 Found 1362 hits with Last Name = 'miller' and Initial = 'p'
Found 1362 hits with Last Name = 'miller' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50326709
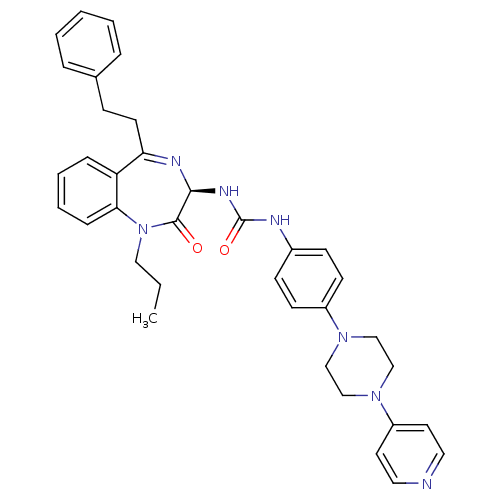
((R)-1-(2-oxo-5-phenethyl-1-propyl-2,3-dihydro-1H-b...)Show SMILES CCCN1c2ccccc2C(CCc2ccccc2)=N[C@@H](NC(=O)Nc2ccc(cc2)N2CCN(CC2)c2ccncc2)C1=O |r,c:20| Show InChI InChI=1S/C36H39N7O2/c1-2-22-43-33-11-7-6-10-31(33)32(17-12-27-8-4-3-5-9-27)39-34(35(43)44)40-36(45)38-28-13-15-29(16-14-28)41-23-25-42(26-24-41)30-18-20-37-21-19-30/h3-11,13-16,18-21,34H,2,12,17,22-26H2,1H3,(H2,38,40,45)/t34-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human bradykinin B1 receptor |
J Med Chem 46: 1803-6 (2003)
Article DOI: 10.1021/jm034020y
BindingDB Entry DOI: 10.7270/Q29P32C6 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM25400

((2R,3R,4S,5R)-2-[6-(cyclopentylamino)-9H-purin-9-y...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NC3CCCC3)ncnc12 Show InChI InChI=1S/C15H21N5O4/c21-5-9-11(22)12(23)15(24-9)20-7-18-10-13(16-6-17-14(10)20)19-8-3-1-2-4-8/h6-9,11-12,15,21-23H,1-5H2,(H,16,17,19)/t9-,11-,12-,15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Affinity for Adenosine A1 receptor determined by [3H]N6-cyclohexyladenosine binding to rat brain membranes |
Bioorg Med Chem Lett 1: 481-486 (1991)
Article DOI: 10.1016/S0960-894X(01)81110-0
BindingDB Entry DOI: 10.7270/Q2K64JJ8 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(RAT) | BDBM50326709

((R)-1-(2-oxo-5-phenethyl-1-propyl-2,3-dihydro-1H-b...)Show SMILES CCCN1c2ccccc2C(CCc2ccccc2)=N[C@@H](NC(=O)Nc2ccc(cc2)N2CCN(CC2)c2ccncc2)C1=O |r,c:20| Show InChI InChI=1S/C36H39N7O2/c1-2-22-43-33-11-7-6-10-31(33)32(17-12-27-8-4-3-5-9-27)39-34(35(43)44)40-36(45)38-28-13-15-29(16-14-28)41-23-25-42(26-24-41)30-18-20-37-21-19-30/h3-11,13-16,18-21,34H,2,12,17,22-26H2,1H3,(H2,38,40,45)/t34-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition against rat Bradykinin receptor B1 |
J Med Chem 46: 1803-6 (2003)
Article DOI: 10.1021/jm034020y
BindingDB Entry DOI: 10.7270/Q29P32C6 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50009552
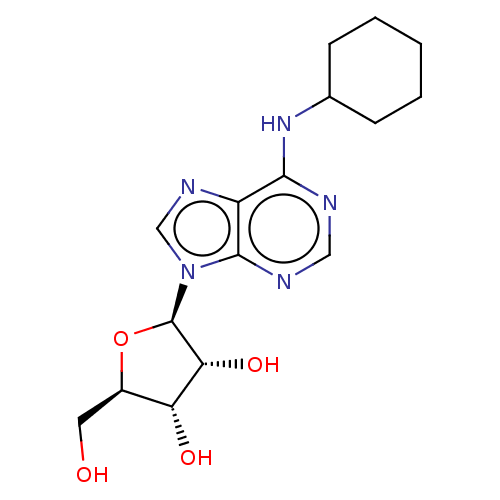
(2-[6-Amino-2-(2-morpholin-4-yl-ethylamino)-purin-9...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NC3CCCCC3)ncnc12 |r| Show InChI InChI=1S/C16H23N5O4/c22-6-10-12(23)13(24)16(25-10)21-8-19-11-14(17-7-18-15(11)21)20-9-4-2-1-3-5-9/h7-10,12-13,16,22-24H,1-6H2,(H,17,18,20)/t10-,12-,13-,16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Affinity for Adenosine A1 receptor determined by [3H]N6-cyclohexyladenosine binding to rat brain membranes |
Bioorg Med Chem Lett 1: 481-486 (1991)
Article DOI: 10.1016/S0960-894X(01)81110-0
BindingDB Entry DOI: 10.7270/Q2K64JJ8 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50370216
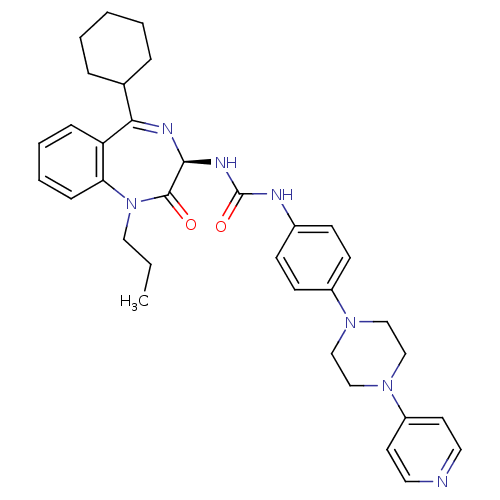
(CHEMBL1744084)Show SMILES CCCN1c2ccccc2C(=N[C@@H](NC(=O)Nc2ccc(cc2)N2CCN(CC2)c2ccncc2)C1=O)C1CCCCC1 |r,c:11| Show InChI InChI=1S/C34H41N7O2/c1-2-20-41-30-11-7-6-10-29(30)31(25-8-4-3-5-9-25)37-32(33(41)42)38-34(43)36-26-12-14-27(15-13-26)39-21-23-40(24-22-39)28-16-18-35-19-17-28/h6-7,10-19,25,32H,2-5,8-9,20-24H2,1H3,(H2,36,38,43)/t32-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human bradykinin B1 receptor |
J Med Chem 46: 1803-6 (2003)
Article DOI: 10.1021/jm034020y
BindingDB Entry DOI: 10.7270/Q29P32C6 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50370213
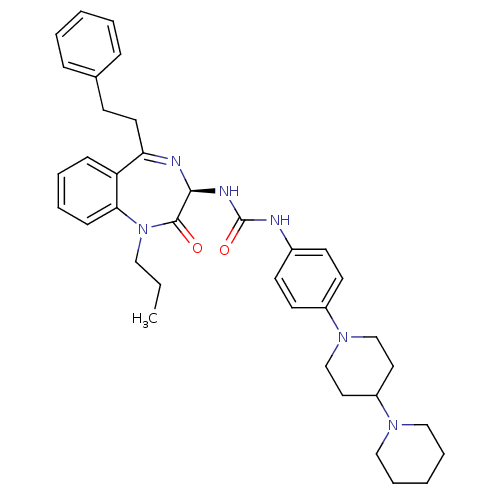
(CHEMBL1744083)Show SMILES CCCN1c2ccccc2C(CCc2ccccc2)=N[C@@H](NC(=O)Nc2ccc(cc2)N2CCC(CC2)N2CCCCC2)C1=O |r,c:20| Show InChI InChI=1S/C37H46N6O2/c1-2-23-43-34-14-8-7-13-32(34)33(20-15-28-11-5-3-6-12-28)39-35(36(43)44)40-37(45)38-29-16-18-30(19-17-29)42-26-21-31(22-27-42)41-24-9-4-10-25-41/h3,5-8,11-14,16-19,31,35H,2,4,9-10,15,20-27H2,1H3,(H2,38,40,45)/t35-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human bradykinin B1 receptor |
J Med Chem 46: 1803-6 (2003)
Article DOI: 10.1021/jm034020y
BindingDB Entry DOI: 10.7270/Q29P32C6 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(RAT) | BDBM50370213

