 Found 273 hits with Last Name = 'moore' and Initial = 'db'
Found 273 hits with Last Name = 'moore' and Initial = 'db' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50267028

(CHEMBL4073525)Show SMILES Cc1cc(OCc2ccccc2)ccc1Oc1ccc(cc1)N1C[C@H](C[C@H]1CC(O)=O)C(F)(F)F |r| Show InChI InChI=1S/C27H26F3NO4/c1-18-13-24(34-17-19-5-3-2-4-6-19)11-12-25(18)35-23-9-7-21(8-10-23)31-16-20(27(28,29)30)14-22(31)15-26(32)33/h2-13,20,22H,14-17H2,1H3,(H,32,33)/t20-,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR40 expressed in CHO-A12 cells assessed as increase in intracellular calcium flux measured for 100 secs by FLIPR assay |
J Med Chem 60: 1417-1431 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01559
BindingDB Entry DOI: 10.7270/Q25X2CD5 |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50267031

(CHEMBL4079930)Show SMILES Cc1c(Cl)cccc1Oc1ccc(cc1)N1C[C@H](C[C@H]1CC(O)=O)C(F)(F)F |r| Show InChI InChI=1S/C20H19ClF3NO3/c1-12-17(21)3-2-4-18(12)28-16-7-5-14(6-8-16)25-11-13(20(22,23)24)9-15(25)10-19(26)27/h2-8,13,15H,9-11H2,1H3,(H,26,27)/t13-,15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR40 expressed in CHO-A12 cells assessed as increase in intracellular calcium flux measured for 100 secs by FLIPR assay |
J Med Chem 60: 1417-1431 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01559
BindingDB Entry DOI: 10.7270/Q25X2CD5 |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50267009
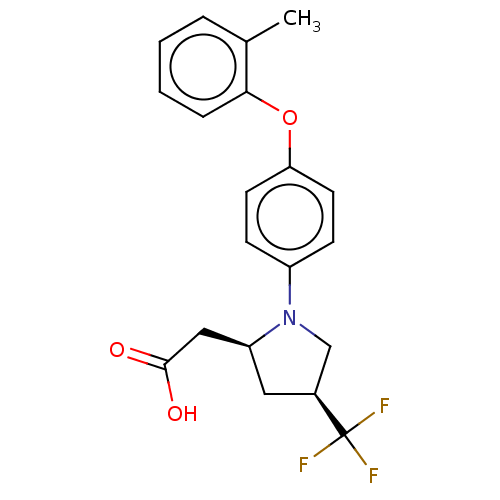
(CHEMBL4089171)Show SMILES Cc1ccccc1Oc1ccc(cc1)N1C[C@H](C[C@H]1CC(O)=O)C(F)(F)F |r| Show InChI InChI=1S/C20H20F3NO3/c1-13-4-2-3-5-18(13)27-17-8-6-15(7-9-17)24-12-14(20(21,22)23)10-16(24)11-19(25)26/h2-9,14,16H,10-12H2,1H3,(H,25,26)/t14-,16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method |
J Med Chem 60: 1417-1431 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01559
BindingDB Entry DOI: 10.7270/Q25X2CD5 |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50267029

(CHEMBL4069191)Show SMILES COc1ccc(Oc2ccc(cc2)N2C[C@H](C[C@H]2CC(O)=O)C(F)(F)F)c(C)c1 |r| Show InChI InChI=1S/C21H22F3NO4/c1-13-9-18(28-2)7-8-19(13)29-17-5-3-15(4-6-17)25-12-14(21(22,23)24)10-16(25)11-20(26)27/h3-9,14,16H,10-12H2,1-2H3,(H,26,27)/t14-,16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method |
J Med Chem 60: 1417-1431 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01559
BindingDB Entry DOI: 10.7270/Q25X2CD5 |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50267010
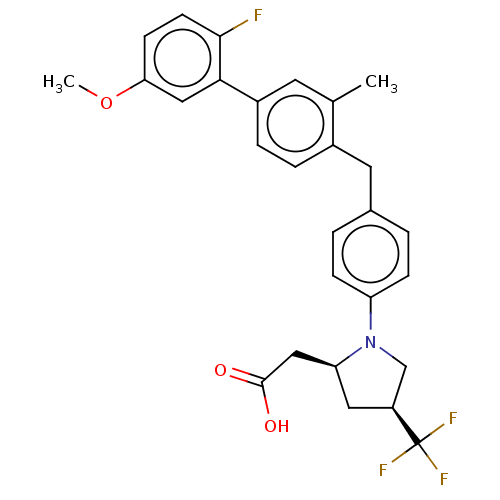
(CHEMBL4082395)Show SMILES COc1ccc(F)c(c1)-c1ccc(Cc2ccc(cc2)N2C[C@H](C[C@H]2CC(O)=O)C(F)(F)F)c(C)c1 |r| Show InChI InChI=1S/C28H27F4NO3/c1-17-11-20(25-15-24(36-2)9-10-26(25)29)6-5-19(17)12-18-3-7-22(8-4-18)33-16-21(28(30,31)32)13-23(33)14-27(34)35/h3-11,15,21,23H,12-14,16H2,1-2H3,(H,34,35)/t21-,23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method |
J Med Chem 60: 1417-1431 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01559
BindingDB Entry DOI: 10.7270/Q25X2CD5 |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50267117

(CHEMBL4060499)Show SMILES COc1ccc(F)c(Cc2cccc(Oc3ccc(cc3)N3C[C@H](C[C@H]3CC(O)=O)C(F)(F)F)c2C)c1 |r| Show InChI InChI=1S/C28H27F4NO4/c1-17-18(12-19-13-24(36-2)10-11-25(19)29)4-3-5-26(17)37-23-8-6-21(7-9-23)33-16-20(28(30,31)32)14-22(33)15-27(34)35/h3-11,13,20,22H,12,14-16H2,1-2H3,(H,34,35)/t20-,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method |
J Med Chem 60: 1417-1431 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01559
BindingDB Entry DOI: 10.7270/Q25X2CD5 |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50267030

