

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50064106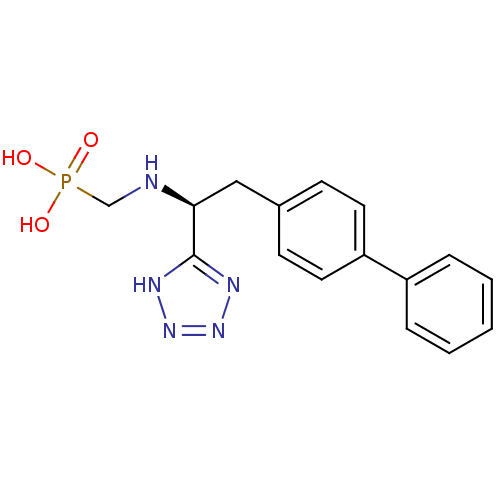 (CGS-26303 | CHEMBL290698 | {[(R)-2-Biphenyl-4-yl-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro inhibition of rat neutral endopeptidase | J Med Chem 43: 488-504 (2000) BindingDB Entry DOI: 10.7270/Q21G0MZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50084905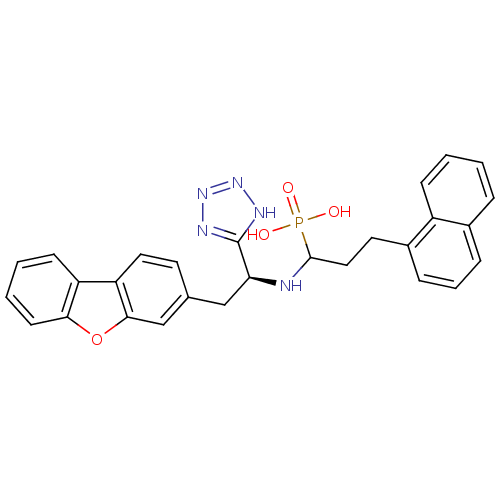 (CHEMBL147964 | {1-[2-Dibenzofuran-3-yl-1-(1H-tetra...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In Vitro inhibition of recombinant human endothelin converting enzyme-1 | J Med Chem 43: 488-504 (2000) BindingDB Entry DOI: 10.7270/Q21G0MZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50084905 (CHEMBL147964 | {1-[2-Dibenzofuran-3-yl-1-(1H-tetra...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In Vitro inhibition of recombinant human endothelin converting enzyme-1 | J Med Chem 43: 488-504 (2000) BindingDB Entry DOI: 10.7270/Q21G0MZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50084897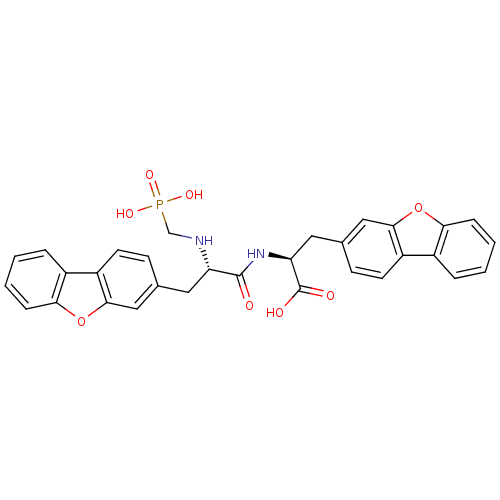 (3-Dibenzofuran-3-yl-2-[3-dibenzofuran-3-yl-2-(phos...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro inhibition of rat neutral endopeptidase | J Med Chem 43: 488-504 (2000) BindingDB Entry DOI: 10.7270/Q21G0MZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM21641 (2-(2-benzyl-3-sulfanylpropanamido)acetic acid | CH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro inhibition of rat neutral endopeptidase | J Med Chem 43: 488-504 (2000) BindingDB Entry DOI: 10.7270/Q21G0MZR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50064109 (CGS-31447 | CHEMBL285619 | {1-[(S)-2-Biphenyl-4-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro inhibition of rat neutral endopeptidase | J Med Chem 43: 488-504 (2000) BindingDB Entry DOI: 10.7270/Q21G0MZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50084903 (CHEMBL148056 | {[2-Dibenzofuran-3-yl-1-(1H-tetrazo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In Vitro inhibition of recombinant human endothelin converting enzyme-1 | J Med Chem 43: 488-504 (2000) BindingDB Entry DOI: 10.7270/Q21G0MZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50084897 (3-Dibenzofuran-3-yl-2-[3-dibenzofuran-3-yl-2-(phos...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In Vitro inhibition of recombinant human endothelin converting enzyme-1 | J Med Chem 43: 488-504 (2000) BindingDB Entry DOI: 10.7270/Q21G0MZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50064110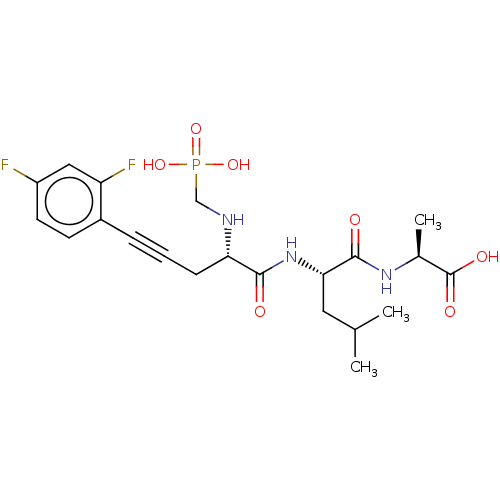 (2-{2-[(S)-5-(2,4-Difluoro-phenyl)-2-(phosphonometh...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In Vitro inhibition of recombinant human endothelin converting enzyme-1 | J Med Chem 43: 488-504 (2000) BindingDB Entry DOI: 10.7270/Q21G0MZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50091882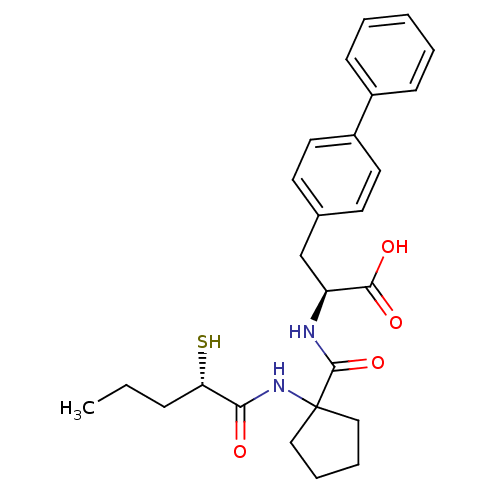 ((S)-3-Biphenyl-4-yl-2-{[1-((S)-2-mercapto-1-oxo-pe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against Endothelin converting enzyme 1 | Bioorg Med Chem Lett 11: 375-8 (2001) BindingDB Entry DOI: 10.7270/Q2HH6JC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50084895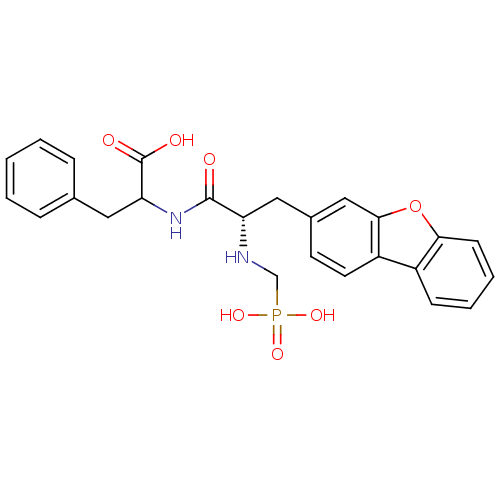 (2-[(S)-3-Dibenzofuran-3-yl-2-(phosphonomethyl-amin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro inhibition of rat neutral endopeptidase | J Med Chem 43: 488-504 (2000) BindingDB Entry DOI: 10.7270/Q21G0MZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50084898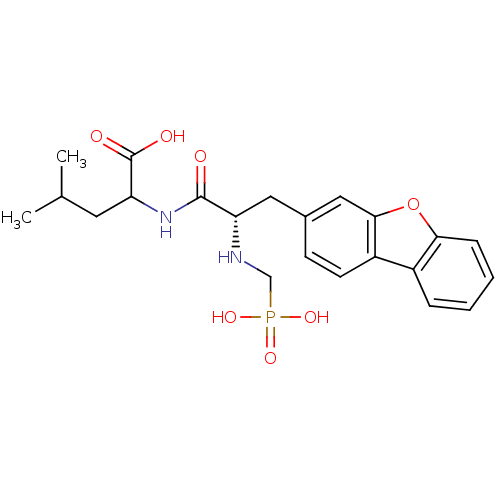 (2-[3-Dibenzofuran-3-yl-2-(phosphonomethyl-amino)-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In Vitro inhibition of recombinant human endothelin converting enzyme-1 | J Med Chem 43: 488-504 (2000) BindingDB Entry DOI: 10.7270/Q21G0MZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50064096 (CHEMBL40028 | {[(S)-4-(2-Fluoro-phenyl)-1-(2H-tetr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro inhibition of rat neutral endopeptidase | J Med Chem 43: 488-504 (2000) BindingDB Entry DOI: 10.7270/Q21G0MZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50064109 (CGS-31447 | CHEMBL285619 | {1-[(S)-2-Biphenyl-4-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In Vitro inhibition of recombinant human endothelin converting enzyme-1 | J Med Chem 43: 488-504 (2000) BindingDB Entry DOI: 10.7270/Q21G0MZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50084893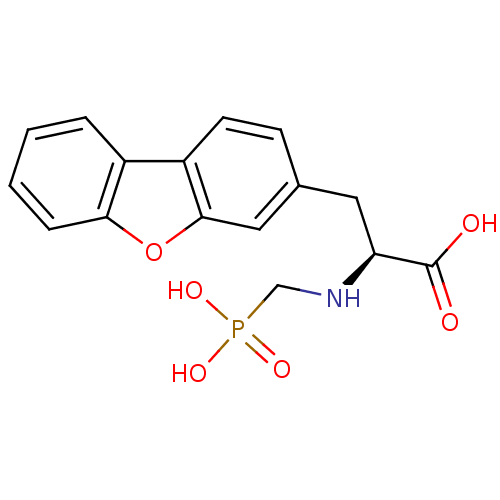 ((S)-3-(dibenzo[b,d]furan-3-yl)-2-(phosphonomethyla...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In Vitro inhibition of recombinant human endothelin converting enzyme-1 | J Med Chem 43: 488-504 (2000) BindingDB Entry DOI: 10.7270/Q21G0MZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50084892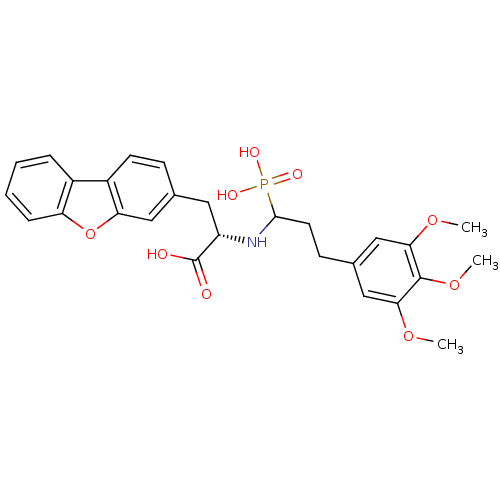 (3-Dibenzofuran-3-yl-2-[1-phosphono-3-(3,4,5-trimet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In Vitro inhibition of recombinant human endothelin converting enzyme-1 | J Med Chem 43: 488-504 (2000) BindingDB Entry DOI: 10.7270/Q21G0MZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50084895 (2-[(S)-3-Dibenzofuran-3-yl-2-(phosphonomethyl-amin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In Vitro inhibition of recombinant human endothelin converting enzyme-1 | J Med Chem 43: 488-504 (2000) BindingDB Entry DOI: 10.7270/Q21G0MZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50084910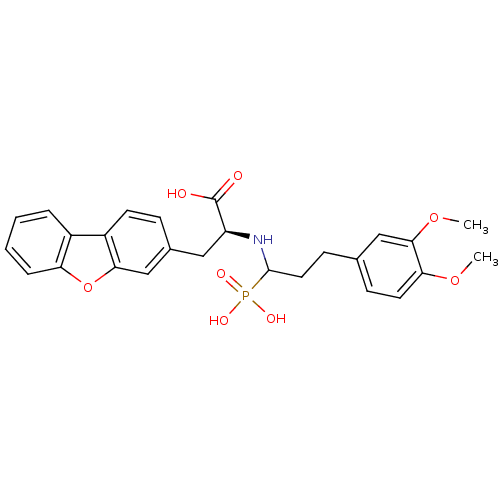 (3-Dibenzofuran-3-yl-2-[3-(3,4-dimethoxy-phenyl)-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In Vitro inhibition of recombinant human endothelin converting enzyme-1 | J Med Chem 43: 488-504 (2000) BindingDB Entry DOI: 10.7270/Q21G0MZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50084910 (3-Dibenzofuran-3-yl-2-[3-(3,4-dimethoxy-phenyl)-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In Vitro inhibition of recombinant human endothelin converting enzyme-1 | J Med Chem 43: 488-504 (2000) BindingDB Entry DOI: 10.7270/Q21G0MZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50084911 (CHEMBL147163 | {[2-(9-Oxo-9H-fluoren-2-yl)-1-(1H-t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In Vitro inhibition of recombinant human endothelin converting enzyme-1 | J Med Chem 43: 488-504 (2000) BindingDB Entry DOI: 10.7270/Q21G0MZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50251742 ((3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro inhibition of rat neutral endopeptidase | J Med Chem 43: 488-504 (2000) BindingDB Entry DOI: 10.7270/Q21G0MZR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50084898 (2-[3-Dibenzofuran-3-yl-2-(phosphonomethyl-amino)-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro inhibition of rat neutral endopeptidase | J Med Chem 43: 488-504 (2000) BindingDB Entry DOI: 10.7270/Q21G0MZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50084912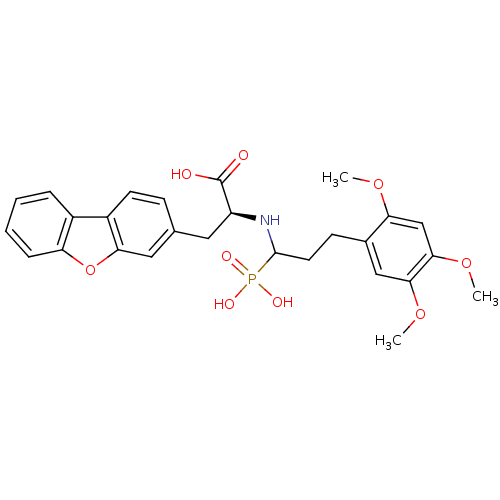 (3-Dibenzofuran-3-yl-2-[1-phosphono-3-(2,4,5-trimet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In Vitro inhibition of recombinant human endothelin converting enzyme-1 | J Med Chem 43: 488-504 (2000) BindingDB Entry DOI: 10.7270/Q21G0MZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50064120 ((S)-2-[(S)-5-(2,4-Difluoro-phenyl)-2-(phosphonomet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In Vitro inhibition of recombinant human endothelin converting enzyme-1 | J Med Chem 43: 488-504 (2000) BindingDB Entry DOI: 10.7270/Q21G0MZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50084900 (3-Dibenzofuran-3-yl-2-(5-methyl-1-phosphono-hexyla...