 Found 1019 hits with Last Name = 'morgan' and Initial = 't'
Found 1019 hits with Last Name = 'morgan' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50437403

(CHEMBL2408771)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12)-[#7]-1-[#6]-[#6]-2-[#6]-[#6]-[#6]-[#7]-[#6]-2-[#6]-1 Show InChI InChI=1S/C26H32N6O3/c1-17(2)11-13-31-22-23(28-25(31)30-14-19-10-7-12-27-20(19)15-30)29(3)26(35)32(24(22)34)16-21(33)18-8-5-4-6-9-18/h4-6,8-9,11,19-20,27H,7,10,12-16H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of porcine DPP4 |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230790

(CHEMBL292892)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(cc1[N+]([O-])=O)C(O)=O Show InChI InChI=1S/C23H23N3O6S/c1-2-3-6-21-24-13-18(10-17(23(29)30)11-19-5-4-9-33-19)25(21)14-16-8-7-15(22(27)28)12-20(16)26(31)32/h4-5,7-10,12-13H,2-3,6,11,14H2,1H3,(H,27,28)(H,29,30)/b17-10+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50427749
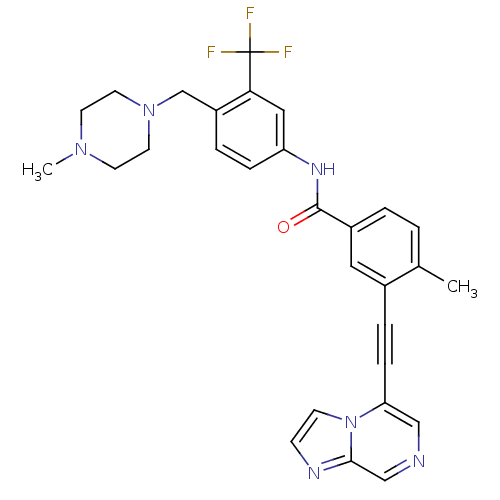
(CHEMBL2324924)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cncc4nccn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-3-4-22(15-21(20)6-8-25-17-33-18-27-34-9-10-38(25)27)28(39)35-24-7-5-23(26(16-24)29(30,31)32)19-37-13-11-36(2)12-14-37/h3-5,7,9-10,15-18H,11-14,19H2,1-2H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl1 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50437403

(CHEMBL2408771)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12)-[#7]-1-[#6]-[#6]-2-[#6]-[#6]-[#6]-[#7]-[#6]-2-[#6]-1 Show InChI InChI=1S/C26H32N6O3/c1-17(2)11-13-31-22-23(28-25(31)30-14-19-10-7-12-27-20(19)15-30)29(3)26(35)32(24(22)34)16-21(33)18-8-5-4-6-9-18/h4-6,8-9,11,19-20,27H,7,10,12-16H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of porcine DPP4 using H-Gly-Pro-AMC as substrate by fluorescence assay |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230779