(CHEMBL4090240)Show SMILES Cc1cccc(Oc2ccc(cc2)N2C[C@H](C[C@H]2CC(O)=O)C(F)(F)F)c1C |r| Show InChI InChI=1S/C21H22F3NO3/c1-13-4-3-5-19(14(13)2)28-18-8-6-16(7-9-18)25-12-15(21(22,23)24)10-17(25)11-20(26)27/h3-9,15,17H,10-12H2,1-2H3,(H,26,27)/t15-,17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method |
J Med Chem 60: 1417-1431 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01559
BindingDB Entry DOI: 10.7270/Q25X2CD5 |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50267044

(CHEMBL4069764)Show SMILES Cc1ccccc1Cc1ccc(cc1)N1C[C@H](C[C@H]1CC(O)=O)C(F)(F)F |r| Show InChI InChI=1S/C21H22F3NO2/c1-14-4-2-3-5-16(14)10-15-6-8-18(9-7-15)25-13-17(21(22,23)24)11-19(25)12-20(26)27/h2-9,17,19H,10-13H2,1H3,(H,26,27)/t17-,19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method |
J Med Chem 60: 1417-1431 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01559
BindingDB Entry DOI: 10.7270/Q25X2CD5 |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50267039

(CHEMBL4090766)Show SMILES Cc1ccccc1Cc1ccc(cc1)N1C[C@@H](F)C[C@H]1CC(O)=O |r| Show InChI InChI=1S/C20H22FNO2/c1-14-4-2-3-5-16(14)10-15-6-8-18(9-7-15)22-13-17(21)11-19(22)12-20(23)24/h2-9,17,19H,10-13H2,1H3,(H,23,24)/t17-,19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method |
J Med Chem 60: 1417-1431 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01559
BindingDB Entry DOI: 10.7270/Q25X2CD5 |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50267035

(CHEMBL4071899)Show SMILES COc1ccc(F)c(c1)-c1ccc(Cc2ccc(cc2)N2CCC[C@H]2CC(O)=O)c(C)c1 |r| Show InChI InChI=1S/C27H28FNO3/c1-18-14-21(25-17-24(32-2)11-12-26(25)28)8-7-20(18)15-19-5-9-22(10-6-19)29-13-3-4-23(29)16-27(30)31/h5-12,14,17,23H,3-4,13,15-16H2,1-2H3,(H,30,31)/t23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method |
J Med Chem 60: 1417-1431 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01559
BindingDB Entry DOI: 10.7270/Q25X2CD5 |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50267049

(CHEMBL4095599)Show SMILES C[C@H]1CCN([C@@H]1CC(O)=O)c1ccc(Cc2ccccc2C)cc1 |r| Show InChI InChI=1S/C21H25NO2/c1-15-5-3-4-6-18(15)13-17-7-9-19(10-8-17)22-12-11-16(2)20(22)14-21(23)24/h3-10,16,20H,11-14H2,1-2H3,(H,23,24)/t16-,20+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR40 expressed in CHO-A12 cells assessed as increase in intracellular calcium flux measured for 100 secs by FLIPR assay |
J Med Chem 60: 1417-1431 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01559
BindingDB Entry DOI: 10.7270/Q25X2CD5 |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50267038

(CHEMBL4061093)Show SMILES C[C@H]1C[C@@H](CC(O)=O)N(C1)c1ccc(Cc2ccccc2C)cc1 |r| Show InChI InChI=1S/C21H25NO2/c1-15-11-20(13-21(23)24)22(14-15)19-9-7-17(8-10-19)12-18-6-4-3-5-16(18)2/h3-10,15,20H,11-14H2,1-2H3,(H,23,24)/t15-,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR40 expressed in CHO-A12 cells assessed as increase in intracellular calcium flux measured for 100 secs by FLIPR assay |
J Med Chem 60: 1417-1431 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01559
BindingDB Entry DOI: 10.7270/Q25X2CD5 |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50267046
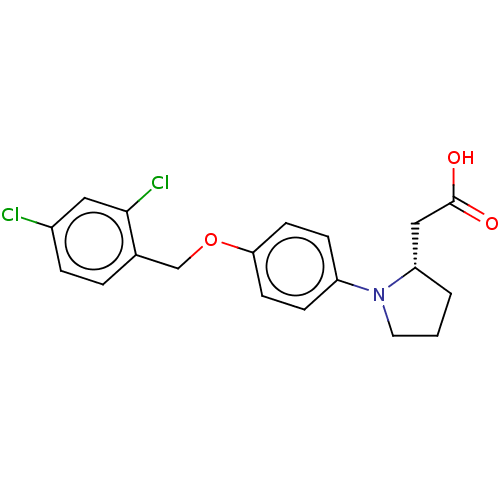
(CHEMBL4103729)Show SMILES OC(=O)C[C@@H]1CCCN1c1ccc(OCc2ccc(Cl)cc2Cl)cc1 |r| Show InChI InChI=1S/C19H19Cl2NO3/c20-14-4-3-13(18(21)10-14)12-25-17-7-5-15(6-8-17)22-9-1-2-16(22)11-19(23)24/h3-8,10,16H,1-2,9,11-12H2,(H,23,24)/t16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR40 expressed in CHO-A12 cells assessed as increase in intracellular calcium flux measured for 100 secs by FLIPR assay |
J Med Chem 60: 1417-1431 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01559
BindingDB Entry DOI: 10.7270/Q25X2CD5 |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50267066
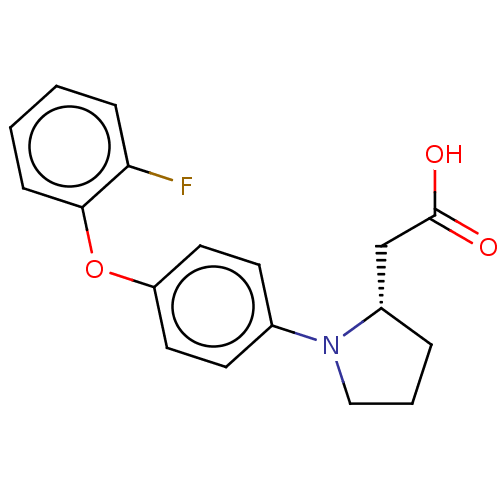
(CHEMBL4067052)Show SMILES OC(=O)C[C@@H]1CCCN1c1ccc(Oc2ccccc2F)cc1 |r| Show InChI InChI=1S/C18H18FNO3/c19-16-5-1-2-6-17(16)23-15-9-7-13(8-10-15)20-11-3-4-14(20)12-18(21)22/h1-2,5-10,14H,3-4,11-12H2,(H,21,22)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method |
J Med Chem 60: 1417-1431 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01559
BindingDB Entry DOI: 10.7270/Q25X2CD5 |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50267118

