 Found 344 hits with Last Name = 'miranda' and Initial = 'k'
Found 344 hits with Last Name = 'miranda' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 11B1, mitochondrial
(Rattus norvegicus) | BDBM50444550

(CHEMBL3099704)Show SMILES Fc1ccc(cc1)-c1cc(ccc1[C@H]1CCCCc2cncn12)C#N |r| Show InChI InChI=1S/C21H18FN3/c22-17-8-6-16(7-9-17)20-11-15(12-23)5-10-19(20)21-4-2-1-3-18-13-24-14-25(18)21/h5-11,13-14,21H,1-4H2/t21-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant CYP11B1 using 11-deoxycortisol as substrate by cell-based assay |
ACS Med Chem Lett 4: 1203-7 (2013)
Article DOI: 10.1021/ml400324c
BindingDB Entry DOI: 10.7270/Q2NZ8938 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50444550

(CHEMBL3099704)Show SMILES Fc1ccc(cc1)-c1cc(ccc1[C@H]1CCCCc2cncn12)C#N |r| Show InChI InChI=1S/C21H18FN3/c22-17-8-6-16(7-9-17)20-11-15(12-23)5-10-19(20)21-4-2-1-3-18-13-24-14-25(18)21/h5-11,13-14,21H,1-4H2/t21-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B1 using 11-deoxycortisol as substrate by cell-based assay |
ACS Med Chem Lett 4: 1203-7 (2013)
Article DOI: 10.1021/ml400324c
BindingDB Entry DOI: 10.7270/Q2NZ8938 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324297

(4-(1-Piperazin-1-yl[2,6]naphthyridin-3-yl)pyridin-...)Show SMILES C1CN(CCN1)c1nc(cc2cnccc12)-c1ccnc(NC2CCOCC2)c1 Show InChI InChI=1S/C22H26N6O/c1-6-25-21(26-18-3-11-29-12-4-18)14-16(1)20-13-17-15-24-5-2-19(17)22(27-20)28-9-7-23-8-10-28/h1-2,5-6,13-15,18,23H,3-4,7-12H2,(H,25,26) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 by TR-FRET assay |
J Med Chem 53: 5400-21 (2010)
Article DOI: 10.1021/jm100075z
BindingDB Entry DOI: 10.7270/Q2T153T9 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50500363

(CHEMBL3747481)Show SMILES FC(F)(F)c1cccc(NC(=O)n2ccc3cc(Oc4ncnc5CNCc45)ccc23)c1 Show InChI InChI=1S/C22H16F3N5O2/c23-22(24,25)14-2-1-3-15(9-14)29-21(31)30-7-6-13-8-16(4-5-19(13)30)32-20-17-10-26-11-18(17)27-12-28-20/h1-9,12,26H,10-11H2,(H,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Tel-fused KDR (unknown origin) expressed in mouse BaF3 cells using poly(Glu,Tyr) as substrate after 10 mins by scintillation counting a... |
J Med Chem 58: 9273-86 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01227
BindingDB Entry DOI: 10.7270/Q26M39T9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50444550

(CHEMBL3099704)Show SMILES Fc1ccc(cc1)-c1cc(ccc1[C@H]1CCCCc2cncn12)C#N |r| Show InChI InChI=1S/C21H18FN3/c22-17-8-6-16(7-9-17)20-11-15(12-23)5-10-19(20)21-4-2-1-3-18-13-24-14-25(18)21/h5-11,13-14,21H,1-4H2/t21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B2 using 11-deoxycorticosterone as substrate by cell-based assay |
ACS Med Chem Lett 4: 1203-7 (2013)
Article DOI: 10.1021/ml400324c
BindingDB Entry DOI: 10.7270/Q2NZ8938 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50500371

(CHEMBL3746941)Show SMILES CC1(CC1)c1cc(NC(=O)n2ccc3cc(Oc4ncnc5CNCc45)ccc23)n[nH]1 Show InChI InChI=1S/C22H21N7O2/c1-22(5-6-22)18-9-19(28-27-18)26-21(30)29-7-4-13-8-14(2-3-17(13)29)31-20-15-10-23-11-16(15)24-12-25-20/h2-4,7-9,12,23H,5-6,10-11H2,1H3,(H2,26,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Tel-fused KDR (unknown origin) expressed in mouse BaF3 cells using poly(Glu,Tyr) as substrate after 10 mins by scintillation counting a... |
J Med Chem 58: 9273-86 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01227
BindingDB Entry DOI: 10.7270/Q26M39T9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324315

(CHEMBL1214998 | Cyclohexyl-[4-(1-piperazin-1-yl[2,...)Show SMILES C1CCC(CC1)Nc1cc(ccn1)-c1cc2cnccc2c(n1)N1CCNCC1 Show InChI InChI=1S/C23H28N6/c1-2-4-19(5-3-1)27-22-15-17(6-9-26-22)21-14-18-16-25-8-7-20(18)23(28-21)29-12-10-24-11-13-29/h6-9,14-16,19,24H,1-5,10-13H2,(H,26,27) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 by TR-FRET assay |
J Med Chem 53: 5400-21 (2010)
Article DOI: 10.1021/jm100075z
BindingDB Entry DOI: 10.7270/Q2T153T9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324315

(CHEMBL1214998 | Cyclohexyl-[4-(1-piperazin-1-yl[2,...)Show SMILES C1CCC(CC1)Nc1cc(ccn1)-c1cc2cnccc2c(n1)N1CCNCC1 Show InChI InChI=1S/C23H28N6/c1-2-4-19(5-3-1)27-22-15-17(6-9-26-22)21-14-18-16-25-8-7-20(18)23(28-21)29-12-10-24-11-13-29/h6-9,14-16,19,24H,1-5,10-13H2,(H,26,27) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 by TR-FRET assay |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50444549

