 Found 484 hits with Last Name = 'mayer' and Initial = 'r'
Found 484 hits with Last Name = 'mayer' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50004774
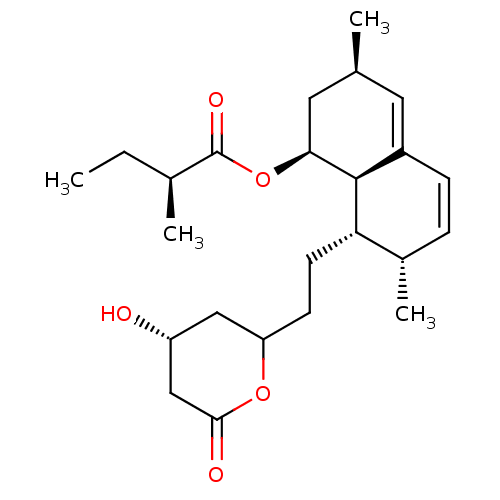
((S)-2-Methyl-butyric acid (1S,3R,7S,8S,8aR)-8-[2-(...)Show SMILES CC[C@H](C)C(=O)O[C@H]1C[C@@H](C)C=C2C=C[C@H](C)[C@H](CCC3C[C@@H](O)CC(=O)O3)[C@@H]12 |c:13,t:11| Show InChI InChI=1S/C24H36O5/c1-5-15(3)24(27)29-21-11-14(2)10-17-7-6-16(4)20(23(17)21)9-8-19-12-18(25)13-22(26)28-19/h6-7,10,14-16,18-21,23,25H,5,8-9,11-13H2,1-4H3/t14-,15-,16-,18+,19?,20-,21-,23-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human HMGCoA reductase |
J Nat Prod 52: 153-161 (1989)
Article DOI: 10.1021/np50061a020
BindingDB Entry DOI: 10.7270/Q28052MW |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50279858
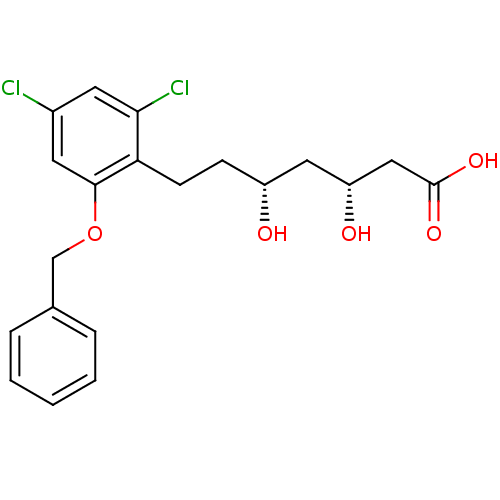
((3R,5R)-7-(2-Benzyloxy-4,6-dichloro-phenyl)-3,5-di...)Show SMILES O[C@H](CCc1c(Cl)cc(Cl)cc1OCc1ccccc1)C[C@@H](O)CC(O)=O Show InChI InChI=1S/C20H22Cl2O5/c21-14-8-18(22)17(7-6-15(23)10-16(24)11-20(25)26)19(9-14)27-12-13-4-2-1-3-5-13/h1-5,8-9,15-16,23-24H,6-7,10-12H2,(H,25,26)/t15-,16-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The inhibitory activity of the compound against purified recombinant human HMG-CoA reductase was evaluated |
Bioorg Med Chem Lett 1: 151-154 (1991)
Article DOI: 10.1016/S0960-894X(01)80788-5
BindingDB Entry DOI: 10.7270/Q21836DP |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50279859
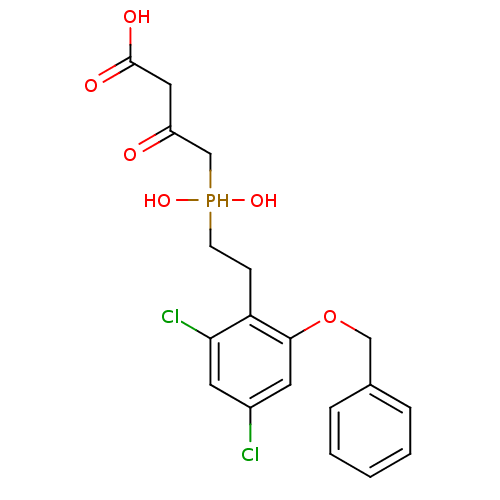
((S)-4-{[2-(2-Benzyloxy-4,6-dichloro-phenyl)-ethyl]...)Show SMILES OC(=O)CC(=O)CP(O)(O)CCc1c(Cl)cc(Cl)cc1OCc1ccccc1 Show InChI InChI=1S/C19H21Cl2O6P/c20-14-8-17(21)16(6-7-28(25,26)12-15(22)10-19(23)24)18(9-14)27-11-13-4-2-1-3-5-13/h1-5,8-9,25-26,28H,6-7,10-12H2,(H,23,24) | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The inhibitory activity of the compound against purified recombinant human HMG-CoA reductase was evaluated |
Bioorg Med Chem Lett 1: 151-154 (1991)
Article DOI: 10.1016/S0960-894X(01)80788-5
BindingDB Entry DOI: 10.7270/Q21836DP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50442673
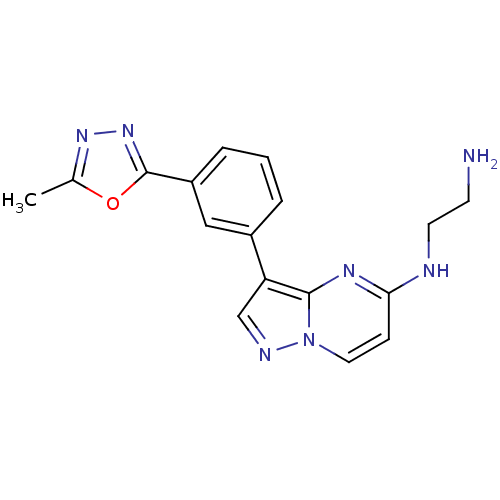
(CHEMBL2442291)Show InChI InChI=1S/C17H17N7O/c1-11-22-23-17(25-11)13-4-2-3-12(9-13)14-10-20-24-8-5-15(19-7-6-18)21-16(14)24/h2-5,8-10H,6-7,18H2,1H3,(H,19,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Pim1 (unknown origin) using STK3 as substrate preincubated for 30 mins followed by substrate and ATP addition after 45 mins by HTRF ass... |
Bioorg Med Chem Lett 23: 6178-82 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.110
BindingDB Entry DOI: 10.7270/Q2MS3V69 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50442690
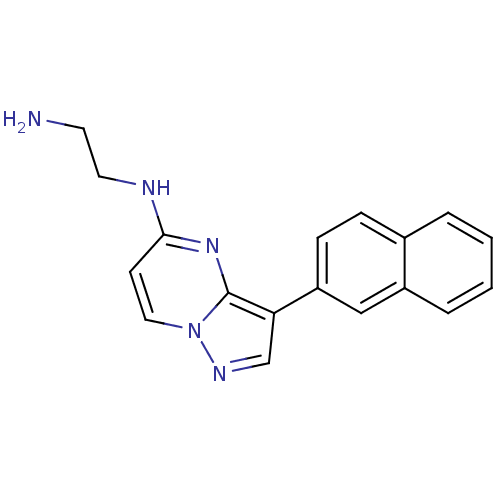
(CHEMBL2442296)Show InChI InChI=1S/C18H17N5/c19-8-9-20-17-7-10-23-18(22-17)16(12-21-23)15-6-5-13-3-1-2-4-14(13)11-15/h1-7,10-12H,8-9,19H2,(H,20,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Pim1 (unknown origin) using STK3 as substrate preincubated for 30 mins followed by substrate and ATP addition after 45 mins by HTRF ass... |
Bioorg Med Chem Lett 23: 6178-82 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.110
BindingDB Entry DOI: 10.7270/Q2MS3V69 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50442685
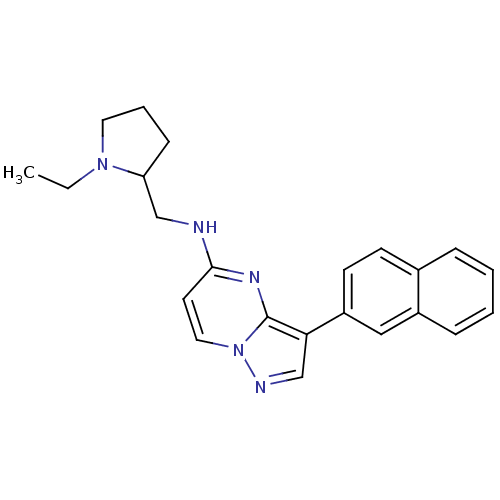
(CHEMBL2442301)Show InChI InChI=1S/C23H25N5/c1-2-27-12-5-8-20(27)15-24-22-11-13-28-23(26-22)21(16-25-28)19-10-9-17-6-3-4-7-18(17)14-19/h3-4,6-7,9-11,13-14,16,20H,2,5,8,12,15H2,1H3,(H,24,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Pim1 (unknown origin) using STK3 as substrate preincubated for 30 mins followed by substrate and ATP addition after 45 mins by HTRF ass... |
Bioorg Med Chem Lett 23: 6178-82 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.110
BindingDB Entry DOI: 10.7270/Q2MS3V69 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50442673

