 Found 223 hits with Last Name = 'miller' and Initial = 'ws'
Found 223 hits with Last Name = 'miller' and Initial = 'ws' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50153608
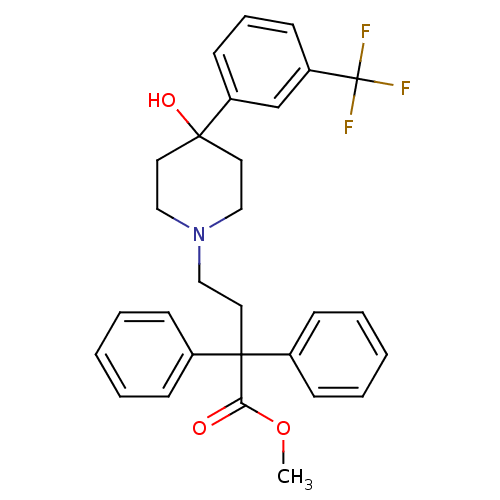
(4-[4-Hydroxy-4-(3-trifluoromethyl-phenyl)-piperidi...)Show SMILES COC(=O)C(CCN1CCC(O)(CC1)c1cccc(c1)C(F)(F)F)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C29H30F3NO3/c1-36-26(34)28(22-9-4-2-5-10-22,23-11-6-3-7-12-23)17-20-33-18-15-27(35,16-19-33)24-13-8-14-25(21-24)29(30,31)32/h2-14,21,35H,15-20H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P.
Curated by ChEMBL
| Assay Description
Binding affinity for recombinant human mu-opioid receptor was determined by using [3H]- diprenophine radioligand |
Bioorg Med Chem Lett 14: 5275-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.032
BindingDB Entry DOI: 10.7270/Q2K073RN |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50017686

(4-[4-(4-Chloro-3-trifluoromethyl-phenyl)-4-hydroxy...)Show SMILES CN(C)C(=O)C(CCN1CCC(O)(CC1)c1ccc(Cl)c(c1)C(F)(F)F)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C30H32ClF3N2O2/c1-35(2)27(37)29(22-9-5-3-6-10-22,23-11-7-4-8-12-23)17-20-36-18-15-28(38,16-19-36)24-13-14-26(31)25(21-24)30(32,33)34/h3-14,21,38H,15-20H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P.
Curated by ChEMBL
| Assay Description
Binding affinity for recombinant human mu-opioid receptor was determined by using [3H]- diprenophine radioligand |
Bioorg Med Chem Lett 14: 5275-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.032
BindingDB Entry DOI: 10.7270/Q2K073RN |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50153614
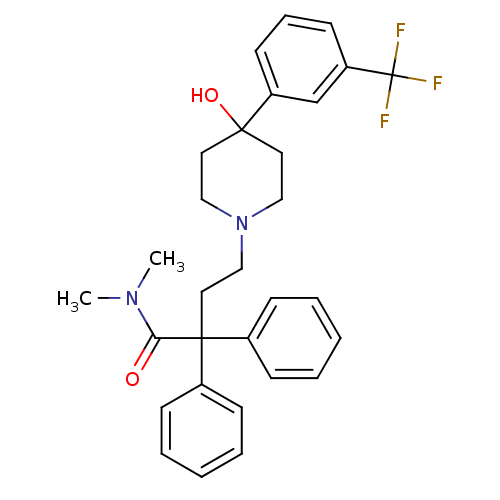
(4-[4-Hydroxy-4-(3-trifluoromethyl-phenyl)-piperidi...)Show SMILES CN(C)C(=O)C(CCN1CCC(O)(CC1)c1cccc(c1)C(F)(F)F)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C30H33F3N2O2/c1-34(2)27(36)29(23-10-5-3-6-11-23,24-12-7-4-8-13-24)18-21-35-19-16-28(37,17-20-35)25-14-9-15-26(22-25)30(31,32)33/h3-15,22,37H,16-21H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P.
Curated by ChEMBL
| Assay Description
Binding affinity for recombinant human mu-opioid receptor was determined by using [3H]- diprenophine radioligand |
Bioorg Med Chem Lett 14: 5275-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.032
BindingDB Entry DOI: 10.7270/Q2K073RN |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50004178
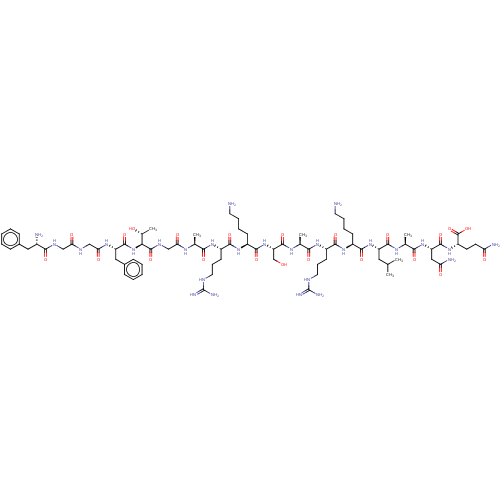
(Nociceptin | Nociceptin/orphanin FQ | ORPHANIN FQ)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)[C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O |r| Show InChI InChI=1S/C79H129N27O22/c1-41(2)33-54(72(122)95-44(5)66(116)103-56(36-59(84)110)73(123)102-53(77(127)128)27-28-58(83)109)104-70(120)49(23-13-15-29-80)100-69(119)52(26-18-32-90-79(87)88)99-65(115)43(4)96-75(125)57(40-107)105-71(121)50(24-14-16-30-81)101-68(118)51(25-17-31-89-78(85)86)98-64(114)42(3)94-61(112)39-93-76(126)63(45(6)108)106-74(124)55(35-47-21-11-8-12-22-47)97-62(113)38-91-60(111)37-92-67(117)48(82)34-46-19-9-7-10-20-46/h7-12,19-22,41-45,48-57,63,107-108H,13-18,23-40,80-82H2,1-6H3,(H2,83,109)(H2,84,110)(H,91,111)(H,92,117)(H,93,126)(H,94,112)(H,95,122)(H,96,125)(H,97,113)(H,98,114)(H,99,115)(H,100,119)(H,101,118)(H,102,123)(H,103,116)(H,104,120)(H,105,121)(H,106,124)(H,127,128)(H4,85,86,89)(H4,87,88,90)/t42-,43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]nociceptin binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50153611

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-2...)Show SMILES COC(=O)C(CCN1CCC(O)(CC1)c1ccc(Cl)cc1)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C28H30ClNO3/c1-33-26(31)28(23-8-4-2-5-9-23,24-10-6-3-7-11-24)18-21-30-19-16-27(32,17-20-30)22-12-14-25(29)15-13-22/h2-15,32H,16-21H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P.
Curated by ChEMBL
| Assay Description
Binding affinity for recombinant human mu-opioid receptor was determined by using [3H]- diprenophine radioligand |
Bioorg Med Chem Lett 14: 5275-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.032
BindingDB Entry DOI: 10.7270/Q2K073RN |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50153613