(CHEMBL4096887)Show InChI InChI=1S/C20H23NO2/c1-15-5-2-3-6-17(15)13-16-8-10-18(11-9-16)21-12-4-7-19(21)14-20(22)23/h2-3,5-6,8-11,19H,4,7,12-14H2,1H3,(H,22,23)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method |
J Med Chem 60: 1417-1431 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01559
BindingDB Entry DOI: 10.7270/Q25X2CD5 |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50267059

(CHEMBL4091552)Show SMILES Cc1ccccc1COc1ccc(cn1)N1CCC[C@H]1CC(O)=O |r| Show InChI InChI=1S/C19H22N2O3/c1-14-5-2-3-6-15(14)13-24-18-9-8-17(12-20-18)21-10-4-7-16(21)11-19(22)23/h2-3,5-6,8-9,12,16H,4,7,10-11,13H2,1H3,(H,22,23)/t16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method |
J Med Chem 60: 1417-1431 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01559
BindingDB Entry DOI: 10.7270/Q25X2CD5 |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50267047
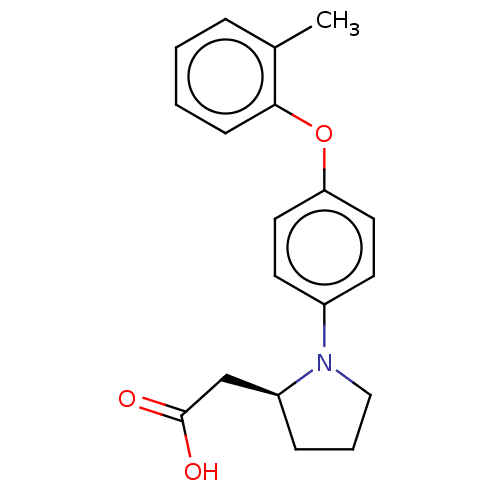
(CHEMBL4078852)Show InChI InChI=1S/C19H21NO3/c1-14-5-2-3-7-18(14)23-17-10-8-15(9-11-17)20-12-4-6-16(20)13-19(21)22/h2-3,5,7-11,16H,4,6,12-13H2,1H3,(H,21,22)/t16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method |
J Med Chem 60: 1417-1431 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01559
BindingDB Entry DOI: 10.7270/Q25X2CD5 |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50267064

(CHEMBL4070703)Show InChI InChI=1S/C18H19NO3/c20-18(21)13-15-5-4-12-19(15)14-8-10-17(11-9-14)22-16-6-2-1-3-7-16/h1-3,6-11,15H,4-5,12-13H2,(H,20,21)/t15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method |
J Med Chem 60: 1417-1431 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01559
BindingDB Entry DOI: 10.7270/Q25X2CD5 |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50267045
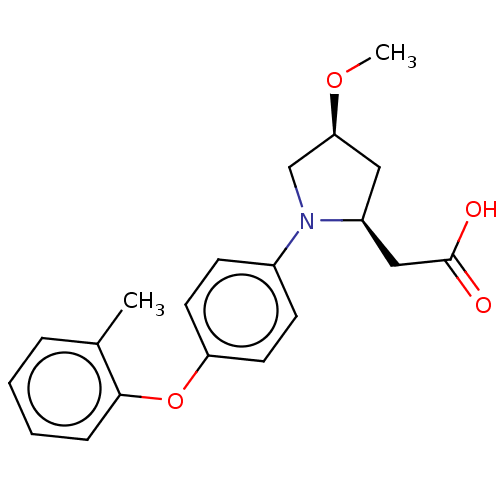
(CHEMBL4063889)Show SMILES CO[C@H]1C[C@@H](CC(O)=O)N(C1)c1ccc(Oc2ccccc2C)cc1 |r| Show InChI InChI=1S/C20H23NO4/c1-14-5-3-4-6-19(14)25-17-9-7-15(8-10-17)21-13-18(24-2)11-16(21)12-20(22)23/h3-10,16,18H,11-13H2,1-2H3,(H,22,23)/t16-,18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method |
J Med Chem 60: 1417-1431 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01559
BindingDB Entry DOI: 10.7270/Q25X2CD5 |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50267048

(CHEMBL4075190)Show SMILES Cc1ccccc1Cc1ccc(cc1)N1CCC[C@@]1(C)CC(O)=O |r| Show InChI InChI=1S/C21H25NO2/c1-16-6-3-4-7-18(16)14-17-8-10-19(11-9-17)22-13-5-12-21(22,2)15-20(23)24/h3-4,6-11H,5,12-15H2,1-2H3,(H,23,24)/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method |
J Med Chem 60: 1417-1431 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01559
BindingDB Entry DOI: 10.7270/Q25X2CD5 |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50267021
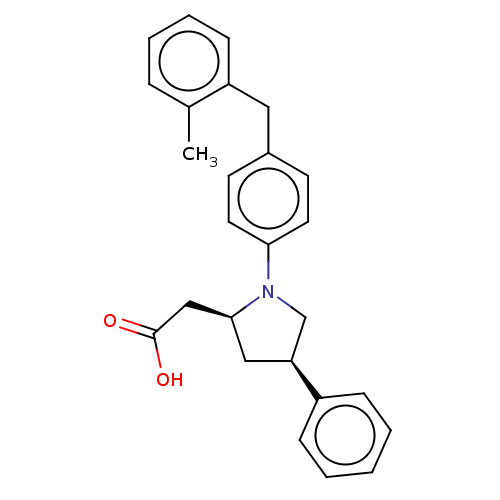
(CHEMBL4091669)Show SMILES Cc1ccccc1Cc1ccc(cc1)N1C[C@H](C[C@H]1CC(O)=O)c1ccccc1 |r| Show InChI InChI=1S/C26H27NO2/c1-19-7-5-6-10-22(19)15-20-11-13-24(14-12-20)27-18-23(16-25(27)17-26(28)29)21-8-3-2-4-9-21/h2-14,23,25H,15-18H2,1H3,(H,28,29)/t23-,25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method |
J Med Chem 60: 1417-1431 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01559
BindingDB Entry DOI: 10.7270/Q25X2CD5 |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50267113
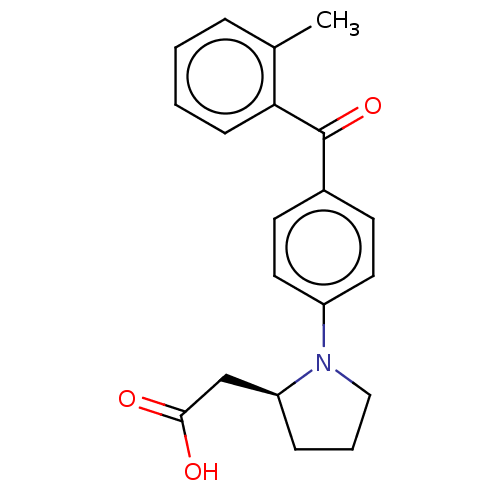
(CHEMBL4105565)Show SMILES Cc1ccccc1C(=O)c1ccc(cc1)N1CCC[C@H]1CC(O)=O |r| Show InChI InChI=1S/C20H21NO3/c1-14-5-2-3-7-18(14)20(24)15-8-10-16(11-9-15)21-12-4-6-17(21)13-19(22)23/h2-3,5,7-11,17H,4,6,12-13H2,1H3,(H,22,23)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method |
J Med Chem 60: 1417-1431 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01559
BindingDB Entry DOI: 10.7270/Q25X2CD5 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50244066