(CHEMBL3099695)Show InChI InChI=1S/C13H10FN3/c14-12-5-9(6-15)1-3-11(12)13-4-2-10-7-16-8-17(10)13/h1,3,5,7-8,13H,2,4H2/t13-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B2 using 11-deoxycorticosterone as substrate by cell-based assay |
ACS Med Chem Lett 4: 1203-7 (2013)
Article DOI: 10.1021/ml400324c
BindingDB Entry DOI: 10.7270/Q2NZ8938 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50270431
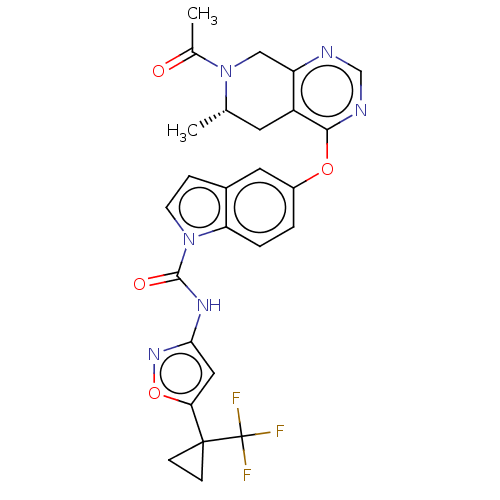
(CHEMBL4076909)Show SMILES C[C@H]1Cc2c(CN1C(C)=O)ncnc2Oc1ccc2n(ccc2c1)C(=O)Nc1cc(on1)C1(CC1)C(F)(F)F |r| Show InChI InChI=1S/C26H23F3N6O4/c1-14-9-18-19(12-35(14)15(2)36)30-13-31-23(18)38-17-3-4-20-16(10-17)5-8-34(20)24(37)32-22-11-21(39-33-22)25(6-7-25)26(27,28)29/h3-5,8,10-11,13-14H,6-7,9,12H2,1-2H3,(H,32,33,37)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of KDR (unknown origin) expressed in mouse Ba/F3 cells |
J Med Chem 61: 1622-1635 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01731
BindingDB Entry DOI: 10.7270/Q22F7QZM |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50500370
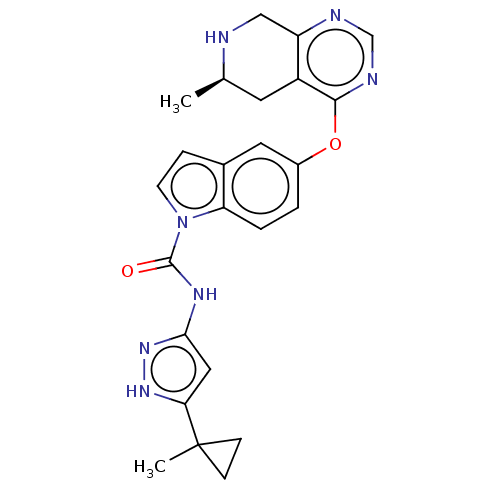
(CHEMBL3746273)Show SMILES C[C@@H]1Cc2c(CN1)ncnc2Oc1ccc2n(ccc2c1)C(=O)Nc1cc([nH]n1)C1(C)CC1 |r| Show InChI InChI=1S/C24H25N7O2/c1-14-9-17-18(12-25-14)26-13-27-22(17)33-16-3-4-19-15(10-16)5-8-31(19)23(32)28-21-11-20(29-30-21)24(2)6-7-24/h3-5,8,10-11,13-14,25H,6-7,9,12H2,1-2H3,(H2,28,29,30,32)/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Tel-fused KDR (unknown origin) expressed in mouse BaF3 cells using poly(Glu,Tyr) as substrate after 10 mins by scintillation counting a... |
J Med Chem 58: 9273-86 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01227
BindingDB Entry DOI: 10.7270/Q26M39T9 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50270424

(CHEMBL3746776)Show SMILES C[C@H]1Cc2c(CN1)ncnc2Oc1ccc2n(ccc2c1)C(=O)Nc1cc([nH]n1)C1(C)CC1 |r| Show InChI InChI=1S/C24H25N7O2/c1-14-9-17-18(12-25-14)26-13-27-22(17)33-16-3-4-19-15(10-16)5-8-31(19)23(32)28-21-11-20(29-30-21)24(2)6-7-24/h3-5,8,10-11,13-14,25H,6-7,9,12H2,1-2H3,(H2,28,29,30,32)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Tel-fused KDR (unknown origin) expressed in mouse BaF3 cells using poly(Glu,Tyr) as substrate after 10 mins by scintillation counting a... |
J Med Chem 58: 9273-86 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01227
BindingDB Entry DOI: 10.7270/Q26M39T9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50444548
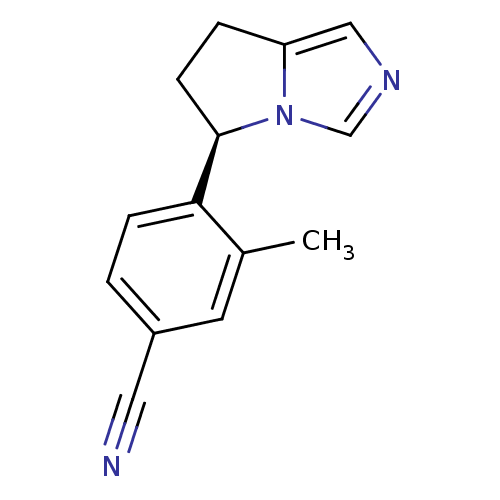
(CHEMBL3099696)Show InChI InChI=1S/C14H13N3/c1-10-6-11(7-15)2-4-13(10)14-5-3-12-8-16-9-17(12)14/h2,4,6,8-9,14H,3,5H2,1H3/t14-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B2 using 11-deoxycorticosterone as substrate by cell-based assay |
ACS Med Chem Lett 4: 1203-7 (2013)
Article DOI: 10.1021/ml400324c
BindingDB Entry DOI: 10.7270/Q2NZ8938 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50270424

(CHEMBL3746776)Show SMILES C[C@H]1Cc2c(CN1)ncnc2Oc1ccc2n(ccc2c1)C(=O)Nc1cc([nH]n1)C1(C)CC1 |r| Show InChI InChI=1S/C24H25N7O2/c1-14-9-17-18(12-25-14)26-13-27-22(17)33-16-3-4-19-15(10-16)5-8-31(19)23(32)28-21-11-20(29-30-21)24(2)6-7-24/h3-5,8,10-11,13-14,25H,6-7,9,12H2,1-2H3,(H2,28,29,30,32)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of KDR (unknown origin) expressed in mouse Ba/F3 cells |
J Med Chem 61: 1622-1635 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01731
BindingDB Entry DOI: 10.7270/Q22F7QZM |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324305