(CHEMBL2442291)Show InChI InChI=1S/C17H17N7O/c1-11-22-23-17(25-11)13-4-2-3-12(9-13)14-10-20-24-8-5-15(19-7-6-18)21-16(14)24/h2-5,8-10H,6-7,18H2,1H3,(H,19,21) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Pim3 (unknown origin) using STK1 as substrate preincubated for 30 mins followed by substrate and ATP addition after 60 mins by HTRF ass... |
Bioorg Med Chem Lett 23: 6178-82 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.110
BindingDB Entry DOI: 10.7270/Q2MS3V69 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50442679

(CHEMBL2442302)Show InChI InChI=1S/C16H15N5S/c17-5-6-18-15-3-7-21-16(20-15)13(10-19-21)11-1-2-14-12(9-11)4-8-22-14/h1-4,7-10H,5-6,17H2,(H,18,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Pim1 (unknown origin) using STK3 as substrate preincubated for 30 mins followed by substrate and ATP addition after 45 mins by HTRF ass... |
Bioorg Med Chem Lett 23: 6178-82 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.110
BindingDB Entry DOI: 10.7270/Q2MS3V69 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50392216
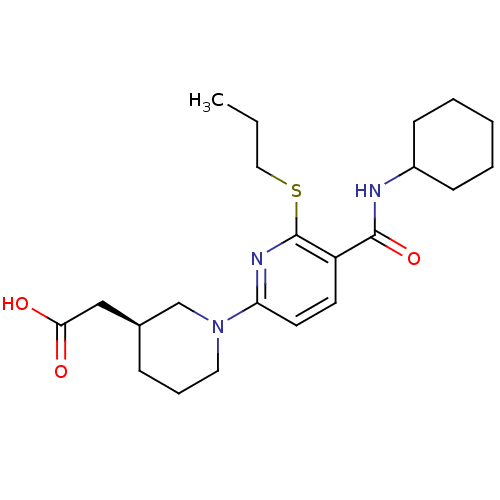
(CHEMBL2153191 | US8673938, 7)Show SMILES CCCSc1nc(ccc1C(=O)NC1CCCCC1)N1CCC[C@@H](CC(O)=O)C1 |r| Show InChI InChI=1S/C22H33N3O3S/c1-2-13-29-22-18(21(28)23-17-8-4-3-5-9-17)10-11-19(24-22)25-12-6-7-16(15-25)14-20(26)27/h10-11,16-17H,2-9,12-15H2,1H3,(H,23,28)(H,26,27)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of 11betaHSD1 in human adipocytes assessed as cortisone to cortisol conversion by scintillation counting method |
J Med Chem 55: 10136-47 (2012)
Article DOI: 10.1021/jm301252n
BindingDB Entry DOI: 10.7270/Q2WM1FJT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50399335

(CHEMBL2177609)Show SMILES CC(C)(C)c1c(cnn1-c1ccc(cc1)C(O)=O)C(=O)NC1C2CC3CC(C2)CC1C3 |TLB:20:21:23.30.24:28.26.27,30:29:27:23.24.25,THB:20:21:27:23.24.25,30:24:21.29.28:27,25:24:21:28.26.27,25:26:21:23.30.24,(26.01,-25.71,;26.64,-24.31,;28.17,-24.15,;27.4,-25.64,;25.74,-23.06,;26.22,-21.6,;24.97,-20.69,;23.72,-21.6,;24.2,-23.06,;23.29,-24.31,;21.76,-24.13,;20.85,-25.38,;21.48,-26.79,;23.02,-26.95,;23.91,-25.7,;20.57,-28.04,;19.04,-27.88,;21.2,-29.44,;27.68,-21.12,;28,-19.62,;28.82,-22.16,;30.29,-21.68,;31.49,-20.41,;32.81,-20.9,;34.21,-20.55,;34.22,-19.02,;32.82,-18.44,;31.48,-18.92,;31.79,-19.67,;31.79,-21.26,;33.2,-21.83,)| Show InChI InChI=1S/C25H31N3O3/c1-25(2,3)22-20(13-26-28(22)19-6-4-16(5-7-19)24(30)31)23(29)27-21-17-9-14-8-15(11-17)12-18(21)10-14/h4-7,13-15,17-18,21H,8-12H2,1-3H3,(H,27,29)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of 11betaHSD1 in human adipocytes assessed as cortisone to cortisol conversion by scintillation counting method |
J Med Chem 55: 10136-47 (2012)
Article DOI: 10.1021/jm301252n
BindingDB Entry DOI: 10.7270/Q2WM1FJT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50392216

(CHEMBL2153191 | US8673938, 7)Show SMILES CCCSc1nc(ccc1C(=O)NC1CCCCC1)N1CCC[C@@H](CC(O)=O)C1 |r| Show InChI InChI=1S/C22H33N3O3S/c1-2-13-29-22-18(21(28)23-17-8-4-3-5-9-17)10-11-19(24-22)25-12-6-7-16(15-25)14-20(26)27/h10-11,16-17H,2-9,12-15H2,1H3,(H,23,28)(H,26,27)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of 11betaHSD1 in human isolated adipocytes using [3H]cortisone as substrate after 6 hrs by flow scintillation analysis |
J Med Chem 55: 5951-64 (2012)
Article DOI: 10.1021/jm300592r
BindingDB Entry DOI: 10.7270/Q24F1RTK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50233576
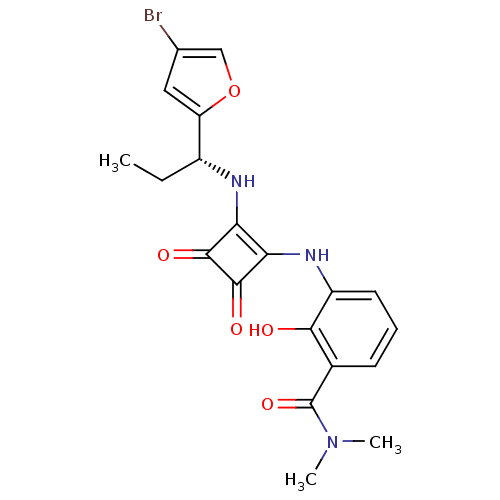
((R)-3-(2-(1-(4-bromofuran-2-yl)propylamino)-3,4-di...)Show SMILES CC[C@@H](Nc1c(Nc2cccc(C(=O)N(C)C)c2O)c(=O)c1=O)c1cc(Br)co1 Show InChI InChI=1S/C20H20BrN3O5/c1-4-12(14-8-10(21)9-29-14)22-15-16(19(27)18(15)26)23-13-7-5-6-11(17(13)25)20(28)24(2)3/h5-9,12,22-23,25H,4H2,1-3H3/t12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CXCR2-mediated chemotaxis in Ba/F3 cells expressing human CXCR2 |
Bioorg Med Chem Lett 18: 1318-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.024
BindingDB Entry DOI: 10.7270/Q2PV6K33 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50004774