(CHEMBL294686)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)c2nn[nH]n2)n1Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H24N6O2S/c1-2-3-6-21-24-14-19(29(21)15-16-7-9-17(10-8-16)23(30)31)12-18(22-25-27-28-26-22)13-20-5-4-11-32-20/h4-5,7-12,14H,2-3,6,13,15H2,1H3,(H,30,31)(H,25,26,27,28)/b18-12+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50427748
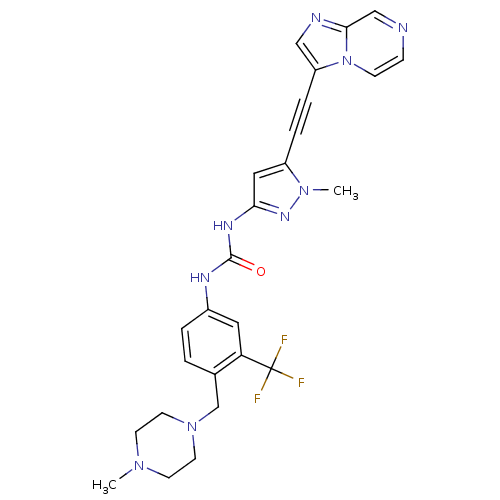
(CHEMBL2324925)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4cnc5cnccn45)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C26H26F3N9O/c1-35-9-11-37(12-10-35)17-18-3-4-19(13-22(18)26(27,28)29)32-25(39)33-23-14-20(36(2)34-23)5-6-21-15-31-24-16-30-7-8-38(21)24/h3-4,7-8,13-16H,9-12,17H2,1-2H3,(H2,32,33,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl1 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230811
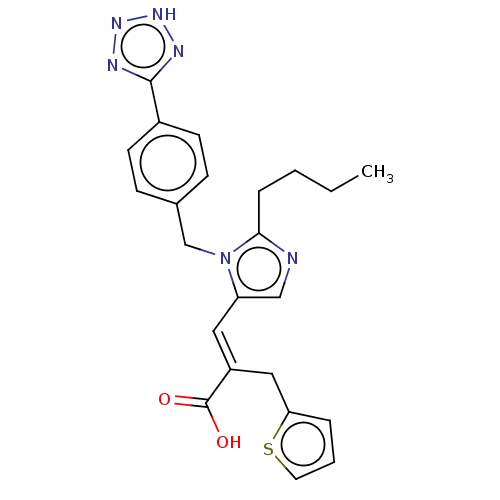
(CHEMBL293091)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(cc1)-c1nn[nH]n1 Show InChI InChI=1S/C23H24N6O2S/c1-2-3-6-21-24-14-19(12-18(23(30)31)13-20-5-4-11-32-20)29(21)15-16-7-9-17(10-8-16)22-25-27-28-26-22/h4-5,7-12,14H,2-3,6,13,15H2,1H3,(H,30,31)(H,25,26,27,28)/b18-12+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230778
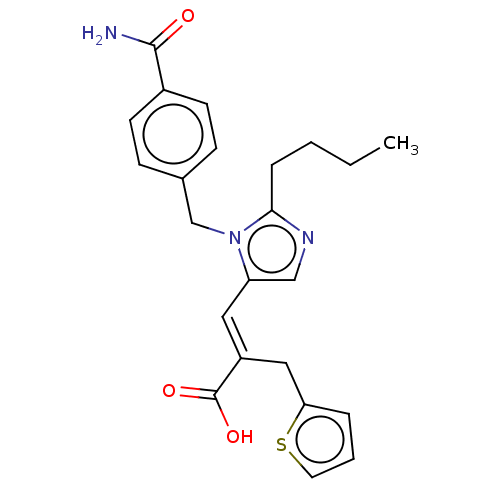
(CHEMBL56211)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(cc1)C(N)=O Show InChI InChI=1S/C23H25N3O3S/c1-2-3-6-21-25-14-19(12-18(23(28)29)13-20-5-4-11-30-20)26(21)15-16-7-9-17(10-8-16)22(24)27/h4-5,7-12,14H,2-3,6,13,15H2,1H3,(H2,24,27)(H,28,29)/b18-12+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230771
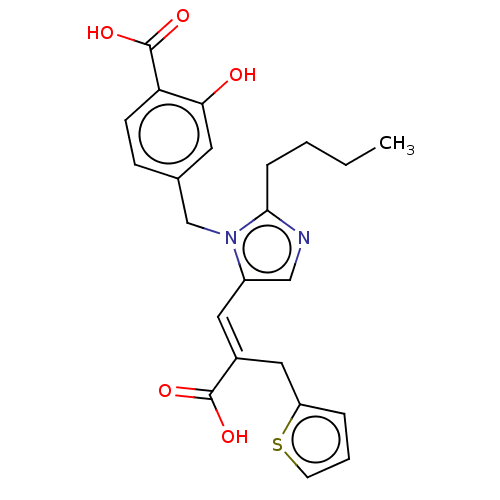
(CHEMBL55510)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(C(O)=O)c(O)c1 Show InChI InChI=1S/C23H24N2O5S/c1-2-3-6-21-24-13-17(11-16(22(27)28)12-18-5-4-9-31-18)25(21)14-15-7-8-19(23(29)30)20(26)10-15/h4-5,7-11,13,26H,2-3,6,12,14H2,1H3,(H,27,28)(H,29,30)/b16-11+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50427749

(CHEMBL2324924)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cncc4nccn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-3-4-22(15-21(20)6-8-25-17-33-18-27-34-9-10-38(25)27)28(39)35-24-7-5-23(26(16-24)29(30,31)32)19-37-13-11-36(2)12-14-37/h3-5,7,9-10,15-18H,11-14,19H2,1-2H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of wild type Abl1 kinase (unknown origin) |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50228407

(8-((R)-3-amino-piperidin-1-yl)-3-methyl-7-(3-methy...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12)-[#7]-1-[#6]-[#6]-[#6]-[#6@@H](-[#7])-[#6]-1 Show InChI InChI=1S/C24H30N6O3/c1-16(2)11-13-29-20-21(26-23(29)28-12-7-10-18(25)14-28)27(3)24(33)30(22(20)32)15-19(31)17-8-5-4-6-9-17/h4-6,8-9,11,18H,7,10,12-15,25H2,1-3H3/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of porcine DPP4 using H-Gly-Pro-AMC as substrate by fluorescence assay |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230806

(CHEMBL294415)Show SMILES CCCCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C25H28N2O4S/c1-2-3-4-5-8-23-26-16-21(14-20(25(30)31)15-22-7-6-13-32-22)27(23)17-18-9-11-19(12-10-18)24(28)29/h6-7,9-14,16H,2-5,8,15,17H2,1H3,(H,28,29)(H,30,31)/b20-14+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50228407

(8-((R)-3-amino-piperidin-1-yl)-3-methyl-7-(3-methy...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12)-[#7]-1-[#6]-[#6]-[#6]-[#6@@H](-[#7])-[#6]-1 Show InChI InChI=1S/C24H30N6O3/c1-16(2)11-13-29-20-21(26-23(29)28-12-7-10-18(25)14-28)27(3)24(33)30(22(20)32)15-19(31)17-8-5-4-6-9-17/h4-6,8-9,11,18H,7,10,12-15,25H2,1-3H3/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50427749

(CHEMBL2324924)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cncc4nccn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-3-4-22(15-21(20)6-8-25-17-33-18-27-34-9-10-38(25)27)28(39)35-24-7-5-23(26(16-24)29(30,31)32)19-37-13-11-36(2)12-14-37/h3-5,7,9-10,15-18H,11-14,19H2,1-2H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl1 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM50427749