(CHEMBL1744083)Show SMILES CCCN1c2ccccc2C(CCc2ccccc2)=N[C@@H](NC(=O)Nc2ccc(cc2)N2CCC(CC2)N2CCCCC2)C1=O |r,c:20| Show InChI InChI=1S/C37H46N6O2/c1-2-23-43-34-14-8-7-13-32(34)33(20-15-28-11-5-3-6-12-28)39-35(36(43)44)40-37(45)38-29-16-18-30(19-17-29)42-26-21-31(22-27-42)41-24-9-4-10-25-41/h3,5-8,11-14,16-19,31,35H,2,4,9-10,15,20-27H2,1H3,(H2,38,40,45)/t35-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition against rat Bradykinin receptor B1 |
J Med Chem 46: 1803-6 (2003)
Article DOI: 10.1021/jm034020y
BindingDB Entry DOI: 10.7270/Q29P32C6 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50370215
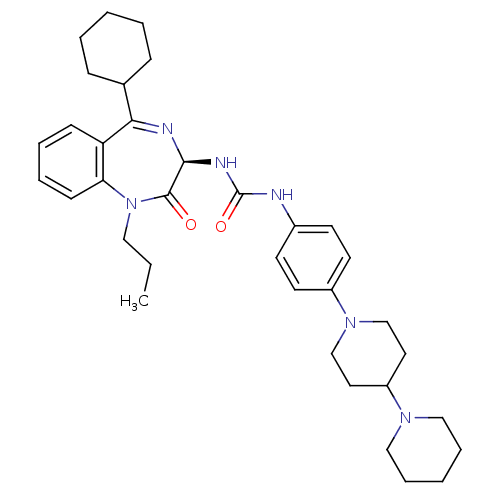
(CHEMBL1744082)Show SMILES CCCN1c2ccccc2C(=N[C@@H](NC(=O)Nc2ccc(cc2)N2CCC(CC2)N2CCCCC2)C1=O)C1CCCCC1 |r,c:11| Show InChI InChI=1S/C35H48N6O2/c1-2-21-41-31-14-8-7-13-30(31)32(26-11-5-3-6-12-26)37-33(34(41)42)38-35(43)36-27-15-17-28(18-16-27)40-24-19-29(20-25-40)39-22-9-4-10-23-39/h7-8,13-18,26,29,33H,2-6,9-12,19-25H2,1H3,(H2,36,38,43)/t33-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human bradykinin B1 receptor |
J Med Chem 46: 1803-6 (2003)
Article DOI: 10.1021/jm034020y
BindingDB Entry DOI: 10.7270/Q29P32C6 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50336997
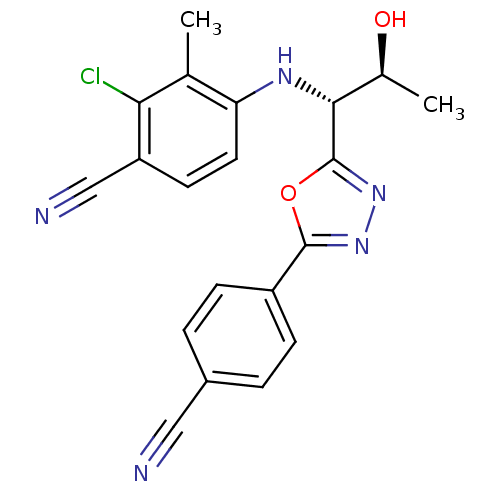
(2-chloro-4-((1R,2S)-1-(5-(4-cyanophenyl)-1,3,4-oxa...)Show SMILES C[C@H](O)[C@@H](Nc1ccc(C#N)c(Cl)c1C)c1nnc(o1)-c1ccc(cc1)C#N |r| Show InChI InChI=1S/C20H16ClN5O2/c1-11-16(8-7-15(10-23)17(11)21)24-18(12(2)27)20-26-25-19(28-20)14-5-3-13(9-22)4-6-14/h3-8,12,18,24,27H,1-2H3/t12-,18+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-tagged R1881 from androgen receptor after 4 hrs by fluorometric assay |
ACS Med Chem Lett 2: 124-129 (2011)
Article DOI: 10.1021/ml1002508
BindingDB Entry DOI: 10.7270/Q2JQ119N |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18161