((S)-2-Methyl-butyric acid (1S,3R,7S,8S,8aR)-8-[2-(...)Show SMILES CC[C@H](C)C(=O)O[C@H]1C[C@@H](C)C=C2C=C[C@H](C)[C@H](CCC3C[C@@H](O)CC(=O)O3)[C@@H]12 |c:13,t:11| Show InChI InChI=1S/C24H36O5/c1-5-15(3)24(27)29-21-11-14(2)10-17-7-6-16(4)20(23(17)21)9-8-19-12-18(25)13-22(26)28-19/h6-7,10,14-16,18-21,23,25H,5,8-9,11-13H2,1-4H3/t14-,15-,16-,18+,19?,20-,21-,23-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human HMGCoA reductase |
J Nat Prod 52: 153-161 (1989)
Article DOI: 10.1021/np50061a020
BindingDB Entry DOI: 10.7270/Q28052MW |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50200886
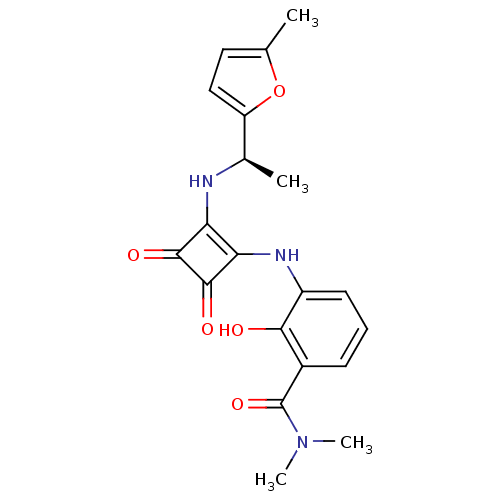
((R)-2-hydroxy-N,N-dimethyl-3-(2-(1-(5-methylfuran-...)Show SMILES C[C@@H](Nc1c(Nc2cccc(C(=O)N(C)C)c2O)c(=O)c1=O)c1ccc(C)o1 |r| Show InChI InChI=1S/C20H21N3O5/c1-10-8-9-14(28-10)11(2)21-15-16(19(26)18(15)25)22-13-7-5-6-12(17(13)24)20(27)23(3)4/h5-9,11,21-22,24H,1-4H3/t11-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CXCL1-induced human neutrophil chemotaxis |
J Med Chem 49: 7603-6 (2006)
Article DOI: 10.1021/jm0609622
BindingDB Entry DOI: 10.7270/Q2KW5GVR |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50200880
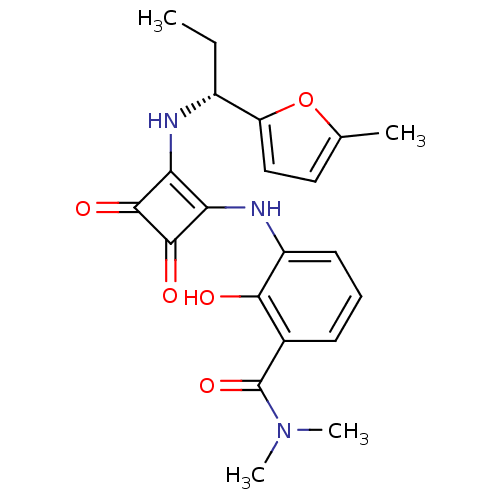
((R)-2-hydroxy-N,N-dimethyl-3-(2-(1-(5-methylfuran-...)Show SMILES CC[C@@H](Nc1c(Nc2cccc(C(=O)N(C)C)c2O)c(=O)c1=O)c1ccc(C)o1 |r| Show InChI InChI=1S/C21H23N3O5/c1-5-13(15-10-9-11(2)29-15)22-16-17(20(27)19(16)26)23-14-8-6-7-12(18(14)25)21(28)24(3)4/h6-10,13,22-23,25H,5H2,1-4H3/t13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]hCXCL8 from human CXCR2 receptor expressed in BaF3 cells |
J Med Chem 49: 7603-6 (2006)
Article DOI: 10.1021/jm0609622
BindingDB Entry DOI: 10.7270/Q2KW5GVR |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50063918

((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-1-methyl...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)[C@H](CSc1cccs1)C(=O)NO Show InChI InChI=1S/C23H31N3O4S2/c1-15(2)12-17(18(22(28)26-30)14-32-20-10-7-11-31-20)21(27)25-19(23(29)24-3)13-16-8-5-4-6-9-16/h4-11,15,17-19,30H,12-14H2,1-3H3,(H,24,29)(H,25,27)(H,26,28)/t17-,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound towards human recombinant fibroblast collagenase |
Bioorg Med Chem Lett 8: 29-34 (1999)
BindingDB Entry DOI: 10.7270/Q2TT4Q3W |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50233586
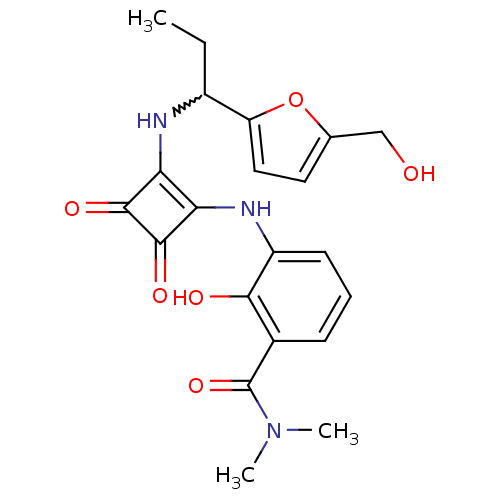
(2-hydroxy-3-(2-(1-(5-(hydroxymethyl)furan-2-yl)pro...)Show SMILES CCC(Nc1c(Nc2cccc(C(=O)N(C)C)c2O)c(=O)c1=O)c1ccc(CO)o1 |w:2.2| Show InChI InChI=1S/C21H23N3O6/c1-4-13(15-9-8-11(10-25)30-15)22-16-17(20(28)19(16)27)23-14-7-5-6-12(18(14)26)21(29)24(2)3/h5-9,13,22-23,25-26H,4,10H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CXCR2 |
Bioorg Med Chem Lett 18: 1318-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.024
BindingDB Entry DOI: 10.7270/Q2PV6K33 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50442692
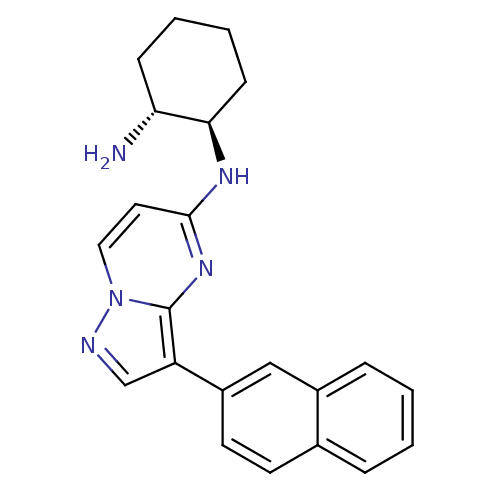
(CHEMBL2442317)Show SMILES N[C@@H]1CCCC[C@H]1Nc1ccn2ncc(-c3ccc4ccccc4c3)c2n1 |r| Show InChI InChI=1S/C22H23N5/c23-19-7-3-4-8-20(19)25-21-11-12-27-22(26-21)18(14-24-27)17-10-9-15-5-1-2-6-16(15)13-17/h1-2,5-6,9-14,19-20H,3-4,7-8,23H2,(H,25,26)/t19-,20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Pim1 (unknown origin) using STK3 as substrate preincubated for 30 mins followed by substrate and ATP addition after 45 mins by HTRF ass... |
Bioorg Med Chem Lett 23: 6178-82 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.110
BindingDB Entry DOI: 10.7270/Q2MS3V69 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50399353
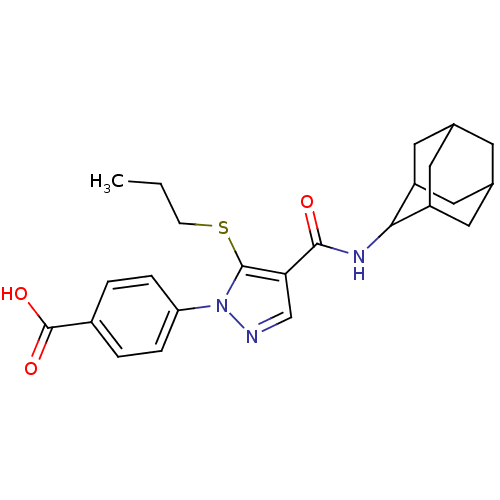
(CHEMBL2177615)Show SMILES CCCSc1c(cnn1-c1ccc(cc1)C(O)=O)C(=O)NC1C2CC3CC(C2)CC1C3 |TLB:30:29:27:23.24.25,20:21:23.30.24:28.26.27,THB:30:24:21.29.28:27,20:21:27:23.24.25,25:24:21:28.26.27,25:26:21:23.30.24,(42.49,-16.8,;40.96,-16.96,;40.05,-15.71,;40.68,-14.31,;39.78,-13.06,;40.26,-11.6,;39.01,-10.69,;37.76,-11.6,;38.24,-13.06,;37.33,-14.3,;35.8,-14.13,;34.89,-15.38,;35.51,-16.79,;37.05,-16.94,;37.95,-15.7,;34.61,-18.03,;33.08,-17.87,;35.24,-19.44,;41.72,-11.12,;42.04,-9.62,;42.86,-12.15,;44.33,-11.68,;45.53,-10.4,;46.85,-10.89,;48.25,-10.55,;48.26,-9.02,;46.86,-8.44,;45.52,-8.92,;45.83,-9.67,;45.83,-11.26,;47.24,-11.82,)| Show InChI InChI=1S/C24H29N3O3S/c1-2-7-31-23-20(13-25-27(23)19-5-3-16(4-6-19)24(29)30)22(28)26-21-17-9-14-8-15(11-17)12-18(21)10-14/h3-6,13-15,17-18,21H,2,7-12H2,1H3,(H,26,28)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-His tagged full length human 11betaHSD1 incubated for 25 mins by HTRF assay |
J Med Chem 55: 10136-47 (2012)
Article DOI: 10.1021/jm301252n
BindingDB Entry DOI: 10.7270/Q2WM1FJT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50442693