(CHEMBL4083365)Show SMILES COc1ccc(F)c(c1)-c1ccc(Cc2ccc(cc2)N2C[C@@H](C[C@@H]2CC(O)=O)C(F)(F)F)c(C)c1 |r| Show InChI InChI=1S/C28H27F4NO3/c1-17-11-20(25-15-24(36-2)9-10-26(25)29)6-5-19(17)12-18-3-7-22(8-4-18)33-16-21(28(30,31)32)13-23(33)14-27(34)35/h3-11,15,21,23H,12-14,16H2,1-2H3,(H,34,35)/t21-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 60: 1417-1431 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01559
BindingDB Entry DOI: 10.7270/Q25X2CD5 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50243975
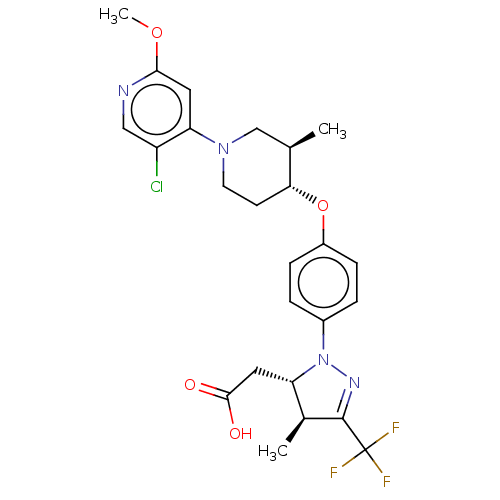
(CHEMBL4080226)Show SMILES COc1cc(N2CC[C@@H](Oc3ccc(cc3)N3N=C([C@@H](C)[C@@H]3CC(O)=O)C(F)(F)F)[C@H](C)C2)c(Cl)cn1 |r,c:18| Show InChI InChI=1S/C25H28ClF3N4O4/c1-14-13-32(20-10-22(36-3)30-12-18(20)26)9-8-21(14)37-17-6-4-16(5-7-17)33-19(11-23(34)35)15(2)24(31-33)25(27,28)29/h4-7,10,12,14-15,19,21H,8-9,11,13H2,1-3H3,(H,34,35)/t14-,15+,19+,21-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50244066

(CHEMBL4083365)Show SMILES COc1ccc(F)c(c1)-c1ccc(Cc2ccc(cc2)N2C[C@@H](C[C@@H]2CC(O)=O)C(F)(F)F)c(C)c1 |r| Show InChI InChI=1S/C28H27F4NO3/c1-17-11-20(25-15-24(36-2)9-10-26(25)29)6-5-19(17)12-18-3-7-22(8-4-18)33-16-21(28(30,31)32)13-23(33)14-27(34)35/h3-11,15,21,23H,12-14,16H2,1-2H3,(H,34,35)/t21-,23-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 (unknown origin) |
J Med Chem 60: 1417-1431 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01559
BindingDB Entry DOI: 10.7270/Q25X2CD5 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50243975

(CHEMBL4080226)Show SMILES COc1cc(N2CC[C@@H](Oc3ccc(cc3)N3N=C([C@@H](C)[C@@H]3CC(O)=O)C(F)(F)F)[C@H](C)C2)c(Cl)cn1 |r,c:18| Show InChI InChI=1S/C25H28ClF3N4O4/c1-14-13-32(20-10-22(36-3)30-12-18(20)26)9-8-21(14)37-17-6-4-16(5-7-17)33-19(11-23(34)35)15(2)24(31-33)25(27,28)29/h4-7,10,12,14-15,19,21H,8-9,11,13H2,1-3H3,(H,34,35)/t14-,15+,19+,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50244066

(CHEMBL4083365)Show SMILES COc1ccc(F)c(c1)-c1ccc(Cc2ccc(cc2)N2C[C@@H](C[C@@H]2CC(O)=O)C(F)(F)F)c(C)c1 |r| Show InChI InChI=1S/C28H27F4NO3/c1-17-11-20(25-15-24(36-2)9-10-26(25)29)6-5-19(17)12-18-3-7-22(8-4-18)33-16-21(28(30,31)32)13-23(33)14-27(34)35/h3-11,15,21,23H,12-14,16H2,1-2H3,(H,34,35)/t21-,23-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
J Med Chem 60: 1417-1431 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01559
BindingDB Entry DOI: 10.7270/Q25X2CD5 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50243975

(CHEMBL4080226)Show SMILES COc1cc(N2CC[C@@H](Oc3ccc(cc3)N3N=C([C@@H](C)[C@@H]3CC(O)=O)C(F)(F)F)[C@H](C)C2)c(Cl)cn1 |r,c:18| Show InChI InChI=1S/C25H28ClF3N4O4/c1-14-13-32(20-10-22(36-3)30-12-18(20)26)9-8-21(14)37-17-6-4-16(5-7-17)33-19(11-23(34)35)15(2)24(31-33)25(27,28)29/h4-7,10,12,14-15,19,21H,8-9,11,13H2,1-3H3,(H,34,35)/t14-,15+,19+,21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of MAO-B (unknown origin) |
J Med Chem 61: 681-694 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00982
BindingDB Entry DOI: 10.7270/Q24T6MTM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50243975