((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])CC(=O)CC[C@]12C |r| Show InChI InChI=1S/C19H30O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h12,14-17,21H,3-11H2,1-2H3/t12-,14-,15-,16-,17-,18-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-tagged R1881 from androgen receptor after 4 hrs by fluorometric assay |
ACS Med Chem Lett 2: 124-129 (2011)
Article DOI: 10.1021/ml1002508
BindingDB Entry DOI: 10.7270/Q2JQ119N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50016697
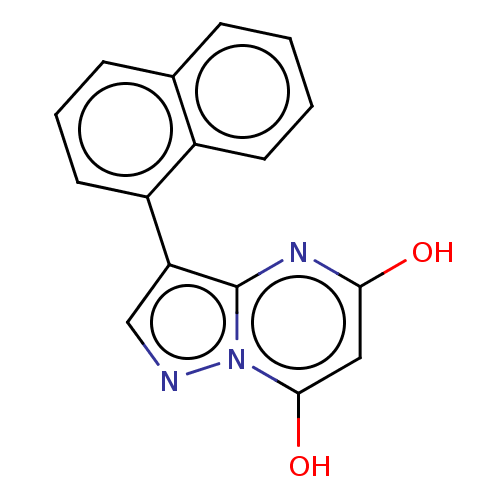
(CHEMBL3277223)Show InChI InChI=1S/C16H11N3O2/c20-14-8-15(21)19-16(18-14)13(9-17-19)12-7-3-5-10-4-1-2-6-11(10)12/h1-9,21H,(H,18,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of bovine milk xanthine oxidase using xanthine as substrate by Lineweaver-Burk plot analysis |
J Med Chem 19: 291-6 (1976)
BindingDB Entry DOI: 10.7270/Q2XP76G9 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM14487

((2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-(hydroxy...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O Show InChI InChI=1S/C10H13N5O4/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(18)6(17)4(1-16)19-10/h2-4,6-7,10,16-18H,1H2,(H2,11,12,13)/t4-,6-,7-,10-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Affinity for Adenosine A1 receptor determined by [3H]N6-cyclohexyladenosine binding to rat brain membranes |
Bioorg Med Chem Lett 1: 481-486 (1991)
Article DOI: 10.1016/S0960-894X(01)81110-0
BindingDB Entry DOI: 10.7270/Q2K64JJ8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50370217

(CHEMBL1744085)Show SMILES CCCN1c2ccccc2C(=N[C@@H](NC(=O)Nc2ccc(cc2)N2CCC(CC2)N2CCCCC2)C1=O)c1ccc(C)cc1 |r,c:11| Show InChI InChI=1S/C36H44N6O2/c1-3-21-42-32-10-6-5-9-31(32)33(27-13-11-26(2)12-14-27)38-34(35(42)43)39-36(44)37-28-15-17-29(18-16-28)41-24-19-30(20-25-41)40-22-7-4-8-23-40/h5-6,9-18,30,34H,3-4,7-8,19-25H2,1-2H3,(H2,37,39,44)/t34-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human bradykinin B1 receptor |
J Med Chem 46: 1803-6 (2003)
Article DOI: 10.1021/jm034020y
BindingDB Entry DOI: 10.7270/Q29P32C6 |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50016697

(CHEMBL3277223)Show InChI InChI=1S/C16H11N3O2/c20-14-8-15(21)19-16(18-14)13(9-17-19)12-7-3-5-10-4-1-2-6-11(10)12/h1-9,21H,(H,18,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of bovine milk xanthine oxidase using hypoxanthine as substrate by Lineweaver-Burk plot analysis |
J Med Chem 19: 291-6 (1976)
BindingDB Entry DOI: 10.7270/Q2XP76G9 |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50016702
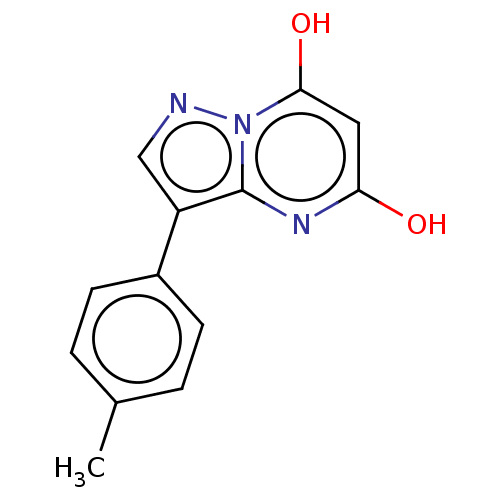
(CHEMBL3276844)Show InChI InChI=1S/C13H11N3O2/c1-8-2-4-9(5-3-8)10-7-14-16-12(18)6-11(17)15-13(10)16/h2-7,18H,1H3,(H,15,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of bovine milk xanthine oxidase using xanthine as substrate by Lineweaver-Burk plot analysis |
J Med Chem 19: 291-6 (1976)
BindingDB Entry DOI: 10.7270/Q2XP76G9 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(RAT) | BDBM50370217

(CHEMBL1744085)Show SMILES CCCN1c2ccccc2C(=N[C@@H](NC(=O)Nc2ccc(cc2)N2CCC(CC2)N2CCCCC2)C1=O)c1ccc(C)cc1 |r,c:11| Show InChI InChI=1S/C36H44N6O2/c1-3-21-42-32-10-6-5-9-31(32)33(27-13-11-26(2)12-14-27)38-34(35(42)43)39-36(44)37-28-15-17-29(18-16-28)41-24-19-30(20-25-41)40-22-7-4-8-23-40/h5-6,9-18,30,34H,3-4,7-8,19-25H2,1-2H3,(H2,37,39,44)/t34-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition against rat Bradykinin receptor B1 |
J Med Chem 46: 1803-6 (2003)
Article DOI: 10.1021/jm034020y
BindingDB Entry DOI: 10.7270/Q29P32C6 |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50016707
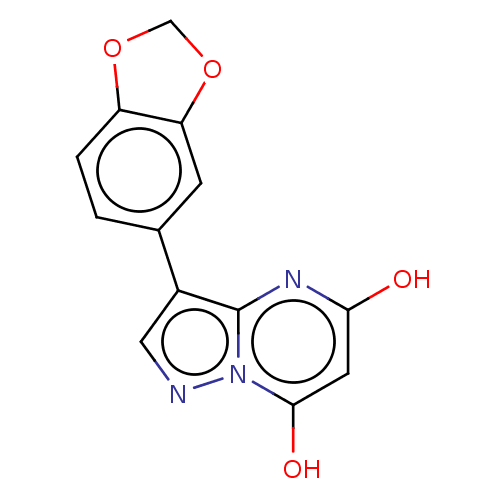
(CHEMBL3276846)Show InChI InChI=1S/C13H9N3O4/c17-11-4-12(18)16-13(15-11)8(5-14-16)7-1-2-9-10(3-7)20-6-19-9/h1-5,18H,6H2,(H,15,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of bovine milk xanthine oxidase using hypoxanthine as substrate by Lineweaver-Burk plot analysis |
J Med Chem 19: 291-6 (1976)
BindingDB Entry DOI: 10.7270/Q2XP76G9 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(RAT) | BDBM50370215

