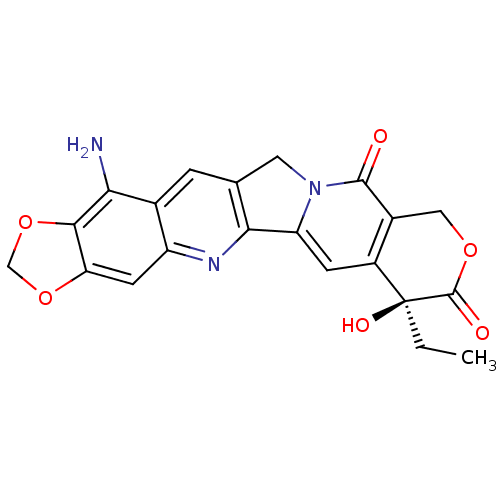 (CHEMBL2114243) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of Topoisomerase I by cleavage complex formation in human HL-60 cells | J Med Chem 36: 2689-700 (1993) BindingDB Entry DOI: 10.7270/Q2KP82RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50045368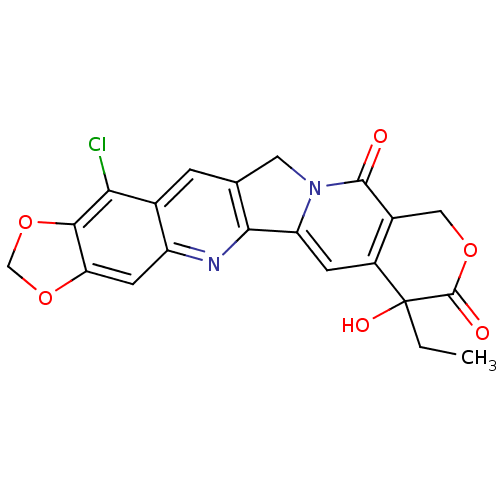 ((20S)-15-chloro-7-ethyl-7-hydroxy-7,8,11,13-tetrah...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of Topoisomerase I by cleavage complex formation in human HL-60 cells | J Med Chem 36: 2689-700 (1993) BindingDB Entry DOI: 10.7270/Q2KP82RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50045377 ((20R)-7-ethyl-7-hydroxy-7,8,11,13-tetrahydro-10H-[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of Topoisomerase I by cleavage complex formation in human HL-60 cells | J Med Chem 36: 2689-700 (1993) BindingDB Entry DOI: 10.7270/Q2KP82RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50045381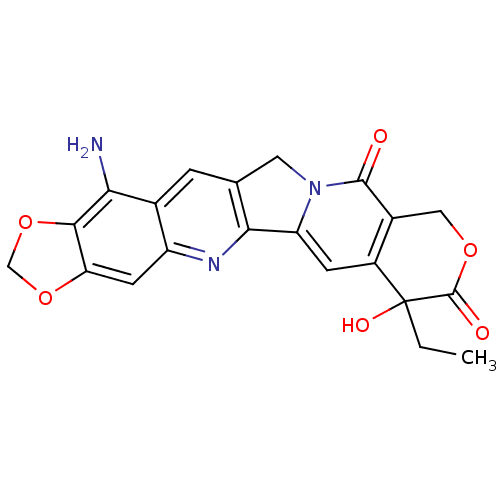 ((20R)-15-amino-7-ethyl-7-hydroxy-7,8,11,13-tetrahy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of Topoisomerase I by cleavage complex formation in intact human HL-60 cells | J Med Chem 36: 2689-700 (1993) BindingDB Entry DOI: 10.7270/Q2KP82RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50045381 ((20R)-15-amino-7-ethyl-7-hydroxy-7,8,11,13-tetrahy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of Topoisomerase I by cleavage complex formation in human HL-60 cells | J Med Chem 36: 2689-700 (1993) BindingDB Entry DOI: 