(CHEMBL4080226)Show SMILES COc1cc(N2CC[C@@H](Oc3ccc(cc3)N3N=C([C@@H](C)[C@@H]3CC(O)=O)C(F)(F)F)[C@H](C)C2)c(Cl)cn1 |r,c:18| Show InChI InChI=1S/C25H28ClF3N4O4/c1-14-13-32(20-10-22(36-3)30-12-18(20)26)9-8-21(14)37-17-6-4-16(5-7-17)33-19(11-23(34)35)15(2)24(31-33)25(27,28)29/h4-7,10,12,14-15,19,21H,8-9,11,13H2,1-3H3,(H,34,35)/t14-,15+,19+,21-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50614666
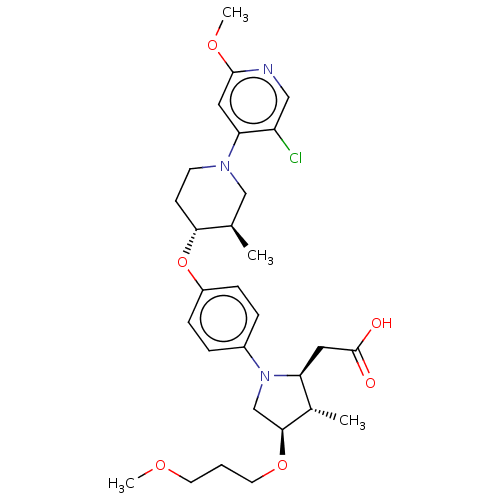
(CHEMBL5285988)Show SMILES COCCCO[C@H]1CN([C@@H](CC(O)=O)[C@@H]1C)c1ccc(O[C@@H]2CCN(C[C@H]2C)c2cc(OC)ncc2Cl)cc1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50243975

(CHEMBL4080226)Show SMILES COc1cc(N2CC[C@@H](Oc3ccc(cc3)N3N=C([C@@H](C)[C@@H]3CC(O)=O)C(F)(F)F)[C@H](C)C2)c(Cl)cn1 |r,c:18| Show InChI InChI=1S/C25H28ClF3N4O4/c1-14-13-32(20-10-22(36-3)30-12-18(20)26)9-8-21(14)37-17-6-4-16(5-7-17)33-19(11-23(34)35)15(2)24(31-33)25(27,28)29/h4-7,10,12,14-15,19,21H,8-9,11,13H2,1-3H3,(H,34,35)/t14-,15+,19+,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50614666

(CHEMBL5285988)Show SMILES COCCCO[C@H]1CN([C@@H](CC(O)=O)[C@@H]1C)c1ccc(O[C@@H]2CCN(C[C@H]2C)c2cc(OC)ncc2Cl)cc1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50614666

(CHEMBL5285988)Show SMILES COCCCO[C@H]1CN([C@@H](CC(O)=O)[C@@H]1C)c1ccc(O[C@@H]2CCN(C[C@H]2C)c2cc(OC)ncc2Cl)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50243975

(CHEMBL4080226)Show SMILES COc1cc(N2CC[C@@H](Oc3ccc(cc3)N3N=C([C@@H](C)[C@@H]3CC(O)=O)C(F)(F)F)[C@H](C)C2)c(Cl)cn1 |r,c:18| Show InChI InChI=1S/C25H28ClF3N4O4/c1-14-13-32(20-10-22(36-3)30-12-18(20)26)9-8-21(14)37-17-6-4-16(5-7-17)33-19(11-23(34)35)15(2)24(31-33)25(27,28)29/h4-7,10,12,14-15,19,21H,8-9,11,13H2,1-3H3,(H,34,35)/t14-,15+,19+,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50243975

(CHEMBL4080226)Show SMILES COc1cc(N2CC[C@@H](Oc3ccc(cc3)N3N=C([C@@H](C)[C@@H]3CC(O)=O)C(F)(F)F)[C@H](C)C2)c(Cl)cn1 |r,c:18| Show InChI InChI=1S/C25H28ClF3N4O4/c1-14-13-32(20-10-22(36-3)30-12-18(20)26)9-8-21(14)37-17-6-4-16(5-7-17)33-19(11-23(34)35)15(2)24(31-33)25(27,28)29/h4-7,10,12,14-15,19,21H,8-9,11,13H2,1-3H3,(H,34,35)/t14-,15+,19+,21-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50614666

(CHEMBL5285988)Show SMILES COCCCO[C@H]1CN([C@@H](CC(O)=O)[C@@H]1C)c1ccc(O[C@@H]2CCN(C[C@H]2C)c2cc(OC)ncc2Cl)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50614666

(CHEMBL5285988)Show SMILES COCCCO[C@H]1CN([C@@H](CC(O)=O)[C@@H]1C)c1ccc(O[C@@H]2CCN(C[C@H]2C)c2cc(OC)ncc2Cl)cc1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50614666