(CHEMBL1744082)Show SMILES CCCN1c2ccccc2C(=N[C@@H](NC(=O)Nc2ccc(cc2)N2CCC(CC2)N2CCCCC2)C1=O)C1CCCCC1 |r,c:11| Show InChI InChI=1S/C35H48N6O2/c1-2-21-41-31-14-8-7-13-30(31)32(26-11-5-3-6-12-26)37-33(34(41)42)38-35(43)36-27-15-17-28(18-16-27)40-24-19-29(20-25-40)39-22-9-4-10-23-39/h7-8,13-18,26,29,33H,2-6,9-12,19-25H2,1H3,(H2,36,38,43)/t33-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition against rat Bradykinin receptor B1 |
J Med Chem 46: 1803-6 (2003)
Article DOI: 10.1021/jm034020y
BindingDB Entry DOI: 10.7270/Q29P32C6 |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50016705
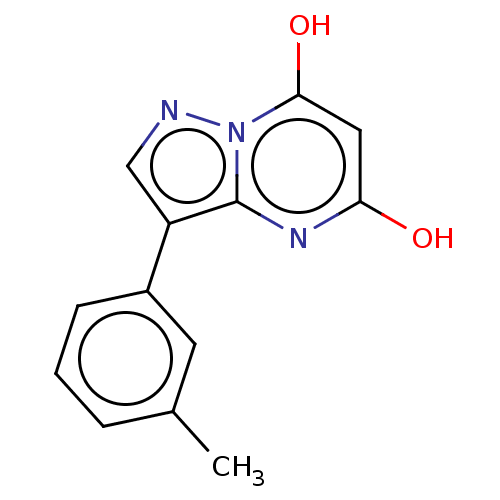
(CHEMBL3276845)Show InChI InChI=1S/C13H11N3O2/c1-8-3-2-4-9(5-8)10-7-14-16-12(18)6-11(17)15-13(10)16/h2-7,18H,1H3,(H,15,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of bovine milk xanthine oxidase using hypoxanthine as substrate by Lineweaver-Burk plot analysis |
J Med Chem 19: 291-6 (1976)
BindingDB Entry DOI: 10.7270/Q2XP76G9 |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50016700
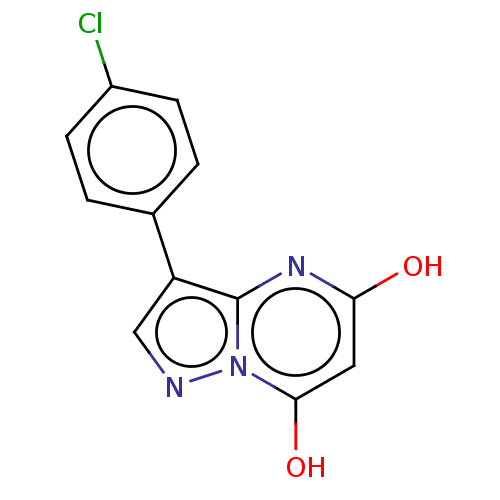
(CHEMBL3276842)Show InChI InChI=1S/C12H8ClN3O2/c13-8-3-1-7(2-4-8)9-6-14-16-11(18)5-10(17)15-12(9)16/h1-6,18H,(H,15,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of bovine milk xanthine oxidase using hypoxanthine as substrate by Lineweaver-Burk plot analysis |
J Med Chem 19: 291-6 (1976)
BindingDB Entry DOI: 10.7270/Q2XP76G9 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50366247
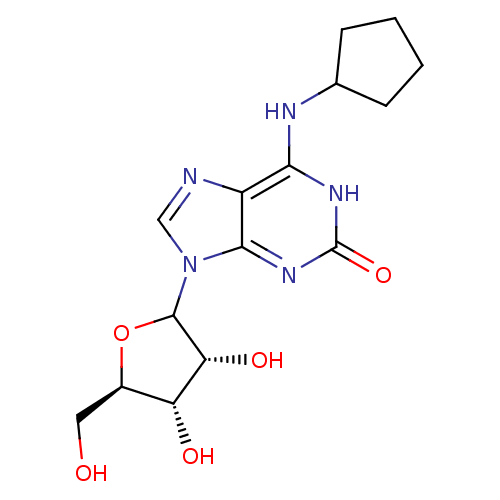
(CHEMBL608168)Show SMILES OC[C@H]1OC([C@H](O)[C@@H]1O)n1cnc2c(NC3CCCC3)[nH]c(=O)nc12 |r| Show InChI InChI=1S/C15H21N5O5/c21-5-8-10(22)11(23)14(25-8)20-6-16-9-12(17-7-3-1-2-4-7)18-15(24)19-13(9)20/h6-8,10-11,14,21-23H,1-5H2,(H2,17,18,19,24)/t8-,10-,11-,14?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Affinity for Adenosine A1 receptor determined by [3H]N6-cyclohexyladenosine binding to rat brain membranes |
Bioorg Med Chem Lett 1: 481-486 (1991)
Article DOI: 10.1016/S0960-894X(01)81110-0
BindingDB Entry DOI: 10.7270/Q2K64JJ8 |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50016705

(CHEMBL3276845)Show InChI InChI=1S/C13H11N3O2/c1-8-3-2-4-9(5-8)10-7-14-16-12(18)6-11(17)15-13(10)16/h2-7,18H,1H3,(H,15,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of bovine milk xanthine oxidase using xanthine as substrate by Lineweaver-Burk plot analysis |
J Med Chem 19: 291-6 (1976)
BindingDB Entry DOI: 10.7270/Q2XP76G9 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50366245

(CHEMBL607881)Show SMILES OC[C@H]1OC([C@H](O)[C@@H]1O)n1cnc2c(NC3CCCC3)nc(I)nc12 |r| Show InChI InChI=1S/C15H20IN5O4/c16-15-19-12(18-7-3-1-2-4-7)9-13(20-15)21(6-17-9)14-11(24)10(23)8(5-22)25-14/h6-8,10-11,14,22-24H,1-5H2,(H,18,19,20)/t8-,10-,11-,14?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Affinity for Adenosine A1 receptor determined by [3H]N6-cyclohexyladenosine binding to rat brain membranes |
Bioorg Med Chem Lett 1: 481-486 (1991)
Article DOI: 10.1016/S0960-894X(01)81110-0
BindingDB Entry DOI: 10.7270/Q2K64JJ8 |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50016700

(CHEMBL3276842)Show InChI InChI=1S/C12H8ClN3O2/c13-8-3-1-7(2-4-8)9-6-14-16-11(18)5-10(17)15-12(9)16/h1-6,18H,(H,15,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of bovine milk xanthine oxidase using xanthine as substrate by Lineweaver-Burk plot analysis |
J Med Chem 19: 291-6 (1976)
BindingDB Entry DOI: 10.7270/Q2XP76G9 |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50016707