(CHEMBL1214711 | N-(2-(pyrrolidin-1-yl)ethyl)-1-(3-...)Show SMILES O=C(NC1CCNCC1)C1CCN(CC1)c1nc(cc2cnccc12)-c1ccnc(NC2CCCCC2)c1 Show InChI InChI=1S/C30H39N7O/c38-30(35-25-7-12-31-13-8-25)21-10-16-37(17-11-21)29-26-9-14-32-20-23(26)18-27(36-29)22-6-15-33-28(19-22)34-24-4-2-1-3-5-24/h6,9,14-15,18-21,24-25,31H,1-5,7-8,10-13,16-17H2,(H,33,34)(H,35,38) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 by TR-FRET assay |
J Med Chem 53: 5400-21 (2010)
Article DOI: 10.1021/jm100075z
BindingDB Entry DOI: 10.7270/Q2T153T9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324346

(CHEMBL1215151 | Cyclohexyl-[6-piperazin-1-yl-4-(1H...)Show SMILES C1CCC(CC1)Nc1cc(ccn1)-c1cc(cc(n1)N1CCNCC1)-c1cn[nH]c1 Show InChI InChI=1S/C23H29N7/c1-2-4-20(5-3-1)28-22-13-17(6-7-25-22)21-12-18(19-15-26-27-16-19)14-23(29-21)30-10-8-24-9-11-30/h6-7,12-16,20,24H,1-5,8-11H2,(H,25,28)(H,26,27) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 by TR-FRET assay |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324347
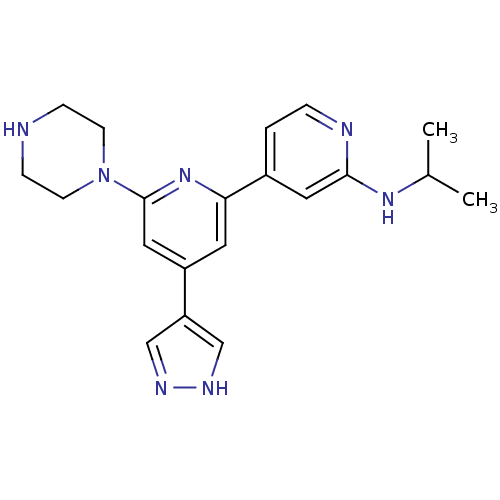
(CHEMBL1215153 | Isopropyl-[6-piperazin-1-yl-4-(1H-...)Show SMILES CC(C)Nc1cc(ccn1)-c1cc(cc(n1)N1CCNCC1)-c1cn[nH]c1 Show InChI InChI=1S/C20H25N7/c1-14(2)25-19-10-15(3-4-22-19)18-9-16(17-12-23-24-13-17)11-20(26-18)27-7-5-21-6-8-27/h3-4,9-14,21H,5-8H2,1-2H3,(H,22,25)(H,23,24) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 by TR-FRET assay |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324348
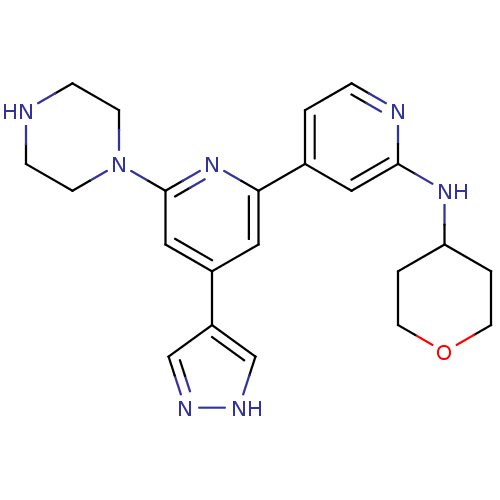
(6-(piperazin-1-yl)-4-(4H-pyrazol-4-yl)-N-(tetrahyd...)Show SMILES C1CN(CCN1)c1cc(cc(n1)-c1ccnc(NC2CCOCC2)c1)-c1cn[nH]c1 Show InChI InChI=1S/C22H27N7O/c1-4-24-21(27-19-2-9-30-10-3-19)12-16(1)20-11-17(18-14-25-26-15-18)13-22(28-20)29-7-5-23-6-8-29/h1,4,11-15,19,23H,2-3,5-10H2,(H,24,27)(H,25,26) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 by TR-FRET assay |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324323
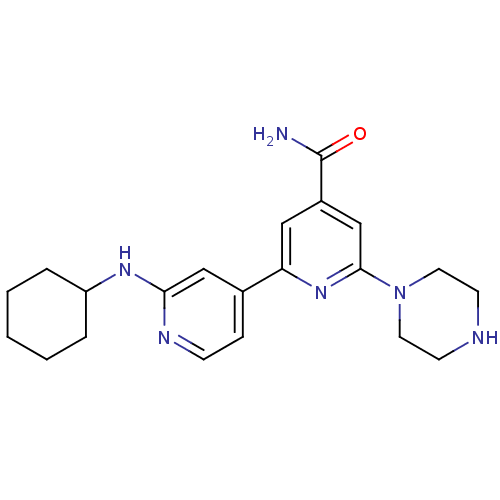
(2'-Cyclohexylamino-6-piperazin-1-yl[2,4']bipyridin...)Show SMILES NC(=O)c1cc(nc(c1)-c1ccnc(NC2CCCCC2)c1)N1CCNCC1 Show InChI InChI=1S/C21H28N6O/c22-21(28)16-12-18(26-20(14-16)27-10-8-23-9-11-27)15-6-7-24-19(13-15)25-17-4-2-1-3-5-17/h6-7,12-14,17,23H,1-5,8-11H2,(H2,22,28)(H,24,25) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 by TR-FRET assay |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324328
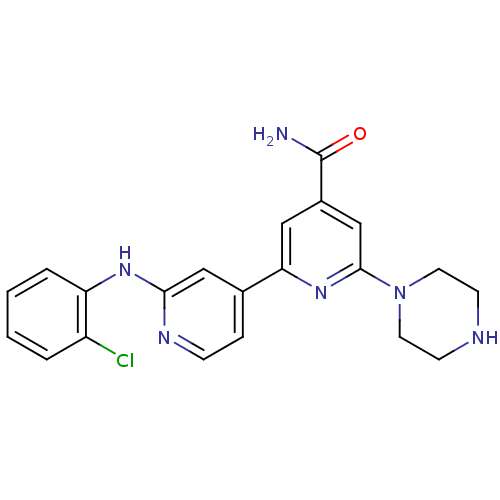
(2'-(2-Chlorophenylamino)-6-piperazin-1-yl[2,4']bip...)Show SMILES NC(=O)c1cc(nc(c1)-c1ccnc(Nc2ccccc2Cl)c1)N1CCNCC1 Show InChI InChI=1S/C21H21ClN6O/c22-16-3-1-2-4-17(16)26-19-12-14(5-6-25-19)18-11-15(21(23)29)13-20(27-18)28-9-7-24-8-10-28/h1-6,11-13,24H,7-10H2,(H2,23,29)(H,25,26) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 by TR-FRET assay |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50500377