(4-[4-(4-Chloro-3-trifluoromethyl-phenyl)-4-hydroxy...)Show SMILES COC(=O)C(CCN1CCC(O)(CC1)c1ccc(Cl)c(c1)C(F)(F)F)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C29H29ClF3NO3/c1-37-26(35)28(21-8-4-2-5-9-21,22-10-6-3-7-11-22)16-19-34-17-14-27(36,15-18-34)23-12-13-25(30)24(20-23)29(31,32)33/h2-13,20,36H,14-19H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P.
Curated by ChEMBL
| Assay Description
Binding affinity for recombinant human mu-opioid receptor was determined by using [3H]- diprenophine radioligand |
Bioorg Med Chem Lett 14: 5275-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.032
BindingDB Entry DOI: 10.7270/Q2K073RN |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50017698
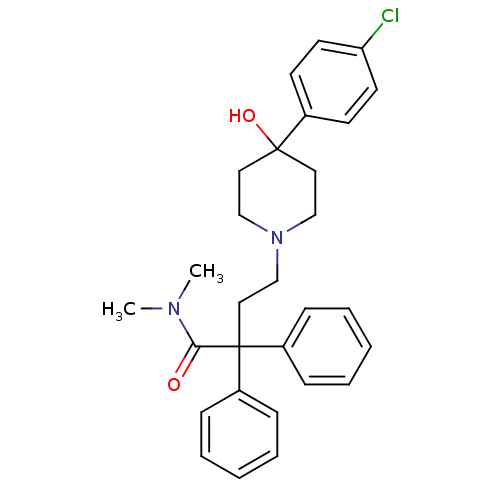
(4-(4-(4-chlorophenyl)-4-hydroxypiperidin-1-yl)-N,N...)Show SMILES CN(C)C(=O)C(CCN1CCC(O)(CC1)c1ccc(Cl)cc1)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C29H33ClN2O2/c1-31(2)27(33)29(24-9-5-3-6-10-24,25-11-7-4-8-12-25)19-22-32-20-17-28(34,18-21-32)23-13-15-26(30)16-14-23/h3-16,34H,17-22H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P.
Curated by ChEMBL
| Assay Description
Binding affinity for recombinant human mu-opioid receptor was determined by using [3H]- diprenophine radioligand |
Bioorg Med Chem Lett 14: 5275-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.032
BindingDB Entry DOI: 10.7270/Q2K073RN |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50132647
(1-(3,3-Diphenyl-propyl)-4-(3-trifluoromethyl-pheny...)Show SMILES OC1(CCN(CCC(c2ccccc2)c2ccccc2)CC1)c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C27H28F3NO/c28-27(29,30)24-13-7-12-23(20-24)26(32)15-18-31(19-16-26)17-14-25(21-8-3-1-4-9-21)22-10-5-2-6-11-22/h1-13,20,25,32H,14-19H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P.
Curated by ChEMBL
| Assay Description
Antagonistic activity against opioid receptor mu1 |
Bioorg Med Chem Lett 13: 3247-52 (2003)
BindingDB Entry DOI: 10.7270/Q2K936XQ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50132647

(1-(3,3-Diphenyl-propyl)-4-(3-trifluoromethyl-pheny...)Show SMILES OC1(CCN(CCC(c2ccccc2)c2ccccc2)CC1)c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C27H28F3NO/c28-27(29,30)24-13-7-12-23(20-24)26(32)15-18-31(19-16-26)17-14-25(21-8-3-1-4-9-21)22-10-5-2-6-11-22/h1-13,20,25,32H,14-19H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P.
Curated by ChEMBL
| Assay Description
Binding affinity for recombinant human mu-opioid receptor was determined by using [3H]- diprenophine radioligand |
Bioorg Med Chem Lett 14: 5275-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.032
BindingDB Entry DOI: 10.7270/Q2K073RN |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50132662
(4-(4-Chloro-3-trifluoromethyl-phenyl)-1-naphthalen...)Show SMILES OC1(CCN(Cc2ccc3ccccc3c2)CC1)c1ccc(Cl)c(c1)C(F)(F)F Show InChI InChI=1S/C23H21ClF3NO/c24-21-8-7-19(14-20(21)23(25,26)27)22(29)9-11-28(12-10-22)15-16-5-6-17-3-1-2-4-18(17)13-16/h1-8,13-14,29H,9-12,15H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P.
Curated by ChEMBL
| Assay Description
Binding affinity for recombinant human mu-opioid receptor was determined by using [3H]- diprenophine radioligand |
Bioorg Med Chem Lett 14: 5275-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.032
BindingDB Entry DOI: 10.7270/Q2K073RN |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153127
(2-{1-[1-(4-Isopropyl-cyclohexyl)-piperidin-4-yl]-2...)Show SMILES CC(C)C1CCC(CC1)N1CCC(CC1)N1c2ccccc2CN(CC(N)=O)S1(=O)=O |(-1.46,-9.19,;-.14,-8.41,;1.2,-9.18,;-.16,-6.87,;-1.5,-6.08,;-1.5,-4.56,;-.16,-3.77,;1.17,-4.56,;1.17,-6.08,;-.16,-2.23,;-1.51,-1.52,;-1.54,.05,;-.23,.84,;1.12,.11,;1.15,-1.45,;-.27,2.4,;-1.61,3.1,;-2.92,2.29,;-4.25,3.04,;-4.3,4.6,;-2.99,5.39,;-1.64,4.64,;-.34,5.44,;1,4.71,;2.31,5.51,;3.67,4.77,;4.98,5.58,;3.7,3.22,;1.04,3.18,;2.53,3.59,;1.45,1.68,)| Show InChI InChI=1S/C23H36N4O3S/c1-17(2)18-7-9-20(10-8-18)25-13-11-21(12-14-25)27-22-6-4-3-5-19(22)15-26(16-23(24)28)31(27,29)30/h3-6,17-18,20-21H,7-16H2,1-2H3,(H2,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]nociceptin binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50132644
(4-(4-Chloro-3-trifluoromethyl-phenyl)-1-(3,3-diphe...)Show SMILES OC1(CCN(CCC(c2ccccc2)c2ccccc2)CC1)c1ccc(Cl)c(c1)C(F)(F)F Show InChI InChI=1S/C27H27ClF3NO/c28-25-12-11-22(19-24(25)27(29,30)31)26(33)14-17-32(18-15-26)16-13-23(20-7-3-1-4-8-20)21-9-5-2-6-10-21/h1-12,19,23,33H,13-18H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P.
Curated by ChEMBL
| Assay Description
Binding affinity for recombinant human mu-opioid receptor was determined by using [3H]- diprenophine radioligand |
Bioorg Med Chem Lett 14: 5275-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.032
BindingDB Entry DOI: 10.7270/Q2K073RN |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50132644