(CHEMBL2442316)Show SMILES N[C@H]1CC[C@@H](CC1)Nc1ccn2ncc(-c3ccc4ccccc4c3)c2n1 |r,wU:1.0,wD:4.7,(62.54,-18.45,;63.88,-17.68,;63.89,-16.14,;65.21,-15.38,;66.54,-16.16,;66.55,-17.69,;65.21,-18.46,;67.88,-15.39,;69.21,-16.16,;69.21,-17.7,;70.54,-18.47,;71.87,-17.7,;73.35,-18.18,;74.26,-16.93,;73.35,-15.67,;73.83,-14.21,;72.8,-13.07,;73.27,-11.61,;74.78,-11.28,;75.24,-9.82,;76.76,-9.51,;77.8,-10.65,;77.32,-12.12,;75.81,-12.43,;75.34,-13.89,;71.87,-16.15,;70.54,-15.39,)| Show InChI InChI=1S/C22H23N5/c23-18-7-9-19(10-8-18)25-21-11-12-27-22(26-21)20(14-24-27)17-6-5-15-3-1-2-4-16(15)13-17/h1-6,11-14,18-19H,7-10,23H2,(H,25,26)/t18-,19- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Pim1 (unknown origin) using STK3 as substrate preincubated for 30 mins followed by substrate and ATP addition after 45 mins by HTRF ass... |
Bioorg Med Chem Lett 23: 6178-82 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.110
BindingDB Entry DOI: 10.7270/Q2MS3V69 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50233554
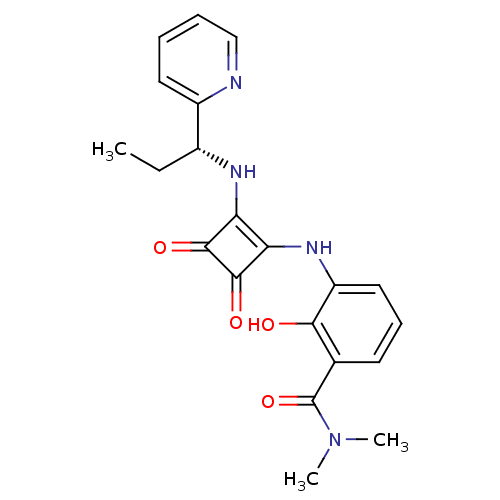
((R)-3-(3,4-dioxo-2-(1-(pyridin-2-yl)propylamino)cy...)Show SMILES CC[C@@H](Nc1c(Nc2cccc(C(=O)N(C)C)c2O)c(=O)c1=O)c1ccccn1 Show InChI InChI=1S/C21H22N4O4/c1-4-13(14-9-5-6-11-22-14)23-16-17(20(28)19(16)27)24-15-10-7-8-12(18(15)26)21(29)25(2)3/h5-11,13,23-24,26H,4H2,1-3H3/t13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CXCR2 |
Bioorg Med Chem Lett 18: 1318-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.024
BindingDB Entry DOI: 10.7270/Q2PV6K33 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50233561
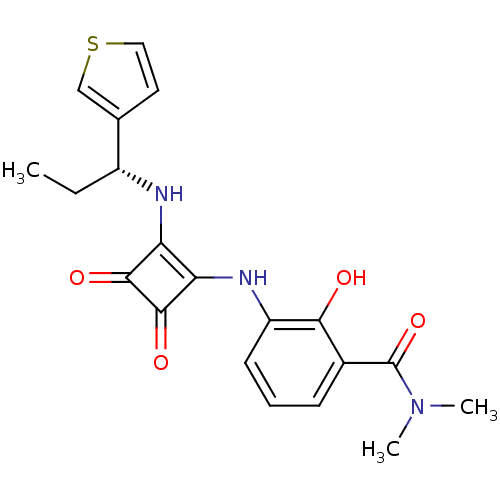
((R)-3-(3,4-dioxo-2-(1-(thiophen-3-yl)propylamino)c...)Show SMILES CC[C@@H](Nc1c(Nc2cccc(C(=O)N(C)C)c2O)c(=O)c1=O)c1ccsc1 Show InChI InChI=1S/C20H21N3O4S/c1-4-13(11-8-9-28-10-11)21-15-16(19(26)18(15)25)22-14-7-5-6-12(17(14)24)20(27)23(2)3/h5-10,13,21-22,24H,4H2,1-3H3/t13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CXCR2 |
Bioorg Med Chem Lett 18: 1318-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.024
BindingDB Entry DOI: 10.7270/Q2PV6K33 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50233560
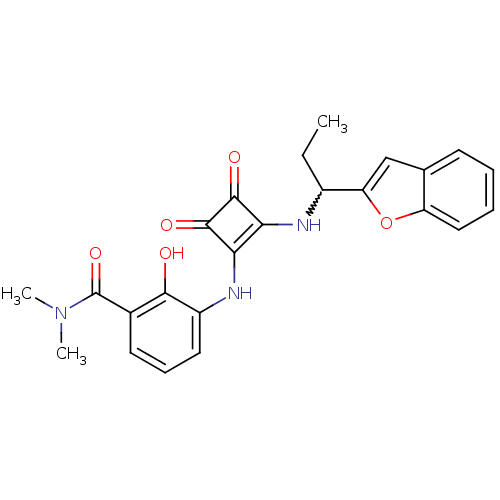
(3-(2-(1-(benzofuran-2-yl)propylamino)-3,4-dioxocyc...)Show SMILES CCC(Nc1c(Nc2cccc(C(=O)N(C)C)c2O)c(=O)c1=O)c1cc2ccccc2o1 |w:2.2| Show InChI InChI=1S/C24H23N3O5/c1-4-15(18-12-13-8-5-6-11-17(13)32-18)25-19-20(23(30)22(19)29)26-16-10-7-9-14(21(16)28)24(31)27(2)3/h5-12,15,25-26,28H,4H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CXCR2 |
Bioorg Med Chem Lett 18: 1318-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.024
BindingDB Entry DOI: 10.7270/Q2PV6K33 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50233576