(CHEMBL3747084)Show SMILES CC(C)(C)c1cc(NC(=O)n2ccc3cc(Oc4ncnc5CNCCc45)ccc23)n[nH]1 Show InChI InChI=1S/C23H25N7O2/c1-23(2,3)19-11-20(29-28-19)27-22(31)30-9-7-14-10-15(4-5-18(14)30)32-21-16-6-8-24-12-17(16)25-13-26-21/h4-5,7,9-11,13,24H,6,8,12H2,1-3H3,(H2,27,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Tel-fused KDR (unknown origin) expressed in mouse BaF3 cells using poly(Glu,Tyr) as substrate after 10 mins by scintillation counting a... |
J Med Chem 58: 9273-86 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01227
BindingDB Entry DOI: 10.7270/Q26M39T9 |
More data for this
Ligand-Target Pair | |
Polycystin-2
(Homo sapiens (Human)) | BDBM50324324

(2'-Phenylamino-6-piperazin-1-yl[2,4']bipyridinyl-4...)Show SMILES NC(=O)c1cc(nc(c1)-c1ccnc(Nc2ccccc2)c1)N1CCNCC1 Show InChI InChI=1S/C21H22N6O/c22-21(28)16-12-18(26-20(14-16)27-10-8-23-9-11-27)15-6-7-24-19(13-15)25-17-4-2-1-3-5-17/h1-7,12-14,23H,8-11H2,(H2,22,28)(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD2 by TR-FRET assay |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
Polycystin-2
(Homo sapiens (Human)) | BDBM50324346

(CHEMBL1215151 | Cyclohexyl-[6-piperazin-1-yl-4-(1H...)Show SMILES C1CCC(CC1)Nc1cc(ccn1)-c1cc(cc(n1)N1CCNCC1)-c1cn[nH]c1 Show InChI InChI=1S/C23H29N7/c1-2-4-20(5-3-1)28-22-13-17(6-7-25-22)21-12-18(19-15-26-27-16-19)14-23(29-21)30-10-8-24-9-11-30/h6-7,12-16,20,24H,1-5,8-11H2,(H,25,28)(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD2 by TR-FRET assay |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Rattus norvegicus) | BDBM50444550

(CHEMBL3099704)Show SMILES Fc1ccc(cc1)-c1cc(ccc1[C@H]1CCCCc2cncn12)C#N |r| Show InChI InChI=1S/C21H18FN3/c22-17-8-6-16(7-9-17)20-11-15(12-23)5-10-19(20)21-4-2-1-3-18-13-24-14-25(18)21/h5-11,13-14,21H,1-4H2/t21-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant CYP11B2 using 11-deoxycorticosterone as substrate by cell-based assay |
ACS Med Chem Lett 4: 1203-7 (2013)
Article DOI: 10.1021/ml400324c
BindingDB Entry DOI: 10.7270/Q2NZ8938 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50444550