(CHEMBL2324924)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cncc4nccn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-3-4-22(15-21(20)6-8-25-17-33-18-27-34-9-10-38(25)27)28(39)35-24-7-5-23(26(16-24)29(30,31)32)19-37-13-11-36(2)12-14-37/h3-5,7,9-10,15-18H,11-14,19H2,1-2H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl2 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50427748

(CHEMBL2324925)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4cnc5cnccn45)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C26H26F3N9O/c1-35-9-11-37(12-10-35)17-18-3-4-19(13-22(18)26(27,28)29)32-25(39)33-23-14-20(36(2)34-23)5-6-21-15-31-24-16-30-7-8-38(21)24/h3-4,7-8,13-16H,9-12,17H2,1-2H3,(H2,32,33,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of wild type Abl1 kinase (unknown origin) |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50377180
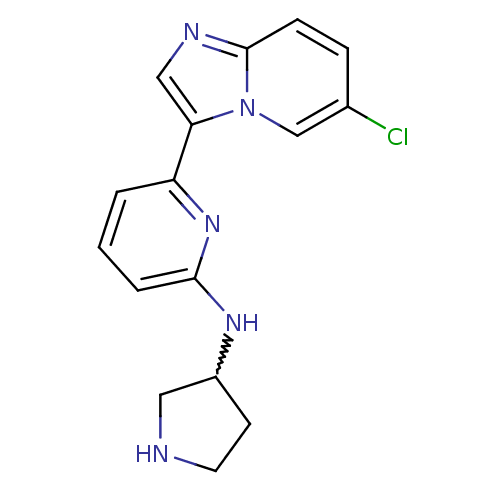
(CHEMBL401633)Show SMILES Clc1ccc2ncc(-c3cccc(NC4CCNC4)n3)n2c1 |w:14.13| Show InChI InChI=1S/C16H16ClN5/c17-11-4-5-16-19-9-14(22(16)10-11)13-2-1-3-15(21-13)20-12-6-7-18-8-12/h1-5,9-10,12,18H,6-8H2,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 |
Bioorg Med Chem Lett 18: 3656-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.042
BindingDB Entry DOI: 10.7270/Q2JH3N2N |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50048078

(4-[2-Butyl-5-((E)-2-carboxy-3-thiophen-2-yl-propen...)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(C(O)=O)c(Cl)c1 Show InChI InChI=1S/C23H23ClN2O4S/c1-2-3-6-21-25-13-17(11-16(22(27)28)12-18-5-4-9-31-18)26(21)14-15-7-8-19(23(29)30)20(24)10-15/h4-5,7-11,13H,2-3,6,12,14H2,1H3,(H,27,28)(H,29,30)/b16-11+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50427747
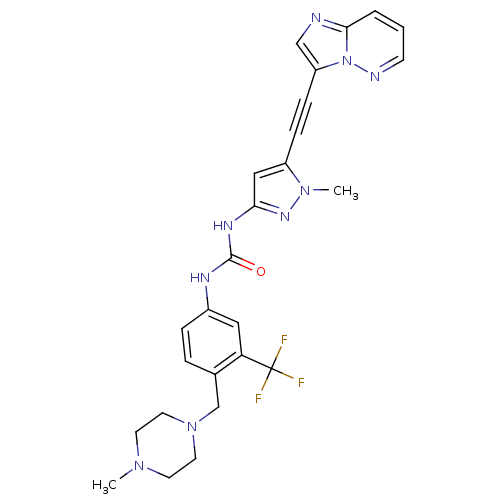
(CHEMBL2324926)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4cnc5cccnn45)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C26H26F3N9O/c1-35-10-12-37(13-11-35)17-18-5-6-19(14-22(18)26(27,28)29)32-25(39)33-23-15-20(36(2)34-23)7-8-21-16-30-24-4-3-9-31-38(21)24/h3-6,9,14-16H,10-13,17H2,1-2H3,(H2,32,33,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of wild type Abl1 kinase (unknown origin) |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50377170
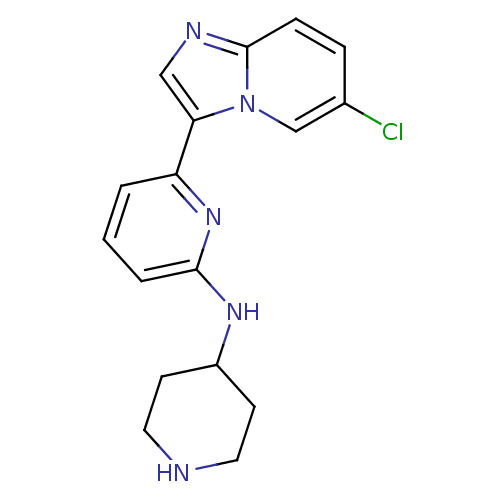
(CHEMBL256570 | US11254667, Compound I-2 | US115422...)Show InChI InChI=1S/C17H18ClN5/c18-12-4-5-17-20-10-15(23(17)11-12)14-2-1-3-16(22-14)21-13-6-8-19-9-7-13/h1-5,10-11,13,19H,6-9H2,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 |
Bioorg Med Chem Lett 18: 3656-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.042
BindingDB Entry DOI: 10.7270/Q2JH3N2N |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50437404
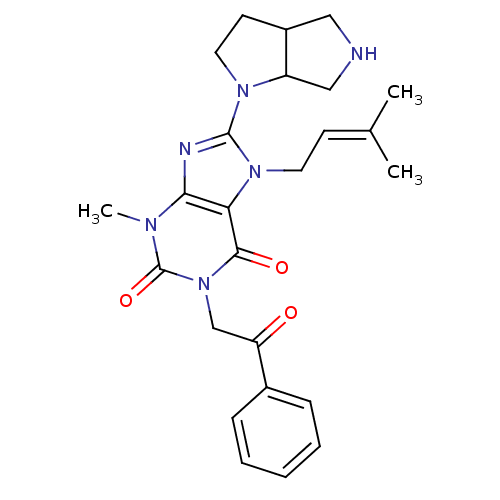
(CHEMBL2408655)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12)-[#7]-1-[#6]-[#6]-[#6]-2-[#6]-[#7]-[#6]-[#6]-1-2 Show InChI InChI=1S/C25H30N6O3/c1-16(2)9-11-30-21-22(27-24(30)29-12-10-18-13-26-14-19(18)29)28(3)25(34)31(23(21)33)15-20(32)17-7-5-4-6-8-17/h4-9,18-19,26H,10-15H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of porcine DPP4 |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50228407