(CHEMBL5285988)Show SMILES COCCCO[C@H]1CN([C@@H](CC(O)=O)[C@@H]1C)c1ccc(O[C@@H]2CCN(C[C@H]2C)c2cc(OC)ncc2Cl)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50243976
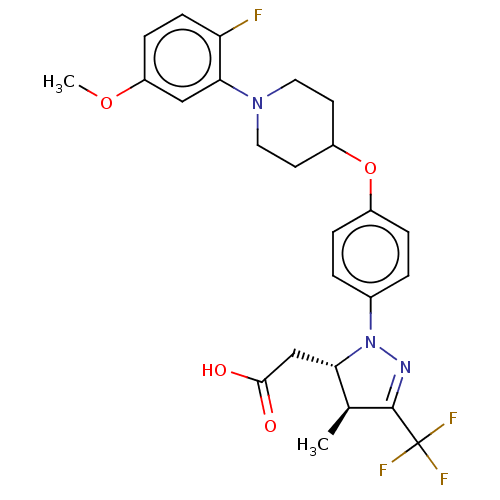
(CHEMBL4105324)Show SMILES COc1ccc(F)c(c1)N1CCC(CC1)Oc1ccc(cc1)N1N=C([C@@H](C)[C@@H]1CC(O)=O)C(F)(F)F |r,c:26| Show InChI InChI=1S/C25H27F4N3O4/c1-15-21(14-23(33)34)32(30-24(15)25(27,28)29)16-3-5-17(6-4-16)36-18-9-11-31(12-10-18)22-13-19(35-2)7-8-20(22)26/h3-8,13,15,18,21H,9-12,14H2,1-2H3,(H,33,34)/t15-,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma (unknown origin) |
J Med Chem 61: 681-694 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00982
BindingDB Entry DOI: 10.7270/Q24T6MTM |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50243983
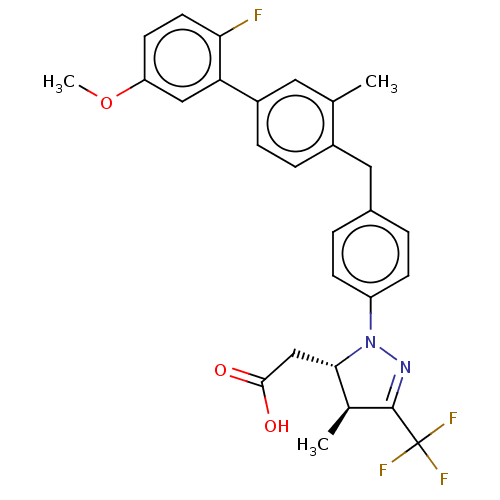
(CHEMBL4086198)Show SMILES COc1ccc(F)c(c1)-c1ccc(Cc2ccc(cc2)N2N=C([C@@H](C)[C@@H]2CC(O)=O)C(F)(F)F)c(C)c1 |r,c:23| Show InChI InChI=1S/C28H26F4N2O3/c1-16-12-20(23-14-22(37-3)10-11-24(23)29)7-6-19(16)13-18-4-8-21(9-5-18)34-25(15-26(35)36)17(2)27(33-34)28(30,31)32/h4-12,14,17,25H,13,15H2,1-3H3,(H,35,36)/t17-,25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma (unknown origin) |
J Med Chem 61: 681-694 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00982
BindingDB Entry DOI: 10.7270/Q24T6MTM |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50243984
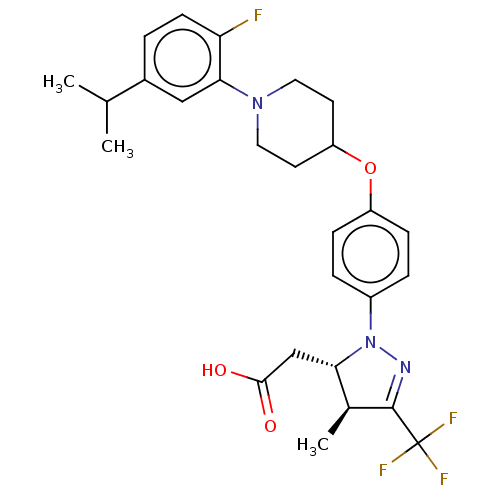
(CHEMBL4063905)Show SMILES CC(C)c1ccc(F)c(c1)N1CCC(CC1)Oc1ccc(cc1)N1N=C([C@@H](C)[C@@H]1CC(O)=O)C(F)(F)F |r,c:27| Show InChI InChI=1S/C27H31F4N3O3/c1-16(2)18-4-9-22(28)24(14-18)33-12-10-21(11-13-33)37-20-7-5-19(6-8-20)34-23(15-25(35)36)17(3)26(32-34)27(29,30)31/h4-9,14,16-17,21,23H,10-13,15H2,1-3H3,(H,35,36)/t17-,23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 600 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR40 expressed in CHOA12 cells assessed as induction of Ca2+ mobilization by FLIPR assay |
J Med Chem 61: 681-694 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00982
BindingDB Entry DOI: 10.7270/Q24T6MTM |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50243997
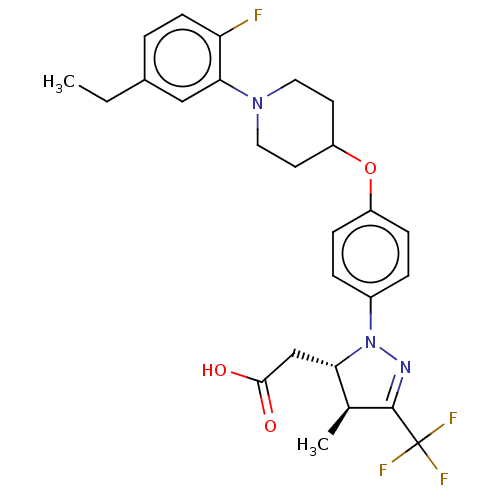
(CHEMBL4092937)Show SMILES CCc1ccc(F)c(c1)N1CCC(CC1)Oc1ccc(cc1)N1N=C([C@@H](C)[C@@H]1CC(O)=O)C(F)(F)F |r,c:26| Show InChI InChI=1S/C26H29F4N3O3/c1-3-17-4-9-21(27)23(14-17)32-12-10-20(11-13-32)36-19-7-5-18(6-8-19)33-22(15-24(34)35)16(2)25(31-33)26(28,29)30/h4-9,14,16,20,22H,3,10-13,15H2,1-2H3,(H,34,35)/t16-,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 450 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR40 expressed in CHOA12 cells assessed as induction of Ca2+ mobilization by FLIPR assay |
J Med Chem 61: 681-694 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00982
BindingDB Entry DOI: 10.7270/Q24T6MTM |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50243998
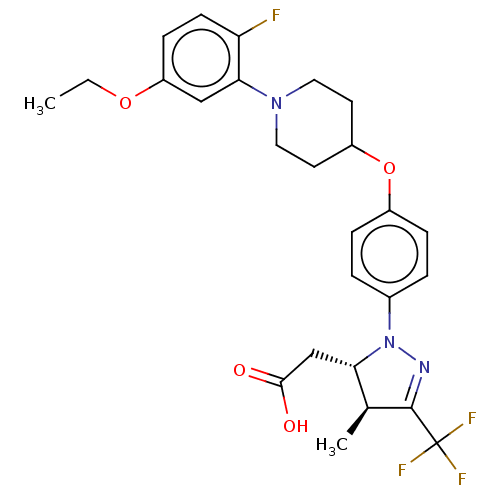