(4-(4-Chloro-3-trifluoromethyl-phenyl)-1-(3,3-diphe...)Show SMILES OC1(CCN(CCC(c2ccccc2)c2ccccc2)CC1)c1ccc(Cl)c(c1)C(F)(F)F Show InChI InChI=1S/C27H27ClF3NO/c28-25-12-11-22(19-24(25)27(29,30)31)26(33)14-17-32(18-15-26)16-13-23(20-7-3-1-4-8-20)21-9-5-2-6-10-21/h1-12,19,23,33H,13-18H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P.
Curated by ChEMBL
| Assay Description
Antagonistic activity against opioid receptor mu1 |
Bioorg Med Chem Lett 13: 3247-52 (2003)
BindingDB Entry DOI: 10.7270/Q2K936XQ |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153124
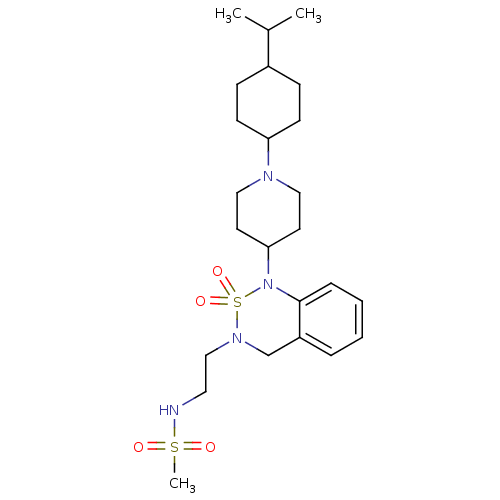
(CHEMBL182967 | N-(2-{1-[1-(4-Isopropyl-cyclohexyl)...)Show SMILES CC(C)C1CCC(CC1)N1CCC(CC1)N1c2ccccc2CN(CCNS(C)(=O)=O)S1(=O)=O |(.52,-9.66,;-.82,-8.88,;-2.15,-9.66,;-.84,-7.34,;.49,-6.56,;.49,-5.02,;-.84,-4.25,;-2.18,-5.02,;-2.18,-6.56,;-.84,-2.69,;-2.2,-1.98,;-2.23,-.41,;-.91,.39,;.44,-.35,;.48,-1.91,;-.94,1.94,;-2.29,2.65,;-3.6,1.84,;-4.94,2.59,;-4.99,4.14,;-3.69,4.93,;-2.32,4.18,;-1.02,4.99,;.34,4.26,;1.65,5.07,;3,4.33,;4.31,5.12,;5.67,4.4,;7.03,3.67,;6.78,5.47,;4.57,3.3,;.37,2.74,;1.86,3.13,;.77,1.24,)| Show InChI InChI=1S/C24H40N4O4S2/c1-19(2)20-8-10-22(11-9-20)26-15-12-23(13-16-26)28-24-7-5-4-6-21(24)18-27(34(28,31)32)17-14-25-33(3,29)30/h4-7,19-20,22-23,25H,8-18H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]nociceptin binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153121
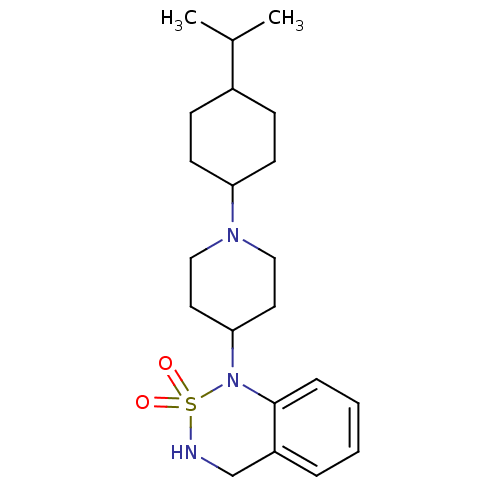
(1-[1-(4-Isopropyl-cyclohexyl)-piperidin-4-yl]-3,4-...)Show SMILES CC(C)C1CCC(CC1)N1CCC(CC1)N1c2ccccc2CNS1(=O)=O |(7.68,-3.52,;7.37,-2.01,;8.54,-.99,;5.92,-1.52,;4.78,-2.53,;3.31,-2.06,;3,-.55,;4.15,.48,;5.61,-.01,;1.55,-.05,;1.24,1.44,;-.23,1.92,;-1.37,.89,;-1.05,-.62,;.41,-1.1,;-2.82,1.37,;-3.81,.18,;-3.27,-1.27,;-4.23,-2.46,;-5.77,-2.21,;-6.31,-.77,;-5.35,.44,;-5.89,1.87,;-4.9,3.06,;-3.37,2.81,;-1.84,2.96,;-3.52,4.37,)| Show InChI InChI=1S/C21H33N3O2S/c1-16(2)17-7-9-19(10-8-17)23-13-11-20(12-14-23)24-21-6-4-3-5-18(21)15-22-27(24,25)26/h3-6,16-17,19-20,22H,7-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]nociceptin binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153132
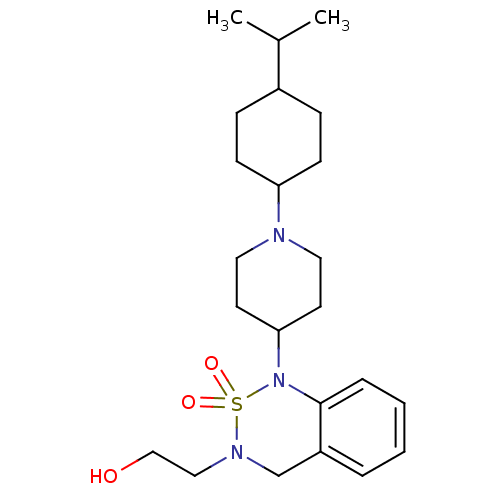
(2-{1-[1-(4-Isopropyl-cyclohexyl)-piperidin-4-yl]-2...)Show SMILES CC(C)C1CCC(CC1)N1CCC(CC1)N1c2ccccc2CN(CCO)S1(=O)=O |(1.33,-9.05,;-.02,-8.29,;-1.35,-9.08,;-.04,-6.75,;-1.38,-5.98,;-1.38,-4.43,;-.04,-3.66,;1.31,-4.43,;1.31,-5.98,;-.04,-2.12,;-1.39,-1.4,;-1.42,.16,;-.11,.96,;1.24,.21,;1.27,-1.33,;-.14,2.49,;-1.49,3.2,;-2.79,2.4,;-4.13,3.14,;-4.17,4.68,;-2.86,5.48,;-1.52,4.74,;-.23,5.53,;1.12,4.82,;2.43,5.6,;3.78,4.87,;5.09,5.69,;1.17,3.28,;2.64,3.69,;1.56,1.8,)| Show InChI InChI=1S/C23H37N3O3S/c1-18(2)19-7-9-21(10-8-19)24-13-11-22(12-14-24)26-23-6-4-3-5-20(23)17-25(15-16-27)30(26,28)29/h3-6,18-19,21-22,27H,7-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]nociceptin binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50132645
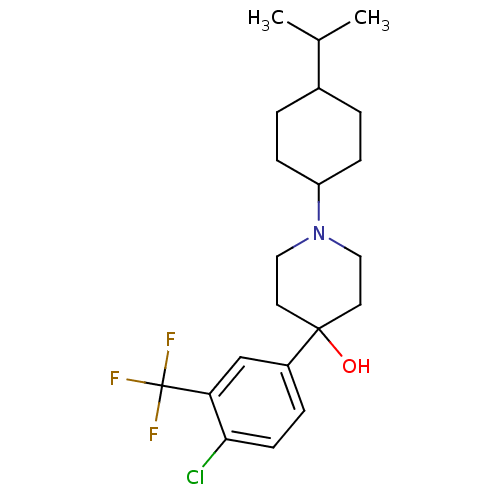
(4-(4-Chloro-3-trifluoromethyl-phenyl)-1-(4-isoprop...)Show SMILES CC(C)C1CCC(CC1)N1CCC(O)(CC1)c1ccc(Cl)c(c1)C(F)(F)F |(16.53,-7.57,;14.99,-7.59,;14.24,-8.94,;14.19,-6.28,;14.94,-4.93,;14.15,-3.6,;12.61,-3.65,;11.86,-4.99,;12.65,-6.3,;11.81,-2.32,;10.27,-2.36,;9.48,-1.03,;10.23,.32,;10.06,1.84,;11.77,.35,;12.56,-.98,;8.73,-.12,;8.35,-1.62,;6.89,-2.03,;5.79,-.96,;4.32,-1.38,;6.16,.53,;7.64,.95,;5.05,1.59,;6.11,2.7,;3.97,.47,;3.93,2.65,)| Show InChI InChI=1S/C21H29ClF3NO/c1-14(2)15-3-6-17(7-4-15)26-11-9-20(27,10-12-26)16-5-8-19(22)18(13-16)21(23,24)25/h5,8,13-15,17,27H,3-4,6-7,9-12H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P.
Curated by ChEMBL
| Assay Description
Antagonistic activity against opioid receptor mu1 |
Bioorg Med Chem Lett 13: 3247-52 (2003)
BindingDB Entry DOI: 10.7270/Q2K936XQ |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50132650