(CHEMBL3099704)Show SMILES Fc1ccc(cc1)-c1cc(ccc1[C@H]1CCCCc2cncn12)C#N |r| Show InChI InChI=1S/C21H18FN3/c22-17-8-6-16(7-9-17)20-11-15(12-23)5-10-19(20)21-4-2-1-3-18-13-24-14-25(18)21/h5-11,13-14,21H,1-4H2/t21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP11B2 in human NCI-H295R cells assessed as inhibition of angiotensin-2-induced aldosterone production after 24 hrs by RIA |
ACS Med Chem Lett 4: 1203-7 (2013)
Article DOI: 10.1021/ml400324c
BindingDB Entry DOI: 10.7270/Q2NZ8938 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324306
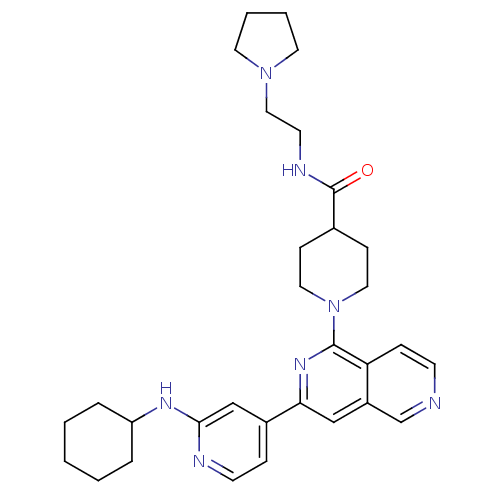
(1-[3-(2-Cyclohexylaminopyridin-4-yl)[2,6]naphthyri...)Show SMILES O=C(NCCN1CCCC1)C1CCN(CC1)c1nc(cc2cnccc12)-c1ccnc(NC2CCCCC2)c1 Show InChI InChI=1S/C31H41N7O/c39-31(34-14-19-37-15-4-5-16-37)23-10-17-38(18-11-23)30-27-9-12-32-22-25(27)20-28(36-30)24-8-13-33-29(21-24)35-26-6-2-1-3-7-26/h8-9,12-13,20-23,26H,1-7,10-11,14-19H2,(H,33,35)(H,34,39) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 by TR-FRET assay |
J Med Chem 53: 5400-21 (2010)
Article DOI: 10.1021/jm100075z
BindingDB Entry DOI: 10.7270/Q2T153T9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324322
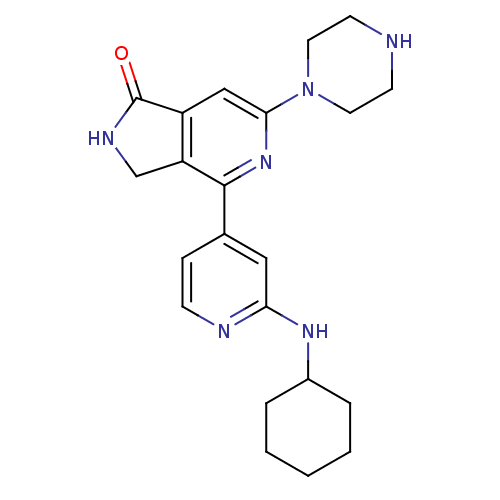
(4-(2-cyclohexylaminopyridin-4-yl)-6-(piperazin-1-y...)Show SMILES O=C1NCc2c1cc(nc2-c1ccnc(NC2CCCCC2)c1)N1CCNCC1 Show InChI InChI=1S/C22H28N6O/c29-22-17-13-20(28-10-8-23-9-11-28)27-21(18(17)14-25-22)15-6-7-24-19(12-15)26-16-4-2-1-3-5-16/h6-7,12-13,16,23H,1-5,8-11,14H2,(H,24,26)(H,25,29) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 by TR-FRET assay |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324296
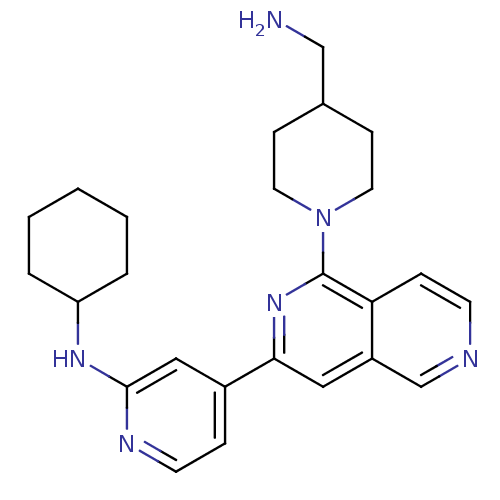
(1-[3-(2-Cyclohexylaminopyridin-4-yl)[2,6]naphthyri...)Show SMILES NCC1CCN(CC1)c1nc(cc2cnccc12)-c1ccnc(NC2CCCCC2)c1 Show InChI InChI=1S/C25H32N6/c26-16-18-8-12-31(13-9-18)25-22-7-10-27-17-20(22)14-23(30-25)19-6-11-28-24(15-19)29-21-4-2-1-3-5-21/h6-7,10-11,14-15,17-18,21H,1-5,8-9,12-13,16,26H2,(H,28,29) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 by TR-FRET assay |
J Med Chem 53: 5400-21 (2010)
Article DOI: 10.1021/jm100075z
BindingDB Entry DOI: 10.7270/Q2T153T9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324298
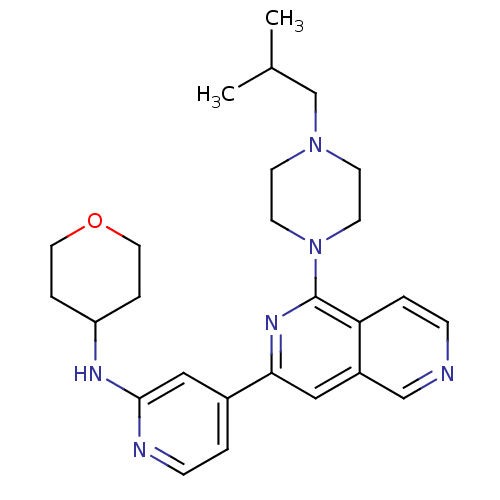
(CHEMBL1215712 | {4-[1-(4-Isobutylpiperazin-1-yl)[2...)Show SMILES CC(C)CN1CCN(CC1)c1nc(cc2cnccc12)-c1ccnc(NC2CCOCC2)c1 Show InChI InChI=1S/C26H34N6O/c1-19(2)18-31-9-11-32(12-10-31)26-23-4-7-27-17-21(23)15-24(30-26)20-3-8-28-25(16-20)29-22-5-13-33-14-6-22/h3-4,7-8,15-17,19,22H,5-6,9-14,18H2,1-2H3,(H,28,29) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 by TR-FRET assay |
J Med Chem 53: 5400-21 (2010)
Article DOI: 10.1021/jm100075z
BindingDB Entry DOI: 10.7270/Q2T153T9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324314
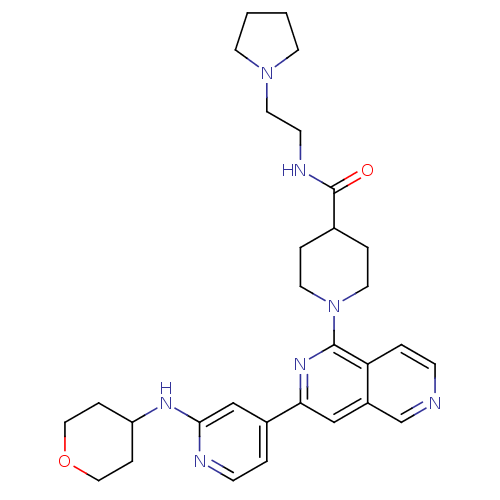
(1-{3-[2-(Tetrahydropyran-4-ylamino)pyridin-4-yl][2...)Show SMILES O=C(NCCN1CCCC1)C1CCN(CC1)c1nc(cc2cnccc12)-c1ccnc(NC2CCOCC2)c1 Show InChI InChI=1S/C30H39N7O2/c38-30(33-11-16-36-12-1-2-13-36)22-5-14-37(15-6-22)29-26-4-9-31-21-24(26)19-27(35-29)23-3-10-32-28(20-23)34-25-7-17-39-18-8-25/h3-4,9-10,19-22,25H,1-2,5-8,11-18H2,(H,32,34)(H,33,38) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 by TR-FRET assay |
J Med Chem 53: 5400-21 (2010)
Article DOI: 10.1021/jm100075z
BindingDB Entry DOI: 10.7270/Q2T153T9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324325
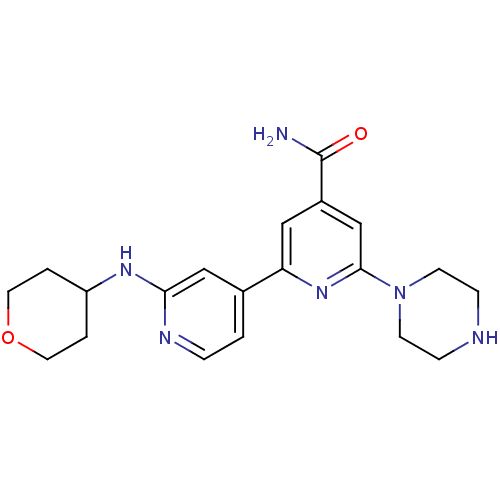
(6-Piperazin-1-yl-2'-(tetrahydropyran-4-ylamino)[2,...)Show SMILES NC(=O)c1cc(nc(c1)-c1ccnc(NC2CCOCC2)c1)N1CCNCC1 Show InChI InChI=1S/C20H26N6O2/c21-20(27)15-11-17(25-19(13-15)26-7-5-22-6-8-26)14-1-4-23-18(12-14)24-16-2-9-28-10-3-16/h1,4,11-13,16,22H,2-3,5-10H2,(H2,21,27)(H,23,24) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 by TR-FRET assay |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324326
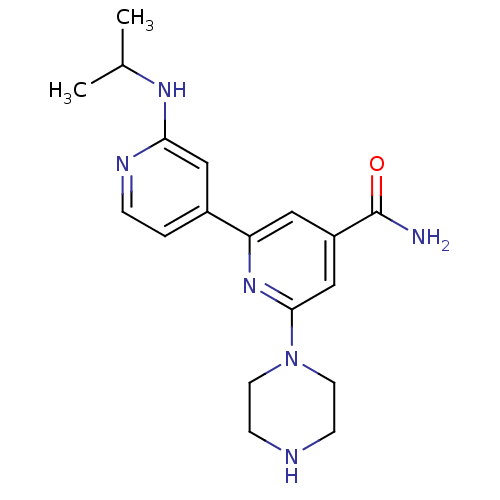
(4'-tert-Butylcarbamoyl-2''-isopropylamino-3,4,5,6-...)Show SMILES CC(C)Nc1cc(ccn1)-c1cc(cc(n1)N1CCNCC1)C(N)=O Show InChI InChI=1S/C18H24N6O/c1-12(2)22-16-10-13(3-4-21-16)15-9-14(18(19)25)11-17(23-15)24-7-5-20-6-8-24/h3-4,9-12,20H,5-8H2,1-2H3,(H2,19,25)(H,21,22) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 by TR-FRET assay |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324324