(8-((R)-3-amino-piperidin-1-yl)-3-methyl-7-(3-methy...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12)-[#7]-1-[#6]-[#6]-[#6]-[#6@@H](-[#7])-[#6]-1 Show InChI InChI=1S/C24H30N6O3/c1-16(2)11-13-29-20-21(26-23(29)28-12-7-10-18(25)14-28)27(3)24(33)30(22(20)32)15-19(31)17-8-5-4-6-9-17/h4-6,8-9,11,18H,7,10,12-15,25H2,1-3H3/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 using H-Gly-Pro-AMC as substrate by fluorescence assay |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50011975

(4-[2-Butyl-5-((E)-2-carboxy-3-thiophen-2-yl-propen...)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(cc1Cl)C(O)=O Show InChI InChI=1S/C23H23ClN2O4S/c1-2-3-6-21-25-13-18(10-17(23(29)30)11-19-5-4-9-31-19)26(21)14-16-8-7-15(22(27)28)12-20(16)24/h4-5,7-10,12-13H,2-3,6,11,14H2,1H3,(H,27,28)(H,29,30)/b17-10+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50437404

(CHEMBL2408655)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12)-[#7]-1-[#6]-[#6]-[#6]-2-[#6]-[#7]-[#6]-[#6]-1-2 Show InChI InChI=1S/C25H30N6O3/c1-16(2)9-11-30-21-22(27-24(30)29-12-10-18-13-26-14-19(18)29)28(3)25(34)31(23(21)33)15-20(32)17-7-5-4-6-8-17/h4-9,18-19,26H,10-15H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of porcine DPP4 using H-Gly-Pro-AMC as substrate by fluorescence assay |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50437403

(CHEMBL2408771)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12)-[#7]-1-[#6]-[#6]-2-[#6]-[#6]-[#6]-[#7]-[#6]-2-[#6]-1 Show InChI InChI=1S/C26H32N6O3/c1-17(2)11-13-31-22-23(28-25(31)30-14-19-10-7-12-27-20(19)15-30)29(3)26(35)32(24(22)34)16-21(33)18-8-5-4-6-9-18/h4-6,8-9,11,19-20,27H,7,10,12-16H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 using H-Gly-Pro-AMC as substrate by fluorescence assay |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50427750
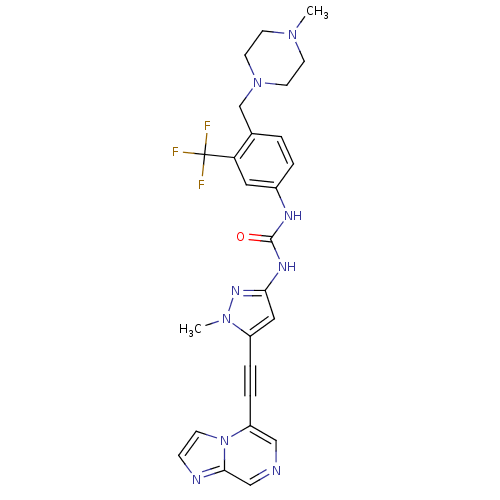
(CHEMBL2324923)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4cncc5nccn45)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C26H26F3N9O/c1-35-9-11-37(12-10-35)17-18-3-4-19(13-22(18)26(27,28)29)32-25(39)33-23-14-20(36(2)34-23)5-6-21-15-30-16-24-31-7-8-38(21)24/h3-4,7-8,13-16H,9-12,17H2,1-2H3,(H2,32,33,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of wild type Abl1 kinase (unknown origin) |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50427750