(CHEMBL4082133)Show SMILES CCOc1ccc(F)c(c1)N1CCC(CC1)Oc1ccc(cc1)N1N=C([C@@H](C)[C@@H]1CC(O)=O)C(F)(F)F |r,c:27| Show InChI InChI=1S/C26H29F4N3O4/c1-3-36-20-8-9-21(27)23(14-20)32-12-10-19(11-13-32)37-18-6-4-17(5-7-18)33-22(15-24(34)35)16(2)25(31-33)26(28,29)30/h4-9,14,16,19,22H,3,10-13,15H2,1-2H3,(H,34,35)/t16-,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 220 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR40 expressed in CHOA12 cells assessed as induction of Ca2+ mobilization by FLIPR assay |
J Med Chem 61: 681-694 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00982
BindingDB Entry DOI: 10.7270/Q24T6MTM |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50243999
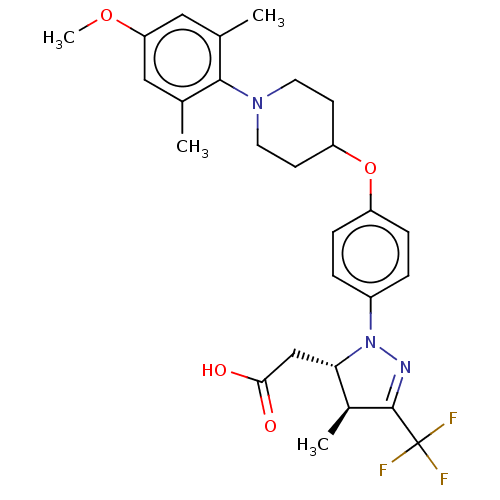
(CHEMBL4061053)Show SMILES COc1cc(C)c(N2CCC(CC2)Oc2ccc(cc2)N2N=C([C@@H](C)[C@@H]2CC(O)=O)C(F)(F)F)c(C)c1 |r,c:23| Show InChI InChI=1S/C27H32F3N3O4/c1-16-13-22(36-4)14-17(2)25(16)32-11-9-21(10-12-32)37-20-7-5-19(6-8-20)33-23(15-24(34)35)18(3)26(31-33)27(28,29)30/h5-8,13-14,18,21,23H,9-12,15H2,1-4H3,(H,34,35)/t18-,23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.06E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR40 expressed in CHOA12 cells assessed as induction of Ca2+ mobilization by FLIPR assay |
J Med Chem 61: 681-694 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00982
BindingDB Entry DOI: 10.7270/Q24T6MTM |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50244000
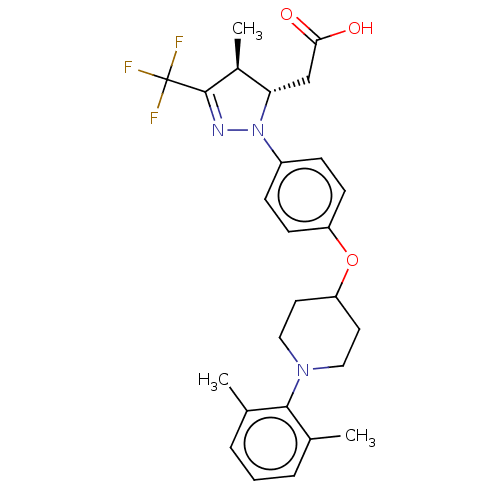
(CHEMBL4072314)Show SMILES C[C@H]1[C@H](CC(O)=O)N(N=C1C(F)(F)F)c1ccc(OC2CCN(CC2)c2c(C)cccc2C)cc1 |r,c:8| Show InChI InChI=1S/C26H30F3N3O3/c1-16-5-4-6-17(2)24(16)31-13-11-21(12-14-31)35-20-9-7-19(8-10-20)32-22(15-23(33)34)18(3)25(30-32)26(27,28)29/h4-10,18,21-22H,11-15H2,1-3H3,(H,33,34)/t18-,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.34E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR40 expressed in CHOA12 cells assessed as induction of Ca2+ mobilization by FLIPR assay |
J Med Chem 61: 681-694 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00982
BindingDB Entry DOI: 10.7270/Q24T6MTM |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50244001
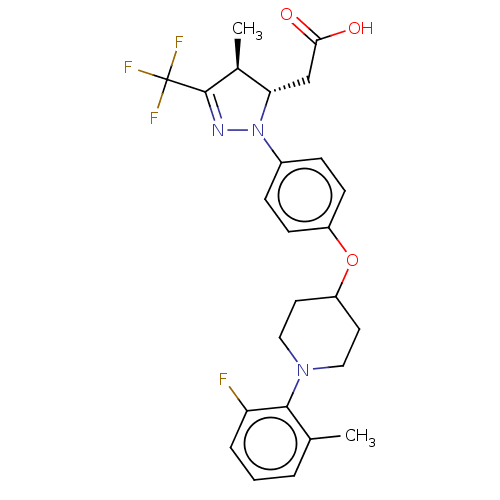
(CHEMBL4076149)Show SMILES C[C@H]1[C@H](CC(O)=O)N(N=C1C(F)(F)F)c1ccc(OC2CCN(CC2)c2c(C)cccc2F)cc1 |r,c:8| Show InChI InChI=1S/C25H27F4N3O3/c1-15-4-3-5-20(26)23(15)31-12-10-19(11-13-31)35-18-8-6-17(7-9-18)32-21(14-22(33)34)16(2)24(30-32)25(27,28)29/h3-9,16,19,21H,10-14H2,1-2H3,(H,33,34)/t16-,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 800 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR40 expressed in CHOA12 cells assessed as induction of Ca2+ mobilization by FLIPR assay |
J Med Chem 61: 681-694 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00982
BindingDB Entry DOI: 10.7270/Q24T6MTM |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50244002