(1-(Decahydro-naphthalen-2-yl)-4-(3-trifluoromethyl...)Show SMILES OC1(CCN(CC1)C1CCC2CCCCC2C1)c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C22H30F3NO/c23-22(24,25)19-7-3-6-18(15-19)21(27)10-12-26(13-11-21)20-9-8-16-4-1-2-5-17(16)14-20/h3,6-7,15-17,20,27H,1-2,4-5,8-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P.
Curated by ChEMBL
| Assay Description
Antagonistic activity against orphan FQ receptor |
Bioorg Med Chem Lett 13: 3247-52 (2003)
BindingDB Entry DOI: 10.7270/Q2K936XQ |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50132639
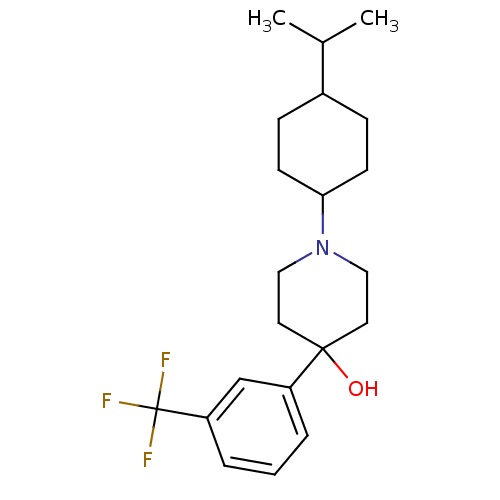
(1-(4-Isopropyl-cyclohexyl)-4-(3-trifluoromethyl-ph...)Show SMILES CC(C)C1CCC(CC1)N1CCC(O)(CC1)c1cccc(c1)C(F)(F)F |(14.24,-8.94,;14.99,-7.59,;16.53,-7.57,;14.19,-6.28,;14.94,-4.93,;14.15,-3.6,;12.61,-3.65,;11.86,-4.99,;12.65,-6.3,;11.81,-2.32,;12.56,-.98,;11.77,.35,;10.23,.32,;10.06,1.84,;9.48,-1.03,;10.27,-2.36,;8.73,-.12,;8.35,-1.62,;6.89,-2.03,;5.79,-.96,;6.16,.53,;7.64,.95,;5.05,1.59,;6.11,2.7,;3.97,.47,;3.93,2.65,)| Show InChI InChI=1S/C21H30F3NO/c1-15(2)16-6-8-19(9-7-16)25-12-10-20(26,11-13-25)17-4-3-5-18(14-17)21(22,23)24/h3-5,14-16,19,26H,6-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P.
Curated by ChEMBL
| Assay Description
Antagonistic activity against orphan FQ receptor |
Bioorg Med Chem Lett 13: 3247-52 (2003)
BindingDB Entry DOI: 10.7270/Q2K936XQ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50153135
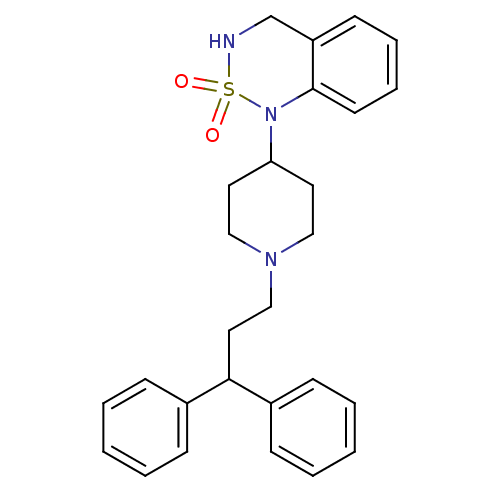
(1-[1-(3,3-Diphenyl-propyl)-piperidin-4-yl]-3,4-dih...)Show SMILES O=S1(=O)NCc2ccccc2N1C1CCN(CCC(c2ccccc2)c2ccccc2)CC1 Show InChI InChI=1S/C27H31N3O2S/c31-33(32)28-21-24-13-7-8-14-27(24)30(33)25-15-18-29(19-16-25)20-17-26(22-9-3-1-4-10-22)23-11-5-2-6-12-23/h1-14,25-26,28H,15-21H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]diprenorphine binding to human Opioid receptor mu 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153137

(3-Butyl-1-[1-(4-isopropyl-cyclohexyl)-piperidin-4-...)Show SMILES CCCCN1Cc2ccccc2N(C2CCN(CC2)C2CCC(CC2)C(C)C)S1(=O)=O |(6.25,4.77,;4.89,5.53,;3.59,4.72,;2.23,5.45,;.92,4.65,;-.44,5.38,;-1.72,4.6,;-3.08,5.33,;-4.39,4.54,;-4.34,2.99,;-3.01,2.24,;-1.7,3.04,;-.34,2.34,;-.32,.8,;-1.63,,;-1.61,-1.57,;-.25,-2.29,;1.06,-1.5,;1.04,.05,;-.25,-3.83,;1.08,-4.61,;1.08,-6.15,;-.25,-6.92,;-1.58,-6.15,;-1.58,-4.61,;-.23,-8.46,;1.13,-9.24,;-1.56,-9.26,;.97,3.13,;2.44,3.55,;1.36,1.64,)| Show InChI InChI=1S/C25H41N3O2S/c1-4-5-16-27-19-22-8-6-7-9-25(22)28(31(27,29)30)24-14-17-26(18-15-24)23-12-10-21(11-13-23)20(2)3/h6-9,20-21,23-24H,4-5,10-19H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]nociceptin binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50132641
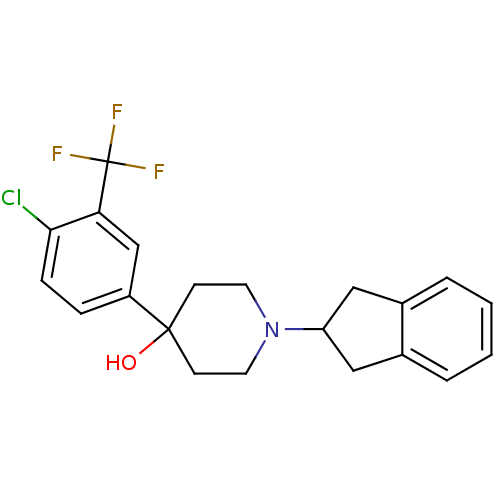
(4-(4-Chloro-3-trifluoromethyl-phenyl)-1-indan-2-yl...)Show SMILES OC1(CCN(CC1)C1Cc2ccccc2C1)c1ccc(Cl)c(c1)C(F)(F)F Show InChI InChI=1S/C21H21ClF3NO/c22-19-6-5-16(13-18(19)21(23,24)25)20(27)7-9-26(10-8-20)17-11-14-3-1-2-4-15(14)12-17/h1-6,13,17,27H,7-12H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P.
Curated by ChEMBL
| Assay Description
Antagonistic activity against opioid receptor mu1 |
Bioorg Med Chem Lett 13: 3247-52 (2003)
BindingDB Entry DOI: 10.7270/Q2K936XQ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50132650