((R)-3-(2-(1-(4-bromofuran-2-yl)propylamino)-3,4-di...)Show SMILES CC[C@@H](Nc1c(Nc2cccc(C(=O)N(C)C)c2O)c(=O)c1=O)c1cc(Br)co1 Show InChI InChI=1S/C20H20BrN3O5/c1-4-12(14-8-10(21)9-29-14)22-15-16(19(27)18(15)26)23-13-7-5-6-11(17(13)25)20(28)24(2)3/h5-9,12,22-23,25H,4H2,1-3H3/t12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CXCR2 |
Bioorg Med Chem Lett 18: 1318-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.024
BindingDB Entry DOI: 10.7270/Q2PV6K33 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50200881
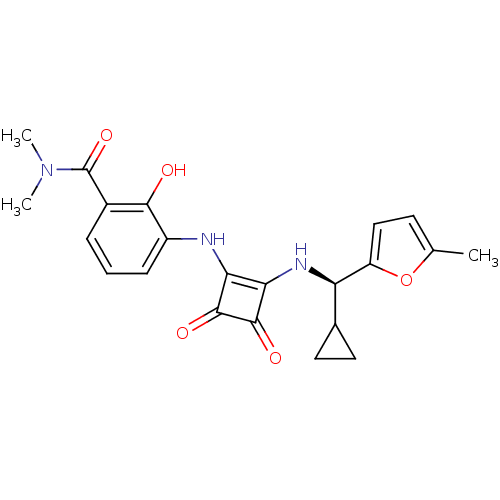
((R)-3-(2-(cyclopropyl(5-methylfuran-2-yl)methylami...)Show SMILES CN(C)C(=O)c1cccc(Nc2c(N[C@H](C3CC3)c3ccc(C)o3)c(=O)c2=O)c1O |r| Show InChI InChI=1S/C22H23N3O5/c1-11-7-10-15(30-11)16(12-8-9-12)24-18-17(20(27)21(18)28)23-14-6-4-5-13(19(14)26)22(29)25(2)3/h4-7,10,12,16,23-24,26H,8-9H2,1-3H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]hCXCL8 from human CXCR2 receptor expressed in BaF3 cells |
J Med Chem 49: 7603-6 (2006)
Article DOI: 10.1021/jm0609622
BindingDB Entry DOI: 10.7270/Q2KW5GVR |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50233574
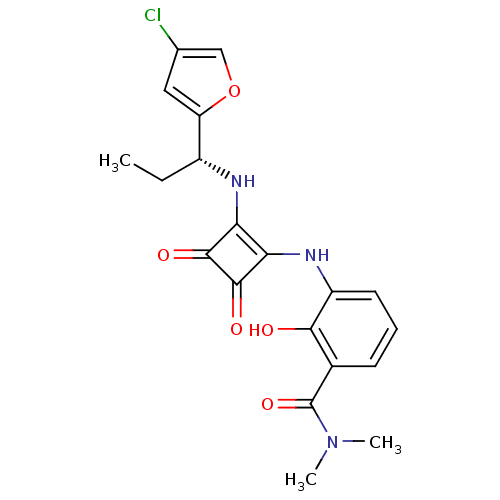
((R)-3-(2-(1-(4-chlorofuran-2-yl)propylamino)-3,4-d...)Show SMILES CC[C@@H](Nc1c(Nc2cccc(C(=O)N(C)C)c2O)c(=O)c1=O)c1cc(Cl)co1 Show InChI InChI=1S/C20H20ClN3O5/c1-4-12(14-8-10(21)9-29-14)22-15-16(19(27)18(15)26)23-13-7-5-6-11(17(13)25)20(28)24(2)3/h5-9,12,22-23,25H,4H2,1-3H3/t12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CXCR2 |
Bioorg Med Chem Lett 18: 1318-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.024
BindingDB Entry DOI: 10.7270/Q2PV6K33 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50200882
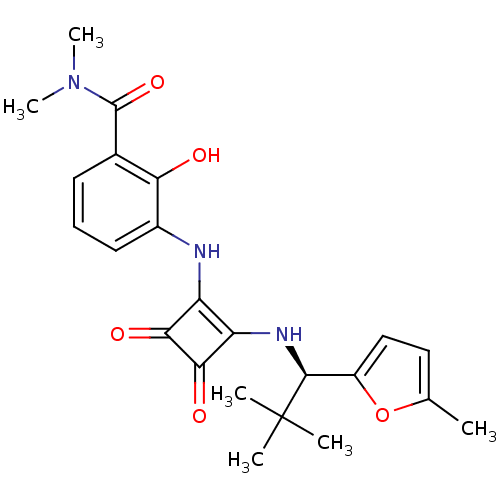
((R)-3-(2-(2,2-dimethyl-1-(5-methylfuran-2-yl)propy...)Show SMILES CN(C)C(=O)c1cccc(Nc2c(N[C@@H](c3ccc(C)o3)C(C)(C)C)c(=O)c2=O)c1O |r| Show InChI InChI=1S/C23H27N3O5/c1-12-10-11-15(31-12)21(23(2,3)4)25-17-16(19(28)20(17)29)24-14-9-7-8-13(18(14)27)22(30)26(5)6/h7-11,21,24-25,27H,1-6H3/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]hCXCL8 from human CXCR2 receptor expressed in BaF3 cells |
J Med Chem 49: 7603-6 (2006)
Article DOI: 10.1021/jm0609622
BindingDB Entry DOI: 10.7270/Q2KW5GVR |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50200878
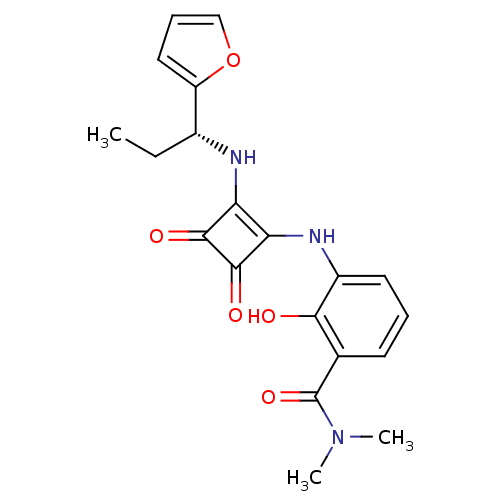
((R)-3-(2-(1-(furan-2-yl)propylamino)-3,4-dioxocycl...)Show SMILES CC[C@@H](Nc1c(Nc2cccc(C(=O)N(C)C)c2O)c(=O)c1=O)c1ccco1 |r| Show InChI InChI=1S/C20H21N3O5/c1-4-12(14-9-6-10-28-14)21-15-16(19(26)18(15)25)22-13-8-5-7-11(17(13)24)20(27)23(2)3/h5-10,12,21-22,24H,4H2,1-3H3/t12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]hCXCL8 from human CXCR2 receptor expressed in BaF3 cells |
J Med Chem 49: 7603-6 (2006)
Article DOI: 10.1021/jm0609622
BindingDB Entry DOI: 10.7270/Q2KW5GVR |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50399348
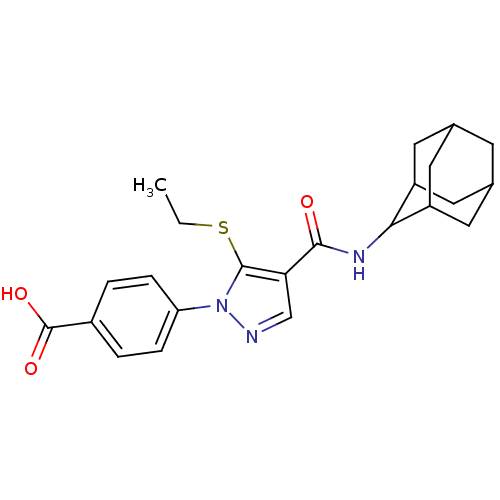
(CHEMBL2177620)Show SMILES CCSc1c(cnn1-c1ccc(cc1)C(O)=O)C(=O)NC1C2CC3CC(C2)CC1C3 |TLB:19:20:22.29.23:27.25.26,29:28:26:22.23.24,THB:19:20:26:22.23.24,29:23:20.28.27:26,24:23:20:27.25.26,24:25:20:22.29.23,(-1.15,-44.09,;-2.05,-42.84,;-1.42,-41.44,;-2.34,-40.19,;-1.85,-38.73,;-3.11,-37.83,;-4.34,-38.73,;-3.88,-40.19,;-4.77,-41.44,;-6.3,-41.27,;-7.22,-42.51,;-6.58,-43.92,;-5.04,-44.07,;-4.15,-42.83,;-7.49,-45.16,;-9.03,-45,;-6.86,-46.57,;-.38,-38.26,;-.07,-36.76,;.76,-39.29,;2.22,-38.82,;3.42,-37.54,;4.75,-38.03,;6.14,-37.69,;6.15,-36.16,;4.76,-35.58,;3.41,-36.06,;3.72,-36.81,;3.72,-38.4,;5.13,-38.96,)| Show InChI InChI=1S/C23H27N3O3S/c1-2-30-22-19(12-24-26(22)18-5-3-15(4-6-18)23(28)29)21(27)25-20-16-8-13-7-14(10-16)11-17(20)9-13/h3-6,12-14,16-17,20H,2,7-11H2,1H3,(H,25,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-His tagged full length human 11betaHSD1 incubated for 25 mins by HTRF assay |
J Med Chem 55: 10136-47 (2012)
Article DOI: 10.1021/jm301252n
BindingDB Entry DOI: 10.7270/Q2WM1FJT |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50233592
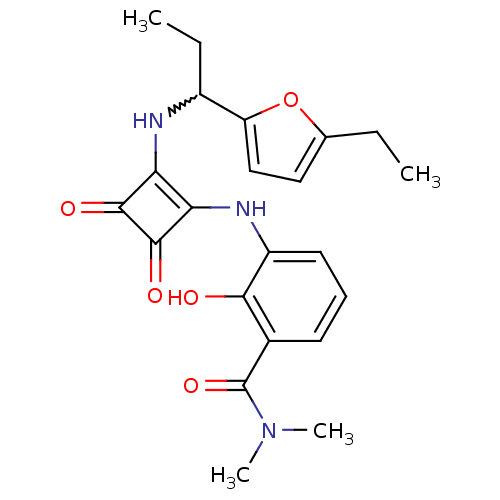
(3-(2-(1-(5-ethylfuran-2-yl)propylamino)-3,4-dioxoc...)Show SMILES CCC(Nc1c(Nc2cccc(C(=O)N(C)C)c2O)c(=O)c1=O)c1ccc(CC)o1 |w:2.2| Show InChI InChI=1S/C22H25N3O5/c1-5-12-10-11-16(30-12)14(6-2)23-17-18(21(28)20(17)27)24-15-9-7-8-13(19(15)26)22(29)25(3)4/h7-11,14,23-24,26H,5-6H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CXCR2 |
Bioorg Med Chem Lett 18: 1318-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.024
BindingDB Entry DOI: 10.7270/Q2PV6K33 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50442681