(2'-Phenylamino-6-piperazin-1-yl[2,4']bipyridinyl-4...)Show SMILES NC(=O)c1cc(nc(c1)-c1ccnc(Nc2ccccc2)c1)N1CCNCC1 Show InChI InChI=1S/C21H22N6O/c22-21(28)16-12-18(26-20(14-16)27-10-8-23-9-11-27)15-6-7-24-19(13-15)25-17-4-2-1-3-5-17/h1-7,12-14,23H,8-11H2,(H2,22,28)(H,24,25) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 by TR-FRET assay |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324327
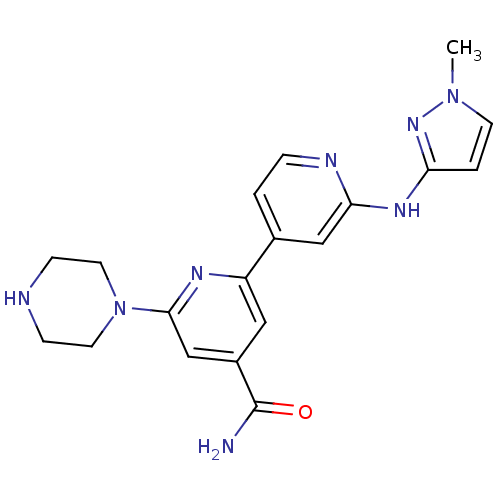
(2'-(1-Methyl-1H-pyrazol-3-ylamino)-6-piperazin-1-y...)Show SMILES Cn1ccc(Nc2cc(ccn2)-c2cc(cc(n2)N2CCNCC2)C(N)=O)n1 Show InChI InChI=1S/C19H22N8O/c1-26-7-3-16(25-26)24-17-11-13(2-4-22-17)15-10-14(19(20)28)12-18(23-15)27-8-5-21-6-9-27/h2-4,7,10-12,21H,5-6,8-9H2,1H3,(H2,20,28)(H,22,24,25) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 by TR-FRET assay |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50270430
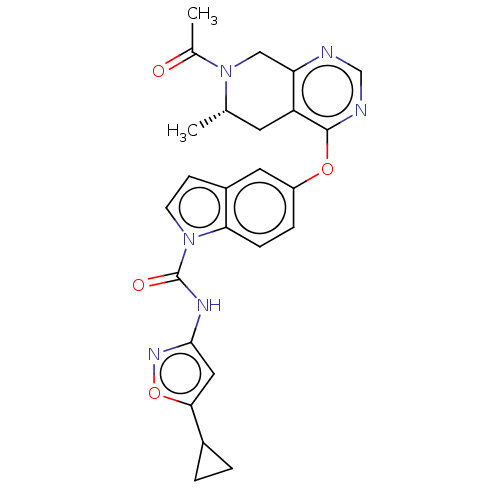
(CHEMBL4077373)Show SMILES C[C@H]1Cc2c(CN1C(C)=O)ncnc2Oc1ccc2n(ccc2c1)C(=O)Nc1cc(on1)C1CC1 |r| Show InChI InChI=1S/C25H24N6O4/c1-14-9-19-20(12-31(14)15(2)32)26-13-27-24(19)34-18-5-6-21-17(10-18)7-8-30(21)25(33)28-23-11-22(35-29-23)16-3-4-16/h5-8,10-11,13-14,16H,3-4,9,12H2,1-2H3,(H,28,29,33)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of KDR (unknown origin) expressed in mouse Ba/F3 cells |
J Med Chem 61: 1622-1635 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01731
BindingDB Entry DOI: 10.7270/Q22F7QZM |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50270425