(CHEMBL3276846)Show InChI InChI=1S/C13H9N3O4/c17-11-4-12(18)16-13(15-11)8(5-14-16)7-1-2-9-10(3-7)20-6-19-9/h1-5,18H,6H2,(H,15,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of bovine milk xanthine oxidase using xanthine as substrate by Lineweaver-Burk plot analysis |
J Med Chem 19: 291-6 (1976)
BindingDB Entry DOI: 10.7270/Q2XP76G9 |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50016699

(CHEMBL3276841)Show InChI InChI=1S/C12H8BrN3O2/c13-8-3-1-7(2-4-8)9-6-14-16-11(18)5-10(17)15-12(9)16/h1-6,18H,(H,15,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of bovine milk xanthine oxidase using hypoxanthine as substrate by Lineweaver-Burk plot analysis |
J Med Chem 19: 291-6 (1976)
BindingDB Entry DOI: 10.7270/Q2XP76G9 |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50016702

(CHEMBL3276844)Show InChI InChI=1S/C13H11N3O2/c1-8-2-4-9(5-3-8)10-7-14-16-12(18)6-11(17)15-13(10)16/h2-7,18H,1H3,(H,15,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of bovine milk xanthine oxidase using hypoxanthine as substrate by Lineweaver-Burk plot analysis |
J Med Chem 19: 291-6 (1976)
BindingDB Entry DOI: 10.7270/Q2XP76G9 |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50016699

(CHEMBL3276841)Show InChI InChI=1S/C12H8BrN3O2/c13-8-3-1-7(2-4-8)9-6-14-16-11(18)5-10(17)15-12(9)16/h1-6,18H,(H,15,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of bovine milk xanthine oxidase using xanthine as substrate by Lineweaver-Burk plot analysis |
J Med Chem 19: 291-6 (1976)
BindingDB Entry DOI: 10.7270/Q2XP76G9 |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50016696

(CHEMBL3276840)Show InChI InChI=1S/C11H8N4O2/c16-9-4-10(17)15-11(14-9)8(6-13-15)7-2-1-3-12-5-7/h1-6,17H,(H,14,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of bovine milk xanthine oxidase using xanthine as substrate by Lineweaver-Burk plot analysis |
J Med Chem 19: 291-6 (1976)
BindingDB Entry DOI: 10.7270/Q2XP76G9 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50127445
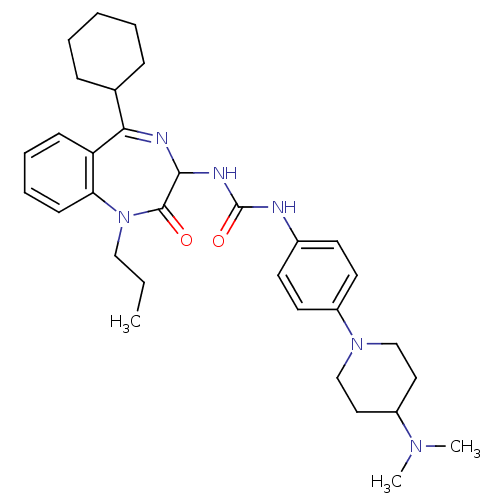
(1-(5-Cyclohexyl-2-oxo-1-propyl-2,3-dihydro-1H-benz...)Show SMILES CCCN1c2ccccc2C(=NC(NC(=O)Nc2ccc(cc2)N2CCC(CC2)N(C)C)C1=O)C1CCCCC1 |c:11| Show InChI InChI=1S/C32H44N6O2/c1-4-20-38-28-13-9-8-12-27(28)29(23-10-6-5-7-11-23)34-30(31(38)39)35-32(40)33-24-14-16-26(17-15-24)37-21-18-25(19-22-37)36(2)3/h8-9,12-17,23,25,30H,4-7,10-11,18-22H2,1-3H3,(H2,33,35,40) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human bradykinin B1 receptor |
J Med Chem 46: 1803-6 (2003)
Article DOI: 10.1021/jm034020y
BindingDB Entry DOI: 10.7270/Q29P32C6 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM8885
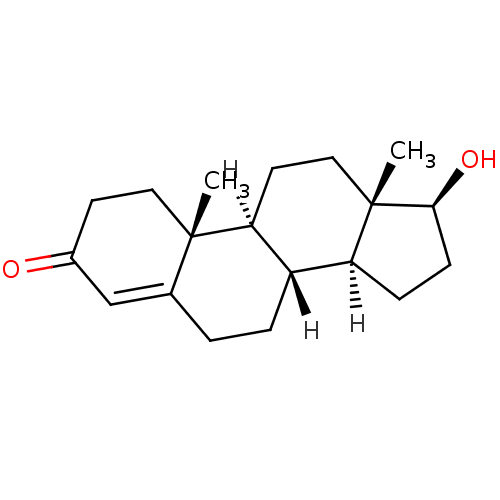
((1S,2R,10R,11S,14S,15S)-14-hydroxy-2,15-dimethylte...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2=CC(=O)CC[C@]12C |r,t:18| Show InChI InChI=1S/C19H28O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h11,14-17,21H,3-10H2,1-2H3/t14-,15-,16-,17-,18-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-tagged R1881 from androgen receptor after 4 hrs by fluorometric assay |
ACS Med Chem Lett 2: 124-129 (2011)
Article DOI: 10.1021/ml1002508
BindingDB Entry DOI: 10.7270/Q2JQ119N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50016696

(CHEMBL3276840)Show InChI InChI=1S/C11H8N4O2/c16-9-4-10(17)15-11(14-9)8(6-13-15)7-2-1-3-12-5-7/h1-6,17H,(H,14,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of bovine milk xanthine oxidase using hypoxanthine as substrate by Lineweaver-Burk plot analysis |
J Med Chem 19: 291-6 (1976)
BindingDB Entry DOI: 10.7270/Q2XP76G9 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50366248

(CHEMBL608742)Show SMILES OC[C@H]1OC([C@H](O)[C@@H]1O)n1cnc2c(NC3CC4CCC3C4)[nH]c(=O)nc12 |r| Show InChI InChI=1S/C17H23N5O5/c23-5-10-12(24)13(25)16(27-10)22-6-18-11-14(20-17(26)21-15(11)22)19-9-4-7-1-2-8(9)3-7/h6-10,12-13,16,23-25H,1-5H2,(H2,19,20,21,26)/t7?,8?,9?,10-,12-,13-,16?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Affinity for Adenosine A1 receptor determined by [3H]N6-cyclohexyladenosine binding to rat brain membranes |
Bioorg Med Chem Lett 1: 481-486 (1991)
Article DOI: 10.1016/S0960-894X(01)81110-0
BindingDB Entry DOI: 10.7270/Q2K64JJ8 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a/A2b
(Rattus norvegicus-Rattus norvegicus (rat)) | BDBM14487