(CHEMBL2324923)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4cncc5nccn45)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C26H26F3N9O/c1-35-9-11-37(12-10-35)17-18-3-4-19(13-22(18)26(27,28)29)32-25(39)33-23-14-20(36(2)34-23)5-6-21-15-30-16-24-31-7-8-38(21)24/h3-4,7-8,13-16H,9-12,17H2,1-2H3,(H2,32,33,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl1 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50377165
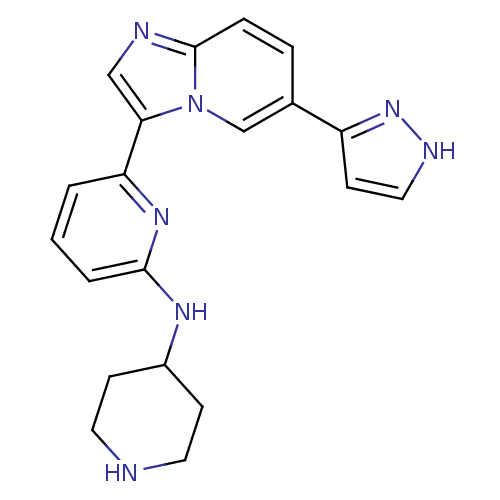
(CHEMBL255867)Show SMILES C1CC(CCN1)Nc1cccc(n1)-c1cnc2ccc(cn12)-c1cc[nH]n1 Show InChI InChI=1S/C20H21N7/c1-2-17(25-19(3-1)24-15-6-9-21-10-7-15)18-12-22-20-5-4-14(13-27(18)20)16-8-11-23-26-16/h1-5,8,11-13,15,21H,6-7,9-10H2,(H,23,26)(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 |
Bioorg Med Chem Lett 18: 3656-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.042
BindingDB Entry DOI: 10.7270/Q2JH3N2N |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM50427749

(CHEMBL2324924)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cncc4nccn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-3-4-22(15-21(20)6-8-25-17-33-18-27-34-9-10-38(25)27)28(39)35-24-7-5-23(26(16-24)29(30,31)32)19-37-13-11-36(2)12-14-37/h3-5,7,9-10,15-18H,11-14,19H2,1-2H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl2 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230787

(CHEMBL54421)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(C(O)=O)c(c1)-c1ccccc1 Show InChI InChI=1S/C29H28N2O4S/c1-2-3-11-27-30-18-23(16-22(28(32)33)17-24-10-7-14-36-24)31(27)19-20-12-13-25(29(34)35)26(15-20)21-8-5-4-6-9-21/h4-10,12-16,18H,2-3,11,17,19H2,1H3,(H,32,33)(H,34,35)/b22-16+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50271563
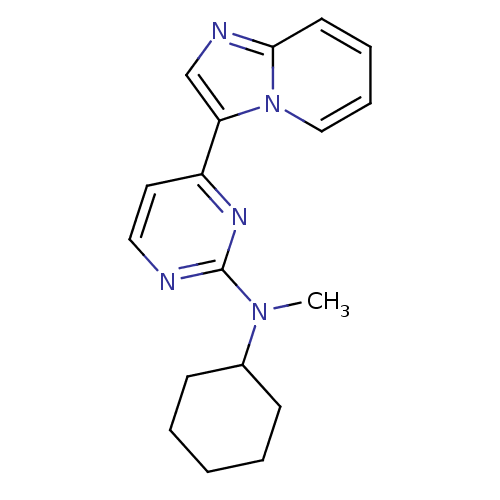
(CHEMBL482708 | N-cyclohexyl-4-(H-imidazo[1,2-a]pyr...)Show InChI InChI=1S/C18H21N5/c1-22(14-7-3-2-4-8-14)18-19-11-10-15(21-18)16-13-20-17-9-5-6-12-23(16)17/h5-6,9-14H,2-4,7-8H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of JNK3 (unknown origin) |
Bioorg Med Chem Lett 18: 3291-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.039
BindingDB Entry DOI: 10.7270/Q2NZ87FX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50377175
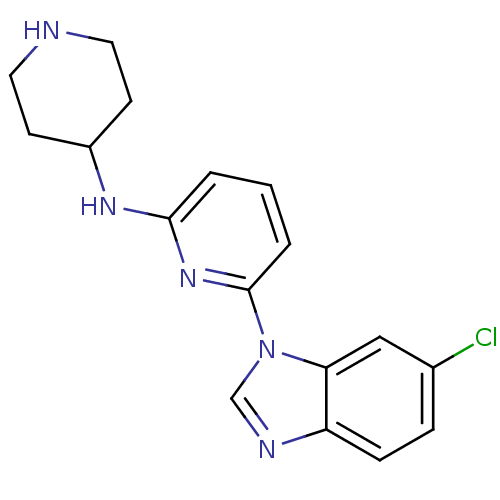
(CHEMBL436653)Show InChI InChI=1S/C17H18ClN5/c18-12-4-5-14-15(10-12)23(11-20-14)17-3-1-2-16(22-17)21-13-6-8-19-9-7-13/h1-5,10-11,13,19H,6-9H2,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 |
Bioorg Med Chem Lett 18: 3656-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.042
BindingDB Entry DOI: 10.7270/Q2JH3N2N |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM50427748