(CHEMBL4080062)Show SMILES OCc1cc(Oc2ccc3c(cccc3c2)C(=O)Nc2cccc(c2)C(F)(F)F)ncn1 Show InChI InChI=1S/C23H16F3N3O3/c24-23(25,26)15-4-2-5-16(10-15)29-22(31)20-6-1-3-14-9-18(7-8-19(14)20)32-21-11-17(12-30)27-13-28-21/h1-11,13,30H,12H2,(H,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of KDR (unknown origin) expressed in mouse Ba/F3 cells |
J Med Chem 61: 1622-1635 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01731
BindingDB Entry DOI: 10.7270/Q22F7QZM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50444552

(CHEMBL3099689)Show SMILES Fc1ccc(COC(=O)[C@@]2(CCc3cncn23)c2ccc(cc2Cl)C#N)cc1 |r| Show InChI InChI=1S/C21H15ClFN3O2/c22-19-9-15(10-24)3-6-18(19)21(8-7-17-11-25-13-26(17)21)20(27)28-12-14-1-4-16(23)5-2-14/h1-6,9,11,13H,7-8,12H2/t21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP11B2 in human NCI-H295R cells assessed as inhibition of angiotensin-2-induced aldosterone production after 24 hrs by RIA |
ACS Med Chem Lett 4: 1203-7 (2013)
Article DOI: 10.1021/ml400324c
BindingDB Entry DOI: 10.7270/Q2NZ8938 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50444548

(CHEMBL3099696)Show InChI InChI=1S/C14H13N3/c1-10-6-11(7-15)2-4-13(10)14-5-3-12-8-16-9-17(12)14/h2,4,6,8-9,14H,3,5H2,1H3/t14-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B1 using 11-deoxycortisol as substrate by cell-based assay |
ACS Med Chem Lett 4: 1203-7 (2013)
Article DOI: 10.1021/ml400324c
BindingDB Entry DOI: 10.7270/Q2NZ8938 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50270439

(CHEMBL4104297)Show SMILES Nc1nccc(Oc2ccc3c(cccc3c2)C(=O)Nc2cccc(c2)C(F)(F)F)n1 Show InChI InChI=1S/C22H15F3N4O2/c23-22(24,25)14-4-2-5-15(12-14)28-20(30)18-6-1-3-13-11-16(7-8-17(13)18)31-19-9-10-27-21(26)29-19/h1-12H,(H,28,30)(H2,26,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of KDR (unknown origin) expressed in mouse Ba/F3 cells |
J Med Chem 61: 1622-1635 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01731
BindingDB Entry DOI: 10.7270/Q22F7QZM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50047262
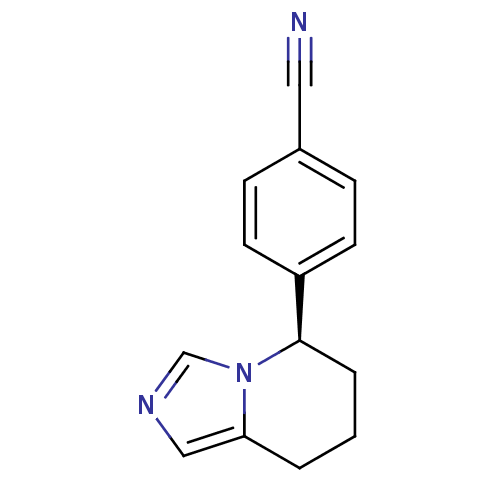
((R)-4-(5,6,7,8-tetrahydroimidazo[1,5-a]pyridin-5-y...)Show InChI InChI=1S/C14H13N3/c15-8-11-4-6-12(7-5-11)14-3-1-2-13-9-16-10-17(13)14/h4-7,9-10,14H,1-3H2/t14-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B2 using 11-deoxycorticosterone as substrate by cell-based assay |
ACS Med Chem Lett 4: 1203-7 (2013)
Article DOI: 10.1021/ml400324c
BindingDB Entry DOI: 10.7270/Q2NZ8938 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324312

(1-{3-[2-(Tetrahydropyran-4-ylamino)pyridin-4-yl][2...)Show SMILES CC(C)CNC(=O)C1CCN(CC1)c1nc(cc2cnccc12)-c1ccnc(NC2CCOCC2)c1 Show InChI InChI=1S/C28H36N6O2/c1-19(2)17-31-28(35)20-5-11-34(12-6-20)27-24-4-9-29-18-22(24)15-25(33-27)21-3-10-30-26(16-21)32-23-7-13-36-14-8-23/h3-4,9-10,15-16,18-20,23H,5-8,11-14,17H2,1-2H3,(H,30,32)(H,31,35) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 by TR-FRET assay |
J Med Chem 53: 5400-21 (2010)
Article DOI: 10.1021/jm100075z
BindingDB Entry DOI: 10.7270/Q2T153T9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324335
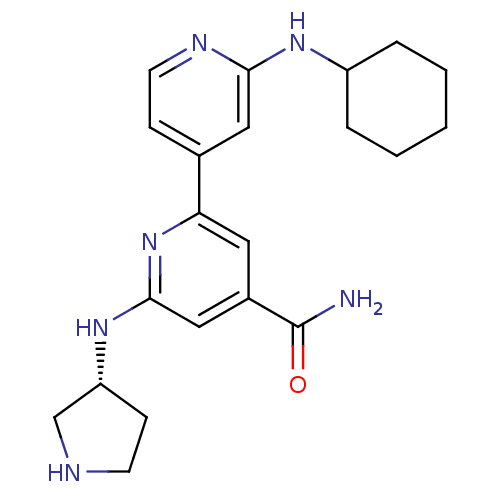
(2'-Cyclohexylamino-6-((R)-pyrrolidin-3-ylamino)[2,...)Show SMILES NC(=O)c1cc(N[C@@H]2CCNC2)nc(c1)-c1ccnc(NC2CCCCC2)c1 |r| Show InChI InChI=1S/C21H28N6O/c22-21(28)15-10-18(27-20(12-15)26-17-7-8-23-13-17)14-6-9-24-19(11-14)25-16-4-2-1-3-5-16/h6,9-12,16-17,23H,1-5,7-8,13H2,(H2,22,28)(H,24,25)(H,26,27)/t17-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 by TR-FRET assay |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50270433