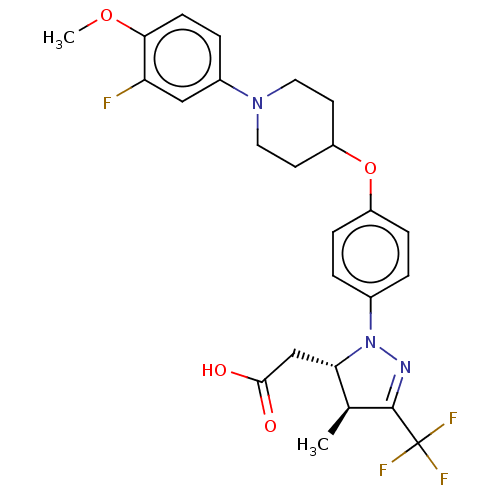
(CHEMBL4061146)Show SMILES COc1ccc(cc1F)N1CCC(CC1)Oc1ccc(cc1)N1N=C([C@@H](C)[C@@H]1CC(O)=O)C(F)(F)F |r,c:26| Show InChI InChI=1S/C25H27F4N3O4/c1-15-21(14-23(33)34)32(30-24(15)25(27,28)29)16-3-6-18(7-4-16)36-19-9-11-31(12-10-19)17-5-8-22(35-2)20(26)13-17/h3-8,13,15,19,21H,9-12,14H2,1-2H3,(H,33,34)/t15-,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.62E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR40 expressed in CHOA12 cells assessed as induction of Ca2+ mobilization by FLIPR assay |
J Med Chem 61: 681-694 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00982
BindingDB Entry DOI: 10.7270/Q24T6MTM |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50244003
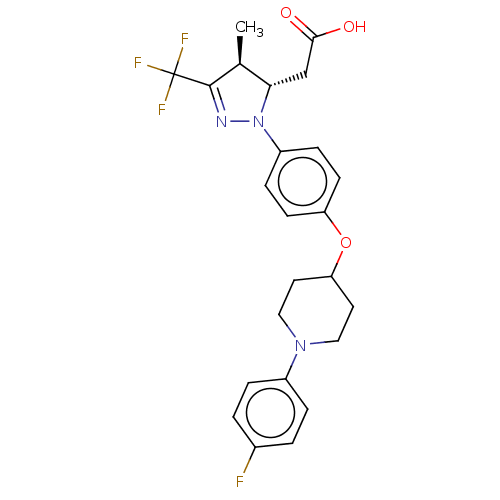
(CHEMBL4067111)Show SMILES C[C@H]1[C@H](CC(O)=O)N(N=C1C(F)(F)F)c1ccc(OC2CCN(CC2)c2ccc(F)cc2)cc1 |r,c:8| Show InChI InChI=1S/C24H25F4N3O3/c1-15-21(14-22(32)33)31(29-23(15)24(26,27)28)18-6-8-19(9-7-18)34-20-10-12-30(13-11-20)17-4-2-16(25)3-5-17/h2-9,15,20-21H,10-14H2,1H3,(H,32,33)/t15-,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.55E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR40 expressed in CHOA12 cells assessed as induction of Ca2+ mobilization by FLIPR assay |
J Med Chem 61: 681-694 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00982
BindingDB Entry DOI: 10.7270/Q24T6MTM |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50244004
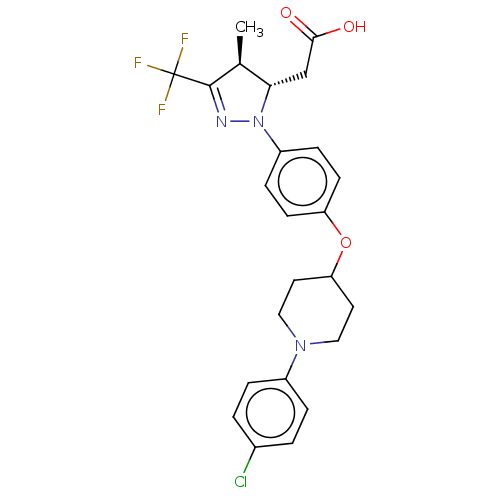
(CHEMBL4088375)Show SMILES C[C@H]1[C@H](CC(O)=O)N(N=C1C(F)(F)F)c1ccc(OC2CCN(CC2)c2ccc(Cl)cc2)cc1 |r,c:8| Show InChI InChI=1S/C24H25ClF3N3O3/c1-15-21(14-22(32)33)31(29-23(15)24(26,27)28)18-6-8-19(9-7-18)34-20-10-12-30(13-11-20)17-4-2-16(25)3-5-17/h2-9,15,20-21H,10-14H2,1H3,(H,32,33)/t15-,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.27E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR40 expressed in CHOA12 cells assessed as induction of Ca2+ mobilization by FLIPR assay |
J Med Chem 61: 681-694 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00982
BindingDB Entry DOI: 10.7270/Q24T6MTM |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50244005
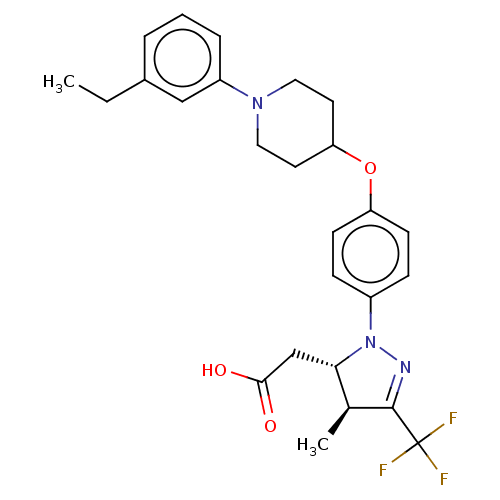
(CHEMBL4080516)Show SMILES CCc1cccc(c1)N1CCC(CC1)Oc1ccc(cc1)N1N=C([C@@H](C)[C@@H]1CC(O)=O)C(F)(F)F |r,c:25| Show InChI InChI=1S/C26H30F3N3O3/c1-3-18-5-4-6-20(15-18)31-13-11-22(12-14-31)35-21-9-7-19(8-10-21)32-23(16-24(33)34)17(2)25(30-32)26(27,28)29/h4-10,15,17,22-23H,3,11-14,16H2,1-2H3,(H,33,34)/t17-,23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.18E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR40 expressed in CHOA12 cells assessed as induction of Ca2+ mobilization by FLIPR assay |
J Med Chem 61: 681-694 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00982
BindingDB Entry DOI: 10.7270/Q24T6MTM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data