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In Vitro inhibition of recombinant human endothelin converting enzyme-1 | J Med Chem 43: 488-504 (2000) BindingDB Entry DOI: 10.7270/Q21G0MZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50084899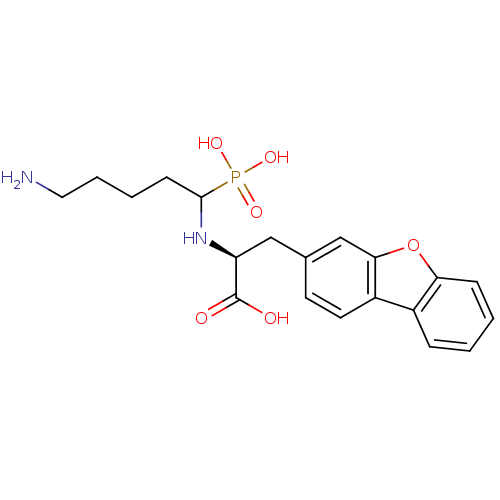 (2-(5-Amino-1-phosphono-pentylamino)-3-dibenzofuran...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In Vitro inhibition of recombinant human endothelin converting enzyme-1 | J Med Chem 43: 488-504 (2000) BindingDB Entry DOI: 10.7270/Q21G0MZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50084894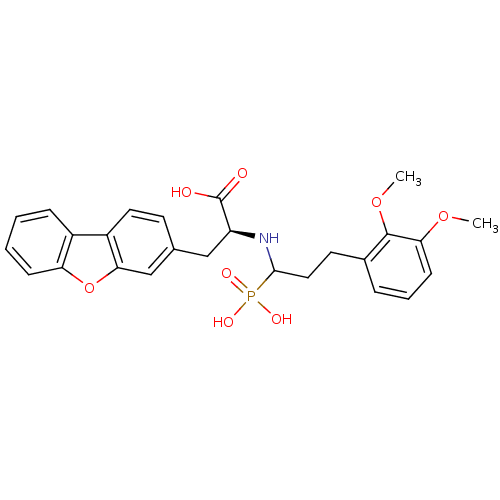 (3-Dibenzofuran-3-yl-2-[3-(2,3-dimethoxy-phenyl)-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In Vitro inhibition of recombinant human endothelin converting enzyme-1 | J Med Chem 43: 488-504 (2000) BindingDB Entry DOI: 10.7270/Q21G0MZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50084894 (3-Dibenzofuran-3-yl-2-[3-(2,3-dimethoxy-phenyl)-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In Vitro inhibition of recombinant human endothelin converting enzyme-1 | J Med Chem 43: 488-504 (2000) BindingDB Entry DOI: 10.7270/Q21G0MZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50084903 (CHEMBL148056 | {[2-Dibenzofuran-3-yl-1-(1H-tetrazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro inhibition of rat neutral endopeptidase | J Med Chem 43: 488-504 (2000) BindingDB Entry DOI: 10.7270/Q21G0MZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50096753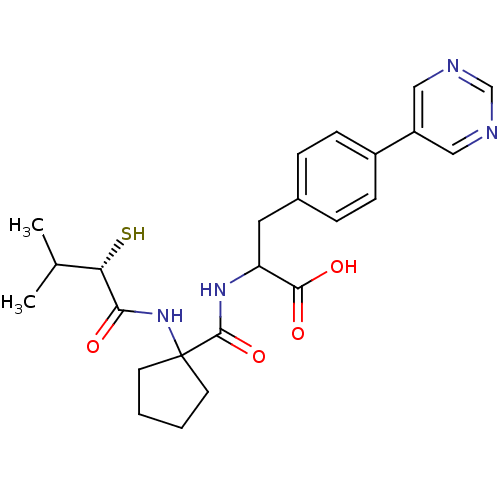 (2-{[1-((S)-2-Mercapto-3-methyl-1-oxo-butylamino)-c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against Endothelin converting enzyme 1 | Bioorg Med Chem Lett 11: 375-8 (2001) BindingDB Entry DOI: 10.7270/Q2HH6JC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50096747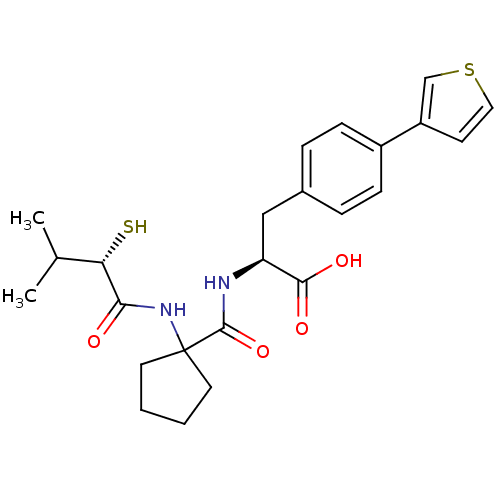 ((S)-2-{[1-((S)-2-Mercapto-3-methyl-1-oxo-butylamin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against Endothelin converting enzyme 1 | Bioorg Med Chem Lett 11: 375-8 (2001) BindingDB Entry DOI: 