10.7270/Q2KP82RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50222090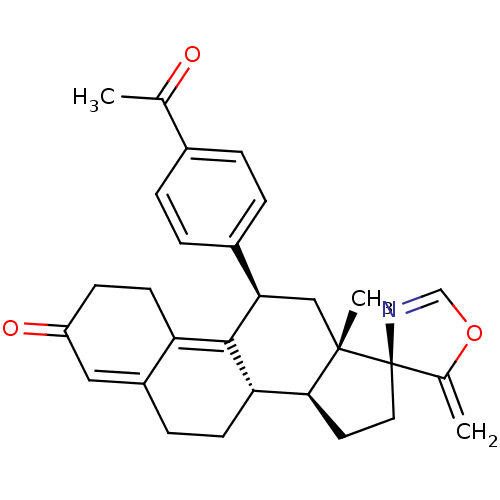 ((3S,10'S,11'S,15'S,17'R)-17'-(4-acetylphenyl)-15'-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Antagonist activity at progesterone receptor expressed in human T47D cells assessed as inhibition of promegestone-induced alkaline phosphatase activi... | Bioorg Med Chem Lett 17: 5754-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.064 BindingDB Entry DOI: 10.7270/Q2Z03905 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50222086 ((3S,10'S,11'S,15'S,17'R)-17'-[4-(dimethylamino)phe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor expressed in A549 cells assessed as inhibition of corticoid-induced gene transcription by luciferase r... | Bioorg Med Chem Lett 17: 5754-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.064 BindingDB Entry DOI: 10.7270/Q2Z03905 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50407001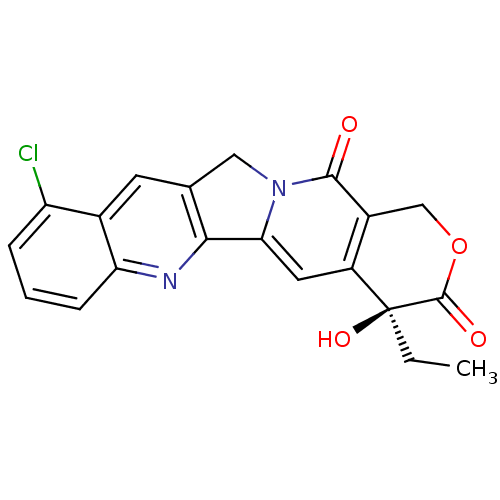 (CHEMBL2114244) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of Topoisomerase I by cleavage complex formation in human HL-60 cells | J Med Chem 36: 2689-700 (1993) BindingDB Entry DOI: 10.7270/Q2KP82RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50045383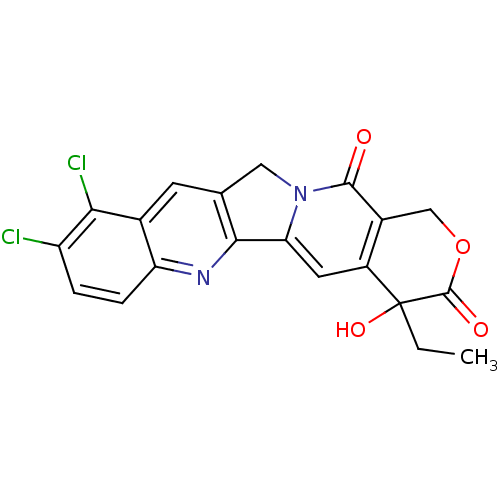 ((20S)-9,10-Dichloro-4-ethyl-4-hydroxy-1,12-dihydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of Topoisomerase I by cleavage complex formation in human HL-60 cells | J Med Chem 36: 2689-700 (1993) BindingDB Entry DOI: 10.7270/Q2KP82RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50008936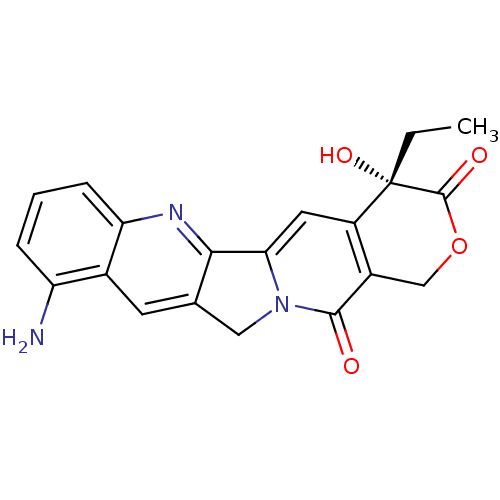 ((S)-10-Amino-4-ethyl-4-hydroxy-1,12-dihydro-4H-2-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of Topoisomerase I by cleavage complex formation in human HL-60 cells | J Med Chem 36: 2689-700 (1993) BindingDB Entry DOI: 10.7270/Q2KP82RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50008922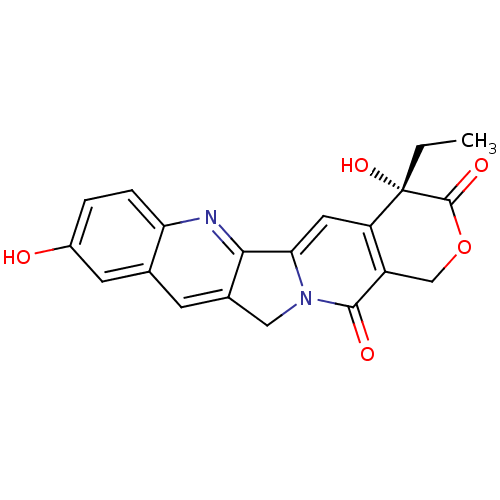 ((20S)-4-Ethyl-4,9-dihydroxy-1,12-dihydro-4H-2-oxa-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of Topoisomerase I by cleavage complex formation in human HL-60 cells | J Med Chem 36: 2689-700 (1993) BindingDB Entry DOI: 10.7270/Q2KP82RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50045398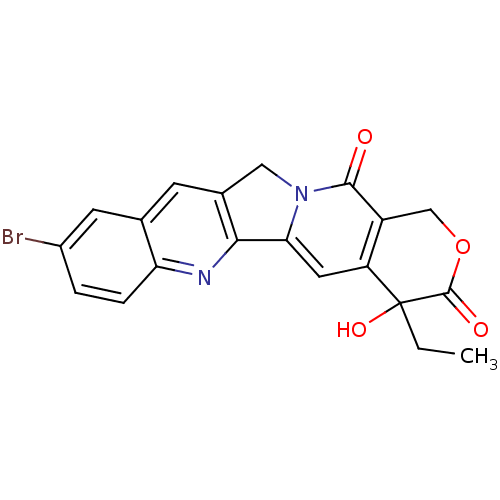 ((20S)-9-Bromo-4-ethyl-4-hydroxy-1,12-dihydro-4H-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of Topoisomerase I by cleavage complex formation in human HL-60 cells | J Med Chem 36: 2689-700 (1993) BindingDB Entry DOI: 10.7270/Q2KP82RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50406990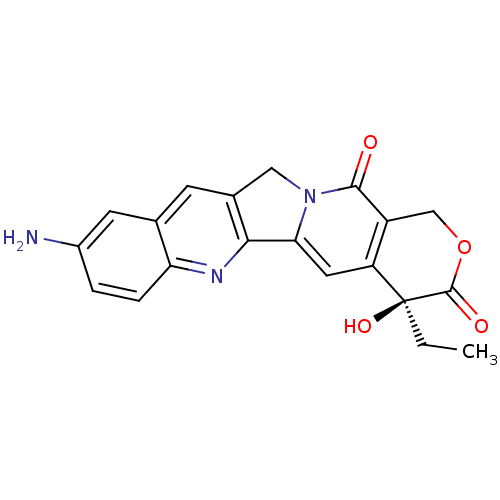 (CHEMBL102252) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of Topoisomerase I by cleavage complex formation in human HL-60 cells | J Med Chem 36: 2689-700 (1993) BindingDB Entry DOI: 10.7270/Q2KP82RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50406995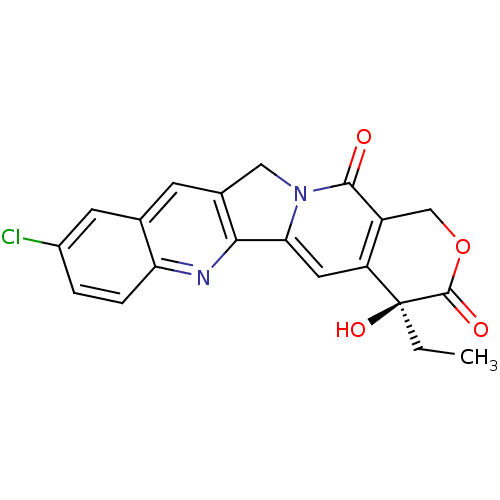 (CHEMBL2115019) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of Topoisomerase I by cleavage complex formation in human HL-60 cells | J Med Chem 36: 2689-700 (1993) BindingDB Entry DOI: 