(CHEMBL2324925)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4cnc5cnccn45)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C26H26F3N9O/c1-35-9-11-37(12-10-35)17-18-3-4-19(13-22(18)26(27,28)29)32-25(39)33-23-14-20(36(2)34-23)5-6-21-15-31-24-16-30-7-8-38(21)24/h3-4,7-8,13-16H,9-12,17H2,1-2H3,(H2,32,33,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl2 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230799
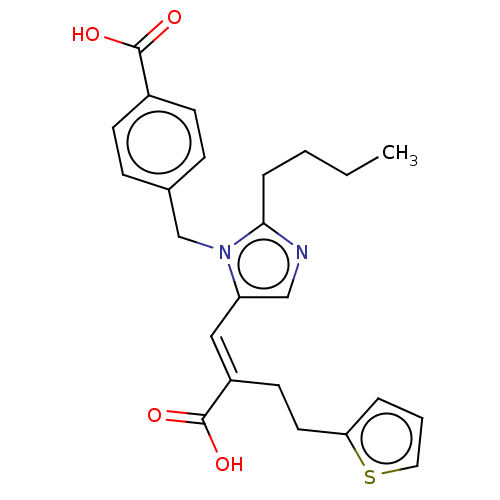
(CHEMBL56333)Show SMILES CCCCc1ncc(\C=C(/CCc2cccs2)C(O)=O)n1Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C24H26N2O4S/c1-2-3-6-22-25-15-20(14-19(24(29)30)11-12-21-5-4-13-31-21)26(22)16-17-7-9-18(10-8-17)23(27)28/h4-5,7-10,13-15H,2-3,6,11-12,16H2,1H3,(H,27,28)(H,29,30)/b19-14+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230792
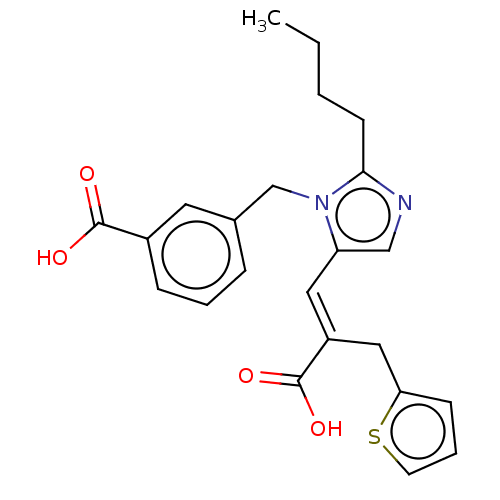
(CHEMBL56497)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1cccc(c1)C(O)=O Show InChI InChI=1S/C23H24N2O4S/c1-2-3-9-21-24-14-19(12-18(23(28)29)13-20-8-5-10-30-20)25(21)15-16-6-4-7-17(11-16)22(26)27/h4-8,10-12,14H,2-3,9,13,15H2,1H3,(H,26,27)(H,28,29)/b18-12+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50377168

(CHEMBL255873)Show InChI InChI=1S/C18H18N6/c19-10-13-4-5-18-21-11-16(24(18)12-13)15-2-1-3-17(23-15)22-14-6-8-20-9-7-14/h1-5,11-12,14,20H,6-9H2,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 |
Bioorg Med Chem Lett 18: 3656-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.042
BindingDB Entry DOI: 10.7270/Q2JH3N2N |
More data for this
Ligand-Target Pair | |
Beta-1/Beta-2/Beta-3 adrenergic receptor
(Homo sapiens (Human)) | BDBM25761
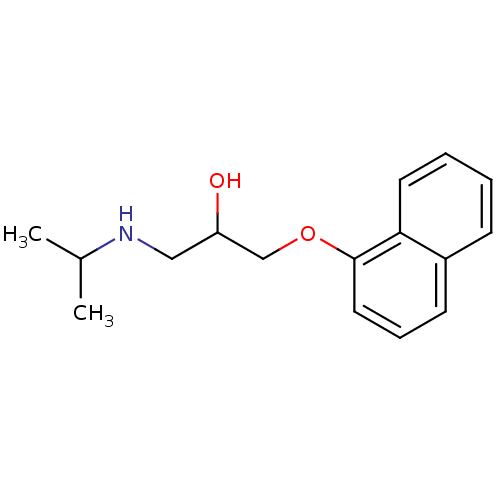
(Anapriline | Avlocardyl | CHEMBL27 | PROPANOLOL(-)...)Show InChI InChI=1S/C16H21NO2/c1-12(2)17-10-14(18)11-19-16-9-5-7-13-6-3-4-8-15(13)16/h3-9,12,14,17-18H,10-11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration effective against displacing [3H]dihydroalprenolol from beta adrenergic receptor from canine ventricular tissue |
J Med Chem 30: 696-704 (1987)
BindingDB Entry DOI: 10.7270/Q2V1271T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230774
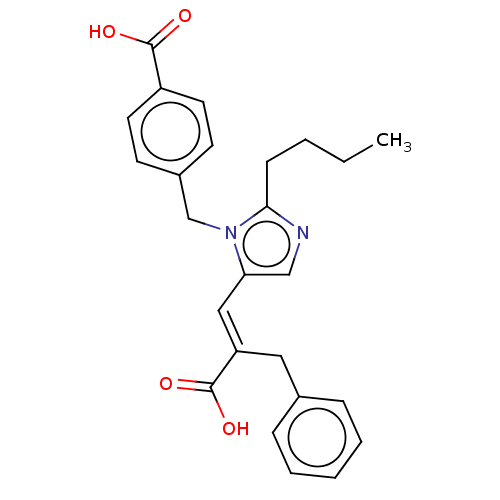
(CHEMBL291955)Show SMILES CCCCc1ncc(\C=C(/Cc2ccccc2)C(O)=O)n1Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C25H26N2O4/c1-2-3-9-23-26-16-22(15-21(25(30)31)14-18-7-5-4-6-8-18)27(23)17-19-10-12-20(13-11-19)24(28)29/h4-8,10-13,15-16H,2-3,9,14,17H2,1H3,(H,28,29)(H,30,31)/b21-15+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230762