(CHEMBL2442290)Show InChI InChI=1S/C18H17N5O/c19-7-8-20-17-6-9-23-18(22-17)15(12-21-23)13-3-1-4-14(11-13)16-5-2-10-24-16/h1-6,9-12H,7-8,19H2,(H,20,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Pim1 (unknown origin) using STK3 as substrate preincubated for 30 mins followed by substrate and ATP addition after 45 mins by HTRF ass... |
Bioorg Med Chem Lett 23: 6178-82 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.110
BindingDB Entry DOI: 10.7270/Q2MS3V69 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50233589
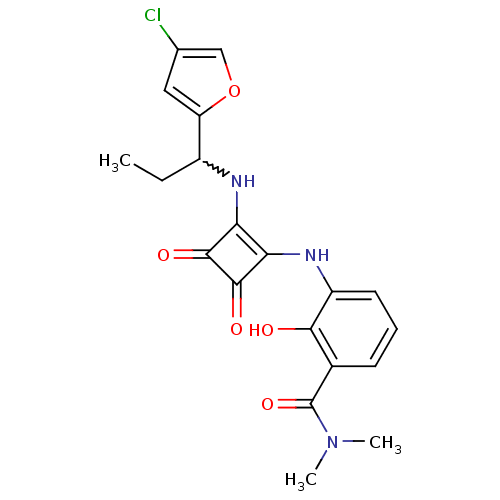
(3-(2-(1-(4-chlorofuran-2-yl)propylamino)-3,4-dioxo...)Show SMILES CCC(Nc1c(Nc2cccc(C(=O)N(C)C)c2O)c(=O)c1=O)c1cc(Cl)co1 |w:2.2| Show InChI InChI=1S/C20H20ClN3O5/c1-4-12(14-8-10(21)9-29-14)22-15-16(19(27)18(15)26)23-13-7-5-6-11(17(13)25)20(28)24(2)3/h5-9,12,22-23,25H,4H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CXCR2 |
Bioorg Med Chem Lett 18: 1318-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.024
BindingDB Entry DOI: 10.7270/Q2PV6K33 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50233554

((R)-3-(3,4-dioxo-2-(1-(pyridin-2-yl)propylamino)cy...)Show SMILES CC[C@@H](Nc1c(Nc2cccc(C(=O)N(C)C)c2O)c(=O)c1=O)c1ccccn1 Show InChI InChI=1S/C21H22N4O4/c1-4-13(14-9-5-6-11-22-14)23-16-17(20(28)19(16)27)24-15-10-7-8-12(18(15)26)21(29)25(2)3/h5-11,13,23-24,26H,4H2,1-3H3/t13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CXCR2-mediated chemotaxis in Ba/F3 cells expressing human CXCR2 |
Bioorg Med Chem Lett 18: 1318-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.024
BindingDB Entry DOI: 10.7270/Q2PV6K33 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50233572
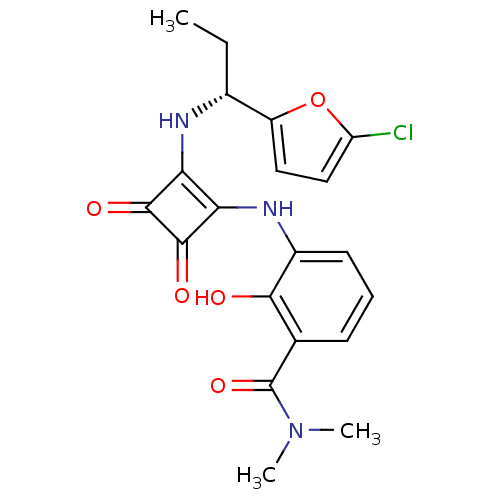
((R)-3-(2-(1-(5-chlorofuran-2-yl)propylamino)-3,4-d...)Show SMILES CC[C@@H](Nc1c(Nc2cccc(C(=O)N(C)C)c2O)c(=O)c1=O)c1ccc(Cl)o1 Show InChI InChI=1S/C20H20ClN3O5/c1-4-11(13-8-9-14(21)29-13)22-15-16(19(27)18(15)26)23-12-7-5-6-10(17(12)25)20(28)24(2)3/h5-9,11,22-23,25H,4H2,1-3H3/t11-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CXCR2 |
Bioorg Med Chem Lett 18: 1318-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.024
BindingDB Entry DOI: 10.7270/Q2PV6K33 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50233588
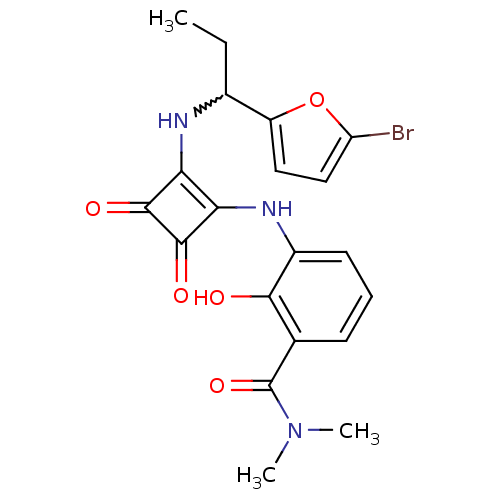
(3-(2-(1-(5-bromofuran-2-yl)propylamino)-3,4-dioxoc...)Show SMILES CCC(Nc1c(Nc2cccc(C(=O)N(C)C)c2O)c(=O)c1=O)c1ccc(Br)o1 |w:2.2| Show InChI InChI=1S/C20H20BrN3O5/c1-4-11(13-8-9-14(21)29-13)22-15-16(19(27)18(15)26)23-12-7-5-6-10(17(12)25)20(28)24(2)3/h5-9,11,22-23,25H,4H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CXCR2 |
Bioorg Med Chem Lett 18: 1318-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.024
BindingDB Entry DOI: 10.7270/Q2PV6K33 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50233581
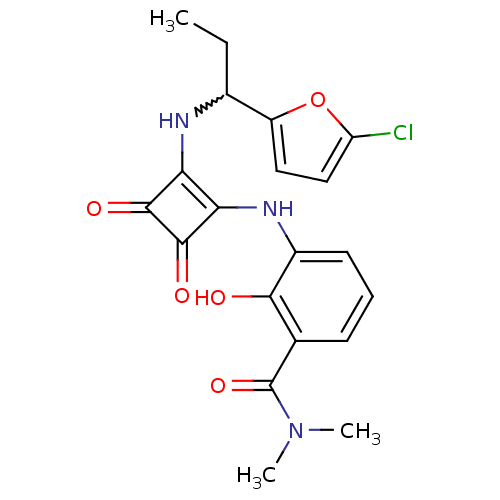
(3-(2-(1-(5-chlorofuran-2-yl)propylamino)-3,4-dioxo...)Show SMILES CCC(Nc1c(Nc2cccc(C(=O)N(C)C)c2O)c(=O)c1=O)c1ccc(Cl)o1 |w:2.2| Show InChI InChI=1S/C20H20ClN3O5/c1-4-11(13-8-9-14(21)29-13)22-15-16(19(27)18(15)26)23-12-7-5-6-10(17(12)25)20(28)24(2)3/h5-9,11,22-23,25H,4H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CXCR2 |
Bioorg Med Chem Lett 18: 1318-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.024
BindingDB Entry DOI: 10.7270/Q2PV6K33 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50200885
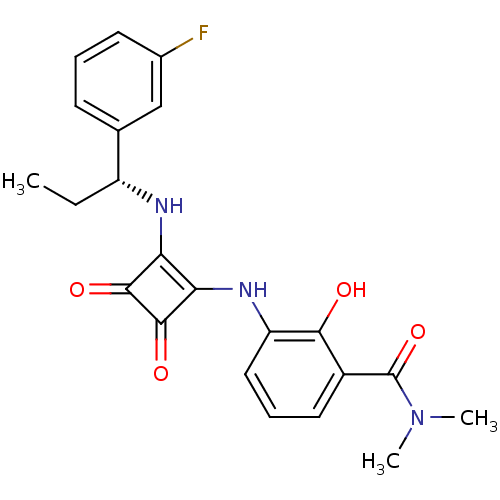
((R)-3-(2-(1-(3-fluorophenyl)propylamino)-3,4-dioxo...)Show SMILES CC[C@@H](Nc1c(Nc2cccc(C(=O)N(C)C)c2O)c(=O)c1=O)c1cccc(F)c1 Show InChI InChI=1S/C22H22FN3O4/c1-4-15(12-7-5-8-13(23)11-12)24-17-18(21(29)20(17)28)25-16-10-6-9-14(19(16)27)22(30)26(2)3/h5-11,15,24-25,27H,4H2,1-3H3/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]hCXCL8 from human CXCR2 receptor expressed in BaF3 cells |
J Med Chem 49: 7603-6 (2006)
Article DOI: 10.1021/jm0609622
BindingDB Entry DOI: 10.7270/Q2KW5GVR |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50233577
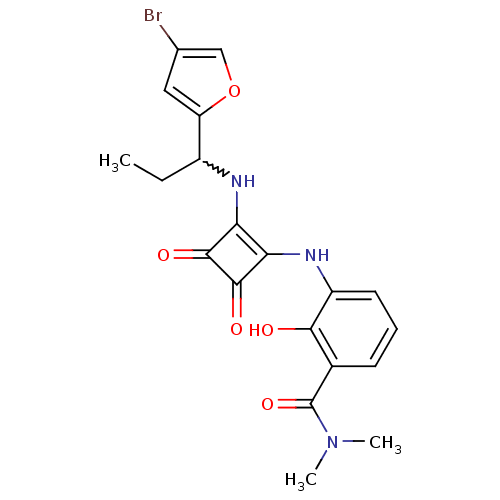
(3-(2-(1-(4-bromofuran-2-yl)propylamino)-3,4-dioxoc...)Show SMILES CCC(Nc1c(Nc2cccc(C(=O)N(C)C)c2O)c(=O)c1=O)c1cc(Br)co1 |w:2.2| Show InChI InChI=1S/C20H20BrN3O5/c1-4-12(14-8-10(21)9-29-14)22-15-16(19(27)18(15)26)23-13-7-5-6-11(17(13)25)20(28)24(2)3/h5-9,12,22-23,25H,4H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CXCR2 |
Bioorg Med Chem Lett 18: 1318-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.024
BindingDB Entry DOI: 10.7270/Q2PV6K33 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50399346