((2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-(hydroxy...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O Show InChI InChI=1S/C10H13N5O4/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(18)6(17)4(1-16)19-10/h2-4,6-7,10,16-18H,1H2,(H2,11,12,13)/t4-,6-,7-,10-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Affinity for Adenosine A2 receptor determined by [3H]NECA binding to rat striatal membranes |
Bioorg Med Chem Lett 1: 481-486 (1991)
Article DOI: 10.1016/S0960-894X(01)81110-0
BindingDB Entry DOI: 10.7270/Q2K64JJ8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50366243

(CHEMBL608747)Show SMILES OC[C@H]1OC([C@H](O)[C@@H]1O)n1cnc2c(NC3CCCCC3)[nH]c(=O)nc12 |r| Show InChI InChI=1S/C16H23N5O5/c22-6-9-11(23)12(24)15(26-9)21-7-17-10-13(19-16(25)20-14(10)21)18-8-4-2-1-3-5-8/h7-9,11-12,15,22-24H,1-6H2,(H2,18,19,20,25)/t9-,11-,12-,15?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Affinity for Adenosine A1 receptor determined by [3H]N6-cyclohexyladenosine binding to rat brain membranes |
Bioorg Med Chem Lett 1: 481-486 (1991)
Article DOI: 10.1016/S0960-894X(01)81110-0
BindingDB Entry DOI: 10.7270/Q2K64JJ8 |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50016669
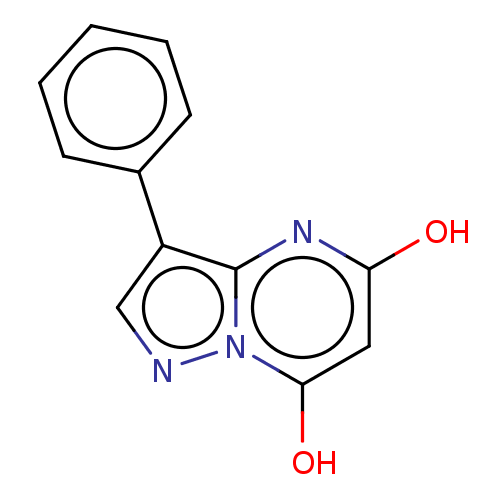
(CHEMBL3276839)Show InChI InChI=1S/C12H9N3O2/c16-10-6-11(17)15-12(14-10)9(7-13-15)8-4-2-1-3-5-8/h1-7,17H,(H,14,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of bovine milk xanthine oxidase using xanthine as substrate by Lineweaver-Burk plot analysis |
J Med Chem 19: 291-6 (1976)
BindingDB Entry DOI: 10.7270/Q2XP76G9 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50366244
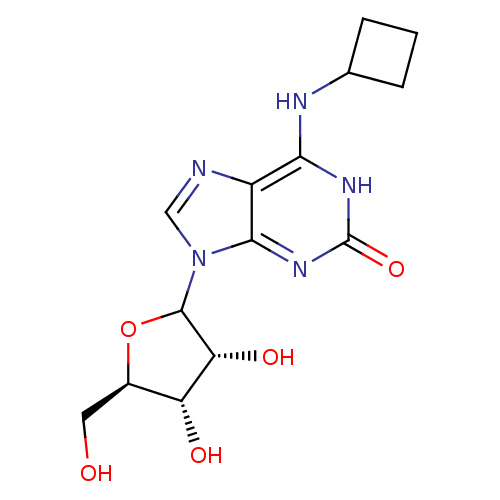
(CHEMBL608445)Show SMILES OC[C@H]1OC([C@H](O)[C@@H]1O)n1cnc2c(NC3CCC3)[nH]c(=O)nc12 |r| Show InChI InChI=1S/C14H19N5O5/c20-4-7-9(21)10(22)13(24-7)19-5-15-8-11(16-6-2-1-3-6)17-14(23)18-12(8)19/h5-7,9-10,13,20-22H,1-4H2,(H2,16,17,18,23)/t7-,9-,10-,13?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Affinity for Adenosine A1 receptor determined by [3H]N6-cyclohexyladenosine binding to rat brain membranes |
Bioorg Med Chem Lett 1: 481-486 (1991)
Article DOI: 10.1016/S0960-894X(01)81110-0
BindingDB Entry DOI: 10.7270/Q2K64JJ8 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50127446
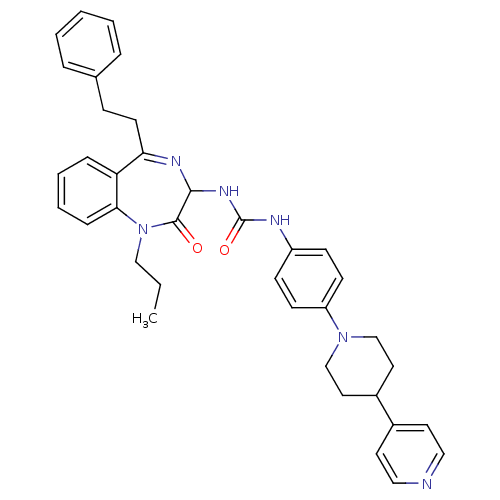
(1-(2-Oxo-5-phenethyl-1-propyl-2,3-dihydro-1H-benzo...)Show SMILES CCCN1c2ccccc2C(CCc2ccccc2)=NC(NC(=O)Nc2ccc(cc2)N2CCC(CC2)c2ccncc2)C1=O |c:20| Show InChI InChI=1S/C37H40N6O2/c1-2-24-43-34-11-7-6-10-32(34)33(17-12-27-8-4-3-5-9-27)40-35(36(43)44)41-37(45)39-30-13-15-31(16-14-30)42-25-20-29(21-26-42)28-18-22-38-23-19-28/h3-11,13-16,18-19,22-23,29,35H,2,12,17,20-21,24-26H2,1H3,(H2,39,41,45) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human bradykinin B1 receptor |
J Med Chem 46: 1803-6 (2003)
Article DOI: 10.1021/jm034020y
BindingDB Entry DOI: 10.7270/Q29P32C6 |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50016669