10.7270/Q2KP82RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50406997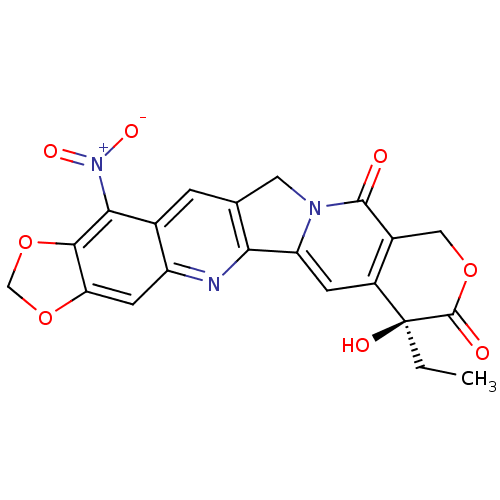 (CHEMBL2114245) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of Topoisomerase I by cleavage complex formation in human HL-60 cells | J Med Chem 36: 2689-700 (1993) BindingDB Entry DOI: 10.7270/Q2KP82RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50045369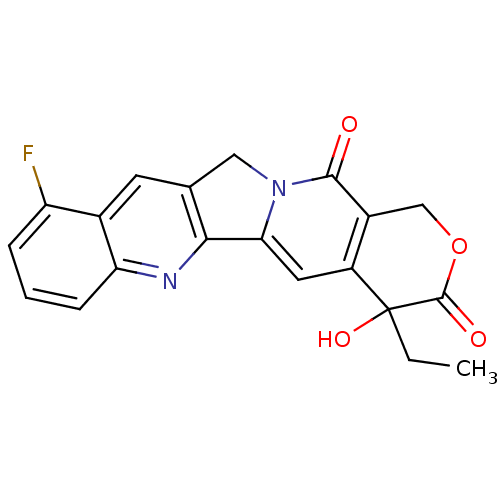 ((20S)-4-Ethyl-10-fluoro-4-hydroxy-1,12-dihydro-4H-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of Topoisomerase I by cleavage complex formation in human HL-60 cells | J Med Chem 36: 2689-700 (1993) BindingDB Entry DOI: 10.7270/Q2KP82RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50045375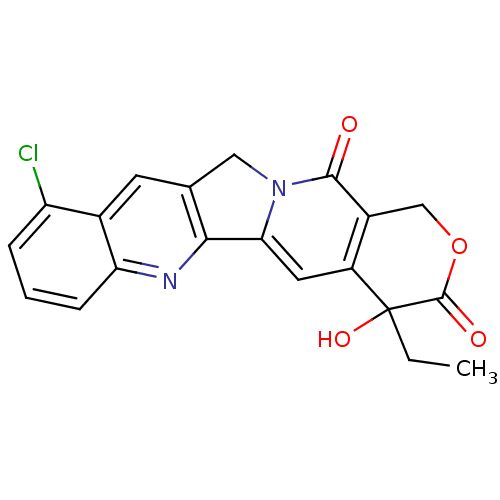 ((20RS)-10-Chloro-4-ethyl-4-hydroxy-1,12-dihydro-4H...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of Topoisomerase I by cleavage complex formation in human HL-60 cells | J Med Chem 36: 2689-700 (1993) BindingDB Entry DOI: 10.7270/Q2KP82RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50222089 ((3S,10'S,11'S,15'S,17'R)-17'-(4-acetylphenyl)-5-et...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor expressed in A549 cells assessed as inhibition of corticoid-induced gene transcription by luciferase r... | Bioorg Med Chem Lett 17: 5754-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.064 BindingDB Entry DOI: 10.7270/Q2Z03905 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50045379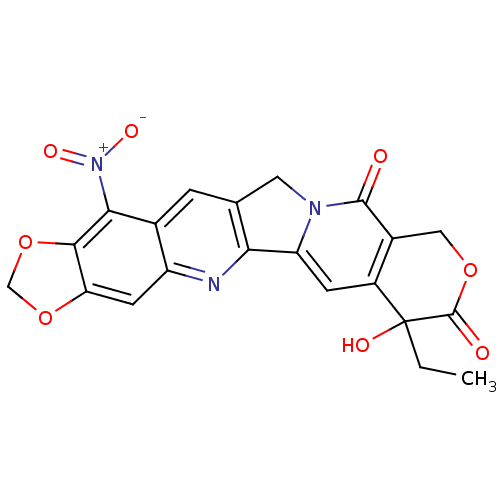 ((20RS)-7-ethyl-7-hydroxy-15-nitro-7,8,11,13-tetrah...