(1-(Decahydro-naphthalen-2-yl)-4-(3-trifluoromethyl...)Show SMILES OC1(CCN(CC1)C1CCC2CCCCC2C1)c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C22H30F3NO/c23-22(24,25)19-7-3-6-18(15-19)21(27)10-12-26(13-11-21)20-9-8-16-4-1-2-5-17(16)14-20/h3,6-7,15-17,20,27H,1-2,4-5,8-14H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P.
Curated by ChEMBL
| Assay Description
Antagonistic activity against opioid receptor mu1 |
Bioorg Med Chem Lett 13: 3247-52 (2003)
BindingDB Entry DOI: 10.7270/Q2K936XQ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50153131
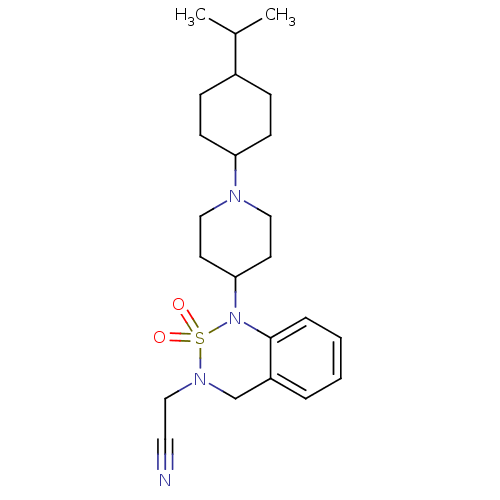
(CHEMBL185415 | {1-[1-(4-Isopropyl-cyclohexyl)-pipe...)Show SMILES CC(C)C1CCC(CC1)N1CCC(CC1)N1c2ccccc2CN(CC#N)S1(=O)=O |(1.34,-9.03,;-.02,-8.25,;-1.35,-9.05,;-.04,-6.71,;-1.37,-5.95,;-1.37,-4.4,;-.04,-3.62,;1.29,-4.4,;1.29,-5.95,;-.04,-2.07,;-1.39,-1.36,;-1.43,.21,;-.11,1.01,;1.25,.27,;1.27,-1.29,;-.14,2.55,;-1.49,3.27,;-2.8,2.46,;-4.13,3.2,;-4.18,4.75,;-2.87,5.54,;-1.51,4.79,;-.23,5.6,;1.13,4.86,;2.44,5.67,;3.8,4.95,;5.14,4.19,;1.17,3.34,;2.65,3.74,;1.57,1.85,)| Show InChI InChI=1S/C23H34N4O2S/c1-18(2)19-7-9-21(10-8-19)25-14-11-22(12-15-25)27-23-6-4-3-5-20(23)17-26(16-13-24)30(27,28)29/h3-6,18-19,21-22H,7-12,14-17H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]diprenorphine binding to human Opioid receptor mu 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153129

(1-[1-(Decahydro-naphthalen-2-yl)-piperidin-4-yl]-3...)Show SMILES O=S1(=O)NCc2ccccc2N1C1CCN(CC1)C1CCC2CCCCC2C1 Show InChI InChI=1S/C22H33N3O2S/c26-28(27)23-16-19-7-3-4-8-22(19)25(28)20-11-13-24(14-12-20)21-10-9-17-5-1-2-6-18(17)15-21/h3-4,7-8,17-18,20-21,23H,1-2,5-6,9-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]nociceptin binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50132645

(4-(4-Chloro-3-trifluoromethyl-phenyl)-1-(4-isoprop...)Show SMILES CC(C)C1CCC(CC1)N1CCC(O)(CC1)c1ccc(Cl)c(c1)C(F)(F)F |(16.53,-7.57,;14.99,-7.59,;14.24,-8.94,;14.19,-6.28,;14.94,-4.93,;14.15,-3.6,;12.61,-3.65,;11.86,-4.99,;12.65,-6.3,;11.81,-2.32,;10.27,-2.36,;9.48,-1.03,;10.23,.32,;10.06,1.84,;11.77,.35,;12.56,-.98,;8.73,-.12,;8.35,-1.62,;6.89,-2.03,;5.79,-.96,;4.32,-1.38,;6.16,.53,;7.64,.95,;5.05,1.59,;6.11,2.7,;3.97,.47,;3.93,2.65,)| Show InChI InChI=1S/C21H29ClF3NO/c1-14(2)15-3-6-17(7-4-15)26-11-9-20(27,10-12-26)16-5-8-19(22)18(13-16)21(23,24)25/h5,8,13-15,17,27H,3-4,6-7,9-12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P.
Curated by ChEMBL
| Assay Description
Antagonistic activity against orphan FQ receptor |
Bioorg Med Chem Lett 13: 3247-52 (2003)
BindingDB Entry DOI: 10.7270/Q2K936XQ |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153131

(CHEMBL185415 | {1-[1-(4-Isopropyl-cyclohexyl)-pipe...)Show SMILES CC(C)C1CCC(CC1)N1CCC(CC1)N1c2ccccc2CN(CC#N)S1(=O)=O |(1.34,-9.03,;-.02,-8.25,;-1.35,-9.05,;-.04,-6.71,;-1.37,-5.95,;-1.37,-4.4,;-.04,-3.62,;1.29,-4.4,;1.29,-5.95,;-.04,-2.07,;-1.39,-1.36,;-1.43,.21,;-.11,1.01,;1.25,.27,;1.27,-1.29,;-.14,2.55,;-1.49,3.27,;-2.8,2.46,;-4.13,3.2,;-4.18,4.75,;-2.87,5.54,;-1.51,4.79,;-.23,5.6,;1.13,4.86,;2.44,5.67,;3.8,4.95,;5.14,4.19,;1.17,3.34,;2.65,3.74,;1.57,1.85,)| Show InChI InChI=1S/C23H34N4O2S/c1-18(2)19-7-9-21(10-8-19)25-14-11-22(12-15-25)27-23-6-4-3-5-20(23)17-26(16-13-24)30(27,28)29/h3-6,18-19,21-22H,7-12,14-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]nociceptin binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50132639

(1-(4-Isopropyl-cyclohexyl)-4-(3-trifluoromethyl-ph...)Show SMILES CC(C)C1CCC(CC1)N1CCC(O)(CC1)c1cccc(c1)C(F)(F)F |(14.24,-8.94,;14.99,-7.59,;16.53,-7.57,;14.19,-6.28,;14.94,-4.93,;14.15,-3.6,;12.61,-3.65,;11.86,-4.99,;12.65,-6.3,;11.81,-2.32,;12.56,-.98,;11.77,.35,;10.23,.32,;10.06,1.84,;9.48,-1.03,;10.27,-2.36,;8.73,-.12,;8.35,-1.62,;6.89,-2.03,;5.79,-.96,;6.16,.53,;7.64,.95,;5.05,1.59,;6.11,2.7,;3.97,.47,;3.93,2.65,)| Show InChI InChI=1S/C21H30F3NO/c1-15(2)16-6-8-19(9-7-16)25-12-10-20(26,11-13-25)17-4-3-5-18(14-17)21(22,23)24/h3-5,14-16,19,26H,6-13H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P.
Curated by ChEMBL
| Assay Description
Antagonistic activity against opioid receptor mu1 |
Bioorg Med Chem Lett 13: 3247-52 (2003)
BindingDB Entry DOI: 10.7270/Q2K936XQ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM21015

((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N(C)[C@@H](Cc1ccccc1)C(=O)NCCO Show InChI InChI=1S/C26H35N5O6/c1-17(30-25(36)21(27)14-19-8-10-20(33)11-9-19)24(35)29-16-23(34)31(2)22(26(37)28-12-13-32)15-18-6-4-3-5-7-18/h3-11,17,21-22,32-33H,12-16,27H2,1-2H3,(H,28,37)(H,29,35)(H,30,36)/t17-,21+,22+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]diprenorphine binding to human Opioid receptor mu 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50153615
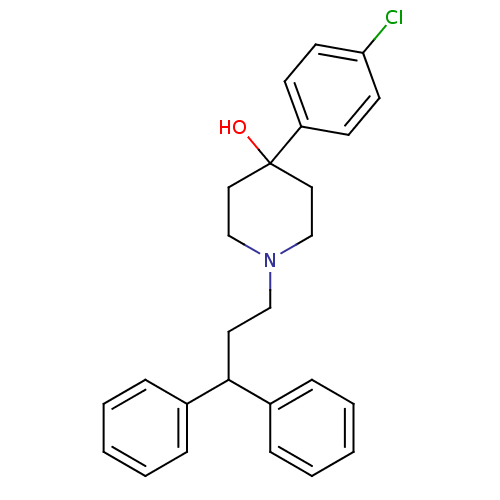
(4-(4-Chloro-phenyl)-1-(3,3-diphenyl-propyl)-piperi...)Show SMILES OC1(CCN(CCC(c2ccccc2)c2ccccc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C26H28ClNO/c27-24-13-11-23(12-14-24)26(29)16-19-28(20-17-26)18-15-25(21-7-3-1-4-8-21)22-9-5-2-6-10-22/h1-14,25,29H,15-20H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P.
Curated by ChEMBL
| Assay Description
Binding affinity for recombinant human mu-opioid receptor was determined by using [3H]- diprenophine radioligand |
Bioorg Med Chem Lett 14: 5275-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.032
BindingDB Entry DOI: 10.7270/Q2K073RN |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50153137