10.7270/Q2HH6JC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50084909 (CHEMBL147041 | {[2-(9-Methyl-9H-carbazol-2-yl)-1-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro inhibition of rat neutral endopeptidase | J Med Chem 43: 488-504 (2000) BindingDB Entry DOI: 10.7270/Q21G0MZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50064096 (CHEMBL40028 | {[(S)-4-(2-Fluoro-phenyl)-1-(2H-tetr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In Vitro inhibition of recombinant human endothelin converting enzyme-1 | J Med Chem 43: 488-504 (2000) BindingDB Entry DOI: 10.7270/Q21G0MZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50096749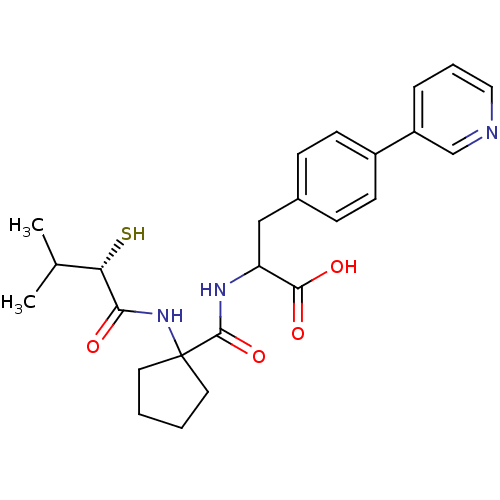 (2-{[1-((S)-2-Mercapto-3-methyl-1-oxo-butylamino)-c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against Endothelin converting enzyme 1 | Bioorg Med Chem Lett 11: 375-8 (2001) BindingDB Entry DOI: 10.7270/Q2HH6JC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50096758 ((S)-2-{[1-((S)-2-Mercapto-3-methyl-1-oxo-butylamin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against Endothelin converting enzyme 1 | Bioorg Med Chem Lett 11: 375-8 (2001) BindingDB Entry DOI: 10.7270/Q2HH6JC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50084896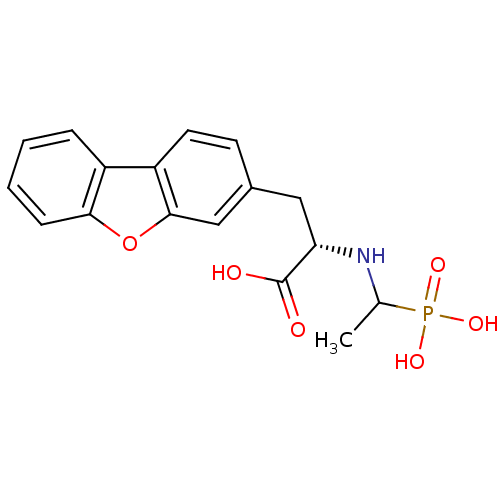 (3-Dibenzofuran-3-yl-2-(1-phosphono-ethylamino)-pro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In Vitro inhibition of recombinant human endothelin converting enzyme-1 | J Med Chem 43: 488-504 (2000) BindingDB Entry DOI: 10.7270/Q21G0MZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50084896 (3-Dibenzofuran-3-yl-2-(1-phosphono-ethylamino)-pro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In Vitro inhibition of recombinant human endothelin converting enzyme-1 | J Med Chem 43: 488-504 (2000) BindingDB Entry DOI: 10.7270/Q21G0MZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50084891 (3-Dibenzofuran-3-yl-2-(1-phosphono-3-quinolin-8-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In Vitro inhibition of recombinant human endothelin converting enzyme-1 | J Med Chem 43: 488-504 (2000) BindingDB Entry DOI: 10.7270/Q21G0MZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50084891 (3-Dibenzofuran-3-yl-2-(1-phosphono-3-quinolin-8-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In Vitro inhibition of recombinant human endothelin converting enzyme-1 | J Med Chem 43: 488-504 (2000) BindingDB Entry DOI: 10.7270/Q21G0MZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50084905 (CHEMBL147964 | {1-[2-Dibenzofuran-3-yl-1-(1H-tetra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro inhibition of rat neutral endopeptidase | J Med Chem 43: 488-504 (2000) BindingDB Entry DOI: 10.7270/Q21G0MZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50084905 (CHEMBL147964 | {1-[2-Dibenzofuran-3-yl-1-(1H-tetra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro inhibition of rat neutral endopeptidase | J Med Chem 43: 488-504 (2000) BindingDB Entry DOI: 10.7270/Q21G0MZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50096756 (2-{[1-((S)-2-Mercapto-3-methyl-1-oxo-butylamino)-c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against Endothelin converting enzyme 1 | Bioorg Med Chem Lett 11: 375-8 (2001) BindingDB Entry DOI: 10.7270/Q2HH6JC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50084911 (CHEMBL147163 | {[2-(9-Oxo-9H-fluoren-2-yl)-1-(1H-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro inhibition of rat neutral endopeptidase | J Med Chem 43: 488-504 (2000) BindingDB Entry DOI: 10.7270/Q21G0MZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50096748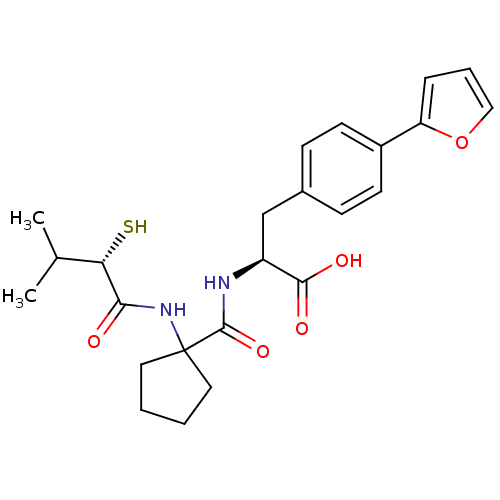 ((S)-3-(4-Furan-2-yl-phenyl)-2-{[1-((S)-2-mercapto-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against Endothelin converting enzyme 1 | Bioorg Med Chem Lett 11: 375-8 (2001) BindingDB Entry DOI: 