(CHEMBL56160)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C22H25N3O4S2/c1-2-3-6-21-24-14-18(12-17(22(26)27)13-19-5-4-11-30-19)25(21)15-16-7-9-20(10-8-16)31(23,28)29/h4-5,7-12,14H,2-3,6,13,15H2,1H3,(H,26,27)(H2,23,28,29)/b17-12+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230810
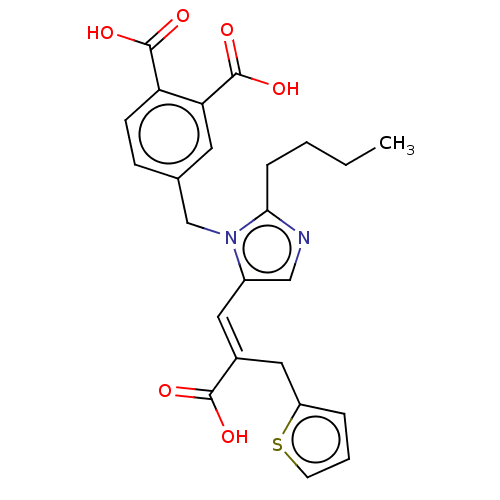
(CHEMBL299064)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(C(O)=O)c(c1)C(O)=O Show InChI InChI=1S/C24H24N2O6S/c1-2-3-6-21-25-13-17(11-16(22(27)28)12-18-5-4-9-33-18)26(21)14-15-7-8-19(23(29)30)20(10-15)24(31)32/h4-5,7-11,13H,2-3,6,12,14H2,1H3,(H,27,28)(H,29,30)(H,31,32)/b16-11+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230764
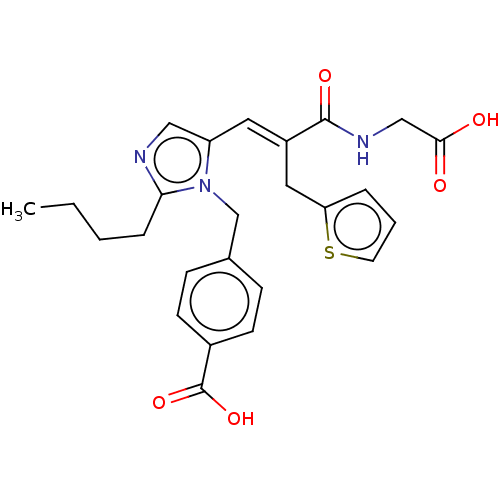
(CHEMBL299954)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(=O)NCC(O)=O)n1Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C25H27N3O5S/c1-2-3-6-22-26-14-20(28(22)16-17-7-9-18(10-8-17)25(32)33)12-19(13-21-5-4-11-34-21)24(31)27-15-23(29)30/h4-5,7-12,14H,2-3,6,13,15-16H2,1H3,(H,27,31)(H,29,30)(H,32,33)/b19-12+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230767
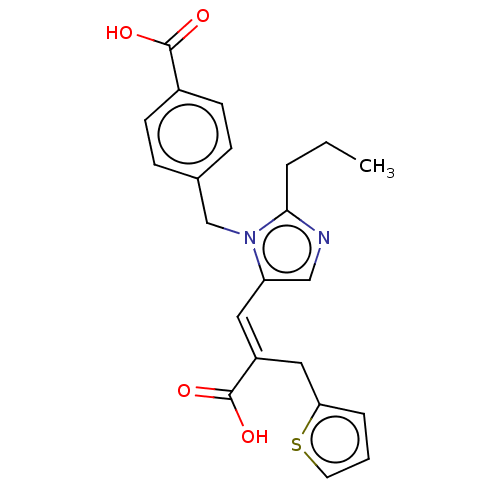
(CHEMBL56522)Show SMILES CCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C22H22N2O4S/c1-2-4-20-23-13-18(11-17(22(27)28)12-19-5-3-10-29-19)24(20)14-15-6-8-16(9-7-15)21(25)26/h3,5-11,13H,2,4,12,14H2,1H3,(H,25,26)(H,27,28)/b17-11+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50377163

(CHEMBL403358)Show InChI InChI=1S/C18H20N6O/c19-18(25)12-4-5-17-21-10-15(24(17)11-12)14-2-1-3-16(23-14)22-13-6-8-20-9-7-13/h1-5,10-11,13,20H,6-9H2,(H2,19,25)(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 |
Bioorg Med Chem Lett 18: 3656-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.042
BindingDB Entry DOI: 10.7270/Q2JH3N2N |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM50427747

(CHEMBL2324926)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4cnc5cccnn45)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C26H26F3N9O/c1-35-10-12-37(13-11-35)17-18-5-6-19(14-22(18)26(27,28)29)32-25(39)33-23-15-20(36(2)34-23)7-8-21-16-30-24-4-3-9-31-38(21)24/h3-6,9,14-16H,10-13,17H2,1-2H3,(H2,32,33,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl2 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50377169