(3-Butyl-1-[1-(4-isopropyl-cyclohexyl)-piperidin-4-...)Show SMILES CCCCN1Cc2ccccc2N(C2CCN(CC2)C2CCC(CC2)C(C)C)S1(=O)=O |(6.25,4.77,;4.89,5.53,;3.59,4.72,;2.23,5.45,;.92,4.65,;-.44,5.38,;-1.72,4.6,;-3.08,5.33,;-4.39,4.54,;-4.34,2.99,;-3.01,2.24,;-1.7,3.04,;-.34,2.34,;-.32,.8,;-1.63,,;-1.61,-1.57,;-.25,-2.29,;1.06,-1.5,;1.04,.05,;-.25,-3.83,;1.08,-4.61,;1.08,-6.15,;-.25,-6.92,;-1.58,-6.15,;-1.58,-4.61,;-.23,-8.46,;1.13,-9.24,;-1.56,-9.26,;.97,3.13,;2.44,3.55,;1.36,1.64,)| Show InChI InChI=1S/C25H41N3O2S/c1-4-5-16-27-19-22-8-6-7-9-25(22)28(31(27,29)30)24-14-17-26(18-15-24)23-12-10-21(11-13-23)20(2)3/h6-9,20-21,23-24H,4-5,10-19H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]diprenorphine binding to human Opioid receptor mu 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153123
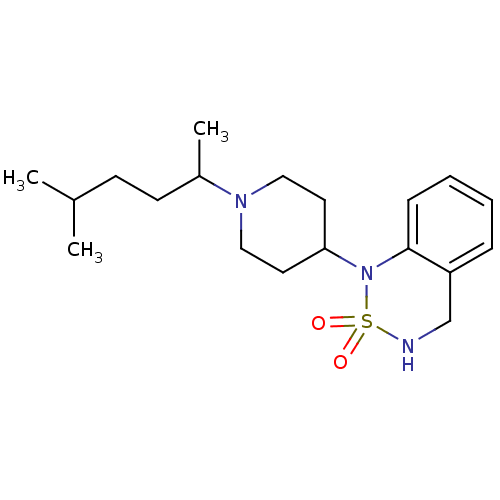
(1-[1-(1,4-Dimethyl-pentyl)-piperidin-4-yl]-3,4-dih...)Show InChI InChI=1S/C19H31N3O2S/c1-15(2)8-9-16(3)21-12-10-18(11-13-21)22-19-7-5-4-6-17(19)14-20-25(22,23)24/h4-7,15-16,18,20H,8-14H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Agonist activity as stimulation of [35S]-GTP-gamma binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153125
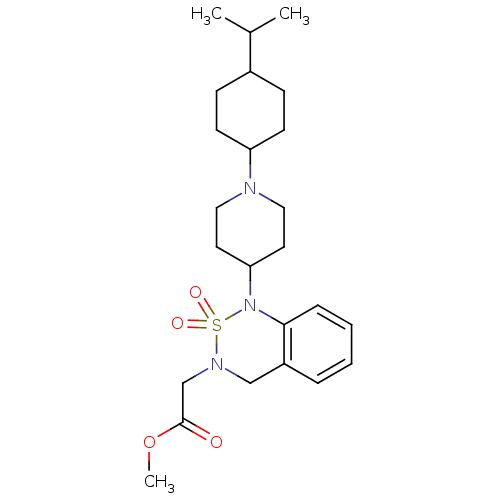
(CHEMBL364844 | {1-[1-(4-Isopropyl-cyclohexyl)-pipe...)Show SMILES COC(=O)CN1Cc2ccccc2N(C2CCN(CC2)C2CCC(CC2)C(C)C)S1(=O)=O |(6.13,4.69,;4.78,5.41,;3.47,4.62,;3.51,3.08,;2.11,5.35,;.8,4.55,;-.55,5.3,;-1.84,4.48,;-3.19,5.23,;-4.5,4.43,;-4.46,2.89,;-3.12,2.14,;-1.82,2.94,;-.46,2.24,;-.44,.69,;-1.74,-.1,;-1.72,-1.67,;-.37,-2.38,;.94,-1.6,;.92,-.05,;-.37,-3.93,;-1.7,-4.71,;-1.7,-6.25,;-.37,-7.02,;.97,-6.25,;.97,-4.71,;-.34,-8.56,;-1.67,-9.35,;1.01,-9.33,;.85,3.03,;2.32,3.43,;1.25,1.53,)| Show InChI InChI=1S/C24H37N3O4S/c1-18(2)19-8-10-21(11-9-19)25-14-12-22(13-15-25)27-23-7-5-4-6-20(23)16-26(32(27,29)30)17-24(28)31-3/h4-7,18-19,21-22H,8-17H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Agonist activity as stimulation of [35S]-GTP-gamma binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153141
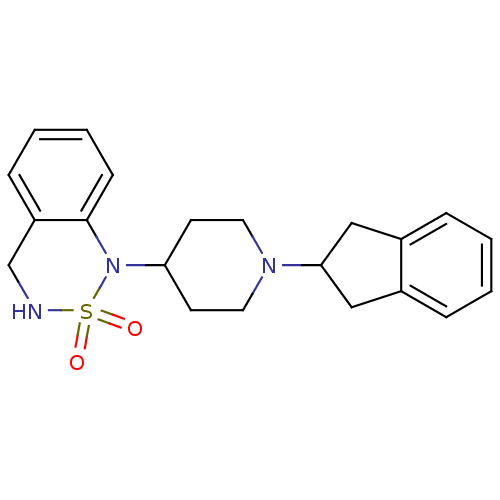
(1-(1-Indan-2-yl-piperidin-4-yl)-3,4-dihydro-1H-ben...)Show SMILES O=S1(=O)NCc2ccccc2N1C1CCN(CC1)C1Cc2ccccc2C1 Show InChI InChI=1S/C21H25N3O2S/c25-27(26)22-15-18-7-3-4-8-21(18)24(27)19-9-11-23(12-10-19)20-13-16-5-1-2-6-17(16)14-20/h1-8,19-20,22H,9-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]nociceptin binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50132642