(CHEMBL3276839)Show InChI InChI=1S/C12H9N3O2/c16-10-6-11(17)15-12(14-10)9(7-13-15)8-4-2-1-3-5-8/h1-7,17H,(H,14,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of bovine milk xanthine oxidase using hypoxanthine as substrate by Lineweaver-Burk plot analysis |
J Med Chem 19: 291-6 (1976)
BindingDB Entry DOI: 10.7270/Q2XP76G9 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50366246
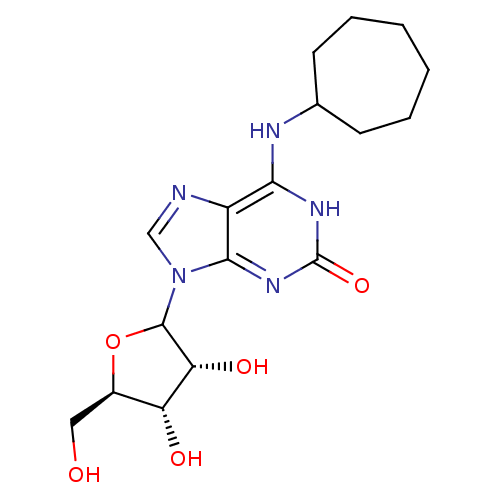
(CHEMBL607606)Show SMILES OC[C@H]1OC([C@H](O)[C@@H]1O)n1cnc2c(NC3CCCCCC3)[nH]c(=O)nc12 |r| Show InChI InChI=1S/C17H25N5O5/c23-7-10-12(24)13(25)16(27-10)22-8-18-11-14(20-17(26)21-15(11)22)19-9-5-3-1-2-4-6-9/h8-10,12-13,16,23-25H,1-7H2,(H2,19,20,21,26)/t10-,12-,13-,16?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Affinity for Adenosine A1 receptor determined by [3H]N6-cyclohexyladenosine binding to rat brain membranes |
Bioorg Med Chem Lett 1: 481-486 (1991)
Article DOI: 10.1016/S0960-894X(01)81110-0
BindingDB Entry DOI: 10.7270/Q2K64JJ8 |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50016701
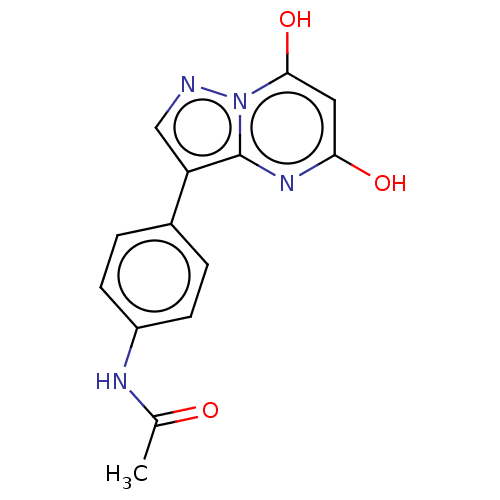
(CHEMBL3276843)Show InChI InChI=1S/C14H12N4O3/c1-8(19)16-10-4-2-9(3-5-10)11-7-15-18-13(21)6-12(20)17-14(11)18/h2-7,21H,1H3,(H,16,19)(H,17,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of bovine milk xanthine oxidase using hypoxanthine as substrate by Lineweaver-Burk plot analysis |
J Med Chem 19: 291-6 (1976)
BindingDB Entry DOI: 10.7270/Q2XP76G9 |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50016701

(CHEMBL3276843)Show InChI InChI=1S/C14H12N4O3/c1-8(19)16-10-4-2-9(3-5-10)11-7-15-18-13(21)6-12(20)17-14(11)18/h2-7,21H,1H3,(H,16,19)(H,17,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of bovine milk xanthine oxidase using xanthine as substrate by Lineweaver-Burk plot analysis |
J Med Chem 19: 291-6 (1976)
BindingDB Entry DOI: 10.7270/Q2XP76G9 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50127442
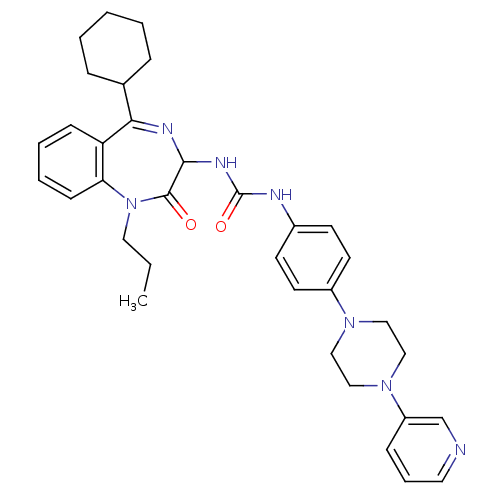
(1-(5-Cyclohexyl-2-oxo-1-propyl-2,3-dihydro-1H-benz...)Show SMILES CCCN1c2ccccc2C(=NC(NC(=O)Nc2ccc(cc2)N2CCN(CC2)c2cccnc2)C1=O)C1CCCCC1 |c:11| Show InChI InChI=1S/C34H41N7O2/c1-2-19-41-30-13-7-6-12-29(30)31(25-9-4-3-5-10-25)37-32(33(41)42)38-34(43)36-26-14-16-27(17-15-26)39-20-22-40(23-21-39)28-11-8-18-35-24-28/h6-8,11-18,24-25,32H,2-5,9-10,19-23H2,1H3,(H2,36,38,43) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human bradykinin B1 receptor |
J Med Chem 46: 1803-6 (2003)
Article DOI: 10.1021/jm034020y
BindingDB Entry DOI: 10.7270/Q29P32C6 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM82031
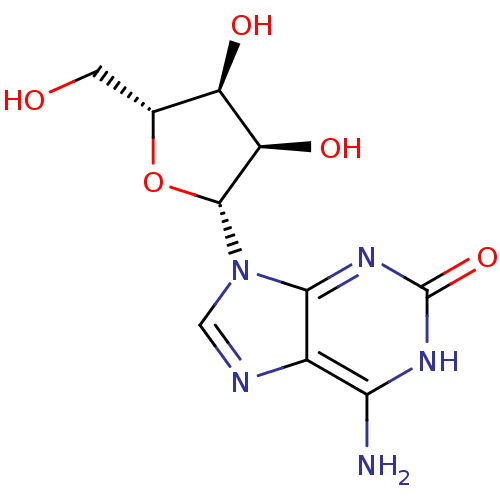
(2-Oxoado (isoguanosine) | CAS_1818-71-9 | isoguano...)Show SMILES Nc1[nH]c(=O)nc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H13N5O5/c11-7-4-8(14-10(19)13-7)15(2-12-4)9-6(18)5(17)3(1-16)20-9/h2-3,5-6,9,16-18H,1H2,(H3,11,13,14,19)/t3-,5-,6-,9-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| Article
| 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Affinity for Adenosine A1 receptor determined by [3H]N6-cyclohexyladenosine binding to rat brain membranes |
Bioorg Med Chem Lett 1: 481-486 (1991)
Article DOI: 10.1016/S0960-894X(01)81110-0
BindingDB Entry DOI: 10.7270/Q2K64JJ8 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50127448
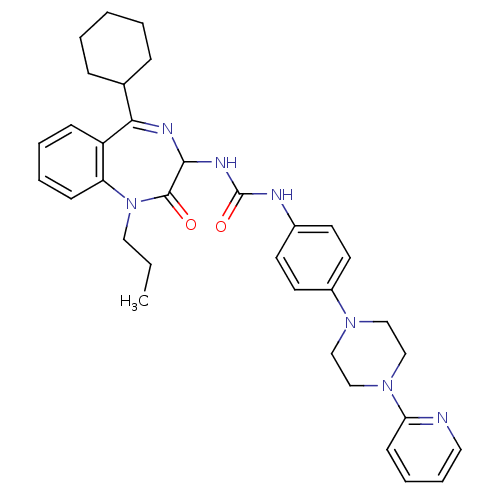
(1-(5-Cyclohexyl-2-oxo-1-propyl-2,3-dihydro-1H-benz...)Show SMILES CCCN1c2ccccc2C(=NC(NC(=O)Nc2ccc(cc2)N2CCN(CC2)c2ccccn2)C1=O)C1CCCCC1 |c:11| Show InChI InChI=1S/C34H41N7O2/c1-2-20-41-29-13-7-6-12-28(29)31(25-10-4-3-5-11-25)37-32(33(41)42)38-34(43)36-26-15-17-27(18-16-26)39-21-23-40(24-22-39)30-14-8-9-19-35-30/h6-9,12-19,25,32H,2-5,10-11,20-24H2,1H3,(H2,36,38,43) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 97.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human bradykinin B1 receptor |
J Med Chem 46: 1803-6 (2003)
Article DOI: 10.1021/jm034020y
BindingDB Entry DOI: 10.7270/Q29P32C6 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50127449