10.7270/Q2HH6JC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50096746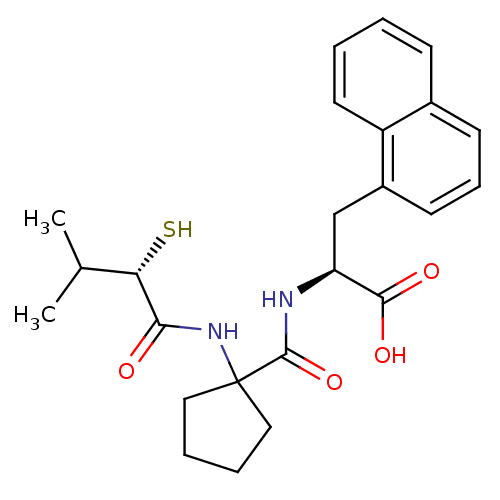 ((S)-2-{[1-((S)-2-Mercapto-3-methyl-1-oxo-butylamin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against Endothelin converting enzyme 1 | Bioorg Med Chem Lett 11: 375-8 (2001) BindingDB Entry DOI: 10.7270/Q2HH6JC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50096752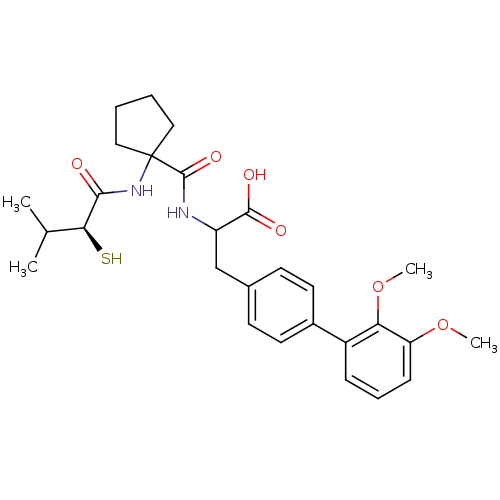 (3-(2',3'-Dimethoxy-biphenyl-4-yl)-2-{[1-((S)-2-mer...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against Endothelin converting enzyme 1 | Bioorg Med Chem Lett 11: 375-8 (2001) BindingDB Entry DOI: 10.7270/Q2HH6JC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50096751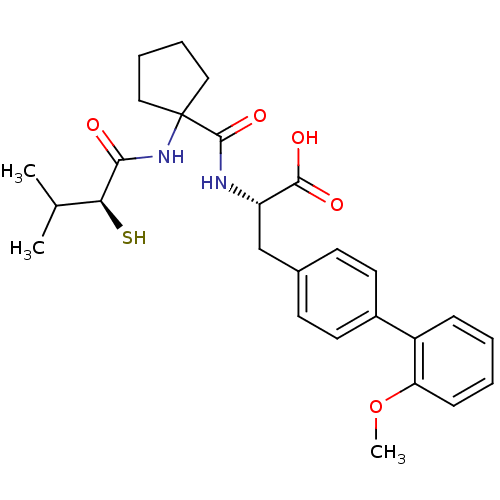 ((S)-2-{[1-((S)-2-Mercapto-3-methyl-1-oxo-butylamin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against Endothelin converting enzyme 1 | Bioorg Med Chem Lett 11: 375-8 (2001) BindingDB Entry DOI: 10.7270/Q2HH6JC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50064106 (CGS-26303 | CHEMBL290698 | {[(R)-2-Biphenyl-4-yl-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In Vitro inhibition of recombinant human endothelin converting enzyme-1 | J Med Chem 43: 488-504 (2000) BindingDB Entry DOI: 10.7270/Q21G0MZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50084906 (CHEMBL343904 | {[2-(9H-Fluoren-2-yl)-1-(1H-tetrazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro inhibition of rat neutral endopeptidase | J Med Chem 43: 488-504 (2000) BindingDB Entry DOI: 10.7270/Q21G0MZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50096759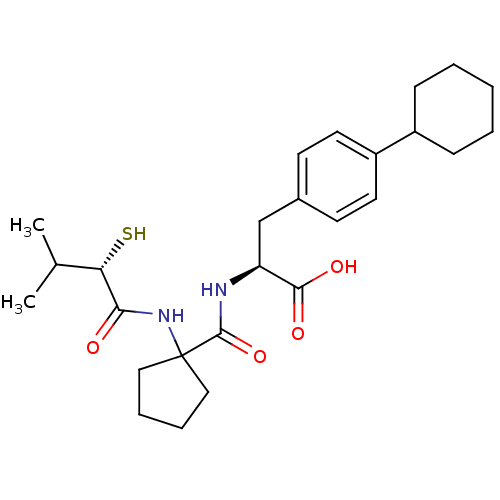 ((S)-3-(4-Cyclohexyl-phenyl)-2-{[1-((S)-2-mercapto-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against Endothelin converting enzyme 1 | Bioorg Med Chem Lett 11: 375-8 (2001) BindingDB Entry DOI: 10.7270/Q2HH6JC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 81 total ) | Next | Last >> |