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of Topoisomerase I by cleavage complex formation in human HL-60 cells | J Med Chem 36: 2689-700 (1993) BindingDB Entry DOI: 10.7270/Q2KP82RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50045360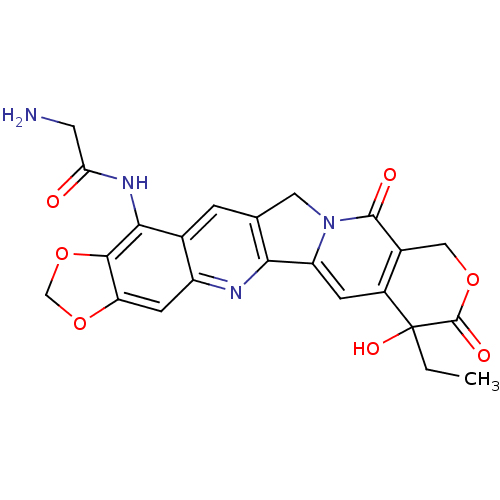 ((20RS)-1N-(7-ethyl-7-hydroxy-8,11-dioxo-7,8,11,13-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of Topoisomerase I by cleavage complex formation in human HL-60 cells | J Med Chem 36: 2689-700 (1993) BindingDB Entry DOI: 10.7270/Q2KP82RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50406991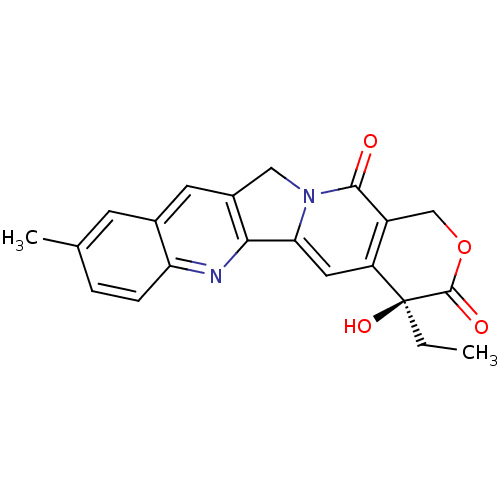 (CHEMBL2115014) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of Topoisomerase I by cleavage complex formation in human HL-60 cells | J Med Chem 36: 2689-700 (1993) BindingDB Entry DOI: 10.7270/Q2KP82RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50045392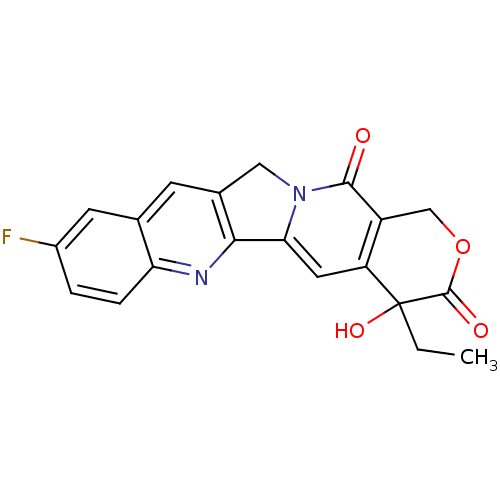 ((20S)-4-Ethyl-9-fluoro-4-hydroxy-1,12-dihydro-4H-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of Topoisomerase I by cleavage complex formation in human HL-60 cells | J Med Chem 36: 2689-700 (1993) BindingDB Entry DOI: 10.7270/Q2KP82RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50045362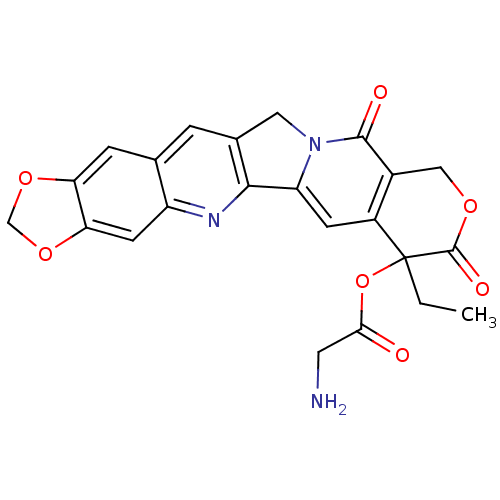 ((20RS)-7-ethyl-8,11-dioxo-7,8,11,13-tetrahydro-10H...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of Topoisomerase I by cleavage complex formation in intact human HL-60 cells | J Med Chem 36: 2689-700 (1993) BindingDB Entry DOI: 10.7270/Q2KP82RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50045367 ((20RS)-10-Amino-4-ethyl-4-hydroxy-1,12-dihydro-4H-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of Topoisomerase I by cleavage complex formation in human HL-60 cells | J Med Chem 36: 2689-700 (1993) BindingDB Entry DOI: 10.7270/Q2KP82RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50222091 ((3S,10'S,11'S,15'S,17'R)-17'-(4-acetylphenyl)-5,15...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor expressed in A549 cells assessed as inhibition of corticoid-induced gene transcription by luciferase r... | Bioorg Med Chem Lett 17: 5754-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.064 BindingDB Entry DOI: 10.7270/Q2Z03905 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50045393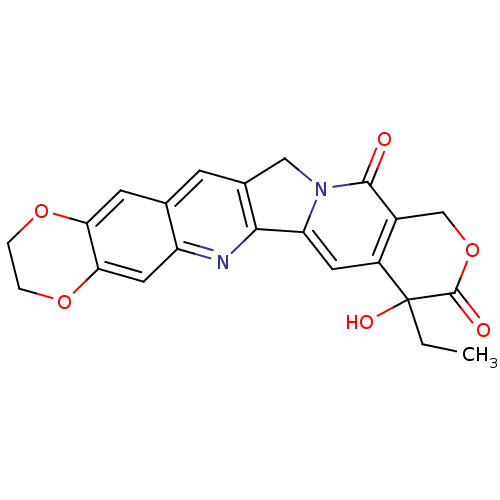 ((20RS)-8-ethyl-8-hydroxy-2,3,8,9,12,14-hexahydro-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of Topoisomerase I by cleavage complex formation in human HL-60 cells | J Med Chem 36: 2689-700 (1993) BindingDB Entry DOI: 10.7270/Q2KP82RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50222085 ((3S,10'S,11'S,15'S,17'R)-17'-[4-(dimethylamino)phe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor expressed in A549 cells assessed as inhibition of corticoid-induced gene transcription by luciferase r... | Bioorg Med Chem Lett 17: 5754-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.064 BindingDB Entry DOI: 10.7270/Q2Z03905 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50045390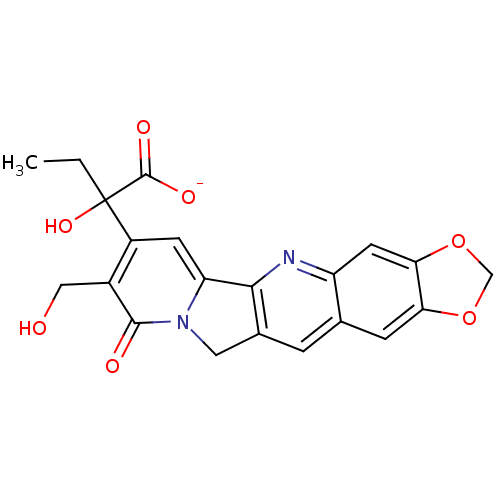 ((20RS)-sodium 2-hydroxy-2-(8-hydroxymethyl-9-oxo-9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of Topoisomerase I by