(CHEMBL2180883)Show SMILES OC(=O)c1ccc(cc1)-n1ncc(C(=O)NC2C3CC4CC(C3)CC2C4)c1SC1CCCC1 |TLB:15:16:18.25.19:23.21.22,25:24:22:18.19.20,THB:15:16:22:18.19.20,25:19:16.24.23:22,20:19:16:23.21.22,20:21:16:18.25.19,(27.7,-42.8,;29.23,-42.96,;29.85,-44.37,;30.13,-41.71,;29.51,-40.3,;30.41,-39.06,;31.95,-39.23,;32.57,-40.63,;31.67,-41.87,;32.85,-37.99,;32.38,-36.52,;33.62,-35.61,;34.87,-36.52,;36.34,-36.05,;36.66,-34.54,;37.48,-37.08,;38.95,-36.61,;40.14,-35.33,;41.47,-35.82,;42.87,-35.47,;42.88,-33.95,;41.48,-33.37,;40.13,-33.85,;40.44,-34.6,;40.45,-36.19,;41.85,-36.75,;34.39,-37.99,;35.3,-39.23,;34.67,-40.64,;33.17,-40.96,;33,-42.49,;34.41,-43.12,;35.44,-41.97,)| Show InChI InChI=1S/C26H31N3O3S/c30-24(28-23-18-10-15-9-16(12-18)13-19(23)11-15)22-14-27-29(25(22)33-21-3-1-2-4-21)20-7-5-17(6-8-20)26(31)32/h5-8,14-16,18-19,21,23H,1-4,9-13H2,(H,28,30)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-His tagged full length human 11betaHSD1 incubated for 25 mins by HTRF assay |
J Med Chem 55: 10136-47 (2012)
Article DOI: 10.1021/jm301252n
BindingDB Entry DOI: 10.7270/Q2WM1FJT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50442686
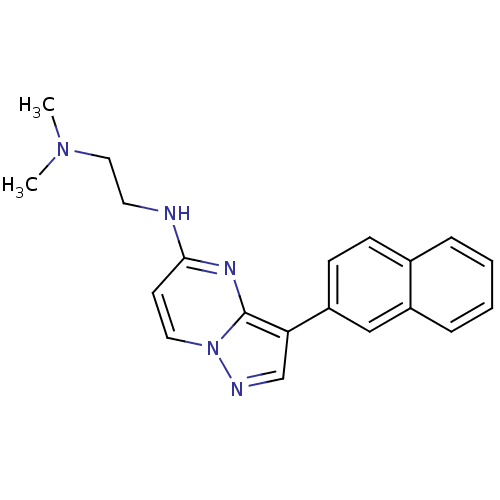
(CHEMBL2442300)Show InChI InChI=1S/C20H21N5/c1-24(2)12-10-21-19-9-11-25-20(23-19)18(14-22-25)17-8-7-15-5-3-4-6-16(15)13-17/h3-9,11,13-14H,10,12H2,1-2H3,(H,21,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Pim1 (unknown origin) using STK3 as substrate preincubated for 30 mins followed by substrate and ATP addition after 45 mins by HTRF ass... |
Bioorg Med Chem Lett 23: 6178-82 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.110
BindingDB Entry DOI: 10.7270/Q2MS3V69 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50063918

((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-1-methyl...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)[C@H](CSc1cccs1)C(=O)NO Show InChI InChI=1S/C23H31N3O4S2/c1-15(2)12-17(18(22(28)26-30)14-32-20-10-7-11-31-20)21(27)25-19(23(29)24-3)13-16-8-5-4-6-9-16/h4-11,15,17-19,30H,12-14H2,1-3H3,(H,24,29)(H,25,27)(H,26,28)/t17-,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human recombinant fibroblast collagenase |
Bioorg Med Chem Lett 8: 23-8 (1999)
BindingDB Entry DOI: 10.7270/Q2ZK5FT6 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50399351
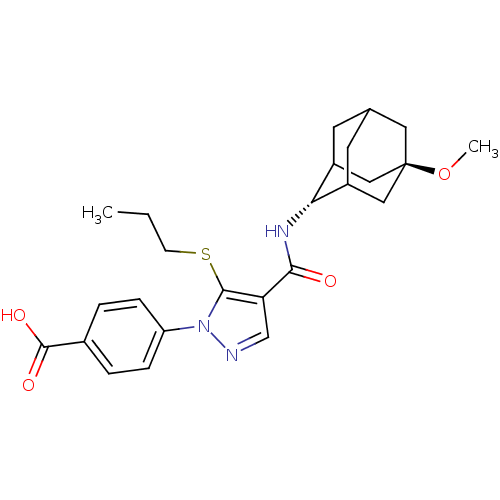
(CHEMBL2177617)Show SMILES CCCSc1c(cnn1-c1ccc(cc1)C(O)=O)C(=O)N[C@H]1C2CC3CC1C[C@](C3)(C2)OC |r,wU:21.22,wD:28.35,TLB:20:21:30.27.28:25.24.23,20:21:23:30.28.29,31:28:21.26.25:23,THB:27:26:23:30.28.29,27:28:21.26.25:23,29:28:21:25.24.23,29:24:21:30.27.28,31:28:21:25.24.23,(6.35,-29.86,;4.82,-30.02,;3.92,-28.78,;4.54,-27.37,;3.64,-26.12,;4.12,-24.66,;2.87,-23.75,;1.63,-24.66,;2.1,-26.12,;1.19,-27.37,;-.35,-27.2,;-1.26,-28.44,;-.62,-29.85,;.92,-30.01,;1.82,-28.76,;-1.54,-31.1,;-3.07,-30.94,;-.9,-32.51,;5.58,-24.19,;5.91,-22.68,;6.73,-25.22,;8.19,-24.75,;9.39,-23.47,;9.38,-21.98,;10.73,-21.51,;9.69,-22.73,;9.69,-24.32,;11.1,-24.89,;12.11,-23.61,;12.12,-22.08,;10.72,-23.96,;13.65,-23.55,;14.47,-24.85,)| Show InChI InChI=1S/C25H31N3O4S/c1-3-8-33-23-20(14-26-28(23)19-6-4-16(5-7-19)24(30)31)22(29)27-21-17-9-15-10-18(21)13-25(11-15,12-17)32-2/h4-7,14-15,17-18,21H,3,8-13H2,1-2H3,(H,27,29)(H,30,31)/t15?,17?,18?,21-,25- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-His tagged full length human 11betaHSD1 incubated for 25 mins by HTRF assay |
J Med Chem 55: 10136-47 (2012)
Article DOI: 10.1021/jm301252n
BindingDB Entry DOI: 10.7270/Q2WM1FJT |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50233563
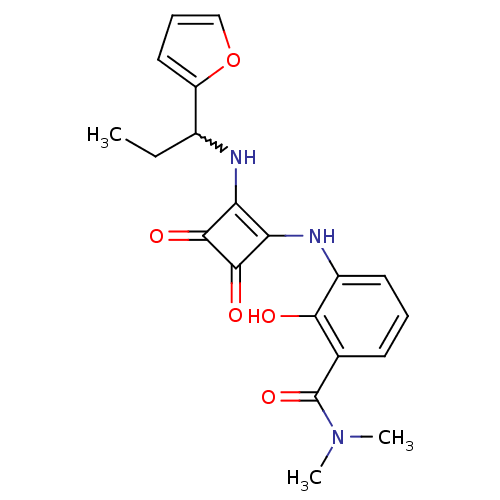
(3-(2-(1-(furan-2-yl)propylamino)-3,4-dioxocyclobut...)Show SMILES CCC(Nc1c(Nc2cccc(C(=O)N(C)C)c2O)c(=O)c1=O)c1ccco1 |w:2.2| Show InChI InChI=1S/C20H21N3O5/c1-4-12(14-9-6-10-28-14)21-15-16(19(26)18(15)25)22-13-8-5-7-11(17(13)24)20(27)23(2)3/h5-10,12,21-22,24H,4H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CXCR2 |
Bioorg Med Chem Lett 18: 1318-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.024
BindingDB Entry DOI: 10.7270/Q2PV6K33 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50200875