(1-(4-Propyl-cyclohexyl)-4-(3-trifluoromethyl-pheny...)Show SMILES CCCC1CCC(CC1)N1CCC(O)(CC1)c1cccc(c1)C(F)(F)F |(12.47,-8.29,;11.67,-6.98,;12.42,-5.62,;11.63,-4.3,;12.37,-2.95,;11.58,-1.63,;10.06,-1.66,;9.31,-3.02,;10.09,-4.32,;9.26,-.35,;10.01,1,;9.2,2.33,;7.66,2.29,;7.5,3.83,;6.91,.95,;7.72,-.38,;6.17,1.86,;5.79,.37,;4.32,-.05,;3.23,1.02,;3.6,2.5,;5.09,2.92,;2.48,3.56,;1.38,4.62,;3.55,4.68,;1.43,2.44,)| Show InChI InChI=1S/C21H30F3NO/c1-2-4-16-7-9-19(10-8-16)25-13-11-20(26,12-14-25)17-5-3-6-18(15-17)21(22,23)24/h3,5-6,15-16,19,26H,2,4,7-14H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P.
Curated by ChEMBL
| Assay Description
Antagonistic activity against opioid receptor mu1 |
Bioorg Med Chem Lett 13: 3247-52 (2003)
BindingDB Entry DOI: 10.7270/Q2K936XQ |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50132643
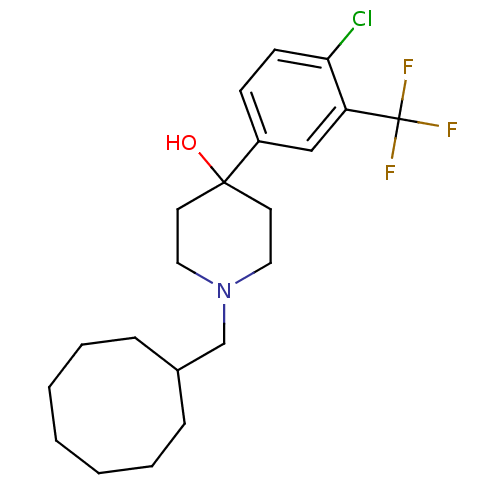
(4-(4-Chloro-3-trifluoromethyl-phenyl)-1-cyclooctyl...)Show SMILES OC1(CCN(CC2CCCCCCC2)CC1)c1ccc(Cl)c(c1)C(F)(F)F Show InChI InChI=1S/C21H29ClF3NO/c22-19-9-8-17(14-18(19)21(23,24)25)20(27)10-12-26(13-11-20)15-16-6-4-2-1-3-5-7-16/h8-9,14,16,27H,1-7,10-13,15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P.
Curated by ChEMBL
| Assay Description
Antagonistic activity against orphan FQ receptor |
Bioorg Med Chem Lett 13: 3247-52 (2003)
BindingDB Entry DOI: 10.7270/Q2K936XQ |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153122
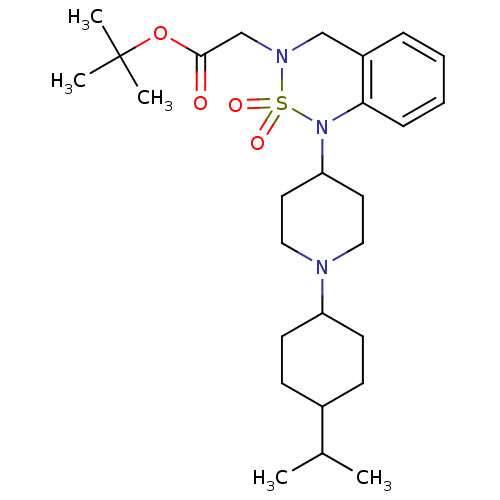
(CHEMBL181545 | {1-[1-(4-Isopropyl-cyclohexyl)-pipe...)Show SMILES CC(C)C1CCC(CC1)N1CCC(CC1)N1c2ccccc2CN(CC(=O)OC(C)(C)C)S1(=O)=O |(-2.24,-9.73,;-.9,-8.94,;.44,-9.71,;-.93,-7.4,;-2.26,-6.62,;-2.26,-5.08,;-.93,-4.31,;.41,-5.08,;.41,-6.62,;-.94,-2.77,;-2.28,-2.04,;-2.31,-.48,;-1,.32,;.36,-.43,;.38,-1.97,;-1.02,1.86,;-2.38,2.56,;-3.69,1.77,;-5.02,2.52,;-5.07,4.06,;-3.76,4.86,;-2.4,4.11,;-1.11,4.9,;.24,4.18,;1.55,4.98,;2.91,4.25,;2.95,2.7,;4.22,5.04,;5.57,4.32,;4.85,2.96,;6.91,3.55,;6.34,5.65,;.29,2.66,;1.76,3.06,;.68,1.16,)| Show InChI InChI=1S/C27H43N3O4S/c1-20(2)21-10-12-23(13-11-21)28-16-14-24(15-17-28)30-25-9-7-6-8-22(25)18-29(35(30,32)33)19-26(31)34-27(3,4)5/h6-9,20-21,23-24H,10-19H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]nociceptin binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50153123

(1-[1-(1,4-Dimethyl-pentyl)-piperidin-4-yl]-3,4-dih...)Show InChI InChI=1S/C19H31N3O2S/c1-15(2)8-9-16(3)21-12-10-18(11-13-21)22-19-7-5-4-6-17(19)14-20-25(22,23)24/h4-7,15-16,18,20H,8-14H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]diprenorphine binding to human Opioid receptor mu 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153608

(4-[4-Hydroxy-4-(3-trifluoromethyl-phenyl)-piperidi...)Show SMILES COC(=O)C(CCN1CCC(O)(CC1)c1cccc(c1)C(F)(F)F)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C29H30F3NO3/c1-36-26(34)28(22-9-4-2-5-10-22,23-11-6-3-7-12-23)17-20-33-18-15-27(35,16-19-33)24-13-8-14-25(21-24)29(30,31)32/h2-14,21,35H,15-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P.
Curated by ChEMBL
| Assay Description
Binding affinity for opioid receptor like 1 expressed in HEK-293 cells |
Bioorg Med Chem Lett 14: 5275-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.032
BindingDB Entry DOI: 10.7270/Q2K073RN |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50132641

(4-(4-Chloro-3-trifluoromethyl-phenyl)-1-indan-2-yl...)Show SMILES OC1(CCN(CC1)C1Cc2ccccc2C1)c1ccc(Cl)c(c1)C(F)(F)F Show InChI InChI=1S/C21H21ClF3NO/c22-19-6-5-16(13-18(19)21(23,24)25)20(27)7-9-26(10-8-20)17-11-14-3-1-2-4-15(14)12-17/h1-6,13,17,27H,7-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P.
Curated by ChEMBL
| Assay Description
Antagonistic activity against orphan FQ receptor |
Bioorg Med Chem Lett 13: 3247-52 (2003)
BindingDB Entry DOI: 10.7270/Q2K936XQ |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153135

(1-[1-(3,3-Diphenyl-propyl)-piperidin-4-yl]-3,4-dih...)Show SMILES O=S1(=O)NCc2ccccc2N1C1CCN(CCC(c2ccccc2)c2ccccc2)CC1 Show InChI InChI=1S/C27H31N3O2S/c31-33(32)28-21-24-13-7-8-14-27(24)30(33)25-15-18-29(19-16-25)20-17-26(22-9-3-1-4-10-22)23-11-5-2-6-12-23/h1-14,25-26,28H,15-21H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]nociceptin binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50153132

(2-{1-[1-(4-Isopropyl-cyclohexyl)-piperidin-4-yl]-2...)Show SMILES CC(C)C1CCC(CC1)N1CCC(CC1)N1c2ccccc2CN(CCO)S1(=O)=O |(1.33,-9.05,;-.02,-8.29,;-1.35,-9.08,;-.04,-6.75,;-1.38,-5.98,;-1.38,-4.43,;-.04,-3.66,;1.31,-4.43,;1.31,-5.98,;-.04,-2.12,;-1.39,-1.4,;-1.42,.16,;-.11,.96,;1.24,.21,;1.27,-1.33,;-.14,2.49,;-1.49,3.2,;-2.79,2.4,;-4.13,3.14,;-4.17,4.68,;-2.86,5.48,;-1.52,4.74,;-.23,5.53,;1.12,4.82,;2.43,5.6,;3.78,4.87,;5.09,5.69,;1.17,3.28,;2.64,3.69,;1.56,1.8,)| Show InChI InChI=1S/C23H37N3O3S/c1-18(2)19-7-9-21(10-8-19)24-13-11-22(12-14-24)26-23-6-4-3-5-20(23)17-25(15-16-27)30(26,28)29/h3-6,18-19,21-22,27H,7-17H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]diprenorphine binding to human Opioid receptor mu 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50153129