(CHEMBL402629)Show InChI InChI=1S/C18H21N5O/c1-24-14-5-6-18-20-11-16(23(18)12-14)15-3-2-4-17(22-15)21-13-7-9-19-10-8-13/h2-6,11-13,19H,7-10H2,1H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 |
Bioorg Med Chem Lett 18: 3656-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.042
BindingDB Entry DOI: 10.7270/Q2JH3N2N |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50377181
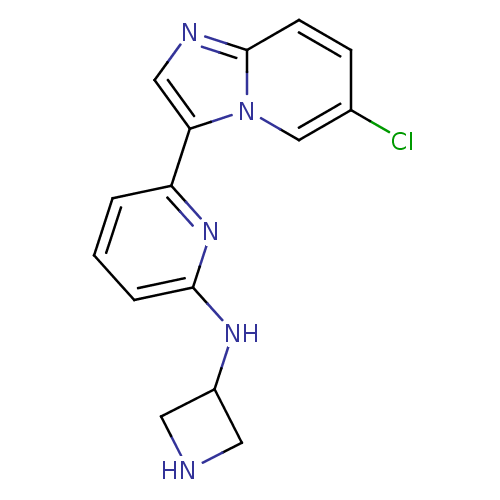
(CHEMBL256963 | US11254667, Compound I-15 | US11542...)Show InChI InChI=1S/C15H14ClN5/c16-10-4-5-15-18-8-13(21(15)9-10)12-2-1-3-14(20-12)19-11-6-17-7-11/h1-5,8-9,11,17H,6-7H2,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 |
Bioorg Med Chem Lett 18: 3656-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.042
BindingDB Entry DOI: 10.7270/Q2JH3N2N |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50437395
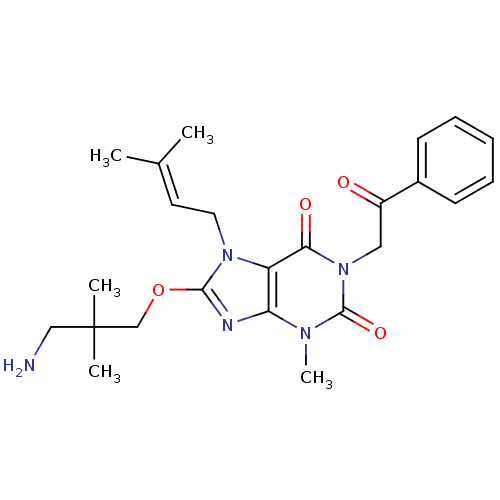
(CHEMBL2408638)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#8]-[#6]C([#6])([#6])[#6]-[#7])nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12 Show InChI InChI=1S/C24H31N5O4/c1-16(2)11-12-28-19-20(26-22(28)33-15-24(3,4)14-25)27(5)23(32)29(21(19)31)13-18(30)17-9-7-6-8-10-17/h6-11H,12-15,25H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of porcine DPP4 using H-Gly-Pro-AMC as substrate by fluorescence assay |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230796

(CHEMBL56690)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(cc1)-c1ccccc1C(O)=O Show InChI InChI=1S/C29H28N2O4S/c1-2-3-10-27-30-18-23(16-22(28(32)33)17-24-7-6-15-36-24)31(27)19-20-11-13-21(14-12-20)25-8-4-5-9-26(25)29(34)35/h4-9,11-16,18H,2-3,10,17,19H2,1H3,(H,32,33)(H,34,35)/b22-16+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50377186

(CHEMBL403433)Show InChI InChI=1S/C16H17N5O/c1-22-12-5-6-16-18-9-14(21(16)10-12)13-3-2-4-15(20-13)19-11-7-17-8-11/h2-6,9-11,17H,7-8H2,1H3,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 |
Bioorg Med Chem Lett 18: 3656-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.042
BindingDB Entry DOI: 10.7270/Q2JH3N2N |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50377166
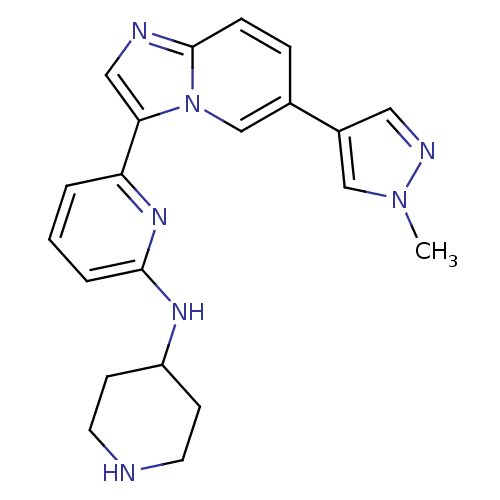
(CHEMBL255446)Show SMILES Cn1cc(cn1)-c1ccc2ncc(-c3cccc(NC4CCNCC4)n3)n2c1 Show InChI InChI=1S/C21H23N7/c1-27-13-16(11-24-27)15-5-6-21-23-12-19(28(21)14-15)18-3-2-4-20(26-18)25-17-7-9-22-10-8-17/h2-6,11-14,17,22H,7-10H2,1H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 |
Bioorg Med Chem Lett 18: 3656-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.042
BindingDB Entry DOI: 10.7270/Q2JH3N2N |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data