((R)-3-(2-(1-(benzo[d][1,3]dioxol-5-yl)propylamino)...)Show SMILES CC[C@@H](Nc1c(Nc2cccc(C(=O)N(C)C)c2O)c(=O)c1=O)c1ccc2OCOc2c1 |r| Show InChI InChI=1S/C23H23N3O6/c1-4-14(12-8-9-16-17(10-12)32-11-31-16)24-18-19(22(29)21(18)28)25-15-7-5-6-13(20(15)27)23(30)26(2)3/h5-10,14,24-25,27H,4,11H2,1-3H3/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]hCXCL8 from human CXCR2 receptor expressed in BaF3 cells |
J Med Chem 49: 7603-6 (2006)
Article DOI: 10.1021/jm0609622
BindingDB Entry DOI: 10.7270/Q2KW5GVR |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50442687
(CHEMBL2442299)Show InChI InChI=1S/C19H19N5/c20-9-3-10-21-18-8-11-24-19(23-18)17(13-22-24)16-7-6-14-4-1-2-5-15(14)12-16/h1-2,4-8,11-13H,3,9-10,20H2,(H,21,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Pim1 (unknown origin) using STK3 as substrate preincubated for 30 mins followed by substrate and ATP addition after 45 mins by HTRF ass... |
Bioorg Med Chem Lett 23: 6178-82 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.110
BindingDB Entry DOI: 10.7270/Q2MS3V69 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50200877

((R)-2-hydroxy-N,N-dimethyl-3-(2-(1-(5-methylthioph...)Show SMILES CC[C@@H](Nc1c(Nc2cccc(C(=O)N(C)C)c2O)c(=O)c1=O)c1ccc(C)s1 |r| Show InChI InChI=1S/C21H23N3O4S/c1-5-13(15-10-9-11(2)29-15)22-16-17(20(27)19(16)26)23-14-8-6-7-12(18(14)25)21(28)24(3)4/h6-10,13,22-23,25H,5H2,1-4H3/t13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]hCXCL8 from human CXCR2 receptor expressed in BaF3 cells |
J Med Chem 49: 7603-6 (2006)
Article DOI: 10.1021/jm0609622
BindingDB Entry DOI: 10.7270/Q2KW5GVR |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50200886

((R)-2-hydroxy-N,N-dimethyl-3-(2-(1-(5-methylfuran-...)Show SMILES C[C@@H](Nc1c(Nc2cccc(C(=O)N(C)C)c2O)c(=O)c1=O)c1ccc(C)o1 |r| Show InChI InChI=1S/C20H21N3O5/c1-10-8-9-14(28-10)11(2)21-15-16(19(26)18(15)25)22-13-7-5-6-12(17(13)24)20(27)23(3)4/h5-9,11,21-22,24H,1-4H3/t11-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]hCXCL8 from human CXCR2 receptor expressed in BaF3 cells |
J Med Chem 49: 7603-6 (2006)
Article DOI: 10.1021/jm0609622
BindingDB Entry DOI: 10.7270/Q2KW5GVR |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50233553
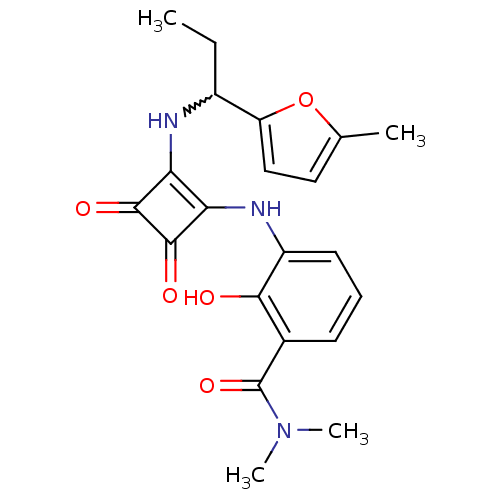
(2-hydroxy-N,N-dimethyl-3-(2-(1-(5-methylfuran-2-yl...)Show SMILES CCC(Nc1c(Nc2cccc(C(=O)N(C)C)c2O)c(=O)c1=O)c1ccc(C)o1 |w:2.2| Show InChI InChI=1S/C21H23N3O5/c1-5-13(15-10-9-11(2)29-15)22-16-17(20(27)19(16)26)23-14-8-6-7-12(18(14)25)21(28)24(3)4/h6-10,13,22-23,25H,5H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CXCR2 |
Bioorg Med Chem Lett 18: 1318-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.024
BindingDB Entry DOI: 10.7270/Q2PV6K33 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50233584
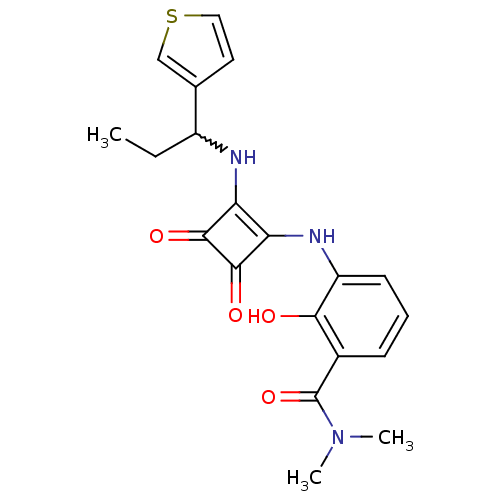
(3-(3,4-dioxo-2-(1-(thiophen-3-yl)propylamino)cyclo...)Show SMILES CCC(Nc1c(Nc2cccc(C(=O)N(C)C)c2O)c(=O)c1=O)c1ccsc1 |w:2.2| Show InChI InChI=1S/C20H21N3O4S/c1-4-13(11-8-9-28-10-11)21-15-16(19(26)18(15)25)22-14-7-5-6-12(17(14)24)20(27)23(2)3/h5-10,13,21-22,24H,4H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CXCR2 |
Bioorg Med Chem Lett 18: 1318-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.024
BindingDB Entry DOI: 10.7270/Q2PV6K33 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50399336

(CHEMBL2177608)Show SMILES CC1(CC1)c1c(cnn1-c1ccc(cc1)C(O)=O)C(=O)NC1C2CC3CC(C2)CC1C3 |TLB:20:21:23.30.24:28.26.27,30:29:27:23.24.25,THB:20:21:27:23.24.25,30:24:21.29.28:27,25:24:21:28.26.27,25:26:21:23.30.24,(10.13,-23.34,;8.59,-23.5,;8.59,-25.04,;7.26,-24.26,;7.69,-22.25,;8.17,-20.79,;6.92,-19.88,;5.68,-20.79,;6.15,-22.25,;5.25,-23.5,;3.71,-23.33,;2.81,-24.57,;3.43,-25.98,;4.97,-26.14,;5.87,-24.89,;2.53,-27.23,;.99,-27.07,;3.15,-28.63,;9.64,-20.31,;9.96,-18.81,;10.78,-21.35,;12.24,-20.87,;13.44,-19.6,;14.77,-20.09,;16.17,-19.74,;16.18,-18.21,;14.78,-17.63,;13.43,-18.11,;13.74,-18.86,;13.75,-20.45,;15.15,-21.02,)| Show InChI InChI=1S/C25H29N3O3/c1-25(6-7-25)22-20(13-26-28(22)19-4-2-16(3-5-19)24(30)31)23(29)27-21-17-9-14-8-15(11-17)12-18(21)10-14/h2-5,13-15,17-18,21H,6-12H2,1H3,(H,27,29)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-His tagged full length human 11betaHSD1 incubated for 25 mins by HTRF assay |
J Med Chem 55: 10136-47 (2012)
Article DOI: 10.1021/jm301252n
BindingDB Entry DOI: 10.7270/Q2WM1FJT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data