(CHEMBL30525 | iN-(4-[1,4']Bipiperidinyl-1'-yl-phen...)Show SMILES O=C(CCCN1c2ccccc2C(=NCC1=O)C1CCCCC1)Nc1ccc(cc1)N1CCC(CC1)N1CCCCC1 |c:13| Show InChI InChI=1S/C35H47N5O2/c41-33(37-28-15-17-29(18-16-28)39-24-19-30(20-25-39)38-21-7-2-8-22-38)14-9-23-40-32-13-6-5-12-31(32)35(36-26-34(40)42)27-10-3-1-4-11-27/h5-6,12-13,15-18,27,30H,1-4,7-11,14,19-26H2,(H,37,41) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 141 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human bradykinin B1 receptor |
J Med Chem 46: 1803-6 (2003)
Article DOI: 10.1021/jm034020y
BindingDB Entry DOI: 10.7270/Q29P32C6 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a/A2b
(Rattus norvegicus-Rattus norvegicus (rat)) | BDBM82031

(2-Oxoado (isoguanosine) | CAS_1818-71-9 | isoguano...)Show SMILES Nc1[nH]c(=O)nc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H13N5O5/c11-7-4-8(14-10(19)13-7)15(2-12-4)9-6(18)5(17)3(1-16)20-9/h2-3,5-6,9,16-18H,1H2,(H3,11,13,14,19)/t3-,5-,6-,9-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| Article
| 331 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Affinity for Adenosine A2 receptor determined by [3H]NECA binding to rat striatal membranes |
Bioorg Med Chem Lett 1: 481-486 (1991)
Article DOI: 10.1016/S0960-894X(01)81110-0
BindingDB Entry DOI: 10.7270/Q2K64JJ8 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a/A2b
(Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50009552

(2-[6-Amino-2-(2-morpholin-4-yl-ethylamino)-purin-9...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NC3CCCCC3)ncnc12 |r| Show InChI InChI=1S/C16H23N5O4/c22-6-10-12(23)13(24)16(25-10)21-8-19-11-14(17-7-18-15(11)21)20-9-4-2-1-3-5-9/h7-10,12-13,16,22-24H,1-6H2,(H,17,18,20)/t10-,12-,13-,16-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
| 363 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Affinity for Adenosine A2 receptor determined by [3H]NECA binding to rat striatal membranes |
Bioorg Med Chem Lett 1: 481-486 (1991)
Article DOI: 10.1016/S0960-894X(01)81110-0
BindingDB Entry DOI: 10.7270/Q2K64JJ8 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a/A2b
(Rattus norvegicus-Rattus norvegicus (rat)) | BDBM25400

((2R,3R,4S,5R)-2-[6-(cyclopentylamino)-9H-purin-9-y...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NC3CCCC3)ncnc12 Show InChI InChI=1S/C15H21N5O4/c21-5-9-11(22)12(23)15(24-9)20-7-18-10-13(16-6-17-14(10)20)19-8-3-1-2-4-8/h6-9,11-12,15,21-23H,1-5H2,(H,16,17,19)/t9-,11-,12-,15-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| 462 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Affinity for Adenosine A2 receptor determined by [3H]NECA binding to rat striatal membranes |
Bioorg Med Chem Lett 1: 481-486 (1991)
Article DOI: 10.1016/S0960-894X(01)81110-0
BindingDB Entry DOI: 10.7270/Q2K64JJ8 |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50016784
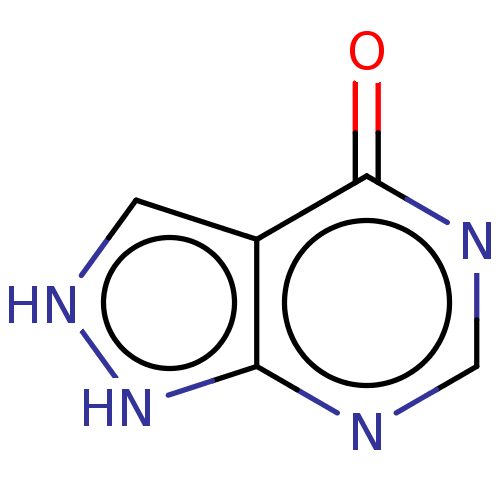
(ALLOPURINOL | BW-56-158 | BW-56158 | CHEBI:40279 |...)Show InChI InChI=1S/C5H2N4O/c10-5-3-1-8-9-4(3)6-2-7-5/h1-2H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of bovine milk xanthine oxidase using xanthine as substrate by Lineweaver-Burk plot analysis |
J Med Chem 19: 291-6 (1976)
BindingDB Entry DOI: 10.7270/Q2XP76G9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data