(CHEMBL4086718)Show SMILES CNCc1cc(Oc2ccc3n(ccc3c2)C(=O)Nc2cc([nH]n2)C2(C)CC2)ncn1 Show InChI InChI=1S/C22H23N7O2/c1-22(6-7-22)18-11-19(28-27-18)26-21(30)29-8-5-14-9-16(3-4-17(14)29)31-20-10-15(12-23-2)24-13-25-20/h3-5,8-11,13,23H,6-7,12H2,1-2H3,(H2,26,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of KDR (unknown origin) expressed in mouse Ba/F3 cells |
J Med Chem 61: 1622-1635 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01731
BindingDB Entry DOI: 10.7270/Q22F7QZM |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50270438
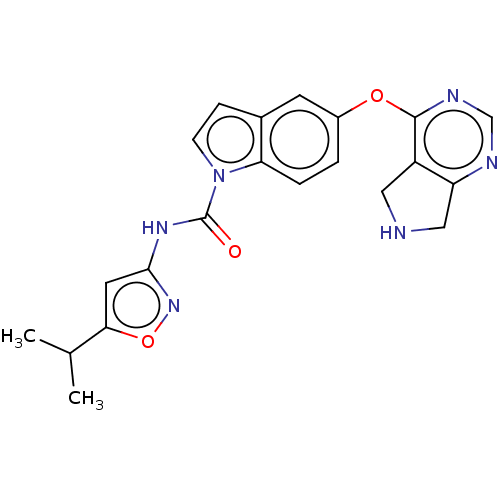
(CHEMBL3747273)Show SMILES CC(C)c1cc(NC(=O)n2ccc3cc(Oc4ncnc5CNCc45)ccc23)no1 Show InChI InChI=1S/C21H20N6O3/c1-12(2)18-8-19(26-30-18)25-21(28)27-6-5-13-7-14(3-4-17(13)27)29-20-15-9-22-10-16(15)23-11-24-20/h3-8,11-12,22H,9-10H2,1-2H3,(H,25,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Tel-fused KDR (unknown origin) expressed in mouse BaF3 cells using poly(Glu,Tyr) as substrate after 10 mins by scintillation counting a... |
J Med Chem 58: 9273-86 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01227
BindingDB Entry DOI: 10.7270/Q26M39T9 |
More data for this
Ligand-Target Pair | |
Polycystin-2
(Homo sapiens (Human)) | BDBM50324347

(CHEMBL1215153 | Isopropyl-[6-piperazin-1-yl-4-(1H-...)Show SMILES CC(C)Nc1cc(ccn1)-c1cc(cc(n1)N1CCNCC1)-c1cn[nH]c1 Show InChI InChI=1S/C20H25N7/c1-14(2)25-19-10-15(3-4-22-19)18-9-16(17-12-23-24-13-17)11-20(26-18)27-7-5-21-6-8-27/h3-4,9-14,21H,5-8H2,1-2H3,(H,22,25)(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD2 by TR-FRET assay |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
Polycystin-2
(Homo sapiens (Human)) | BDBM50324348

(6-(piperazin-1-yl)-4-(4H-pyrazol-4-yl)-N-(tetrahyd...)Show SMILES C1CN(CCN1)c1cc(cc(n1)-c1ccnc(NC2CCOCC2)c1)-c1cn[nH]c1 Show InChI InChI=1S/C22H27N7O/c1-4-24-21(27-19-2-9-30-10-3-19)12-16(1)20-11-17(18-14-25-26-15-18)13-22(28-20)29-7-5-23-6-8-29/h1,4,11-15,19,23H,2-3,5-10H2,(H,24,27)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD2 by TR-FRET assay |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324309

(1-{3-[2-(Tetrahydropyran-4-ylamino)pyridin-4-yl][2...)Show SMILES NC(=O)C1CCN(CC1)c1nc(cc2cnccc12)-c1ccnc(NC2CCOCC2)c1 Show InChI InChI=1S/C24H28N6O2/c25-23(31)16-3-9-30(10-4-16)24-20-2-7-26-15-18(20)13-21(29-24)17-1-8-27-22(14-17)28-19-5-11-32-12-6-19/h1-2,7-8,13-16,19H,3-6,9-12H2,(H2,25,31)(H,27,28) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 by TR-FRET assay |
J Med Chem 53: 5400-21 (2010)
Article DOI: 10.1021/jm100075z
BindingDB Entry DOI: 10.7270/Q2T153T9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50444564

(CHEMBL3099682)Show InChI InChI=1S/C20H20N2/c1-2-8-16(9-3-1)18-11-5-6-12-19(18)20-13-7-4-10-17-14-21-15-22(17)20/h1-3,5-6,8-9,11-12,14-15,20H,4,7,10,13H2/t20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP11B2 in human NCI-H295R cells assessed as inhibition of angiotensin-2-induced aldosterone production after 24 hrs by RIA |
ACS Med Chem Lett 4: 1203-7 (2013)
Article DOI: 10.1021/ml400324c
BindingDB Entry DOI: 10.7270/Q2NZ8938 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50444565

(CHEMBL3099604)Show InChI InChI=1S/C20H19FN2/c21-16-11-9-15(10-12-16)18-6-2-3-7-19(18)20-8-4-1-5-17-13-22-14-23(17)20/h2-3,6-7,9-14,20H,1,4-5,8H2/t20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP11B2 in human NCI-H295R cells assessed as inhibition of angiotensin-2-induced aldosterone production after 24 hrs by RIA |
ACS Med Chem Lett 4: 1203-7 (2013)
Article DOI: 10.1021/ml400324c
BindingDB Entry DOI: 10.7270/Q2NZ8938 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324294

(CHEMBL1215643 | Cyclohexyl-{4-[1-(4-cyclopropylmet...)Show SMILES C(C1CC1)N1CCN(CC1)c1nc(cc2cnccc12)-c1ccnc(NC2CCCCC2)c1 Show InChI InChI=1S/C27H34N6/c1-2-4-23(5-3-1)30-26-17-21(8-11-29-26)25-16-22-18-28-10-9-24(22)27(31-25)33-14-12-32(13-15-33)19-20-6-7-20/h8-11,16-18,20,23H,1-7,12-15,19H2,(H,29,30) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 by TR-FRET assay |
J Med Chem 53: 5400-21 (2010)
Article DOI: 10.1021/jm100075z
BindingDB Entry DOI: 10.7270/Q2T153T9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data