(1-[1-(Decahydro-naphthalen-2-yl)-piperidin-4-yl]-3...)Show SMILES O=S1(=O)NCc2ccccc2N1C1CCN(CC1)C1CCC2CCCCC2C1 Show InChI InChI=1S/C22H33N3O2S/c26-28(27)23-16-19-7-3-4-8-22(19)25(28)20-11-13-24(14-12-20)21-10-9-17-5-1-2-6-18(17)15-21/h3-4,7-8,17-18,20-21,23H,1-2,5-6,9-16H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]diprenorphine binding to human Opioid receptor mu 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50153127

(2-{1-[1-(4-Isopropyl-cyclohexyl)-piperidin-4-yl]-2...)Show SMILES CC(C)C1CCC(CC1)N1CCC(CC1)N1c2ccccc2CN(CC(N)=O)S1(=O)=O |(-1.46,-9.19,;-.14,-8.41,;1.2,-9.18,;-.16,-6.87,;-1.5,-6.08,;-1.5,-4.56,;-.16,-3.77,;1.17,-4.56,;1.17,-6.08,;-.16,-2.23,;-1.51,-1.52,;-1.54,.05,;-.23,.84,;1.12,.11,;1.15,-1.45,;-.27,2.4,;-1.61,3.1,;-2.92,2.29,;-4.25,3.04,;-4.3,4.6,;-2.99,5.39,;-1.64,4.64,;-.34,5.44,;1,4.71,;2.31,5.51,;3.67,4.77,;4.98,5.58,;3.7,3.22,;1.04,3.18,;2.53,3.59,;1.45,1.68,)| Show InChI InChI=1S/C23H36N4O3S/c1-17(2)18-7-9-20(10-8-18)25-13-11-21(12-14-25)27-22-6-4-3-5-19(22)15-26(16-23(24)28)31(27,29)30/h3-6,17-18,20-21H,7-16H2,1-2H3,(H2,24,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]diprenorphine binding to human Opioid receptor mu 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50132652

(1-Cyclooctylmethyl-4-(3-trifluoromethyl-phenyl)-pi...)Show InChI InChI=1S/C21H30F3NO/c22-21(23,24)19-10-6-9-18(15-19)20(26)11-13-25(14-12-20)16-17-7-4-2-1-3-5-8-17/h6,9-10,15,17,26H,1-5,7-8,11-14,16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P.
Curated by ChEMBL
| Assay Description
Antagonistic activity against orphan FQ receptor |
Bioorg Med Chem Lett 13: 3247-52 (2003)
BindingDB Entry DOI: 10.7270/Q2K936XQ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50132651
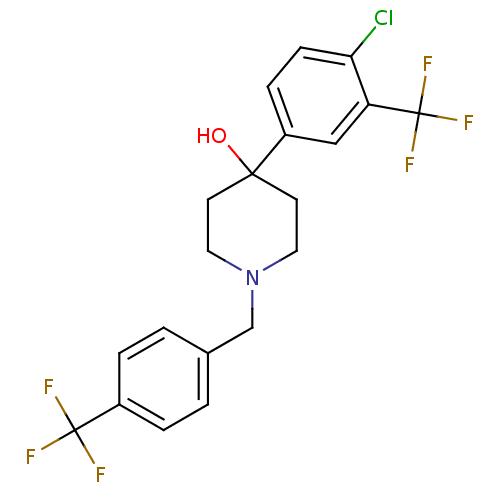
(4-(4-Chloro-3-trifluoromethyl-phenyl)-1-(4-trifluo...)Show SMILES OC1(CCN(Cc2ccc(cc2)C(F)(F)F)CC1)c1ccc(Cl)c(c1)C(F)(F)F Show InChI InChI=1S/C20H18ClF6NO/c21-17-6-5-15(11-16(17)20(25,26)27)18(29)7-9-28(10-8-18)12-13-1-3-14(4-2-13)19(22,23)24/h1-6,11,29H,7-10,12H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 104 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P.
Curated by ChEMBL
| Assay Description
Binding affinity for recombinant human mu-opioid receptor was determined by using [3H]- diprenophine radioligand |
Bioorg Med Chem Lett 14: 5275-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.032
BindingDB Entry DOI: 10.7270/Q2K073RN |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50153124

(CHEMBL182967 | N-(2-{1-[1-(4-Isopropyl-cyclohexyl)...)Show SMILES CC(C)C1CCC(CC1)N1CCC(CC1)N1c2ccccc2CN(CCNS(C)(=O)=O)S1(=O)=O |(.52,-9.66,;-.82,-8.88,;-2.15,-9.66,;-.84,-7.34,;.49,-6.56,;.49,-5.02,;-.84,-4.25,;-2.18,-5.02,;-2.18,-6.56,;-.84,-2.69,;-2.2,-1.98,;-2.23,-.41,;-.91,.39,;.44,-.35,;.48,-1.91,;-.94,1.94,;-2.29,2.65,;-3.6,1.84,;-4.94,2.59,;-4.99,4.14,;-3.69,4.93,;-2.32,4.18,;-1.02,4.99,;.34,4.26,;1.65,5.07,;3,4.33,;4.31,5.12,;5.67,4.4,;7.03,3.67,;6.78,5.47,;4.57,3.3,;.37,2.74,;1.86,3.13,;.77,1.24,)| Show InChI InChI=1S/C24H40N4O4S2/c1-19(2)20-8-10-22(11-9-20)26-15-12-23(13-16-26)28-24-7-5-4-6-21(24)18-27(34(28,31)32)17-14-25-33(3,29)30/h4-7,19-20,22-23,25H,8-18H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]diprenorphine binding to human Opioid receptor mu 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50132652

(1-Cyclooctylmethyl-4-(3-trifluoromethyl-phenyl)-pi...)Show InChI InChI=1S/C21H30F3NO/c22-21(23,24)19-10-6-9-18(15-19)20(26)11-13-25(14-12-20)16-17-7-4-2-1-3-5-8-17/h6,9-10,15,17,26H,1-5,7-8,11-14,16H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 113 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P.
Curated by ChEMBL
| Assay Description
Antagonistic activity against opioid receptor mu1 |
Bioorg Med Chem Lett 13: 3247-52 (2003)
BindingDB Entry DOI: 10.7270/Q2K936XQ |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50132647

(1-(3,3-Diphenyl-propyl)-4-(3-trifluoromethyl-pheny...)Show SMILES OC1(CCN(CCC(c2ccccc2)c2ccccc2)CC1)c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C27H28F3NO/c28-27(29,30)24-13-7-12-23(20-24)26(32)15-18-31(19-16-26)17-14-25(21-8-3-1-4-9-21)22-10-5-2-6-11-22/h1-13,20,25,32H,14-19H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 116 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P.
Curated by ChEMBL
| Assay Description
Antagonistic activity against orphan FQ receptor |
Bioorg Med Chem Lett 13: 3247-52 (2003)
BindingDB Entry DOI: 10.7270/Q2K936XQ |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50132647

(1-(3,3-Diphenyl-propyl)-4-(3-trifluoromethyl-pheny...)Show SMILES OC1(CCN(CCC(c2ccccc2)c2ccccc2)CC1)c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C27H28F3NO/c28-27(29,30)24-13-7-12-23(20-24)26(32)15-18-31(19-16-26)17-14-25(21-8-3-1-4-9-21)22-10-5-2-6-11-22/h1-13,20,25,32H,14-19H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 116 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P.
Curated by ChEMBL
| Assay Description
Binding affinity for opioid receptor like 1 expressed in HEK-293 cells |
Bioorg Med Chem Lett 14: 5275-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.032
BindingDB Entry DOI: 10.7270/Q2K073RN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data