cleavage complex formation in human HL-60 cells | J Med Chem 36: 2689-700 (1993) BindingDB Entry DOI: 10.7270/Q2KP82RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50045384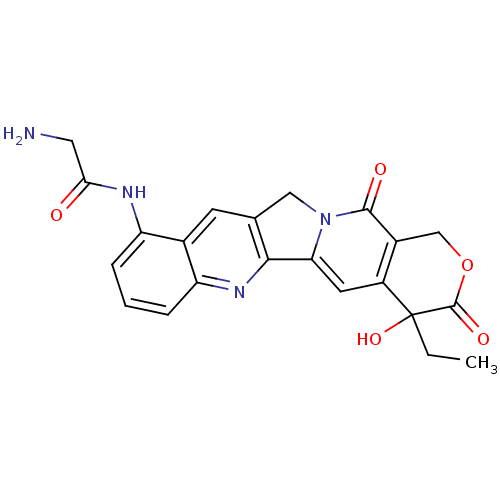 ((20RS)-2-Amino-N-(4-ethyl-4-hydroxy-3,13-dioxo-3,4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of Topoisomerase I by cleavage complex formation in human HL-60 cells | J Med Chem 36: 2689-700 (1993) BindingDB Entry DOI: 10.7270/Q2KP82RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50406993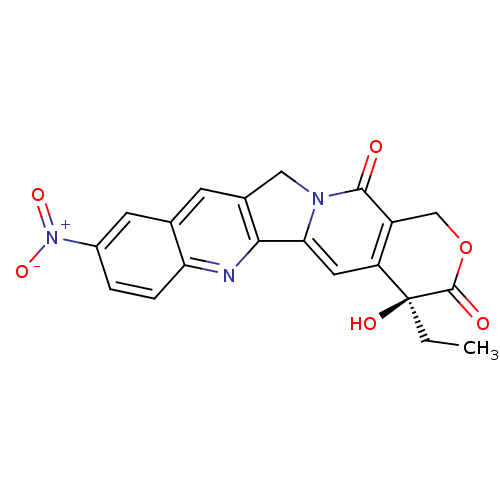 (CHEMBL2115022) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of Topoisomerase I by cleavage complex formation in human HL-60 cells | J Med Chem 36: 2689-700 (1993) BindingDB Entry DOI: 10.7270/Q2KP82RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50222087 ((3S,10'S,11'S,15'S,17'R)-17'-(3-fluoro-4-methoxyph...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor expressed in A549 cells assessed as inhibition of corticoid-induced gene transcription by luciferase r... | Bioorg Med Chem Lett 17: 5754-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.064 BindingDB Entry DOI: 10.7270/Q2Z03905 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50008923 ((S)-4-ethyl-4-hydroxy-1,12-dihydro-4H-2-oxa-6,12a-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of Topoisomerase I by cleavage complex formation in human HL-60 cells | J Med Chem 36: 2689-700 (1993) BindingDB Entry DOI: 10.7270/Q2KP82RH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50045367 ((20RS)-10-Amino-4-ethyl-4-hydroxy-1,12-dihydro-4H-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of Topoisomerase I by cleavage complex formation in intact human HL-60 cells | J Med Chem 36: 2689-700 (1993) BindingDB Entry DOI: 10.7270/Q2KP82RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50407000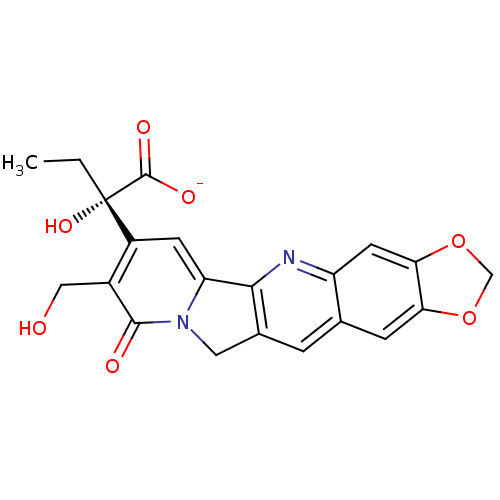 (CHEMBL2114241) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of Topoisomerase I by cleavage complex formation in human HL-60 cells | J Med Chem 36: 2689-700 (1993) BindingDB Entry DOI: 10.7270/Q2KP82RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50406996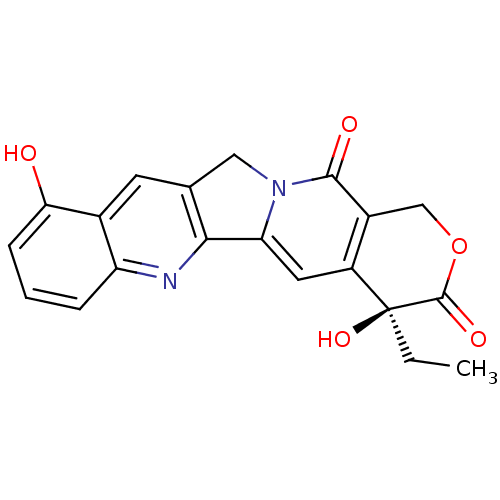 (CHEMBL87791) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of Topoisomerase I by cleavage complex formation in human HL-60 cells | J Med Chem 36: 2689-700 (1993) BindingDB Entry DOI: 10.7270/Q2KP82RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50045372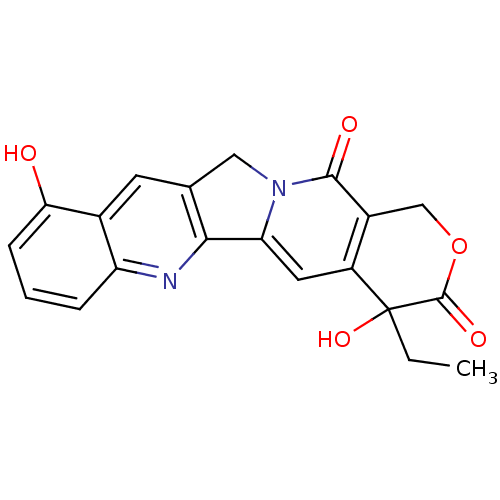 ((20RS)-4-Ethyl-4,10-dihydroxy-1,12-dihydro-4H-2-ox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of Topoisomerase I by cleavage complex formation in human HL-60 cells | J Med Chem 36: 2689-700 (1993) BindingDB Entry DOI: 10.7270/Q2KP82RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50008935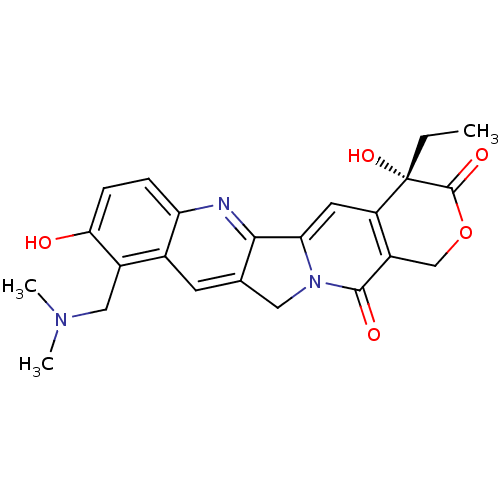 ((20S)-10-Dimethylaminomethyl-4-ethyl-4,9-dihydroxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of Topoisomerase I by cleavage complex formation in human HL-60 cells | J Med Chem 36: 2689-700 (1993) BindingDB Entry DOI: 10.7270/Q2KP82RH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 97 total ) | Next | Last >> |